Gaps in Monitoring Leave Northern Australian Mammal Fauna with Uncertain Futures
Abstract
:1. Introduction
2. Mammal Decline in Northern Australia
2.1. Background
2.2. Recent Changes
2.3. Taxonomic Revisions and Imminent Extinctions
2.4. Northern Quoll as an Important Conservation Case Study
3. Causes: Cattle, Cats, Climate Change, Cane Toads, Diseases, and Fire?
3.1. Resurgence of Fire Application
3.2. Unsupported Assumptions Related to Fire Management
3.3. Introduced Animals
3.4. Diseases
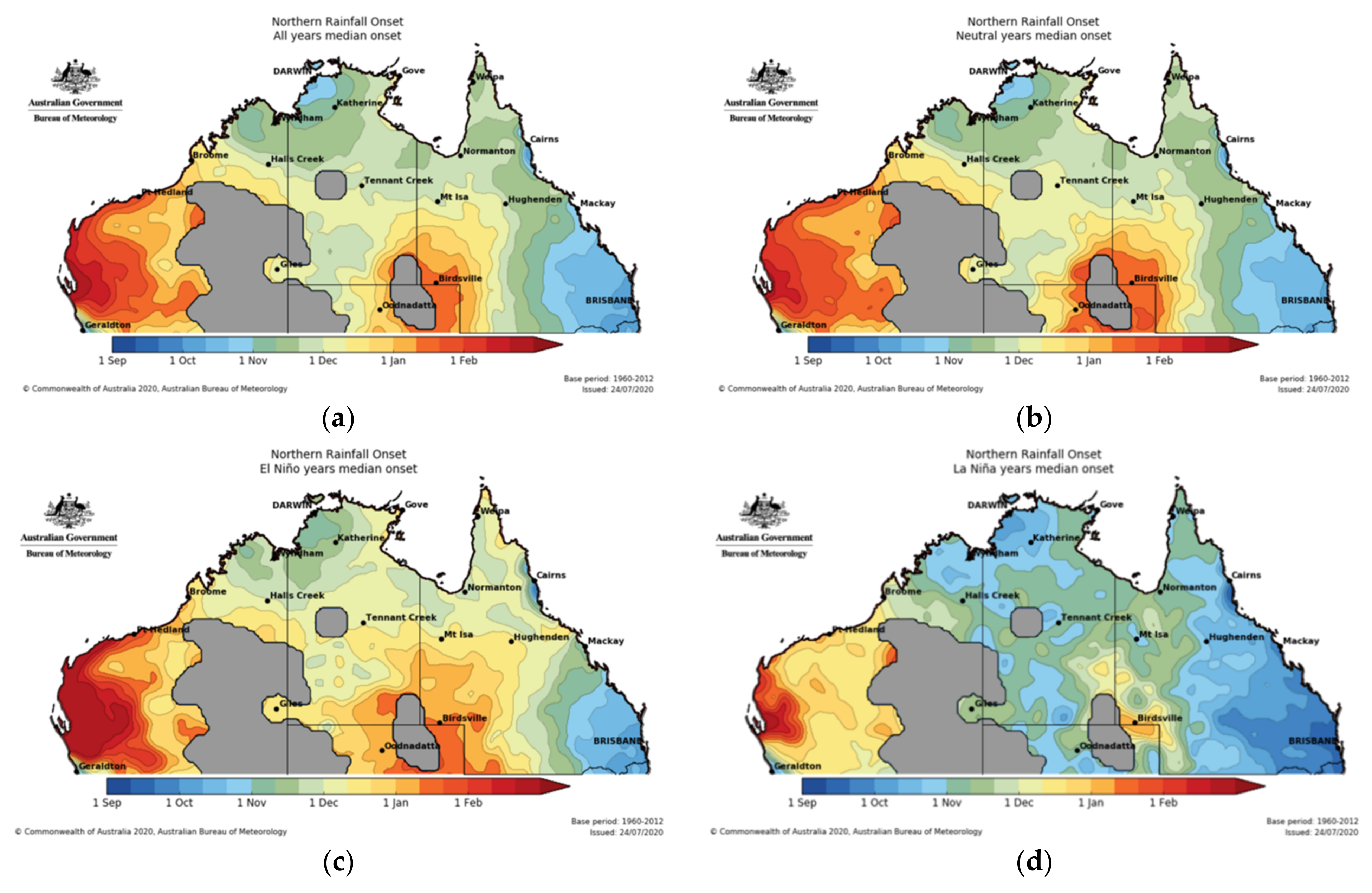
3.5. Climate Change and Habitat Loss
4. Filling the Monitoring Gaps
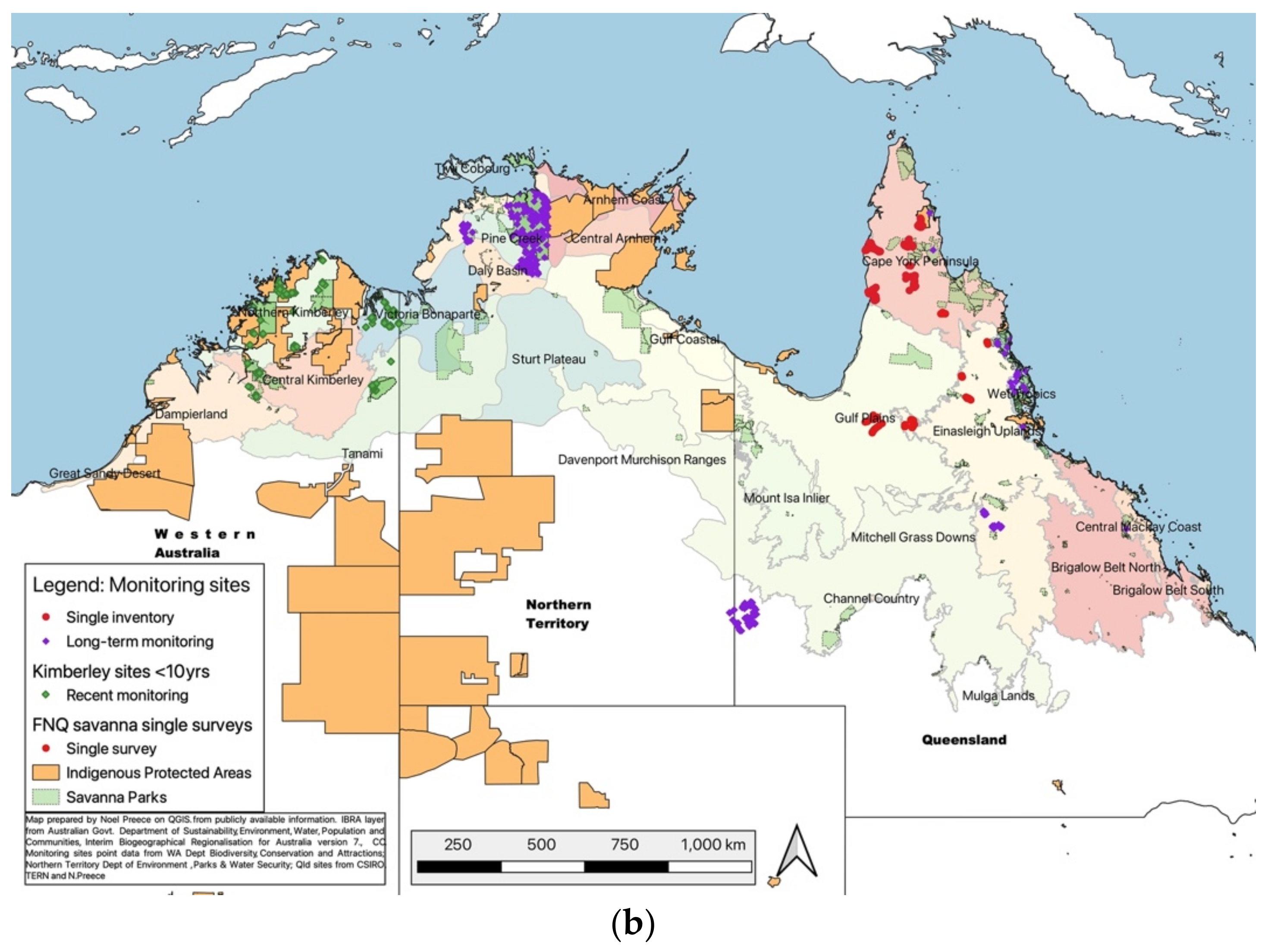
4.1. Broadening the Research and Monitoring Network
- Very few systematic studies of fauna exist that establish baselines and monitor trends in abundance and distribution over time;
- Most studies are short-term, poorly designed, or incompatible, survey limited areas of habitat (poor coverage), are poorly coordinated, do not make data available adequately, do not report adequately, do not link well with management, and do not adequately examine demographics [242];
- Studies have concentrated in only a few areas and on a few species, leaving very large areas and most species unstudied.
4.2. Adjusting Monitoring Methods
- High confidence in the results of monitoring (statistical power of 0.8) could be achieved for moderate to large declines in only the most common and easily detected species;
- It was relatively poor for species with moderate occupancy and detectability, unless simulated declines were very large;
- For species with very low occupancy and detectability, no monitoring was able to detect even severe declines [13].
4.3. Scientific Permit Requirements for Live Trapping or Interference with Fauna
5. Monitoring and Regional Employment
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ceballos, G.; Ehrlich, P.R.; Raven, P.H. Vertebrates on the brink as indicators of biological annihilation and the sixth mass extinction. Proc. Natl. Acad. Sci. USA 2020, 117, 13596–13602. [Google Scholar] [CrossRef]
- Bergstrom, D.M.; Wienecke, B.C.; van den Hoff, J.; Hughes, L.; Lindenmayer, D.B.; Ainsworth, T.D.; Baker, C.M.; Bland, L.; Bowman, D.; Brooks, S.T.; et al. Combating ecosystem collapse from the tropics to the Antarctic. Glob. Chang. Biol. 2021, 27, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.J.A.; Ehrlich, P.R.; Beattie, A.; Ceballos, G.; Crist, E.; Diamond, J.; Dirzo, R.; Ehrlich, A.H.; Harte, J.; Harte, M.E.; et al. Underestimating the challenges of avoiding a ghastly future. Front. Conserv. Sci. 2021, 1, 615419. [Google Scholar] [CrossRef]
- IPBES. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar]
- Xu, H.; Cao, Y.; Yu, D.; Cao, M.; He, Y.; Gill, M.; Pereira, H.M. Ensuring effective implementation of the post-2020 global biodiversity targets. Nat. Ecol. Evol. 2021, 5, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Rounsevell, M.D.A.; Harfoot, M.; Harrison, P.A.; Newbold, T.; Gregory, R.D.; Mace, G.M. A biodiversity target based on species extinctions. Science 2020, 368, 1193–1195. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Braby, M.F.; Burbidge, A.A.; Coates, D.; Garnett, S.T.; Fensham, R.J.; Legge, S.M.; McKenzie, N.L.; Silcock, J.L.; Murphy, B.P. Reading the black book: The number, timing, distribution and causes of listed extinctions in Australia. Biol. Conserv. 2019, 239, 108261. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z. The illusion of nature: Perception and the reality of natural landscapes, as illustrated by vertebrate fauna in the Northern Territory, Australia. Ecol. Manag. Restor. 2014, 15, 30–33. [Google Scholar] [CrossRef]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M.; et al. Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 2016, 7, 12558. [Google Scholar] [CrossRef] [Green Version]
- Plumptre, A.J.; Baisero, D.; Belote, R.T.; Vázquez-Domínguez, E.; Faurby, S.; Jȩdrzejewski, W.; Kiara, H.; Kühl, H.; Benítez-López, A.; Luna-Aranguré, C.; et al. Where might we find ecologically intact communities? Front. For. Glob. Chang. 2021, 4, 626635. [Google Scholar] [CrossRef]
- Legg, C.J.; Nagy, L. Why most conservation monitoring is, but need not be, a waste of time. J. Environ. Manag. 2006, 78, 194–199. [Google Scholar] [CrossRef]
- Legge, S.; Lindenmayer, D.; Robinson, N.; Scheele, B.; Southwell, D.; Wintle, B. Monitoring Threatened Species and Ecological Communities; CSIRO Publishing: Melbourne, Australia, 2018. [Google Scholar]
- Einoder, L.D.; Southwell, D.M.; Gillespie, G.R.; Fisher, A.; Lahoz-Monfort, J.J.; Wintle, B.A. Optimising broad-scale monitoring for trend detection: Review and re-design of a long-term program in northern Australia. In Monitoring Threatened Species and Ecological Communities; Legge, S., Lindenmayer, D., Robinson, N., Scheele, B., Southwell, D., Wintle, B., Eds.; CSIRO Publishing: Melbourne, Australia, 2018; pp. 165–178. [Google Scholar]
- Edwards, A.; Archer, R.; De Bruyn, P.; Evans, J.; Lewis, B.; Vigilante, T.; Whyte, S.; Russell-Smith, J. Transforming fire management in northern Australia through successful implementation of savanna burning emissions reductions projects. J. Environ. Manag. 2021, 290, 112568. [Google Scholar] [CrossRef] [PubMed]
- TERN. TERN Ecosystem Surveillance. Available online: https://www.tern.org.au/tern-observatory/tern-ecosystem-surveillance/ (accessed on 2 January 2022).
- Wintle, B.A.; Cadenhead, N.C.R.; Morgain, R.A.; Legge, S.M.; Bekessy, S.A.; Cantele, M.; Possingham, H.P.; Watson, J.E.M.; Maron, M.; Keith, D.A.; et al. Spending to save: What will it cost to halt Australia’s extinction crisis? Conserv. Lett. 2019, 12, e12682. [Google Scholar] [CrossRef]
- Australian Conservation Foundation. Federal Government Spending on Australia’s Environment and Climate; Australian Conservation Foundation: Melbourne, Australia, 2021. [Google Scholar]
- Willacy, M.; Blucher, A.; Queensland’s Former LNP Government Used ‘Pain Ranking’ to Help Decide Environment Budget Cuts. ABC News 14 February 2017. Available online: https://www.abc.net.au/news/2017-02-14/queensland-lnp-government-used-pain-ranking-for-budget-cuts/8266762 (accessed on 22 January 2022).
- Woinarski, J.C.Z.; Burbidge, A.A.; Harrison, P.L. The extent and adequacy of monitoring for Australian threatened mammal species. In Monitoring Threatened Species and Ecological Communities; Legge, S., Lindenmayer, D., Robinson, N., Scheele, B., Southwell, D., Wintle, B., Eds.; CSIRO Publishing: Melbourne, Australia, 2018; pp. 21–41. [Google Scholar]
- McKenzie, N.L.; Burbidge, A.A.; Baynes, A.; Brereton, R.N.; Dickman, C.R.; Gordon, G.; Gibson, L.A.; Menkhorst, P.W.; Robinson, A.C.; Williams, M.R.; et al. Analysis of factors implicated in the recent decline of Australia’s mammal fauna. J. Biogeogr. 2007, 34, 597–611. [Google Scholar] [CrossRef]
- Fitzsimons, J.; Legge, S.; Traill, B.; Woinarski, J. Into Oblivion? The Disappearing Native Mammals of Northern Australia; The Nature Conservancy: Melbourne, Australia, 2010. [Google Scholar]
- Woinarski, J.C.Z.; Legge, S.; Fitzsimons, J.A.; Traill, B.J.; Burbidge, A.A.; Fisher, A.; Firth, R.S.C.; Gordon, I.J.; Griffiths, A.D.; Johnson, C.N.; et al. The disappearing mammal fauna of northern Australia: Context, cause and response. Conserv. Lett. 2011, 4, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Ziembicki, M.R.; Woinarski, J.C.Z.; Webb, J.K.; Vanderduys, E.; Tuft, K.; Smith, J.; Ritchie, E.G.; Reardon, T.B.; Radford, I.J.; Preece, N.D.; et al. Stemming the tide: Progress towards resolving the causes of decline and implementing management responses for the disappearing mammal fauna of northern Australia. Therya 2015, 6, 169–225. [Google Scholar] [CrossRef] [Green Version]
- Corey, B.; Andersen, A.N.; Legge, S.; Woinarski, J.C.Z.; Radford, I.J.; Perry, J.J. Better biodiversity accounting is needed to prevent bioperversity and maximize co-benefits from savanna burning. Conserv. Lett. 2019, 13, e12685. [Google Scholar] [CrossRef]
- Johnson, C.N.; Isaac, J.L.; Fisher, D.O. Rarity of a top predator triggers continent-wide collapse of mammal prey: Dingoes and marsupials in Australia. Proc. R. Soc. B Biol. Sci. 2007, 274, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Woinarski, J.C.Z.; Milne, D.J.; Wanganeen, G. Changes in mammal populations in relatively intact landscapes in Kakadu National Park, Northern Territory, Australia. Austral Ecol. 2001, 26, 360–370. [Google Scholar] [CrossRef]
- Price, O.; Rankmore, B.; Milne, D.; Brock, C.; Tynan, C.; Kean, L.; Roeger, L. Regional patterns of mammal abundance and their relationship to landscape variables in eucalypt woodlands near Darwin, northern Australia. Wildl. Res. 2005, 32, 435–446. [Google Scholar] [CrossRef]
- Start, A.N.; Burbidge, A.A.; McDowell, M.C.; McKenzie, N.L. The status of non-volant mammals along a rainfall gradient in the south-west Kimberley, Western Australia. Austral. Mammal. 2012, 34, 36–48. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Armstrong, M.; Brennan, K.; Fisher, A.; Griffiths, A.D.; Hill, B.; Milne, D.J.; Palmer, C.; Ward, S.; Watson, M.; et al. Monitoring indicates rapid and severe decline of native small mammals in Kakadu National Park, northern Australia. Wildl. Res. 2010, 37, 116–126. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Edwards, A.C.; Woinarski, J.C.; McCartney, J.; Kerin, S.; Winderlich, S.; Murphy, B.P.; Watt, F.A. Fire and biodiversity monitoring for conservation managers: A 10-year assessment of the ‘Three Parks’ (Kakadu, Litchfield and Nitmiluk) program. In Culture, Ecology and Economy of Savanna Fire Management in Northern Australia: Rekindling the Wurrk Tradition; Russell-Smith, J., Whitehead, P.J., Cooke, P., Eds.; CSIRO Publishing: Melbourne, Australia, 2009; pp. 257–285. [Google Scholar]
- Einoder, L.D.; Gillespie, G.R.; Buckley, K.A. Terrestrial Fauna Monitoring in Kakadu National Park: Final Report; Northern Territory Department of Environment, Parks and Water Security: Darwin, Australia, 2021. Available online: https://www.nespnorthern.edu.au/wp-content/uploads/2021/11/Terrestrial-fauna-monitoring-in-Kakadu-National-Park-final-report.pdf (accessed on 22 January 2022).
- Legge, S.; Murphy, S.; Heathcote, J.; Flaxman, E.; Augusteyn, J.; Crossman, M. The short-term effects of an extensive and high-intensity fire on vertebrates in the tropical savannas of the central Kimberley, northern Australia. Wildl. Res. 2008, 35, 33–43. [Google Scholar] [CrossRef]
- Legge, S.; Kennedy, M.S.; Lloyd, R.A.Y.; Murphy, S.A.; Fisher, A. Rapid recovery of mammal fauna in the central Kimberley, northern Australia, following the removal of introduced herbivores. Austral Ecol. 2011, 36, 791–799. [Google Scholar] [CrossRef]
- Leahy, L.; Legge, S.M.; Tuft, K.; McGregor, H.W.; Barmuta, L.A.; Jones, M.E.; Johnson, C.N. Amplified predation after fire suppresses rodent populations in Australia’s tropical savannas. Wildl. Res. 2016, 42, 705–716. [Google Scholar] [CrossRef]
- Tuft, K.; Legge, S.; Frank, A.S.K.; James, A.I.; May, T.; Page, E.; Radford, I.J.; Woinarski, J.C.Z.; Fisher, A.; Lawes, M.J.; et al. Cats are a key threatening factor to the survival of local populations of native small mammals in Australia’s tropical savannas: Evidence from translocation trials with Rattus tunneyi. Wildl. Res. 2021, 48, 654–662. [Google Scholar] [CrossRef]
- Ibbett, M.; Woinarski, J.C.Z.; Oakwood, M. Declines in the mammal assemblage of a rugged sandstone environment in Kakadu National Park, Northern Territory, Australia. Austral. Mammal. 2018, 40, 181–187. [Google Scholar] [CrossRef]
- Winter, J.W.; Allison, F.R. The native mammals of Cape York Peninsula: Changes in status since the 1948 Archbold Expedition. In Contemporary Cape York; Stevens, N.C., Bailey, A., Eds.; Royal Society of Queensland: Brisbane, Australia, 1980; pp. 31–47. [Google Scholar]
- Dickman, C.R.; Leung, L.K.-P.; Van Dyck, S.M. Status, ecological attributes and conservation of native rodents in Queensland. Wildl. Res. 2000, 27, 333–346. [Google Scholar] [CrossRef]
- Perry, J.J.; Vanderduys, E.P.; Kutt, A.S. More famine than feast: Pattern and variation in a potentially degenerating mammal fauna on Cape York Peninsula. Wildl. Res. 2015, 42, 475–487. [Google Scholar] [CrossRef]
- Kutt, A.S.; Vanderduys, E.P.; Perry, J.J.; Perkins, G.C.; Kemp, J.E.; Bateman, B.L.; Kanowski, J.; Jensen, R. Signals of change in tropical savanna woodland vertebrate fauna 5 years after cessation of livestock grazing. Wildl. Res. 2012, 39, 386–396. [Google Scholar] [CrossRef]
- Braithwaite, R.W.; Brady, P. The delicate mouse, Pseudomys delicatulus: A continuous breeder waiting for the good times. Austral. Mammal. 1993, 16, 94–98. [Google Scholar] [CrossRef]
- Burbidge, A.A.; McKenzie, N.L. Patterns in the modern decline of Western Australia’s vertebrate fauna: Causes and conservation implications. Biol. Conserv. 1989, 50, 143–198. [Google Scholar] [CrossRef]
- Fisher, D.O.; Johnson, C.N.; Lawes, M.J.; Fritz, S.A.; McCallum, H.; Blomberg, S.P.; VanDerWal, J.; Abbott, B.; Frank, A.; Legge, S.; et al. The current decline of tropical marsupials in Australia: Is history repeating? Glob. Ecol. Biogeogr. 2014, 23, 181–190. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z. Critical-weight-range marsupials in northern Australia are declining: A commentary on Fisher et al. (2014) ‘The current decline of tropical marsupials in Australia: Is history repeating?’. Glob. Ecol. Biogeogr. 2015, 24, 118–122. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Legge, S. The impacts of fire on birds in Australia’s tropical savannas. Emu-Austral Ornithol. 2013, 113, 319–352. [Google Scholar] [CrossRef] [Green Version]
- Woinarski, J.C.Z.; Fisher, A.; Armstrong, M.; Brennan, K.; Griffiths, A.D.; Hill, B.; Choy, J.L.; Milne, D.; Stewart, A.; Young, S.; et al. Monitoring indicates greater resilience for birds than for mammals in Kakadu National Park, Northern Australia. Wildl. Res. 2012, 39, 397–407. [Google Scholar] [CrossRef]
- Geyle, H.M.; Woinarski, J.C.Z.; Baker, G.B.; Dickman, C.R.; Dutson, G.; Fisher, D.O.; Ford, H.; Holdsworth, M.; Jones, M.E.; Kutt, A.; et al. Quantifying extinction risk and forecasting the number of impending Australian bird and mammal extinctions. Pac. Conserv. Biol. 2018, 24, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Reside, A.E.; Cosgrove, A.J.; Pointon, R.; Trezise, J.; Watson, J.E.M.; Maron, M. How to send a finch extinct. Environ. Sci. Pol. 2019, 94, 163–173. [Google Scholar] [CrossRef]
- Geyle, H.M.; Tingley, R.; Amey, A.P.; Cogger, H.; Couper, P.J.; Cowan, M.; Craig, M.D.; Doughty, P.; Driscoll, D.A.; Ellis, R.J.; et al. Reptiles on the brink: Identifying the Australian terrestrial snake and lizard species most at risk of extinction. Pac. Conserv. Biol. 2021, 27, 3–12. [Google Scholar] [CrossRef]
- Chapple, D.G.; Roll, U.; Böhm, M.; Aguilar, R.; Amey, A.P.; Austin, C.C.; Baling, M.; Barley, A.J.; Bates, M.F.; Bauer, A.M.; et al. Conservation status of the world’s skinks (Scincidae): Taxonomic and geographic patterns in extinction risk. Biol. Conserv. 2021, 257, 109101. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Burbidge, A.A.; Harrison, P.L. The Action Plan for Australian Mammals 2012; CSIRO Publishing: Melbourne, Australia, 2014. [Google Scholar]
- Westcott, D.A.; Caley, P.; Heersink, D.K.; McKeown, A. A state-space modelling approach to wildlife monitoring with application to flying-fox abundance. Sci. Rep. 2018, 8, 4038. [Google Scholar] [CrossRef] [Green Version]
- Davies, H.F.; McCarthy, M.A.; Firth, R.S.C.; Woinarski, J.C.Z.; Gillespie, G.R.; Andersen, A.N.; Rioli, W.; Puruntatameri, J.; Roberts, W.; Kerinaiua, C.; et al. Declining populations in one of the last refuges for threatened mammal species in northern Australia. Austral Ecol. 2018, 43, 602–612. [Google Scholar] [CrossRef]
- Threatened Species Scientific Committee. Conservation Advice Mesembriomys gouldii rattoides Black-Footed Tree-Rat (North Queensland); Department of the Environment: Canberra, Australia, 2015.
- Davies, H.F.; McCarthy, M.A.; Firth, R.S.C.; Woinarski, J.C.Z.; Gillespie, G.R.; Andersen, A.N.; Geyle, H.M.; Nicholson, E.; Murphy, B.P. Top-down control of species distributions: Feral cats driving the regional extinction of a threatened rodent in northern Australia. Divers. Distrib. 2017, 23, 272–283. [Google Scholar] [CrossRef]
- Gynther, I.; Waller, N.; Leung, L.K.-P. Confirmation of the Extinction of the Bramble Cay Melomys Melomys rubicola on Bramble Cay, Torres Strait: Results and Conclusions from a Comprehensive Survey in August–September 2014; Department of Environment and Heritage Protection: Brisbane, Australia, 2016; Unpublished report.
- Woinarski, J.C.Z.; Garnett, S.T.; Legge, S.M.; Lindenmayer, D.B. The contribution of policy, law, management, research, and advocacy failings to the recent extinctions of three Australian vertebrate species. Conserv. Biol. 2017, 31, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Latch, P. Recovery Plan for the Bramble Cay Melomys Melomys rubicola. Report to Department of the Environment, Water, Heritage and the Arts, Canberra; Environment Protection Agency: Brisbane, Australia, 2008.
- Corlett, R.T. Climate change in the tropics: The end of the world as we know it? Biol. Conserv. 2012, 151, 22–25. [Google Scholar] [CrossRef]
- Ziembicki, M.; Lockie, S. Implications of an Expanding and Intensifying Tropical Zone for the Sustainable Development Agenda. GSDR Science Brief—2016 Update; Working Paper; Sustainable Development Knowledge Platform: New York, NY, USA, 2016. [Google Scholar]
- Radford, I.J.; Dickman, C.R.; Start, A.N.; Palmer, C.; Carnes, K.; Everitt, C.; Fairman, R.; Graham, G.; Partridge, T.; Thomson, A. Mammals of Australia’s tropical savannas: A conceptual model of assemblage structure and regulatory factors in the Kimberley region. PLoS ONE 2014, 9, e92341. [Google Scholar]
- Radford, I.J.; Gibson, L.A.; Corey, B.; Carnes, K.; Fairman, R. Influence of fire mosaics, habitat characteristics and cattle disturbance on mammals in fire-prone savanna landscapes of the northern Kimberley. PLoS ONE 2015, 10, e0130721. [Google Scholar]
- Start, A.N.; Burbidge, A.A.; McKenzie, N.L.; Palmer, C. The status of mammals in the North Kimberley, Western Australia. Austral. Mammal. 2007, 29, 1–16. [Google Scholar] [CrossRef]
- McKenzie, N.L. Mammals of the Phanerozoic South-West Kimberley, Western Australia: Biogeography and recent changes. J. Biogeogr. 1981, 8, 263–280. [Google Scholar] [CrossRef]
- Gibson, L.A.; McKenzie, N.L. Occurrence of non-volant mammals on islands along the Kimberley coast of Western Australia. Rec. West. Austral. Mus. 2012, 81, 15–39. [Google Scholar] [CrossRef] [Green Version]
- Ziembicki, M.R.; Woinarski, J.C.Z.; Mackey, B. Evaluating the status of species using Indigenous knowledge: Novel evidence for major native mammal declines in northern Australia. Biol. Conserv. 2013, 157, 78–92. [Google Scholar] [CrossRef]
- WWF-Australia. WWF-Australia Kimberley Program Update for the WWF-Australia Board, August 2018; Worldwide Fund for Nature: Sydney, Australia, 2018. [Google Scholar]
- Travouillon, K.J.; Phillips, M.J. Total evidence analysis of the phylogenetic relationships of bandicoots and bilbies (Marsupialia: Peramelemorphia): Reassessment of two species and description of a new species. Zootaxa 2018, 4378, 224–256. [Google Scholar] [CrossRef] [PubMed]
- Woolley, P.A.; Krajewski, C.; Westerman, M. Phylogenetic relationships within Dasyurus (Dasyuromorphia: Dasyuridae): Quoll systematics based on molecular evidence and male characteristics. J. Mammal. 2015, 96, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Romero, J.; Kenndard, M.; Cattarino, L.; Pressey, R.; Adams, V.; Anthony, M.; Carwardine, J.; Doherty, T.; Eyre, T.; Firth, R.; et al. Persistence of Northern Australian Aquatic and Terrestrial Vertebrate Species under Different Threat Levels. James Cook University Dataset. 2021. Available online: https://research.jcu.edu.au/data/published/a73bb85bf4ba026b5b3e6c952d9bcee4/ (accessed on 22 January 2022). [CrossRef]
- Garnett, S.; Szabo, J.K.; Dutson, G. The Action Plan for Australian Birds 2010; CSIRO Publishing: Melbourne, Australia, 2011. [Google Scholar]
- Humane Society International. Spectacled Flying-Fox (Pteropus conspicillatus) Nomination to Change the Conservation Status of a Species under the Queensland Nature Conservation Act 1992; Humane Society International: Sydney, Australia, 2021. [Google Scholar]
- Woinarski, J.C.Z.; Burbidge, A.A.; Harrison, P.L. Ongoing unraveling of a continental fauna: Decline and extinction of Australian mammals since European settlement. Proc. Natl. Acad. Sci. USA 2015, 112, 4531–4540. [Google Scholar] [CrossRef] [Green Version]
- Westerman, M.; Woolley, P.A. Comment on a research note reporting new populations of the northern quoll in Western Australia. Austral. Mammal. 2016, 38, 124–126. [Google Scholar] [CrossRef]
- Atlas of Living Australia. Dasyurus hallucatus Gould, 1842; Atlas of Living Australia. 2021. Available online: https://bie.ala.org.au/species/urn:lsid:biodiversity.org.au:afd.taxon:5d7aeda8-2ec1-4111-830e-847f34321f88#overview (accessed on 19 December 2021).
- Gillespie, G.R.; Risler, J.A.; Einoder, L.; Stokeld, D. Factors Influencing Patterns of Native Mammal Diversity at Fish River Station. Report to the Indigenous Land Corporation and the Nature Conservancy; Flora and Fauna Division, Department of Land Resource Management: Darwin, Australia, 2016.
- Heiniger, J.; Gillespie, G. High variation in camera trap-model sensitivity for surveying mammal species in northern Australia. Wildl. Res. 2018, 45, 578–585. [Google Scholar] [CrossRef]
- Doody, J.S.; McHenry, C.; Rhind, D.; Gray, C.; Clulow, S. Impacts of invasive cane toads on an Endangered marsupial predator and its prey. Endang. Spec. Res. 2021, 46, 269–277. [Google Scholar] [CrossRef]
- Anindilyakwa Land Council. Quarantine and Biosecurity. Available online: https://www.anindilyakwa.com.au/land-sea/quarantine-and-biosecurity (accessed on 19 December 2021).
- Moore, H.A.; Dunlop, J.A.; Jolly, C.J.; Kelly, E.; Woinarski, J.C.Z.; Ritchie, E.G.; Burnett, S.; van Leeuwen, S.; Valentine, L.E.; Cowan, M.A.; et al. A brief history of the northern quoll (Dasyurus hallucatus): A systematic review. Austral. Mammal. 2021. [Google Scholar] [CrossRef]
- Rio Tinto Iron Ore. Koodaideri Iron Ore Project Northern Quoll Management Plan; Rio Tinto Iron Ore: Perth, Australia, 2018. [Google Scholar]
- Dunlop, J. Pilbara Northern Quoll Research Program; Department of Parks and Wildlife: Bentley, WA, USA, 2017.
- Hernandez-Santin, L.; Henderson, M.; Molloy, S.W.; Dunlop, J.A.; Davis, R.A. Spatial ecology of an endangered carnivore, the Pilbara northern quoll. Austral. Mammal. 2021, 43, 235–242. [Google Scholar] [CrossRef]
- Moore, H.A.; Michael, D.R.; Ritchie, E.G.; Dunlop, J.A.; Valentine, L.E.; Hobbs, R.J.; Nimmo, D.G. A rocky heart in a spinifex sea: Occurrence of an endangered marsupial predator is multiscale dependent in naturally fragmented landscapes. Landsc. Ecol. 2021, 36, 1359–1376. [Google Scholar] [CrossRef]
- Hohnen, R.D.; Tuft, K.; Legge, S.; Hillyer, M.; Spencer, P.B.S.; Radford, I.J.; Johnson, C.N.; Burridge, C.P. Rainfall and topography predict gene flow among populations of the declining northern quoll (Dasyurus hallucatus). Conserv. Genet. 2016, 17, 1213–1228. [Google Scholar] [CrossRef]
- Burnett, S.; Piza-Roca, C.; Nugent, D. Mt Emerald Wind Farm Fauna Monitoring Final Report; University of the Sunshine Coast: Sippy Downs, Australia, 2019. [Google Scholar]
- Burnett, S.; Shimizu, Y.; Middleton, J. Distribution and Abundance of the Northern Quoll (Dasyurus hallucatus) in Far North Queensland; Report for Ratch Australasia; University of the Sunshine Coast: Sippy Downs, Australia, 2013. [Google Scholar]
- Thomas, A.J. Habitat Preferences of the Terrestrial Vertebrate Fauna of Weipa, Cape York Peninsula. Master’s Thesis, James Cook University, Townsville, Australia, 2004. [Google Scholar]
- Cardoso, M.J.; Eldridge, M.D.B.; Oakwood, M.; Rankmore, B.; Sherwin, W.B.; Firestone, K.B. Effects of founder events on the genetic variation of translocated island populations: Implications for conservation management of the northern quoll. Conserv. Genet. 2009, 10, 1719–1733. [Google Scholar] [CrossRef]
- Cremona, T.; Crowther, M.S.; Webb, J.K. High mortality and small population size prevents population recovery of a reintroduced mesopredator. Anim. Conserv. 2017, 20, 555–563. [Google Scholar] [CrossRef]
- Cremona, T.; Spencer, P.; Shine, R.; Webb, J.K. Avoiding the last supper: Parentage analysis indicates multi-generational survival of re-introduced ‘toad-smart’ lineage. Conserv. Genet. 2017, 18, 1475–1480. [Google Scholar] [CrossRef]
- Griffiths, A.D.; Rankmore, B.; Brennan, K.; Woinarski, J.C.Z. Demographic evaluation of translocating the threatened northern quoll to two Australian islands. Wildl. Res. 2017, 44, 238–247. [Google Scholar] [CrossRef]
- Kelly, E.; Kenbi Traditional Owners and Rangers; Jolly, C.J.; Indigo, N.; Smart, A.; Webb, J.; Phillips, B. No outbreeding depression in a trial of targeted gene flow in an endangered Australian marsupial. Conserv. Genet. 2021, 22, 23–33. [Google Scholar] [CrossRef]
- Jolly, C.J.; Webb, J.K.; Phillips, B.L. The perils of paradise: An endangered species conserved on an island loses antipredator behaviours within 13 generations. Biol. Lett. 2018, 14, 20180222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IUCN/SSC. Guidelines for Reintroductions and Other Conservation Translocations; Version 1.0.; IUCN Species Survival Commission: Gland, Switzerland, 2013. [Google Scholar]
- Fisher, D.O.; Johnson, C.N.; Lawes, M.J.; Fritz, S.A.; McCallum, H.; Blomberg, S.P.; Van Der Wal, J.; Abbott, B.; Frank, A.; Legge, S.; et al. Response to commentary by Woinarski (Critical-weight-range marsupials in northern Australia are declining: A commentary on Fisher et al. (2014) ‘The current decline of tropical marsupials in Australia: Is history repeating?’). Global Ecol. Biogeogr. 2015, 24, 123–125. [Google Scholar] [CrossRef]
- Lawes, M.J.; Fisher, D.O.; Johnson, C.N.; Blomberg, S.P.; Frank, A.S.K.; Fritz, S.A.; McCallum, H.; VanDerWal, J.; Abbott, B.N.; Legge, S.; et al. Correlates of recent declines of rodents in northern and southern Australia: Habitat structure is critical. PLoS ONE 2015, 10, e0130626. [Google Scholar]
- Lawes, M.J.; Murphy, B.P.; Fisher, A.; Woinarski, J.C.Z.; Edwards, A.C.; Russell-Smith, J. Small mammals decline with increasing fire extent in northern Australia: Evidence from long-term monitoring in Kakadu National Park. Int. J. Wildland Fire 2015, 24, 712–722. [Google Scholar] [CrossRef]
- Radford, I.J.; Woolley, L.-A.; Dickman, C.R.; Corey, B.; Trembath, D.; Fairman, R. Invasive anuran driven trophic cascade: An alternative hypothesis for recent critical weight range mammal collapses across northern Australia. Biol. Invas. 2020, 22, 1967–1982. [Google Scholar] [CrossRef]
- Andersen, A.N. Faunal responses to fire in Australian tropical savannas: Insights from field experiments and their lessons for conservation management. Divers. Distrib. 2021, 27, 828–843. [Google Scholar] [CrossRef]
- Allek, A.; Assis, A.S.; Eiras, N.; Amaral, T.P.; Williams, B.; Butt, N.; Renwick, A.R.; Bennett, J.R.; Beyer, H.L. The threats endangering Australia’s at-risk fauna. Biol. Conserv. 2018, 222, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Yibarbuk, D.; Whitehead, P.J.; Russell-Smith, J.; Jackson, D.; Fisher, A.; Cooke, P.; Choquenot, D.; Bowman, D.J.M.S. Fire ecology and Aboriginal land management in central Arnhem Land, northern Australia: A tradition of ecosystem management. J. Biogeogr. 2000, 28, 325–343. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Risler, J.; Kean, L. Response of vegetation and vertebrate fauna to 23 years of fire exclusion in a tropical Eucalyptus open forest, Northern Territory, Australia. Austral Ecol. 2004, 29, 156–176. [Google Scholar] [CrossRef]
- Andersen, A.N.; Cook, G.D.; Corbett, L.K.; Douglas, M.M.; Eager, R.W.; Russell-Smith, J.; Setterfield, S.A.; Williams, R.J.; Woinarski, J.C.Z. Fire frequency and biodiversity conservation in Australian tropical savannas: Implications from the Kapalga fire experiment. Austral Ecol. 2005, 30, 155–167. [Google Scholar] [CrossRef]
- Russell-Smith, J. Fire in the Kimberley and Inland Regions of WA—Issues Paper; Environmental Protection Authority: Perth, Australia, 2005.
- Murphy, S.A.; Legge, S.M.; Heathcote, J.; Mulder, E. The effects of early and late-season fires on mortality, dispersal, physiology and breeding of red-backed fairy-wrens (Malurus melanocephalus). Wildl. Res. 2010, 37, 145–155. [Google Scholar] [CrossRef]
- Kutt, A.S.; Woinarski, J.C.Z. The effects of grazing and fire on vegetation and the vertebrate assemblage in a tropical savanna woodland in north-eastern Australia. J. Trop. Ecol. 2007, 23, 95–106. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Whitehead, P.; Cooke, P. (Eds.) Culture, Ecology and Economy of Savanna Fire Management in Northern Australia: Rekindling the Wurrk Tradition; CSIRO Publishing: Melbourne, Australia, 2009. [Google Scholar]
- Evans, J.; Russell-Smith, J. Delivering effective savanna fire management for defined biodiversity conservation outcomes: An Arnhem Land case study. Int. J. Wildland Fire 2020, 29, 386–400. [Google Scholar] [CrossRef] [Green Version]
- Preece, N. Aboriginal fires in monsoonal Australia from historical accounts. J. Biogeogr. 2002, 29, 321–336. [Google Scholar] [CrossRef]
- Preece, N.D. Traditional and ecological fires and effects of bushfire laws in north Australian savannas. Int. J. Wildland Fire 2007, 16, 378–389. [Google Scholar] [CrossRef]
- Ritchie, D. Things fall apart: The end of an era of systematic Indigenous fire management. In Culture, Ecology and Economy of Savanna Fire Management in Northern Australia: Rekindling the Wurrk Tradition; Russell-Smith, J., Whitehead, P.J., Cooke, P., Eds.; CSIRO Publishing: Melbourne, Australia, 2009; pp. 23–40. [Google Scholar]
- Wohling, M. The problem of scale in Indigenous knowledge: A perspective from Northern Australia. Ecol. Soc. 2009, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- Fitzsimons, J.; Russell-Smith, J.; James, G.; Vigilante, T.; Lipsett-Moore, G.; Morrison, J.; Looker, M. Insights into the biodiversity and social benchmarking components of the Northern Australian fire management and carbon abatement programmes. Ecol. Manage. Restor. 2012, 13, 51–57. [Google Scholar] [CrossRef]
- Australian Government. Carbon Credits (Carbon Farming Initiative—Savanna Fire Management—Emissions Avoidance) Methodology Determination 2018; Australian Government: Canberra, Australia, 2018. Available online: https://www.legislation.gov.au/Details/F2018L00560 (accessed on 22 January 2022).
- Trauernicht, C.; Brook, B.W.; Murphy, B.P.; Williamson, G.J.; Bowman, D.M. Local and global pyrogeographic evidence that Indigenous fire management creates pyrodiversity. Ecol. Evol. 2015, 5, 1908–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, J.J.; Sinclair, M.; Wikmunea, H.; Wolmby, S.; Martin, D.; Martin, B. The divergence of traditional Aboriginal and contemporary fire management practices on Wik traditional lands, Cape York Peninsula, Northern Australia. Ecol. Manag. Restor. 2018, 19, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Wunambal Gaambera Aboriginal Corporation. Land of Wandjina and Wunggurr: Ngauwudu Management Plan Wunambal People: Mitchell Plateau North-West Australia; Wunambal Gaambera Aboriginal Corporation: Broome, Australia, 2001. [Google Scholar]
- Dambimangari Aboriginal Corporation. Dambimangari Healthy Country Plan 2012–2022; Dambimangari Aboriginal Corporation: Derby, Australia, 2012. [Google Scholar]
- Wilinggin Aboriginal Corporation. Wilinggin Healthy Country Plan—Looking after Ngarinyin Country 2010–2022; Wilinggin Aboriginal Corporation: Derby, Australia, 2012. [Google Scholar]
- Walalakoo Aboriginal Corporation. Walalakoo Healthy Country Plan 2017–2027; Walalakoo Aboriginal Corporation: Derby, Australia, 2016. [Google Scholar]
- Olkola Aboriginal Corporation. Olkola Healthy Country Strategic Plan; Olkola Aboriginal Corporation: Cairns, Australia, 2018. [Google Scholar]
- Perry, J.J.; Vanderduys, E.P.; Kutt, A.S. Shifting fire regimes from late to early dry-season fires to abate greenhouse emissions does not completely equate with terrestrial vertebrate biodiversity co-benefits on Cape York Peninsula, Australia. Int. J. Wildland Fire 2016, 25, 742–752. [Google Scholar] [CrossRef]
- Petty, A.M.; de Koninck, V.; Orlove, B. Cleaning, protecting, or abating? Making Indigenous fire management “work” in northern Australia. J. Ethnobiol. 2015, 35, 140–162. [Google Scholar] [CrossRef] [Green Version]
- Russell-Smith, J.; Murphy, B.P.; Meyer, C.P.; Cook, G.D.; Maier, S.; Edwards, A.C.; Schatz, J.; Brocklehurst, P. Improving estimates of savanna burning emissions for greenhouse accounting in northern Australia: Limitations, challenges, applications. Int. J. Wildland Fire 2009, 18, 1–18. [Google Scholar] [CrossRef]
- Russell-Smith, J. Key Research to Assist the Development of Carbon Sequestration Methods for Savanna Fire Management in Northern Australia; Meat and Livestock Australia: Sydney, Australia, 2020. [Google Scholar]
- Crowley, G.; Garnett, S.; Shephard, S. Impact of storm-burning on Melaleuca viridiflora invasion of grasslands and grassy woodlands on Cape York Peninsula, Australia. Austral Ecol. 2009, 34, 196–209. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Yates, C.; Evans, J.; Desailly, M. Developing a savanna burning emissions abatement methodology for tussock grasslands in high rainfall regions of northern Australia. Trop. Grassl–Forrajes Trop. 2014, 2, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Perry, J.J.; Cook, G.D.; Graham, E.; Meyer, C.P.; Murphy, H.T.; VanDerWal, J. Regional seasonality of fire size and fire weather conditions across Australia’s northern savanna. Int. J. Wildland Fire 2020, 29, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bureau of Meteorology. Northern Territory in 2020: Fifth-Warmest Year on Record; Close to Average Rainfall. Available online: http://www.bom.gov.au/climate/current/annual/nt/summary.shtml (accessed on 2 May 2021).
- Parr, C.L.; Andersen, A.N. Patch mosaic burning for biodiversity conservation: A critique of the pyrodiversity paradigm. Conserv. Biol. 2006, 20, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Bradstock, R.A.; Bedward, M.; Gill, A.M.; Cohn, J.S. Which mosaic? A landscape ecological approach for evaluating interactions between fire regimes, habitat and animals. Wildl. Res. 2005, 32, 409–423. [Google Scholar] [CrossRef] [Green Version]
- Murphy, B.P.; Liedloff, A.C.; Cook, G.D. Does fire limit tree biomass in Australian savannas? Int. J. Wildland Fire 2015, 24, 1–13. [Google Scholar] [CrossRef]
- Taylor, R.S.; Watson, S.J.; Nimmo, D.G.; Kelly, L.T.; Bennett, A.F.; Clarke, M.F. Landscape-scale effects of fire on bird assemblages: Does pyrodiversity beget biodiversity? Divers. Distrib. 2012, 18, 519–529. [Google Scholar] [CrossRef]
- Andersen, A.N.; Woinarski, J.C.Z.; Parr, C.L. Savanna burning for biodiversity: Fire management for faunal conservation in Australian tropical savannas. Austral Ecol. 2012, 37, 658–667. [Google Scholar] [CrossRef]
- Davies, H.F.; McCarthy, M.A.; Rioli, W.; Puruntatameri, J.; Roberts, W.; Kerinaiua, C.; Kerinauia, V.; Womatakimi, K.B.; Andersen, A.N.; Murphy, B.P. An experimental test of whether pyrodiversity promotes mammal diversity in a northern Australian savanna. J. Appl. Ecol. 2018, 55, 2124–2134. [Google Scholar] [CrossRef]
- Farnsworth, L.M.; Nimmo, D.G.; Kelly, L.T.; Bennett, A.F.; Clarke, M.F. Does pyrodiversity beget alpha, beta or gamma diversity? A case study using reptiles from semi-arid Australia. Divers. Distrib. 2014, 20, 663–673. [Google Scholar] [CrossRef]
- Broken-Brow, J.; Hitch, A.T.; Armstrong, K.N.; Leung, L.K.-P. Effect of fire on insectivorous bat activity in northern Australia: Does fire intensity matter on a local scale? Austral. J. Zool. 2019, 67, 260–268. [Google Scholar] [CrossRef]
- Shaw, R.E. The Genetic Consequences of Demography and Disturbance in Small Mammal Populations. Ph.D. Thesis, Australian National University, Canberra, Australia, 2018. [Google Scholar]
- Radford, I.J.; Corey, B.; Hatherley, E.; Vigilante, T.; Wunambal Gaambera Aboriginal Corporation; Fairman, R.; Carnes, K.; Start, T.; Pollock, K.H. Prescribed burning benefits threatened mammals in northern Australia. Biodiv. Conserv. 2020, 29, 2985–3007. [Google Scholar] [CrossRef]
- Firth, R.S.C.; Brook, B.W.; Woinarski, J.C.Z.; Fordham, D.A. Decline and likely extinction of a northern Australian native rodent, the Brush-tailed Rabbit-rat Conilurus penicillatus. Biol. Conserv. 2010, 143, 1193–1201. [Google Scholar] [CrossRef]
- Hohnen, R.; Tuft, K.D.; Legge, S.; Radford, I.J.; Carver, S.; Johnson, C.N. Post-fire habitat use of the golden-backed tree-rat (Mesembriomys macrurus) in the northwest Kimberley, Western Australia. Austral Ecol. 2015, 40, 941–952. [Google Scholar] [CrossRef]
- Hohnen, R.; Tuft, K.; Legge, S.; Walters, N.; Johanson, L.; Carver, S.; Radford, I.J.; Johnson, C.N. The significance of topographic complexity in habitat selection and persistence of a declining marsupial in the Kimberley region of Western Australia. Austral. J. Zool. 2016, 64, 198–216. [Google Scholar] [CrossRef]
- Scott, K.; Setterfield, S.A.; Douglas, M.M.; Parr, C.L.; Schatz, J.O.N.; Andersen, A.N. Does long-term fire exclusion in an Australian tropical savanna result in a biome shift? A test using the reintroduction of fire. Austral Ecol. 2012, 37, 693–711. [Google Scholar] [CrossRef]
- Radford, I.J.; Corey, B.; Carnes, K.; Shedley, E.; McCaw, L.; Woolley, L.-A. Landscape-scale effects of fire, cats, and feral livestock on threatened savanna mammals: Unburnt habitat matters more than pyrodiversity. Front. Ecol. Evol. 2021, 9, 739817. [Google Scholar] [CrossRef]
- Williams, R.J.; Woinarski, J.C.Z.; Andersen, A.N. Fire experiments in northern Australia: Contributions to ecological understanding and biodiversity conservation in tropical savannas. Int. J. Wildland Fire 2003, 12, 391–402. [Google Scholar] [CrossRef]
- Griffiths, A.D.; Brook, B.W. Fire impacts recruitment more than survival of small-mammals in a tropical savanna. Ecosphere 2015, 6, art99. [Google Scholar] [CrossRef] [Green Version]
- Gurevitch, J.; Padilla, D.K. Are invasive species a major cause of extinctions? Trends Ecol. Evol. 2004, 19, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Woinarski, J.C.Z.; Ash, A.J. Responses of vertebrates to pastoralism, military land use and landscape position in an Australian tropical savanna. Austral Ecol. 2002, 27, 311–323. [Google Scholar] [CrossRef]
- Franklin, D.C.; Whitehead, P.J.; Pardon, G.; Matthews, J.; McMahon, P.; McIntyre, D. Geographic patterns and correlates of the decline of granivorous birds in northern Australia. Wildl. Res. 2005, 32, 399–408. [Google Scholar] [CrossRef]
- Neilly, H.; Vanderwal, J.; Schwarzkopf, L. Balancing biodiversity and food production: A better understanding of wildlife response to grazing will inform off-reserve conservation on rangelands. Rangel. Ecol. Manag. 2016, 69, 430–436. [Google Scholar] [CrossRef]
- Neilly, H.; Nordberg, E.; VanDerWal, J.; Schwarzkopf, L. Arboreality increases reptile community resistance to disturbance from livestock grazing. J. Appl. Ecol. 2017, 55, 786–799. [Google Scholar] [CrossRef]
- Legge, S.; Smith, J.G.; James, A.; Tuft, K.D.; Webb, T.; Woinarski, J.C.Z. Interactions among threats affect conservation management outcomes: Livestock grazing removes the benefits of fire management for small mammals in Australian tropical savannas. Conserv. Sci. Pract. 2019, 1, e52. [Google Scholar] [CrossRef]
- Neilly, H.; Schwarzkopf, L. The impact of cattle grazing regimes on tropical savanna bird assemblages. Austral Ecol. 2019, 44, 187–198. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Oakwood, M.; Winter, J.; Burnett, S.; Milne, D.; Foster, P.; Myles, H.; Holmes, B. Surviving the Toads: Patterns of Persistence of the Northern Quoll Dasyurus hallucatus in Queensland; Report Submitted to the Natural Heritage Trust Strategic Reserve Program, as a Component of Project 2005/162: Monitoring & Management of Cane Toad Impact in the Northern Territory; Department of Natural Resources, Environment and the Arts: Palmerston, Australia, 2008.
- Frank, A.S.K.; Johnson, C.N.; Potts, J.M.; Fisher, A.; Lawes, M.J.; Woinarski, J.C.Z.; Tuft, K.; Radford, I.J.; Gordon, I.J.; Collis, M.-A.; et al. Experimental evidence that feral cats cause local extirpation of small mammals in Australia’s tropical savannas. J. Appl. Ecol. 2014, 51, 1486–1493. [Google Scholar] [CrossRef] [Green Version]
- Burnett, S. Colonizing Cane Toads cause population declines in native predators: Reliable anecdotal information and management implications. Pac. Conserv. Biol. 1997, 3, 65–72. [Google Scholar]
- Van Dam, R.A.; Walden, D.J.; Begg, G.W. A Preliminary Risk Assessment of Cane Toads in Kakadu National Park; Supervising Scientist Report 164; Supervising Scientist: Darwin, Australia, 2002.
- Letnic, M.; Webb, J.K.; Shine, R. Invasive cane toads (Bufo marinus) cause mass mortality of freshwater crocodiles (Crocodylus johnstoni) in tropical Australia. Biol. Conserv. 2008, 141, 1773–1782. [Google Scholar] [CrossRef]
- Legge, S.; Murphy, B.P.; McGregor, H.; Woinarski, J.C.Z.; Augusteyn, J.; Ballard, G.; Baseler, M.; Buckmaster, T.; Dickman, C.R.; Doherty, T.; et al. Enumerating a continental-scale threat: How many feral cats are in Australia? Biol. Conserv. 2017, 206, 293–303. [Google Scholar] [CrossRef]
- Doherty, T.S.; Davis, R.A.; van Etten, E.J.B.; Algar, D.; Collier, N.; Dickman, C.R.; Edwards, G.; Masters, P.; Palmer, R.; Robinson, S.; et al. A continental-scale analysis of feral cat diet in Australia. J. Biogeogr. 2015, 42, 964–975. [Google Scholar]
- Woinarski, J.C.Z.; Murphy, B.P.; Legge, S.M.; Garnett, S.T.; Lawes, M.J.; Comer, S.; Dickman, C.R.; Doherty, T.S.; Edwards, G.; Nankivell, A.; et al. How many birds are killed by cats in Australia? Biolog. Conserv. 2017, 214, 76–87. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Murphy, B.P.; Palmer, R.; Legge, S.M.; Dickman, C.R.; Doherty, T.S.; Edwards, G.; Nankivell, A.; Read, J.L.; Stokeld, D. How many reptiles are killed by cats in Australia? Wildl. Res. 2018, 45, 247–266. [Google Scholar] [CrossRef]
- Doherty, T.S.; Dickman, C.R.; Johnson, C.N.; Legge, S.M.; Ritchie, E.G.; Woinarski, J.C.Z. Impacts and management of feral cats Felis catus in Australia. Mammal Rev. 2017, 47, 83–97. [Google Scholar] [CrossRef]
- Smith, A.P.; Quin, D.G. Patterns and causes of extinction and decline in Australian conilurine rodents. Biol. Conserv. 1996, 77, 243–267. [Google Scholar] [CrossRef]
- Geffen, E.; Rowe, K.C.; Yom-Tov, Y. Reproductive rates in Australian rodents are related to phylogeny. PLoS ONE 2011, 6, e19199. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, J.B.; Whittington, A.; Dickman, C.R.; Leung, L.K.P. Perfect storm: Demographic responses of an irruptive desert mammal to prescribed burns following flooding rain. Austral Ecol. 2013, 38, 765–776. [Google Scholar] [CrossRef]
- Stokeld, D.; Fisher, A.; Gentles, T.; Hill, B.M.; Woinarski, J.C.Z.; Young, S.; Gillespie, G.R. Rapid increase of Australian tropical savanna reptile abundance following exclusion of feral cats. Biol. Conserv. 2018, 225, 213–221. [Google Scholar] [CrossRef]
- Stokeld, D.; Gentles, T.; Young, S.; Hill, B.; Fisher, A.; Woinarski, J.; Gillespie, G. Experimental Evaluation of the Role of Feral Cat Predation in the Decline of Small Mammals in Kakadu National Park; Department of Environment and Natural Resources: Darwin, Australia, 2016.
- Brook, L.; Johnson, C.; Ritchie, E. Effects of predator control on behaviour of an apex predator and indirect consequences for mesopredator suppression. J. Appl. Ecol. 2012, 49, 1278–1286. [Google Scholar] [CrossRef]
- Johnson, C.N.; Ritchie, E.G. The dingo and biodiversity conservation: Response to Fleming et al. (2012). Austral. Mammal. 2013, 35, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Cairns, K.M.; Crowther, M.S.; Nesbitt, B.; Letnic, M. The myth of wild dogs in Australia: Are there any out there? Austral. Mammal. 2022, 44, 67–75. [Google Scholar] [CrossRef]
- Kennedy, M.; Phillips, B.L.; Legge, S.; Murphy, S.A.; Faulkner, R.A. Do dingoes suppress the activity of feral cats in northern Australia? Austral Ecol. 2012, 37, 134–139. [Google Scholar] [CrossRef]
- Stokeld, D.; Fisher, A.; Gentles, T.; Hill, B.; Triggs, B.; Woinarski, J.C.Z.; Gillespie, G.R. What do predator diets tell us about mammal declines in Kakadu National Park? Wildl. Rese. 2018, 45, 92–101. [Google Scholar] [CrossRef]
- Read, J.L.; Cunningham, R. Relative impacts of cattle grazing and feral animals on an Australian arid zone reptile and small mammal assemblage. Austral Ecol. 2010, 35, 314–324. [Google Scholar] [CrossRef]
- Doherty, T.S.; Driscoll, D.A.; Nimmo, D.G.; Ritchie, E.G.; Spencer, R.J. Conservation or politics? Australia’s target to kill 2 million cats. Conserv. Lett. 2019, 12, e12633. [Google Scholar] [CrossRef] [Green Version]
- Australian Government. Threatened Species Strategy; Australian Government: Canberra, Australia, 2015.
- Australian Government. Threatened Species Strategy 2021–2031; Department of Agriculture, Water and the Environment: Canberra, Australia, 2021.
- Doherty, T.S.; Ritchie, E.G. Stop jumping the gun: A call for evidence-based invasive predator management. Conserv. Lett. 2017, 10, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Januchowski-Hartley, S.R.; Adams, V.M.; Hermoso, V. The need for spatially explicit quantification of benefits in invasive-species management. Conserv. Biol. 2018, 32, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Paltridge, R.; Ward, N.N.; West, J.T.; Crossing, K. Is cat hunting by Indigenous tracking experts an effective way to reduce cat impacts on threatened species? Wildl. Res. 2020, 47, 709–719. [Google Scholar] [CrossRef]
- Hardman, B.; Moro, D.; Calver, M. Direct evidence implicates feral cat predation as the primary cause of failure of a mammal reintroduction programme. Ecol. Manag. Restor. 2016, 17, 152–158. [Google Scholar] [CrossRef]
- Nordberg, E.J.; Macdonald, S.; Zimny, G.; Hoskins, A.; Zimny, A.; Somaweera, R.; Ferguson, J.; Perry, J. An evaluation of nest predator impacts and the efficacy of plastic meshing on marine turtle nests on the western Cape York Peninsula, Australia. Biol. Conserv. 2019, 238, 108201. [Google Scholar] [CrossRef]
- Johnson, C. Australia’s Mammal Extinctions: A 50,000 Year History; Cambridge University Press: Melbourne, Australia, 2006. [Google Scholar]
- Bettiol, S.; Kettlewell, J.; Davies, N.; Goldsmid, J. Giardiasis in native marsupials of Tasmania. J. Wildl. Dis. 1997, 33, 352–354. [Google Scholar] [CrossRef] [Green Version]
- Warren, K.S.; Swan, R.A.; Morgan-Ryan, U.M.; Friend, J.A.; Elliot, A. Cryptosporidium muris infection in bilbies (Macrotis lagotis). Austral. Vet. J. 2003, 81, 739–741. [Google Scholar] [CrossRef]
- Appelbee, A.J.; Thompson, R.C.A.; Olson, M.E. Giardia and Cryptosporidium in mammalian wildlife–current status and future needs. Trends Parasitol. 2005, 21, 370–376. [Google Scholar] [CrossRef]
- Skerratt, L.F. Sarcoptes scabiei: An important exotic pathogen of wombats. Microbiol. Austral. 2005, 26, 79–81. [Google Scholar] [CrossRef]
- Abbott, I. Mammalian faunal collapse in Western Australia, 1875–1925: The hypothesised role of epizootic disease and a conceptual model of its origin, introduction, transmission and spread. Austral. Zool. 2006, 33, 530–561. [Google Scholar] [CrossRef]
- Wyatt, K.B.; Campos, P.F.; Gilbert, M.T.P.; Kolokotronis, S.-O.; Hynes, W.H.; DeSalle, R.; Ball, S.J.; Daszak, P.; MacPhee, R.D.E.; Greenwood, A.D. Historical mammal extinction on Christmas Island (Indian Ocean) correlates with Introduced Infectious Disease. PLoS ONE 2008, 3, e3602. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.C.A.; Conlan, J.V. Emerging issues and parasite zoonoses in the SE Asian and Australasian region. Vet. Parasitol. 2011, 181, 69–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, R.C.A.; Lymbery, A.J.; Smith, A. Parasites, emerging disease and wildlife conservation. Intern. J. Parasitol. 2010, 40, 1163–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buettner, P.G.; Westcott, D.A.; Maclean, J.; Brown, L.; McKeown, A.; Johnson, A.; Wilson, K.; Blair, D.; Luly, J.; Skerratt, L.; et al. Tick paralysis in Spectacled Flying-Foxes (Pteropus conspicillatus) in North Queensland, Australia: Impact of a ground-dwelling ectoparasite finding an arboreal host. PLoS ONE 2013, 8, e73078. [Google Scholar] [CrossRef]
- Wayne, A.F.; Maxwell, M.A.; Ward, C.G.; Vellios, C.V.; Ward, B.G.; Liddelow, G.L.; Wilson, I.; Wayne, J.C.; Williams, M.R. Importance of getting the numbers right: Quantifying the rapid and substantial decline of an abundant marsupial, Bettongia penicillata. Wildl. Res. 2013, 40, 169–183. [Google Scholar] [CrossRef]
- Wayne, A.F.; Maxwell, M.A.; Ward, C.G.; Vellios, C.V.; Wilson, I.; Wayne, J.C.; Williams, M.R. Sudden and rapid decline of the abundant marsupial Bettongia penicillata in Australia. Oryx 2013, 49, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Preece, N.D.; Abell, S.E.; Grogan, L.; Wayne, A.; Skerratt, L.F.; van Oosterzee, P.; Shima, A.L.; Daszak, P.; Field, H.; Reiss, A.; et al. A guide for ecologists: Detecting the role of disease in faunal declines and managing population recovery. Biol. Conserv. 2017, 214, 136–146. [Google Scholar] [CrossRef]
- Thompson, R.C.A.; Smith, A.; Lymbery, A.J.; Averis, S.; Morris, K.D.; Wayne, A.F. Giardia in Western Australian wildlife. Vet. Parasitol. 2010, 170, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Fenwick, S.; Potter, A.; Ng, J.; Ryan, U. Identification of novel Cryptosporidium genotypes in kangaroos from Western Australia. Vet. Parasit. 2011, 179, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legge, S.; Taggart, P.L.; Dickman, C.R.; Read, J.L.; Woinarski, J.C.Z. Cat-dependent diseases cost Australia AU$6 billion per year through impacts on human health and livestock production. Wildl. Res. 2020, 47, 731–746. [Google Scholar] [CrossRef]
- Hillman, A.E.; Lymbery, A.J.; Thompson, R.C. Is Toxoplasma gondii a threat to the conservation of free-ranging Australian marsupial populations? Int. J. Parasitol. Parasites Wildl. 2016, 5, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Wobeser, G.; Wobeser, A.G. Carcass detectability and estimation of mortality in a simulated die-off of small birds. J. Wildl. Dis. 1992, 28, 548–554. [Google Scholar] [CrossRef]
- Santos, S.M.; Carvalho, F.; Mira, A. How long do the dead survive on the road? Carcass persistence probability and implications for road-kill monitoring surveys. PLoS ONE 2011, 6, e25383. [Google Scholar] [CrossRef] [Green Version]
- Barrientos, R.; Martins, R.C.; Ascensão, F.; D’Amico, M.; Moreira, F.; Borda-de-Água, L. A review of searcher efficiency and carcass persistence in infrastructure-driven mortality assessment studies. Biol. Conserv. 2018, 222, 146–153. [Google Scholar] [CrossRef]
- Stephenson, E.B.; Peel, A.J.; Reid, S.A.; Jansen, C.C.; McCallum, H. The non-human reservoirs of Ross River virus: A systematic review of the evidence. Parasit. Vectors 2018, 11, 188. [Google Scholar] [CrossRef] [Green Version]
- Peel, A.J.; Field, H.E.; Aravena, M.R.; Edson, D.; McCallum, H.; Plowright, R.K.; Prada, D. Coronaviruses and Australian bats: A review in the midst of a pandemic. Austral. J. Zool. 2019, 67, 346–360. [Google Scholar] [CrossRef]
- Hussain-Yusuf, H.; Stenos, J.; Vincent, G.; Shima, A.; Abell, S.; Preece, N.D.; Tadepalli, M.; Hii, S.F.; Bowie, N.; Mitram, K.; et al. Screening for Rickettsia, Coxiella and Borrelia Species in Ticks from Queensland, Australia. Pathogens 2020, 9, 1016. [Google Scholar] [CrossRef]
- Iglesias, R.; Cox-Witton, K.; Field, H.; Skerratt, L.F.; Barrett, J. Australian Bat Lyssavirus: Analysis of National Bat Surveillance Data from 2010 to 2016. Viruses 2021, 13, 189. [Google Scholar] [CrossRef]
- Ong, O.T.W.; Skinner, E.B.; Johnson, B.J.; Old, J.M. Mosquito-borne viruses and non-human vertebrates in Australia: A review. Viruses 2021, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Brook, B.W.; Sodhi, N.S.; Bradshaw, C.J.A. Synergies among extinction drivers under global change. Trends Ecol. Evol. 2008, 23, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.W.; Jackson, S.T. Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Environ. 2007, 5, 475–482. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Moise, A.; Abbs, D.; Bhend, J.; Chiew, F.; Church, J.; Ekström, M.; Kirono, D.; Lenton, A.; Lucas, C.; McInnes, K.; et al. Monsoonal North Cluster Report. In Climate Change in Australia Projections for Australia’s Natural Resource Management Regions: Cluster Reports; Ekström, M., Whetton, P., Gerbing, C., Grose, M., Webb, L., Risbey, J., Eds.; CSIRO and Bureau of Meteorology: Melbourne, Australia, 2015. [Google Scholar]
- National Environmental Science Program. Our Changing Climate. How Will Rainfall Change in Northern Australia over this Century? Earth Systems and Climate Change Hub, National Environmental Science Program: Canberra, Australia, 2018.
- Crowley, G.M. Climate Change in the Southern Gulf Region: A Background Paper to Inform the Southern Gulf Natural Resource Management Plan; Southern Gulf Natural Resource Management: Mount Isa, Australia, 2016. [Google Scholar]
- Hughes, L. Climate change and Australia: Trends, projections and impacts. Austral Ecol. 2003, 28, 423–443. [Google Scholar] [CrossRef]
- CSIRO; Bureau of Meteorology. Monsoonal North Brochure. In Climate Change in Australia. Projections for Australia’s NRM Regions; CSIRO & Bureau of Meteorology: Melbourne, Australia, 2015. [Google Scholar]
- CSIRO; Bureau of Meteorology. Australia’s Changing Climate; CSIRO and Bureau of Meteorology: Canberra, Australia, 2016.
- State of Queensland. Climate Change in the Cape York Region, Version 1; Department of Environment and Science: Brisbane, Australia, 2019.
- State of Queensland. Climate Change in the Gulf Region, Version 1; Department of Environment and Science: Brisbane, Australia, 2019.
- Australian Academy of Science. The Risks to Australia of a 3 °C Warmer World; Australian Academy of Science: Canberra, Australia, 2021. [Google Scholar]
- Brodie, J.F.; Lieberman, S.; Moehrenschlager, A.; Redford, K.H.; Rodríguez, J.P.; Schwartz, M.; Seddon, P.J.; Watson, J.E.M. Global policy for assisted colonization of species. Science 2021, 372, 456–458. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, K.S. Climate Change in the Tropics: Ecological and Evolutionary Responses at Low Latitudes. Ann. Rev. Ecol. Evol. Syst. 2019, 50, 303–333. [Google Scholar] [CrossRef]
- Brook, B.W. Synergies between climate change, extinctions and invasive vertebrates. Wildl. Res. 2008, 35, 249–252. [Google Scholar] [CrossRef]
- Meynecke, J.-O. Effects of global climate change on geographic distributions of vertebrates in North Queensland. Ecol. Model. 2004, 174, 347–357. [Google Scholar] [CrossRef]
- Wet Tropics Management Authority. State of Wet Tropics Report 2015–2016. Ancient, Endemic, Rare and Threatened Vertebrates of the Wet Tropics; Wet Tropics Management Authority: Cairns, Australia, 2016.
- Hoffmann, A.A.; Rymer, P.D.; Byrne, M.; Ruthrof, K.X.; Whinam, J.; McGeoch, M.; Bergstrom, D.M.; Guerin, G.R.; Sparrow, B.; Joseph, L.; et al. Impacts of recent climate change on terrestrial flora and fauna: Some emerging Australian examples. Austral Ecol. 2019, 44, 3–27. [Google Scholar] [CrossRef] [Green Version]
- Duke, N.C.; Kovacs, J.M.; Griffiths, A.D.; Preece, L.; Hill, D.J.E.; van Oosterzee, P.; Mackenzie, J.; Morning, H.S.; Burrows, D. Large-scale dieback of mangroves in Australia’s Gulf of Carpentaria: A severe ecosystem response, coincidental with an unusually extreme weather event. Mar. Freshw. Res. 2017, 68, 1816–1829. [Google Scholar] [CrossRef]
- Hosking, E.J. Land Clearing in the Northern Territory; Report 24/2002; Northern Territory Department of Infrastructure, Planning & Environment: Darwin, Australia, 2002.
- Lawes, M.J.; Greiner, R.; Leiper, I.A.; Ninnis, R.; Pearson, D.; Boggs, G. The effects of a moratorium on land-clearing in the Douglas-Daly region, Northern Territory, Australia. Rangel. J. 2015, 37, 399–408. [Google Scholar] [CrossRef]
- Maron, M.; Laurance, B.; Pressey, B.; Catterall, C.P.; McAlpine, C.; Possingham, H.; Watson, J.; Rhodes, J.; Wilson, K.; Hockings, M. Queensland land clearing is undermining Australia’s environmental progress. The Conversation. 22 February 2016. Available online: https://theconversation.com/queensland-land-clearing-is-undermining-australias-environmental-progress-54882 (accessed on 22 January 2022).
- Fisher, A.; Hunt, L.; Kutt, A.; Mazzer, T. Biodiversity Monitoring in the Rangelands: A Way Forward. Volume 2: Case Studies; Desert Knowledge CRC: Alice Springs, Australia, 2006. [Google Scholar]
- Lindenmayer, D.B.; Likens, G.E. Adaptive monitoring: A new paradigm for long-term research and monitoring. Trends Ecol. Evol. 2009, 24, 482–486. [Google Scholar] [CrossRef]
- McDonald-Madden, E.; Baxter, P.W.J.; Fuller, R.A.; Martin, T.G.; Game, E.T.; Montambault, J.; Possingham, H.P. Monitoring does not always count. Trends Ecol. Evol. 2010, 25, 547–550. [Google Scholar] [CrossRef]
- Lindenmayer, D.; Burns, E.; Thurgate, N.; Lowe, A. (Eds.) Biodiversity and Environmental Change: Monitoring, Challenges and Direction; CSIRO Publishing: Melbourne, Australia, 2014. [Google Scholar]
- Gillespie, G.R.; Low Choy, J. Evaluation of Vertebrate Monitoring Methods for Fish River Station; Report to the Indigenous Land Corporation; Department of Land Resource Management: Darwin, Australia, 2016.
- Walters, C.J.; Holling, C.S. Large-scale management experiments and learning by doing. Ecology 1990, 71, 2060–2068. [Google Scholar] [CrossRef]
- Sutherland, W.J. Predicting the ecological consequences of environmental change: A review of the methods. J. Appl. Ecol. 2006, 43, 599–616. [Google Scholar] [CrossRef]
- Burns, E.; Lindenmayer, D. Policy Handbook: Learning from Long-Term Research to Better Manage Biodiversity in Australia; CSIRO Publishing: Collingwood, Australia, 2014. [Google Scholar]
- Scheele, B.C.; Legge, S.; Armstrong, D.P.; Copley, P.; Robinson, N.; Southwell, D.; Westgate, M.J.; Lindenmayer, D.B. How to improve threatened species management: An Australian perspective. J. Environ. Manag. 2018, 223, 668–675. [Google Scholar] [CrossRef]
- Australian National Audit Office. Administration of the Biodiversity Fund Program; ANAO Report No. 10 2014–15; Australian National Audit Office: Canberra, Australia, 2014.
- Woinarski, J.C.Z. A framework for evaluating the adequacy of monitoring programs for threatened species. In Monitoring Threatened Species and Ecological Communities; Legge, S., Lindenmayer, D., Robinson, N., Scheele, B., Southwell, D., Wintle, B., Eds.; CSIRO Publishing: Melbourne, Australia, 2018; pp. 13–20. [Google Scholar]
- Russell-Smith, J.; Edwards, A.C.; Woinarski, J.; Fisher, A.; Murphy, B.P.; Lawes, M.J.; Crase, B.; Thurgate, N. North Australian tropical savannas: The Three Parks Savanna Fire-Effects Plot Network. In Biodiversity and Environmental Change: Monitoring, Challenges and Direction; Lindenmayer, D., Burns, E., Thurgate, N., Lowe, A., Eds.; CSIRO Publishing: Melbourne, Australia, 2014; pp. 335–378. [Google Scholar]
- Fleming, P.A.; Bateman, P.W. The good, the bad, and the ugly: Which Australian terrestrial mammal species attract most research? Mammal Rev. 2016, 46, 241–254. [Google Scholar] [CrossRef]
- Department of the Environment Water Heritage and the Arts. Assessment of Australia’s Terrestrial Biodiversity 2008; Report prepared by the Biodiversity Assessment Working Group of the National Land and Water Resources Audit for the Australian Government, Canberra; van Oosterzee, P., Ed.; Department of the Environment, Water, Heritage and the Arts: Canberra, Australia, 2009; p. 316.
- Jackson, W. Australia State of the Environment 2016: Drivers, Independent Report to the Australian Government Minister for the Environment and Energy; Australian Government Department of the Environment and Energy: Canberra, Australia, 2016.
- Jackson, W.; Argent, R.; Bax, N.; Clark, G.; Coleman, S.; Cresswell, I.; Emmerson, K.; Evans, K.; Hibberd, M.; Johnston, E.; et al. Australia State of the Environment 2016: Overview, Independent Report to the Australian Government Minister for the Environment and Energy; Australian Government Department of the Environment and Energy: Canberra, Australia, 2016.
- Lindenmayer, D.; Burns, E.L.; Dickman, C.R.; Green, P.T.; Hoffmann, A.A.; Keith, D.A.; Morgan, J.W.; Russell-Smith, J.; Wardle, G.M.; Gillespie, G.R.; et al. Save Australia’s ecological research. Science 2017, 357, 557. [Google Scholar] [CrossRef]
- Einoder, L.D.; Southwell, D.M.; Lahoz-Monfort, J.J.; Gillespie, G.R.; Fisher, A.; Wintle, B.A. Occupancy and detectability modelling of vertebrates in northern Australia using multiple sampling methods. PLoS ONE 2018, 13, e0203304. [Google Scholar] [CrossRef] [Green Version]
- Fisher, A.; Kutt, A. Biodiversity and Land Condition in Tropical Savanna Rangelands: Technical Report; Tropical Savannas CRC: Darwin, Australia, 2007. [Google Scholar]
- Kutt, A.; Eyre, T.; Fisher, A.; Hunt, L. A Biodiversity Monitoring Program for Australian Rangelands; Department of the Environment, Water, Heritage and the Arts: Canberra, Australia, 2009.
- Northern Territory Environment Protection Authority. Guidelines for Assessment of Impacts on Terrestrial Biodiversity; Version 2.0.; Northern Territory Environment Protection Authority: Darwin, Australia, 2013.
- Preece, N.D.; van Oosterzee, P.; Preece, L.D.; Newton, M. Fauna Survey Kimba Range Killarney Station; Olkola Aboriginal Corporation: Cooktown, Australia, 2014. [Google Scholar]
- Shellberg, J.; Preece, N.; van Oosterzee, P.; Grimes, K.; Turpin, G.; Newton, M.; Carroll, J.; Coates, J.; Preece, L.; Hogbin, A.; et al. Kimba Plateau Physical and Biological Diversity, Olkola Country, Cape York Peninsula; Funding from the Queensland Government’s Indigenous Land and Sea Grants Program through the Department of Environment and Heritage Protection; Olkola Aboriginal Corporation: Cooktown, Australia, 2014. [Google Scholar]
- Preece, N.; van Oosterzee, P.; Anthony, M.; Wargent, S.; Kapteyn, M.; Lacey, T.; Shioji, K. Biodiversity Survey 2018 Talaroo Station & Indigenous Protected Area; Ewamian Aboriginal Corporation: Mareeba, Australia, 2018. [Google Scholar]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J. Designing studies to detect differences in species occupancy: Power analysis under imperfect detection. Meth. Ecol. Evol. 2012, 3, 860–869. [Google Scholar] [CrossRef]
- Mistry, J.; Berardi, A. Bridging Indigenous and scientific knowledge. Science 2016, 352, 1274–1275. [Google Scholar] [CrossRef] [Green Version]
- Morrison, J.; Yu, P.; George, M. 2-Way Country–Challenges for inclusive, equitable, and prosperous development in North Australia. In Sustainable Land Sector Development in Northern Australia: Indigenous Rights, Aspirations, and Cultural Responsibilities; Russell-Smith, J., James, G., Pedersen, H., Sangha, K.K., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018. [Google Scholar]
- Ward-Fear, G.; Rangers, B.; Pearson, D.; Bruton, M.; Shine, R. Sharper eyes see shyer lizards: Collaboration with Indigenous peoples can alter the outcomes of conservation research. Conserv. Lett. 2019, 12, e12643. [Google Scholar] [CrossRef] [Green Version]
- Skroblin, A.; Carboon, T.; Bidu, G.; Chapman, N.; Miller, M.; Taylor, K.; Taylor, W.; Game, E.T.; Wintle, B.A. Including Indigenous knowledge in species distribution modelling for increased ecological insights. Conserv. Biol. 2021, 35, 587–597. [Google Scholar] [CrossRef]
- Ward-Fear, G.; Pauly, G.B.; Vendetti, J.E.; Shine, R. Authorship protocols must change to credit citizen scientists. Trends Ecol. Evol. 2020, 35, 187–190. [Google Scholar] [CrossRef]
- Austin, B.J.; Robinson, C.J.; Fitzsimons, J.A.; Sandford, M.; Ens, E.J.; Macdonald, J.M.; Hockings, M.; Hinchley, D.G.; McDonald, F.B.; Corrigan, C.; et al. Integrated measures of Indigenous land and sea management effectiveness challenges and opportunities for improved conservation partnerships in Australia. Conserv. Soc. 2018, 16, 372–384. [Google Scholar] [CrossRef]
- Woinarski, J.; Green, J.; Fisher, A.; Ensbey, M.; Mackey, B. The effectiveness of conservation reserves: Land tenure Impacts upon biodiversity across extensive natural landscapes in the tropical savannahs of the Northern Territory, Australia. Land 2013, 2, 20–36. [Google Scholar] [CrossRef] [Green Version]
- Fitzsimons, J.A. Private protected areas in Australia: Current status and future directions. Nat. Conserv. 2015, 10, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Moritz, C.; Ens, E.; Potter, S.; Catullo, R. The Australian monsoonal tropics: An opportunity to protect unique biodiversity and secure benefits for Aboriginal communities. Pac. Conserv. Biol. 2013, 19, 343–355. [Google Scholar] [CrossRef]
- Leiper, I.; Zander, K.K.; Robinson, C.J.; Carwadine, J.; Moggridge, B.J.; Garnett, S.T. Quantifying current and potential contributions of Australian Indigenous peoples to threatened species management. Conserv. Biol. 2018, 32, 1038–1047. [Google Scholar] [CrossRef]
- Ens, E.J.; Towler, G.M.; Daniels, C.; Rangers, Y.M.; Rangers, M. Looking back to move forward: Collaborative ecological monitoring in remote Arnhem Land. Ecol. Manag. Restor. 2012, 13, 26–35. [Google Scholar] [CrossRef]
- Partridge, R.; Skroblin, A. Threatened species monitoring on Aboriginal land: Finding the common ground between Kuka, Jukurrpa, Ranger work and science. In Monitoring Threatened Species and Ecological Communities; Legge, S., Lindenmayer, D., Robinson, N., Scheele, B., Southwell, D., Wintle, B., Eds.; CSIRO Publishing: Melbourne, Australia, 2018; pp. 321–332. [Google Scholar]
- Moorcroft, H.; Ignjic, E.; Cowell, S.; Goonack, J.; Mangolomara, S.; Oobagooma, J.; Karadada, R.; Williams, D.; Waina, N. Conservation planning in a cross-cultural context: The Wunambal Gaambera Healthy Country Project in the Kimberley, Western Australia. Ecol. Manag. Restor. 2012, 13, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Preece, N.; Locke, J.; Turpin, G. Perspectives on research protocols on Indigenous conservation lands: Outcomes from a talking circle. Ecol. Manag. Restor. 2022, 23, 5–9. [Google Scholar] [CrossRef]
- Heiner, M.; Hinchley, D.; Fitzsimons, J.; Weisenberger, F.; Bergmann, W.; McMahon, T.; Milgin, J.; Nardea, L.; Oakleaf, J.; Parriman, D.; et al. Moving from reactive to proactive development planning to conserve Indigenous community and biodiversity values. Environ. Impact Assess. Rev. 2019, 74, 1–13. [Google Scholar] [CrossRef]
- Australian National Audit Office. The Conservation and Protection of National Threatened Species and Ecological Communities; Audit Report No.31 2006–07; Australian National Audit Office: Canberra, Australia, 2007.
- Queensland Audit Office. Conserving Threatened Species; Report 7: 2018–19; Queensland Audit Office: Brisbane, Australia, 2018.
- Preece, N.D.; Eyre, T.; Sparrow, B.; Horton, B.; Foster, A.; Wills, T.; Burns, E.; Wardle, G.; Andersen, A.; Mele, P.; et al. Taking our Environmental Pulse. A Strategy for Monitoring Ecosystems in Australia; Ecosystem Science Council: Sydney, Australia, 2020. [Google Scholar]
- Einoder, L.D.; Gillespie, G.R.; Southwell, D.M.; Fisher, A. Evaluation and Redesign of the Northern Territory Top End National Parks Ecological Monitoring Program; Technical Report to the Northern Territory Parks and Wildlife Commission; Flora and Fauna Division, Department of Environment and Natural Resources: Darwin, Australia, 2018.
- Caravaggi, A.; Banks, P.B.; Burton, A.C.; Finlay, C.M.V.; Haswell, P.M.; Hayward, M.W.; Rowcliffe, M.J.; Wood, M.D. A review of camera trapping for conservation behaviour research. Remote Sens. Ecol. Conserv. 2017, 3, 109–122. [Google Scholar] [CrossRef]
- De Bondi, N.; White, J.G.; Stevens, M.; Cooke, R. A comparison of the effectiveness of camera trapping and live trapping for sampling terrestrial small-mammal communities. Wildl. Res. 2010, 37, 456–465. [Google Scholar] [CrossRef]
- Diete, R.L.; Meek, P.D.; Dixon, K.M.; Dickman, C.R.; Leung, L.K.-P. Best bait for your buck: Bait preference for camera trapping north Australian mammals. Austral. J. Zool. 2016, 63, 376–382. [Google Scholar] [CrossRef]
- Driessen, M.M.; Jarman, P.J.; Troy, S.; Callander, S. Animal detections vary among commonly used camera trap models. Wildl. Res. 2017, 44, 291–297. [Google Scholar] [CrossRef]
- Ens, E.; Scott, M.L.; Rangers, Y.M.; Moritz, C.; Pirzl, R. Putting Indigenous conservation policy into practice delivers biodiversity and cultural benefits. Biodivers. Conserv. 2016, 25, 2889–2906. [Google Scholar] [CrossRef]
- Gillespie, G.; Risler, J.; Gentles, T.; Hill, B.; Stokeld, D.; Mahney, T.; Young, S.; Buckley, K. Camera Traps: Guidelines for use of Reconyx Hyperfire cameras in Biodiversity and Monitoring Projects. Standard Operating Procedures; Northern Territory Department of Land Resource Management: Darwin, Australia, 2017.
- Gillespie, G.R.; Brennan, K.; Gentles, T.; Hill, B.; Low Choy, J.; Mahney, T.; Stevens, A.; Stokeld, D. A Guide for the Use of Remote Cameras for Wildlife Survey in Northern Australia; Charles Darwin University: Casuarina, NT, Australia, 2015. [Google Scholar]
- Hoskin, C.; Grigg, G.; Stewart, D.; MacDonald, S. Frogs of Australia (1.1 (4614)); Mobile Application Software; Ug Media: Townsville, Australia, 2015. [Google Scholar]
- Jumeau, J.; Petrod, L.; Handrich, Y. A comparison of camera trap and permanent recording video camera efficiency in wildlife underpasses. Ecol. Evol. 2017, 7, 7399–7407. [Google Scholar] [CrossRef] [Green Version]
- Lepard, C.C.; Moll, R.J.; Cepek, J.D.; Lorch, P.D.; Dennis, P.M.; Robison, T.; Montgomery, R.A. The influence of the delay-period setting on camera-trap data storage, wildlife detections and occupancy models. Wildl. Res. 2019, 46, 37–53. [Google Scholar] [CrossRef]
- Marcus Rowcliffe, J. Key frontiers in camera trapping research. Remote Sens. Ecol. Conserv. 2017, 3, 107–108. [Google Scholar] [CrossRef]
- Meek, P.; Ballard, G.; Fleming, P.; Falzon, G. Are we getting the full picture? Animal responses to camera traps and implications for predator studies. Ecol. Evol. 2016, 6, 3216–3225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meek, P.D.; Ballard, G.-A.; Fleming, P.J.S. The pitfalls of wildlife camera trapping as a survey tool in Australia. Austral. Mammal. 2015, 37, 13–22. [Google Scholar] [CrossRef]
- Morcombe, M.; Stewart, D. The Michael Morcombe eGuide to the Birds of Australia; PDA Solutions T/A/MyDigitalEarth: Johannesburg, South Africa, 2011. [Google Scholar]
- Pizzey, G.; Knight, F. Birds of Australia; Digital Edition Version 1.5; Gibbon Multimedia: Melbourne, Australia, 2013. [Google Scholar]
- Potter, L.C.; Brady, C.J.; Murphy, B.P. Accuracy of identifications of mammal species from camera trap images: A northern Australian case study. Austral Ecol. 2018, 44, 473–483. [Google Scholar] [CrossRef]
- Randler, C.; Kalb, N. Distance and size matters: A comparison of six wildlife camera traps and their usefulness for wild birds. Ecol. Evol. 2018, 8, 7151–7163. [Google Scholar] [CrossRef] [Green Version]
- Richardson, E.; Nimmo, D.G.; Avitabile, S.; Tworkowski, L.; Watson, S.J.; Welbourne, D.; Leonard, S.W.J. Camera traps and pitfalls: An evaluation of two methods for surveying reptiles in a semiarid ecosystem. Wildl. Rese. 2017, 44, 637–647. [Google Scholar] [CrossRef]
- Roe, P.; Eichinski, P.; Fuller, R.A.; McDonald, P.G.; Schwarzkopf, L.; Towsey, M.; Truskinger, A.; Tucker, D.; Watson, D.M. The Australian Acoustic Observatory. Methods Ecol. Evol. 2021, 12, 1802–1808. [Google Scholar] [CrossRef]
- Stokeld, D.; Frank, A.S.K.; Hill, B.; Choy, J.L.; Mahney, T.; Stevens, A.; Young, S.; Rangers, D.; Rangers, W.; Gillespie, G. Multiple cameras required to reliably detect feral cats in northern Australian tropical savanna: An evaluation of sampling design when using camera traps. Wildl. Res. 2016, 42, 642–649. [Google Scholar] [CrossRef]

| Scientific Name | Common Name | Distribution | Conservation Status: National or International Level | Conservation Status: State/Territory Level | Summed Monitoring Score (1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| EPBCA | IUCN | APAM | WA | NT | Qld | Score + (Naïve Score) *** | |||
| TACHYGLOSSIDAE | |||||||||
| Tachyglossus aculeatus | Short-beaked echidna | RX | LC | LC | SLC | 28 | |||
| Zaglossus bruijnii | Western long-beaked echidna | X | CR | EX | |||||
| ORNITHORHYNCHIDAE | |||||||||
| Ornithorhynchus anatinus | Platypus | R | LC > NT | NT | 25 | ||||
| DASYURIDAE | |||||||||
| Antechinomys laniger | Kultarr | R | LC | LC | LC | n | |||
| Antechinus bellus | Fawn antechinus | VU | LC > VU | VU | EN | 19 | |||
| Antechinus leo | Cinnamon antechinus | LC | LC | LC | n | ||||
| Pseudantechinus bilarni | Sandstone antechinus | NT > LC | LC | LC | 20 | ||||
| Pseudantechinus mimulus | Carpentarian antechinus | VU > nl | EN > NT | NT | LC | 15 | |||
| Pseudantechinus ningbing | Ningbing antechinus | LC | LC | n | |||||
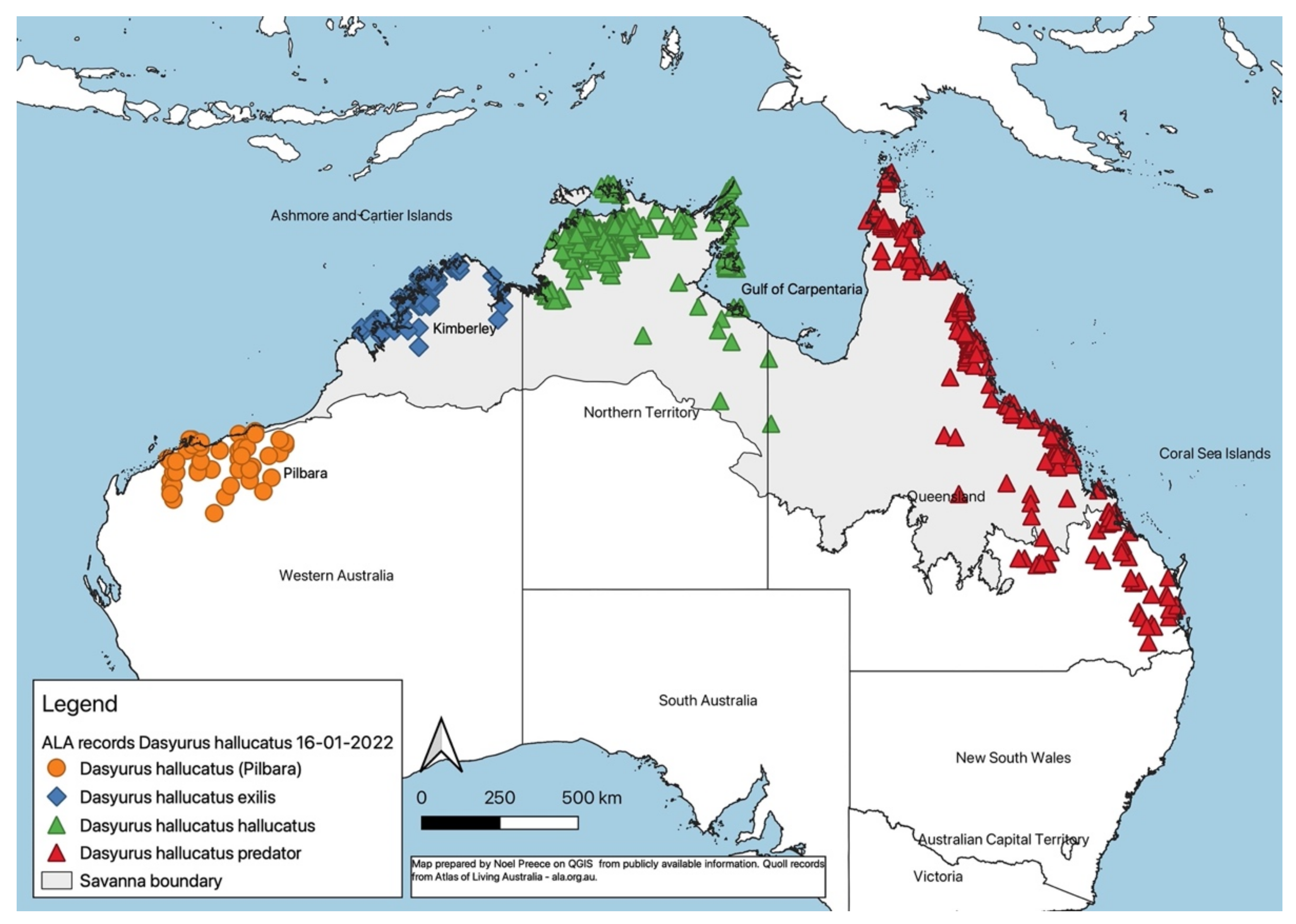
| Dasyurus hallucatus | Northern quoll | R | EN | EN | EN | EN | CR | LC | 28 |
| Dasyurus hallucatus hallucatus | Northern quoll (NT) | nl | nl | nl | 28 ** | ||||
| Dasyurus hallucatus exilis | Northern quoll (Kimberley, WA) | nl | nl | nl | n (28) | ||||
| Dasyurus hallucatus predator | Northern quoll (Cape York, Qld) | nl | nl | nl | n (12) | ||||
| Dasyurus hallucatus (Pilbara) | Northern quoll (Pilbara, WA) | nl | nl | nl | n (28) | ||||
| Dasyurus maculatus gracilis | Spotted-tailed quoll (northern subspecies) | nl | EN | EN | 24 | ||||
| Phascogale pirata | Northern brush-tailed phascogale | VU | VU | VU | EN | 9 | |||
| Phascogale tapoatafa kimberleyensis | Brush-tailed phascogale | R | NT > nl | NT | EN | 0 | |||
| Planigale ingrami | Long-tailed planigale | R | LC | LC | n | ||||
| Planigale maculata | Common planigale | R | LC | LC | LC | n | |||
| Sminthopsis archeri | Chestnut dunnart | X | DD | NT | NT | 0 | |||
| Sminthopsis bindi | Kakadu dunnart | LC > NT | NT | 13 | |||||
| Sminthopsis butleri | Butler’s dunnart | VU | VU | VU | EN > VU | NT > VU | 14 | ||
| Sminthopsis douglasi | Julia Creek dunnart | EN > VU | NT | NT | EN | 16 | |||
| Sminthopsis macroura | Stripe-faced dunnart | R | LC | LC | LC | n | |||
| Sminthopsis virginiae | Red-cheeked dunnart | X | LC | LC | n | ||||
| PERAMELIDAE | |||||||||
| Echymipera rufescens | Long-nosed echymipera | X | LC | LC | LC | n | |||
| Isoodon auratus auratus | Golden bandicoot | R | VU * | VU > nl | VU | EN * > VU | EN | 25 | |
| Isoodon macrourus | Northern brown bandicoot | RX | LC | LC | LC | n | |||
| Isoodon peninsulae | Cape York brown bandicoot | LC | LC | n | |||||
| Perameles pallescens | Northern Long-nosed bandicoot | LC | LC | n | |||||
| THYLACOMYIDAE | |||||||||
| Macrotis lagotis | Greater bilby | R | VU | VU | VU | EN > VU | VU | EN | 25 |
| PHASCOLARCTIDAE | |||||||||
| Phascolarctos cinereus | Koala | R | (VU) > EN | LC > VU | VU | VU | 28 | ||
| VOMBATIDAE | |||||||||
| Lasiorhinus krefftii | Northern hairy-nosed wombat | R | EN > CR | CR | CR | EN > CR | 41 | ||
| PETAURIDAE | |||||||||
| Dactylopsila trivirgata | Striped possum | RX | LC | LC | LC | n | |||
| Petaurus australis | Yellow-bellied glider | R | LC > NT | NT | 24 | ||||
| Petaurus australis unnamed subsp. | Yellow-bellied glider (northern subspecies) | EN | n | ||||||
| Petaurus breviceps | Sugar glider | RX | LC | LC | LC | n | |||
| Petaurus gracilis | Mahogany glider | EN | EN | EN | EN | 18 | |||
| Petaurus norfolcensis | Squirrel glider | R | LC | LC | LC | n | |||
| PSEUDOCHEIRIDAE | |||||||||
| Petauroides minor | Northern greater glider | nl as sp. | VU | n | |||||
| Petauroides volans | Greater glider (southern) | R | VU | LC > VU | VU | VU | 19 | ||
| Petropseudes dahli | Rock ringtail possum | LC | LC | P3 | LC | n | |||
| ACROBATIDAE | |||||||||
| Acrobates pygmaeus | Feathertail glider | R | LC | LC | LC | n | |||
| PHALANGERIDAE | |||||||||
| Spilocuscus maculatus | Common spotted cuscus | RX | LC | LC | LC | n | |||
| Phalanger mimicus | Southern common cuscus | RX | LC | LC | LC | n | |||
| Trichosurus vulpecula | Common brushtail possum | R | LC | LC | LC | n | |||
| Trichosurus vulpecula arnhemensis | Common brushtail possum (Arnhem subsp.) | nl | nl | 16 | |||||
| Trichosurus vulpecula vulpecula | Common brushtail possum (NT and WA) | nl | EN | n | |||||
| Trichosurus vulpecula eburacensis | Common brushtail possum (Cape York) | nl | n (0) | ||||||
| Wyulda squamicaudata | Scaly-tailed possum | DD > NT | NT | P4 (NT) | 24 | ||||
| POTOROIDAE | |||||||||
| Aepyprymnus rufescens | Rufous bettong | R | LC | LC | LC | n | |||
| Bettongia lesueur | Boodie | R | NT | NT (CD) | 33 | ||||
| Bettongia lesueur graii | Boodie | R | NT | NT (CD) | EN > EX | EX | n | ||
| Bettongia tropica | Northern bettong | EN | EN | EN | EN | 23 | |||
| MACROPODIDAE | |||||||||
| Lagorchestes conspicillatus | Spectacled hare-wallaby | RX | LC | NT | LC | 0 | |||
| Macropus agilis | Agile wallaby | RX | LC | LC | n | ||||
| Osphranter(Macropus) antilopinus | Antilopine wallaroo | LC | LC | n | |||||
| Osphranter(Macropus) bernardus | Black wallaroo | LC | NT | 0 | |||||
| Notamacropus (Macropus) dorsalis | Black-striped wallaby | R | LC | LC | |||||
| Macropus giganteus | Eastern grey kangaroo | R | LC | LC | |||||
| Notamacropus (Macropus) parryi | Whiptail wallaby | R | LC | LC | |||||
| Macropus robustus | Euro | R | LC | LC | |||||
| Osphranter (Macropus) rufus | Red kangaroo | R | LC | LC | |||||
| Onychogalea fraenata | Bridled nailtail wallaby | R | EN | EN > VU | VU | EN | 38 | ||
| Onychogalea unguifera | Northern nailtail wallaby | LC | LC | LC | n | ||||
| Petrogale assimilis | Allied rock-wallaby | LC | LC | LC | n | ||||
| Petrogale brachyotis | Western short-eared rock-wallaby | LC | LC | 0 | |||||
| Petrogale burbidgei | Monjon | NT | NT | P4 (NT) | 18 | ||||
| Petrogale coenensis | Cape York rock-wallaby | EN | NT > EN | EN | VU | 0 | |||
| Petrogale concinna | Nabarlek | DD > EN | NT | VU | 14 | ||||
| Petrogale concinna canescens | Nabarlek (Top End) | VU > EN | nl | VU | EN | 0 | |||
| Petrogale concinna concinna | Nabarlek (Victoria River district) | EN | nl | CR (poss. EX) | CR (poss. EX) | 0 | |||
| Petrogale concinna monastria | Kimberley nabarlek | EN | nl | NT | EN | 15 | |||
| Petrogale godmani | Godman’s rock-wallaby | LC > NT | NT | LC | 0 | ||||
| Petrogale herberti | Herbert’s rock-wallaby | LC | LC | LC | n | ||||
| Petrogale inornata | Unadorned rock-wallaby | LC | LC | LC | n | ||||
| Petrogale lateralis | Black-footed rock-wallaby | R | EN > nl | NT | EN | VU | 25 | ||
| Petrogale lateralis kimberleyensis | Black-footed rock-wallaby | R | EN | NT | EN | EN | 10 | ||
| Petrogale mareeba | Mareeba rock-wallaby | LC > NT | NT | LC | 0 | ||||
| Petrogale penicillata | Brush-tailed rock-wallaby | VU | VU | n | |||||
| Petrogale persephone | Proserpine rock-wallaby | EN | EN | EN | EN | 32 | |||
| Petrogale purpureicollis | Purple-necked rock-wallaby | LC > NT | NT | VU | 0 | ||||
| Petrogale sharmani | Mount Claro rock-wallaby | NT > VU | VU | VU | 28 | ||||
| Petrogale wilkinsi | Eastern short-eared rock-wallaby | LC > nl | LC | LC | n | ||||
| Thylogale stigmatica | Red-legged pademelon | RX | LC | LC | LC | n | |||
| Wallabia bicolor | Swamp wallaby | R | LC | LC | LC | n | |||
| NOTORYCTIDAE | |||||||||
| Notoryctes caurinus | Kakarratul | R | EN > nl | DD > LC | LC | EN > P4 (NT) | n (0) | ||
| PTEROPODIDAE | |||||||||
| Dobsonia magna | Bare-backed fruit bat | X | LC | LC | LC | n | |||
| Macroglossus minimus | Northern blossom bat | RX | LC | LC | LC | n | |||
| Nyctimene robinsoni | Eastern tube-nosed bat | R | LC | LC | LC | n | |||
| Pteropus alecto | Black flying-fox | RX | LC | LC | LC | n | |||
| Pteropus conspicillatus | Spectacled flying-fox | RX | VU > EN | LC > EN | NT (CD) | EN | 28 | ||
| Pteropus macrotis | Large-eared flying-fox | X | LC | LC | n | ||||
| Pteropus scapulatus | Little red flying-fox | R | LC | LC | LC | n | |||
| Syconycteris australis | Eastern blossom bat | RX | LC | LC | LC | n | |||
| MEGADERMATIDAE | |||||||||
| Macroderma gigas | Ghost bat | R | VU | VU | VU | NT > VU | NT | EN | 19 |
| RHINOLOPHIDAE | |||||||||
| Rhinolophus megaphyllus | Eastern horseshoe-bat | RX | LC | LC | n | ||||
| Rhinolophus ‘intermediate’ | Lesser large-eared horseshoe-bat | VU | 0 | ||||||
| Rhinolophus philippinensis (robertsi) | Greater large-eared horseshoe-bat | VU | VU >LC | NT | EN | 0 | |||
| HIPPOSIDERIDAE | |||||||||
| Hipposideros ater | Dusky leaf-nosed bat | X | LC | LC | n | ||||
| Hipposideros cervinus | Fawn leaf-nosed Nat | X | LC | NT | VU | 9 | |||
| Hipposideros diadema reginae | Diadem leaf-nosed bat | X | LC (as H. diadema) | NT | VU | 0 | |||
| Hipposideros inornatus | Arnhem leaf-nosed bat | EN | VU | EN | VU | 0 | |||
| Hipposideros semoni | Semon’s leaf-nosed bat | RX | EN > VU | DD > LC | NT | EN | 13 | ||
| Hipposideros stenotis | Northern leaf-nosed bat | LC > VU | NT | NT > P2 | VU | 0 | |||
| Rhinonicteris aurantia | Orange leaf-nosed bat | R | LC | LC | EN > P4 (NT) | VU | n | ||
| EMBALLONURIDAE | |||||||||
| Saccolaimus flaviventris | Yellow-bellied sheath-tailed bat | R | LC | LC | LC | n | |||
| Saccolaimus mixtus | Cape York sheath-tailed bat | X | DD > NT | NT | LC | 0 | |||
| Saccolaimus saccolaimus nudicluniatus | Bare-rumped sheath-tailed bat | X | LC | NT | NT | EN | 0 | ||
| Taphozous australis | Coastal sheath-tailed bat | RX | NT | NT | NT | 0 | |||
| Taphozous georgianus | Common sheath-tailed bat | R | LC | LC | LC | n | |||
| Taphozous kapalgensis | Arnhem sheath-tailed bat | LC | LC | n | |||||
| Taphazous troughtoni | Troughton’s sheath-tailed bat | DD > nl | LC | LC | n | ||||
| MOLOSSIDAE | |||||||||
| Austronomus australis | White-striped free-tailed bat | R | LC | LC | LC | n | |||
| Chaerephon jobensis | Greater northern free-tailed bat | RX | LC | LC | LC | n | |||
| Setirostris (Mormopterus) eleryi | Bristle-faced free-tailed bat | R | LC | LC | LC | 15 | |||
| Ozimops (Mormopterus) lumsdenae | Northern free-tailed bat | R | LC | LC | n | ||||
| Mormopterus ridei | Eastern free-tailed bat | R | LC | LC | n | ||||
| Ozimops (Mormopterus) halli | Cape York free-tailed bat | DD | LC | 0 | |||||
| Ozimops (Mormopterus) cobourgianus | North-western free-tailed bat | R | LC | n | |||||
| MINIOPTERIDAE | |||||||||
| Miniopterus australis | Little bent-winged bat | RX | LC | LC | LC | ||||
| Miniopterus orianae | Common bent-wing bat | RX | NT > nl | LC | 36 | ||||
| VESPERTILIONIDAE | |||||||||
| Chalinolobus gouldii | Gould’s wattled bat | R | LC | LC | LC | n | |||
| Chalinolobus nigrogriseus | Hoary wattled bat | RX | LC | LC | LC | n | |||
| Murina florium | Flute-nosed bat | RX | LC | NT | VU | 13 | |||
| Myotis macropus | Large-footed myotis | RX | LC | LC | LC | n | |||
| Nyctophilus arnhemensis | Northern long-eared bat | R | LC | LC | LC | n | |||
| Nyctophilus bifax | Eastern long-eared bat | RX | LC | LC | LC | n | |||
| Nyctophilus daedalus | Pallid long-eared bat | LC | LC | n | |||||
| Nyctophilus geoffroyi | Lesser long-eared bat | R | LC | LC | LC | n | |||
| Nyctophilus gouldi | Gould’s long-eared bat | R | LC | LC | LC | n | |||
| Nyctophilus walkeri | Pygmy long-eared bat | LC | LC | LC | n | ||||
| Phoniscus papuensis | Golden-tipped bat | RX | LC | LC | LC | n | |||
| Pipistrellus adamsi | Cape York pipistrelle | LC | LC | LC | n | ||||
| Pipistrellus westralis | Northern pipistrelle | LC | LC | LC | n | ||||
| Scotorepens balstoni | Inland broad-nosed bat | R | LC | LC | LC | n | |||
| Scotorepens greyii | Little broad-nosed bat | R | LC | LC | LC | n | |||
| Scotorepens sanborni | Northern broad-nosed bat | RX | LC | LC | LC | n | |||
| Vespadelus caurinus | Western cave-bat | LC | LC | LC | n | ||||
| Vespadelus douglasorum | Yellow-lipped cave bat | LC | LC | NT > P2 | n | ||||
| Vespadelus finlaysoni | Inland cave bat | R | LC | LC | LC | n | |||
| Vespadelus troughtoni | Eastern cave bat | R | LC | LC | LC | n | |||
| MURIDAE | |||||||||
| Conilurus capricornensis | Capricornian rabbit-rat | R | EX | EX | n | ||||
| Conilurus penicillatus | Brush-tailed rabbit-rat | X | VU | NT > VU | VU | EN | VU | 13 | |
| Conilurus penicillatus melibius | Brush-tailed rabbit-rat (Tiwi Islands) | nl | nl | VU | 24 | ||||
| Conilurus penicillatus penicillatus | Brush-tailed rabbit-rat (Kimberley, Top End) | nl | nl | VU | VU | 18 | |||
| Hydromys chrysogaster | Water-rat | RX | LC | LC | NT | LC | n | ||
| Leggadina lakedownensis | Northern short-tailed mouse | R | LC | LC | NT | LC | n | ||
| Melomys burtoni | Grassland melomys | R | LC | LC | LC | n | |||
| Melomys capensis | Cape York melomys | LC | LC | LC | n | ||||
| Melomys cervinipes | Fawn-footed melomys | R | LC | LC | LC | n | |||
| Melomys rubicola | Bramble Cay melomys | EN > EX | CR > EX | CR (PE) | EXW | n | |||
| Mesembriomys gouldii | Black-footed tree-rat | NT > VU | VU | 16 | |||||
| Mesembriomys gouldii gouldii (Kimberley and mainland NT) | Black-footed tree-rat | nl > EN | nl | VU | EN | LC | 16 | ||
| Mesembriomys gouldii melvillensis (Melville Island) | Black-footed tree-rat | nl > VU | nl | VU | VU | LC | 22 | ||
| Mesembriomys gouldii rattoides (north Queensland) | Black-footed tree-rat | nl > VU | nl | VU | LC | 0 | |||
| Mesembriomys macrurus | Golden-backed tree-rat | VU > nl | LC > NT | NT | P4 (NT) | CR | 24 | ||
| Notomys alexis | Spinifex hopping-mouse | R | LC | LC | LC | n | |||
| Notomys aquilo | Northern hopping-mouse | VU | VU | EN | VU | VU | VU | 16 | |
| Notomys fuscus | Dusky hopping-mouse | nl > VU | EN | 20 | |||||
| Pogonomys sp. | Tree mouse | R | LC | LC | n | ||||
| Pseudomys calabyi | Kakadu pebble-mouse | VU | NT | NT | 18 | ||||
| Pseudomys delicatulus | Delicate mouse | RX | LC | LC | LC | n | |||
| Pseudomys desertor | Desert mouse | R | LC | LC | LC | n | |||
| Pseudomys gracilicaudatus | Eastern chestnut mouse | R | LC | LC | LC | n | |||
| Pseudomys johnsoni | Central pebble-mouse | R | LC | LC | LC | n | |||
| Pseudomys nanus | Western chestnut Mouse | LC | LC | LC | n | ||||
| Pseudomys patrius | Eastern pebble-mouse | R | LC | LC | LC | n | |||
| Rattus colletti | Dusky rat | LC | LC | LC | n | ||||
| Rattus fuscipes | Bush rat | R | LC | LC | LC | n | |||
| Rattus leucopus | Cape York rat | RX | LC | LC | LC | n | |||
| Rattus lutreolus | Swamp rat | R | LC | LC | LC | n | |||
| Rattus sordidus | Canefield rat | R | LC | LC | CR > nl | LC | n | ||
| Rattus tunneyi | Pale field-rat | R | LC | LC | VU | LC | 18 | ||
| Rattus villosissimus | Long-haired rat | R | LC | LC | LC | n | |||
| Uromys caudimaculatus | Giant white-tailed rat | RX | LC | LC | LC | n | |||
| Xeromys myoides | Water mouse | RX | VU | VU | VU | DD | VU | 21 | |
| Zyzomys argurus | Common rock-rat | R | LC | LC | LC | n | |||
| Zyzomys maini | Arnhem rock-rat | VU | VU | NT > VU | VU | VU | 25 | ||
| Zyzomys palatalis | Carpentarian rock-rat | EN | EN | CR | CR | CR > EN | 24 | ||
| Zyzomys woodwardi | Kimberley rock-rat | LC | LC | n | |||||
| CANIDAE | |||||||||
| Canis dingo | Dingo | R | LC | NT | 17 | ||||
| Rank (Out of 20 for Each Taxon) | Mammals | Extinction Likelihood |
|---|---|---|
| 2 | Northern hopping-mouse, Notomys aquilo | 0.48 |
| 3 | Carpentarian rock-rat, Zyzomys palatalis | 0.44 |
| 5 | Black-footed tree-rat (Kimberley and mainland NT), Mesembriomys gouldii gouldii | 0.39 |
| 8 | Nabarlek (Top End), Petrogale concinna canescens | 0.29 |
| 9 | Brush-tailed phascogale (Kimberley), Phascogale tapoatafa kimberleyensis | 0.28 |
| 10 | Brush-tailed rabbit-rat (Kimberley and Top End), Conilurus penicillatus penicillatus | 0.25 |
| 12 | Northern brush-tailed phascogale, Phascogale pirata | 0.23 |
| 15 | Brush-tailed rabbit-rat (Tiwi Islands), Conilurus penicillatus melibius | 0.21 |
| 19 | Northern bettong, Bettongia tropica | 0.14 |
| Birds | ||
| 20 | Alligator Rivers yellow chat, Epthianura crocea tunneyi | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preece, N.; Fitzsimons, J. Gaps in Monitoring Leave Northern Australian Mammal Fauna with Uncertain Futures. Diversity 2022, 14, 158. https://doi.org/10.3390/d14030158
Preece N, Fitzsimons J. Gaps in Monitoring Leave Northern Australian Mammal Fauna with Uncertain Futures. Diversity. 2022; 14(3):158. https://doi.org/10.3390/d14030158
Chicago/Turabian StylePreece, Noel, and James Fitzsimons. 2022. "Gaps in Monitoring Leave Northern Australian Mammal Fauna with Uncertain Futures" Diversity 14, no. 3: 158. https://doi.org/10.3390/d14030158
APA StylePreece, N., & Fitzsimons, J. (2022). Gaps in Monitoring Leave Northern Australian Mammal Fauna with Uncertain Futures. Diversity, 14(3), 158. https://doi.org/10.3390/d14030158






