Impacts of Anthropogenic Disturbances on Diatom Diversity in a Shallow Spring-Fed Pool
Abstract
:1. Introduction
2. Materials and Methods
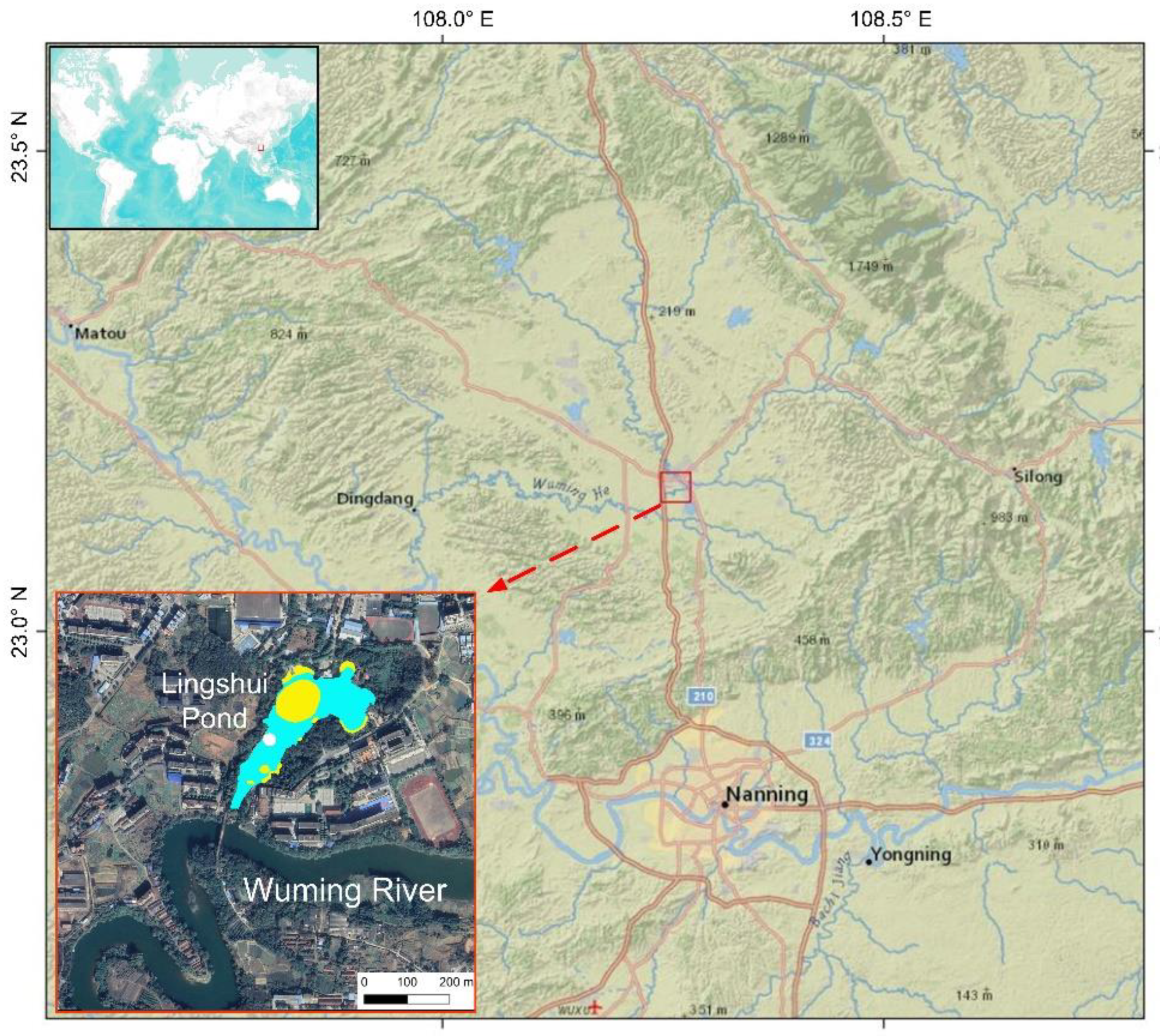
2.1. The Study Site, Sampling, and Chronology
2.2. Proxy Analyses
2.3. Data Analyses
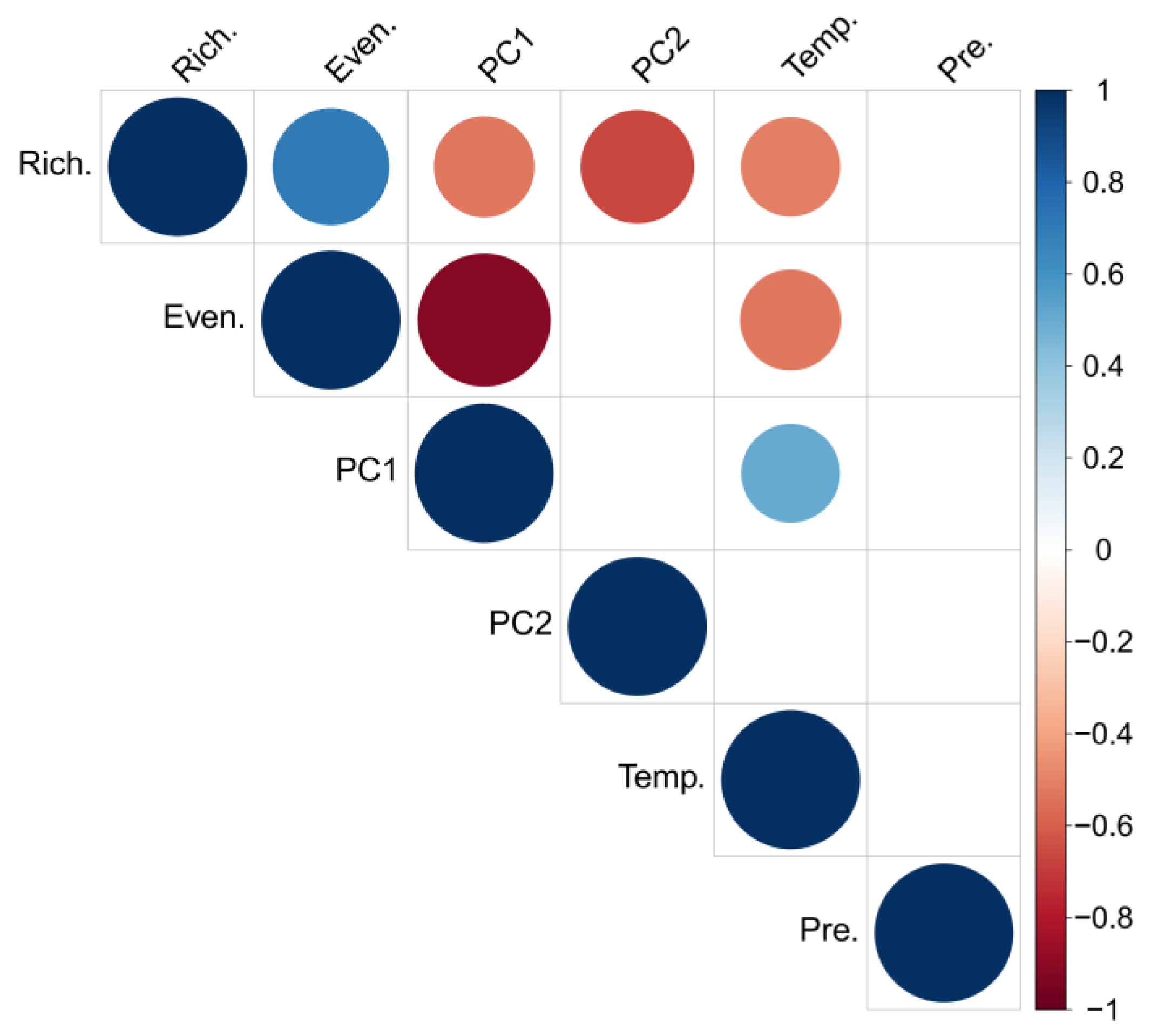
3. Results
3.1. Sediment Chronology
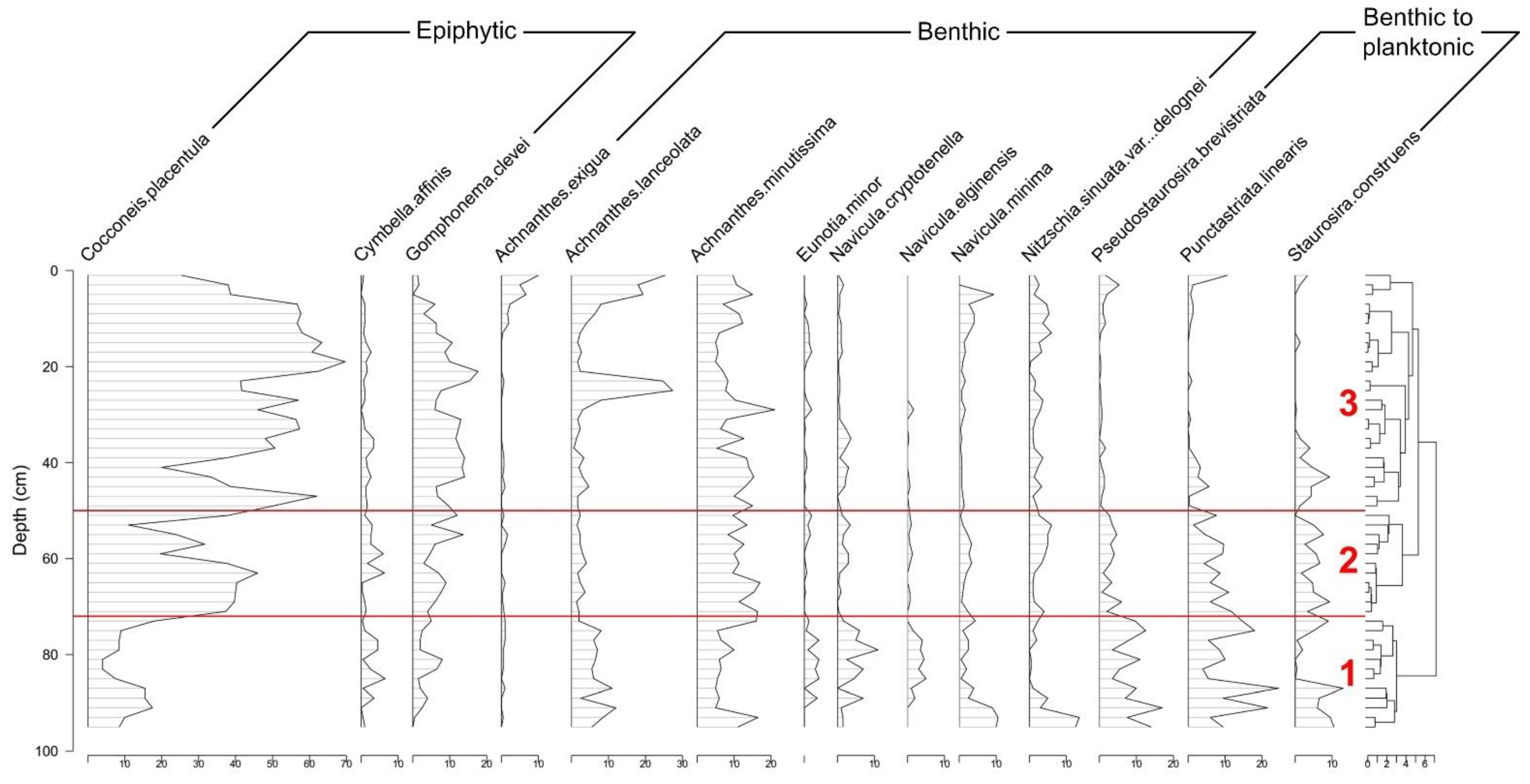
3.2. Sediment Geochemistry, Diatom Stratigraphy, and Diversity
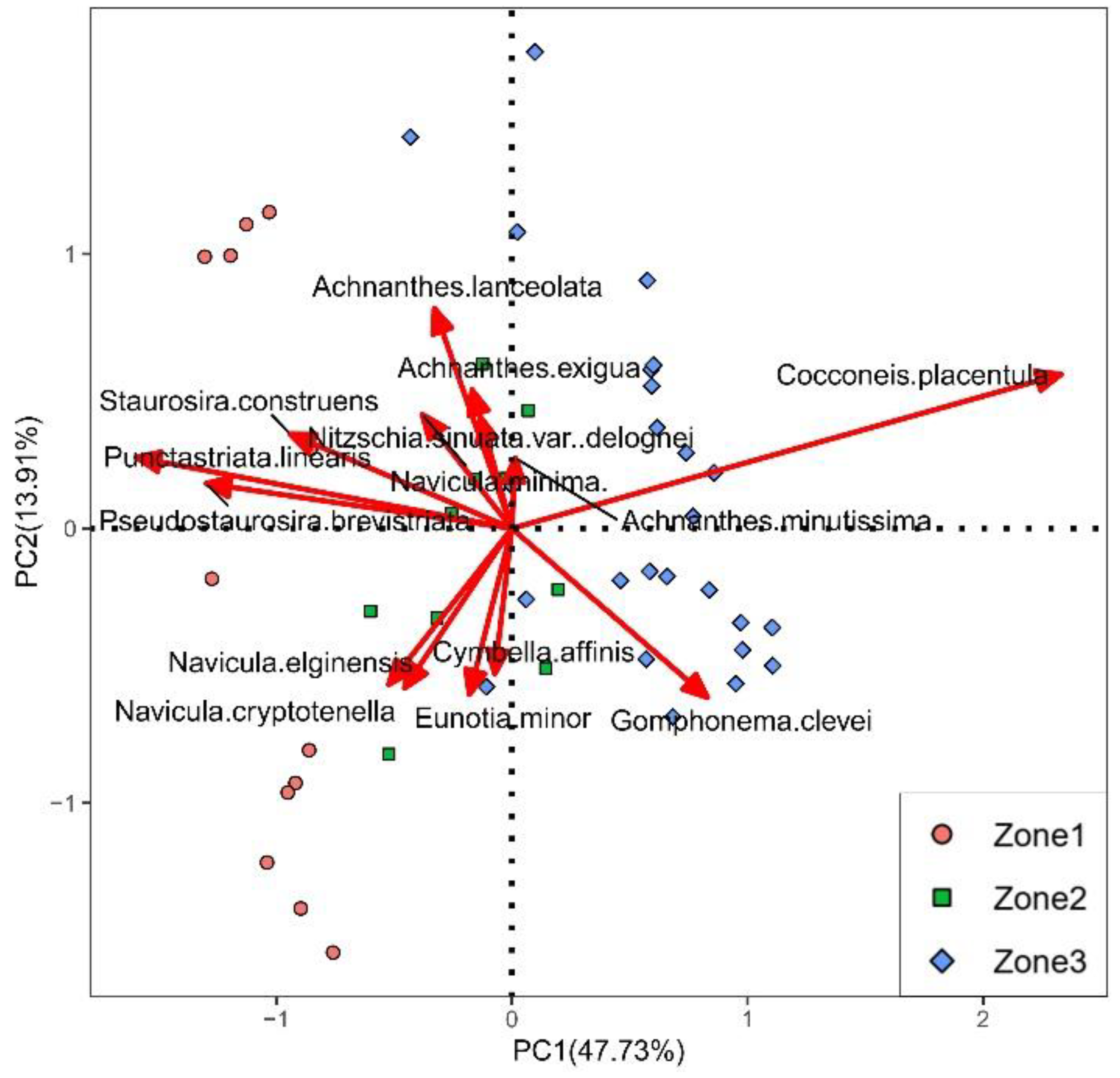
3.3. PCA Results
4. Discussion
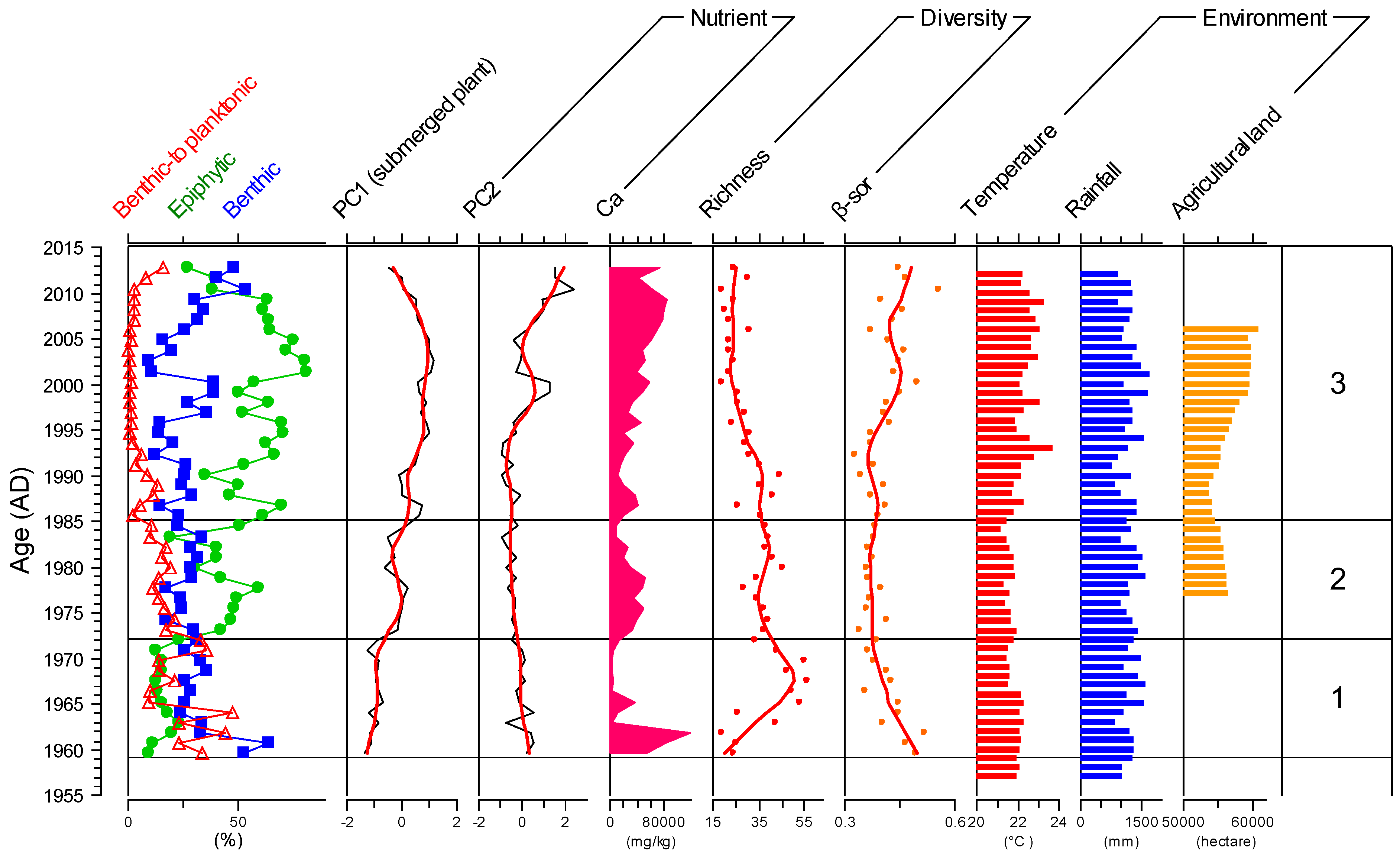
4.1. Reconstruction of the Status of LSP since 1960
4.2. Causes of Diatom-Diversity Changes and Strategies for Lake Ecosystem Restoration
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, F.; Jiang, G.; Zhao, H.; Polk, J.; Liu, S. Physicochemical Parameters and Phytoplankton as Indicators of the Aquatic Environment in Karstic Springs of South China. Sci. Total Environ. 2019, 659, 74–83. [Google Scholar] [CrossRef]
- Zhao, M.; Zeng, C.; Liu, Z.; Wang, S. Effect of Different Land Use/Land Cover on Karst Hydrogeochemistry: A Paired Catchment Study of Chenqi and Dengzhanhe, Puding, Guizhou, SW China. J. Hydrol. 2010, 388, 121–130. [Google Scholar] [CrossRef]
- Qicai, L. Influence of Dams on River Ecosystem and Its Countermeasures. J. Water Resour. Prot. 2011, 3, 60–66. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, J.; Pei, Z.; Lu, X.; Xu, S.; Wang, X. Effect of Dam Construction on Nutrient Deposition from a Small Agricultural Karst Catchment. Ecol. Indic. 2019, 107, 105548. [Google Scholar] [CrossRef]
- Guo, F.; Jiang, G.; Polk, J.S.; Huang, X.; Huang, S. Resilience of Groundwater Impacted by Land Use and Climate Change in a Karst Aquifer, South China. Water Environ. Res. 2015, 87, 1990–1998. [Google Scholar] [CrossRef]
- Guo, F.; Jiang, G. Hydro-Ecological Processes of Hyporheic Zone in a Karst Spring-Fed Pool: Effects of Groundwater Decline and River Backflow. J. Hydrol. 2020, 587, 124987. [Google Scholar] [CrossRef]
- Wang, L.-C.; Chou, Y.-M.; Chen, H.-F.; Chang, Y.-P.; Chiang, H.-W.; Yang, T.-N.; Shiau, L.-J.; Chen, Y.-G. Paleolimnological Evidence for Lacustrine Environmental Evolution and Paleo-Typhoon Records during the Late Holocene in Eastern Taiwan. J. Paleolimnol. 2021, 1–17. [Google Scholar] [CrossRef]
- Wang, L.-C. Using Paleoecological Data to Inform the Conservation Strategy for Floristic Diversity and Isoetes Taiwanensis in Northern Taiwan. Diversity 2021, 13, 395. [Google Scholar] [CrossRef]
- Thevenon, F.; Guédron, S.; Chiaradia, M.; Loizeau, J.-L.; Poté, J. (Pre-) Historic Changes in Natural and Anthropogenic Heavy Metals Deposition Inferred from Two Contrasting Swiss Alpine Lakes. Quat. Sci. Rev. 2011, 30, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-C.; Tang, Z.-W.; Chen, H.-F.; Li, H.-C.; Shiau, L.-J.; Huang, J.-J.S.; Wei, K.-Y.; Chuang, C.-K.; Chou, Y.-M. Late Holocene Vegetation, Climate, and Natural Disturbance Records from an Alpine Pond in Central Taiwan. Quat. Int. 2019, 528, 63–72. [Google Scholar] [CrossRef]
- Wang, L.-C.; Chang, Y.-P.; Li, H.-C.; Chen, S.-H.; Wu, J.-T.; Lee, T.-Q.; Shiau, L.-J. Revealing the Vegetation, Fire and Human Activities in the Lowland of Eastern Taiwan during Late Holocene. Quat. Int. 2020, 544, 32–40. [Google Scholar] [CrossRef]
- Birks, H.H.; Birks, H.J.B. Multi-Proxy Studies in Palaeolimnology. Veg. Hist. Archaeobot. 2006, 15, 235–251. [Google Scholar] [CrossRef] [Green Version]
- Xue, H.; Zheng, B.; Meng, F.; Wang, Y.; Zhang, L.; Cheng, P. Assessment of Aquatic Ecosystem Health of the Wutong River Based on Benthic Diatoms. Water 2019, 11, 727. [Google Scholar] [CrossRef] [Green Version]
- Wojtal, A.Z.; Sobczyk, Ł. The Influence of Substrates and Physicochemical Factors on the Composition of Diatom Assemblages in Karst Springs and Their Applicability in Water-Quality Assessment. Hydrobiologia 2012, 695, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-C.; Behling, H.; Chen, Y.-M.; Huang, M.-S.; Chen, C.-T.A.; Lou, J.-Y.; Chang, Y.-P.; Li, H.-C. Holocene Monsoonal Climate Changes Tracked by Multiproxy Approach from a Lacustrine Sediment Core of the Subalpine Retreat Lake in Taiwan. Quat. Int. 2014, 333, 69–76. [Google Scholar] [CrossRef]
- Li, H.-C.; Bischoff, J.L.; Ku, T.-L.; Zhu, Z.-Y. Climate and Hydrology of the Last Interglaciation (MIS 5) in Owens Basin, California: Isotopic and Geochemical Evidence from Core OL-92. Quat. Sci. Rev. 2004, 23, 49–63. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, L.; Xu, H.; Yan, Y.; Zhang, J.; Liu, Y.; Li, P.; Peng, Z.; Gu, Z.; Lu, H. Do Changes in Water Depth and Water Level Influence the Diatom Diversity of Yunlong Lake, in Yunnan Province, Southwest China? J. Paleolimnol. 2020, 64, 273–291. [Google Scholar] [CrossRef]
- Huh, C.-A.; Chen, W.; Hsu, F.-H.; Su, C.-C.; Chiu, J.-K.; Lin, S.; Liu, C.-S.; Huang, B.-J. Modern (<100 Years) Sedimentation in the Taiwan Strait: Rates and Source-to-Sink Pathways Elucidated from Radionuclides and Particle Size Distribution. Cont. Shelf Res. 2011, 31, 47–63. [Google Scholar] [CrossRef]
- von Gunten, L.; Grosjean, M.; Beer, J.; Grob, P.; Morales, A.; Urrutia, R. Age Modeling of Young Non-Varved Lake Sediments: Methods and Limits. Examples from Two Lakes in Central Chile. J. Paleolimnol. 2009, 42, 401–412. [Google Scholar] [CrossRef]
- Wang, L.-C.; Lee, T.-Q.; Chen, S.-H.; Wu, J.-T. Diatoms in Liyu Lake, Eastern Taiwan. Taiwania 2010, 55, 228–242. [Google Scholar]
- Watanabe, T.; Ohtsuka, T.; Tuji, A.; Houki, A. Picture Book and Ecology of the Freshwater Diatoms; Uchidarokakuho: Tokyo, Japan, 2005. [Google Scholar]
- Wu, J.-T.; Babu, B.; Chou, C.-L.; Saraswathi, S.J. Freshwater Diatom Flora of Taiwan; Biodiversity Research Center, Academia Sinica: Taipei, Taiwan, 2011; p. 747. [Google Scholar]
- Wang, Q.; Zhi, C.; Hamilton, P.; Kang, F. Diatom Distributions and Species Optima for Phosphorus and Current Velocity in Rivers from ZhuJiang Watershed within a Karst Region of South-Central China. Fundam. Appl. Limnol. 2009, 175, 125. [Google Scholar] [CrossRef]
- Tan, X.; Sheldon, F.; Bunn, S.E.; Zhang, Q. Using Diatom Indices for Water Quality Assessment in a Subtropical River, China. Environ. Sci. Pollut. Res. 2013, 20, 4164–4175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayasi, H.; Idei, M.; Mayama, S.; Nagumo, T.; Osada, K.H. Kobayasi’s Atlas of Japanese Diatoms Based on Electron Microscopy; Uchidarokakuho: Tokyo, Japan, 2006. [Google Scholar]
- Juggins, S. Rioja: Analysis of Quaternary Science Data, R Package Version (0.9–26). 2020. Available online: https://cran.r-project.org/package=rioja (accessed on 17 January 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Juggins, S. C2 Version 1.5: Software for Ecological and Palaeoecological Data Analysis and Visualisation; University of Newcastle: Newcastle, Australia, 2005. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’Hara, R.G.; Simpson, G.L.; Solymos, P.; Stevens, M.; Wagner, H. Multivariate Analysis of Ecological Communities in R: Vegan Tutorial. R Package Version 1.7. 2013. Available online: https://CRAN.R-project.org/package=vegan (accessed on 17 January 2022).
- Baselga, A.; Orme, C.D.L. Betapart: An R Package for the Study of Beta Diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Harrell, F.E.; Dupont, C. Hmisc: Harrell Miscellaneous. R Package Version 4.1-1. 2018. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 17 January 2022).
- Aliff, M.N.; Reavie, E.D.; Post, S.P.; Zanko, L.M. Anthropocene Geochemistry of Metals in Sediment Cores from the Laurentian Great Lakes. PeerJ 2020, 8, e9034. [Google Scholar] [CrossRef] [PubMed]
- Grimm, E.C. CONISS: A FORTRAN 77 Program for Stratigraphically Constrained Cluster Analysis by the Method of Incremental Sum of Squares. Comput. Geosci. 1987, 13, 13–35. [Google Scholar] [CrossRef]
- Pielou, E.C. Species-Diversity and Pattern-Diversity in the Study of Ecological Succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef]
- Song, B.; Wang, R.; Wang, Q.; Kong, L.; Hu, Z.; Zheng, W.; Yang, X. Pollen and Diatom Record Long-Term Complex Relationships between Diversity and Stability in a Lake and Nearby Vegetation from Tingming Lake in Yunnan, SW China. Quat. Int. 2021, 580, 87–94. [Google Scholar] [CrossRef]
- Jantz, N.; Behling, H. A Holocene Environmental Record Reflecting Vegetation, Climate, and Fire Variability at the Páramo of Quimsacocha, Southwestern Ecuadorian Andes. Veg. Hist. Archaeobot. 2012, 21, 169–185. [Google Scholar] [CrossRef] [Green Version]
- Rojas, L.A.; Hassan, G.S. Distribution of Epiphytic Diatoms on Five Macrophytes from a Pampean Shallow Lake: Host-Specificity and Implications for Paleoenvironmental Reconstructions. Diatom Res. 2017, 32, 263–275. [Google Scholar] [CrossRef]
- Wang, P.X.; Wang, B.; Cheng, H.; Fasullo, J.; Guo, Z.T.; Kiefer, T.; Liu, Z.Y. The Global Monsoon across Timescales: Coherent Variability of Regional Monsoons. Clim. Past 2014, 10, 2007–2052. [Google Scholar] [CrossRef] [Green Version]
- Chin, Z.W.; Arumugam, K.; Ashari, S.E.; Faizal Wong, F.W.; Tan, J.S.; Ariff, A.B.; Mohamed, M.S. Enhancement of Biomass and Calcium Carbonate Biomineralization of Chlorella Vulgaris through Plackett–Burman Screening and Box–Behnken Optimization Approach. Molecules 2020, 25, 3416. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-F.; Wei, K.-Y.; Huang, J.-J.S.; Lin, C.-C.; Su, C.-C.; Song, G.-S.; Li, H.-C.; Lee, T.-Q.; Song, S.-R.; Pan, H.-J. High-Resolution Records of Anthropogenic Activity and Geohazards from the Reservoir of Sun Moon Lake, Central Taiwan. Elem. Sci. Anthr. 2021, 9, 00150. [Google Scholar] [CrossRef]
- Chuan, O.M.; Yunus, K. Metals Pollution in Tropical Wetlands. In Wetlands Management-Assessing Risk and Sustainable Solutions; IntechOpen: London, UK, 2019; pp. 29–44. [Google Scholar]
- Soininen, J.; Passy, S.; Hillebrand, H. The Relationship between Species Richness and Evenness: A Meta-Analysis of Studies across Aquatic Ecosystems. Oecologia 2012, 169, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Zorzal-Almeida, S.; Bartozek, E.C.R.; Bicudo, D.C. Homogenization of Diatom Assemblages Is Driven by Eutrophication in Tropical Reservoirs. Environ. Pollut. 2021, 288, 117778. [Google Scholar] [CrossRef]
- Cantonati, M.; Gerecke, R.; Bertuzzi, E. Springs of the Alps–Sensitive Ecosystems to Environmental Change: From Biodiversity Assessments to Long-Term Studies. Hydrobiologia 2006, 562, 59–96. [Google Scholar] [CrossRef]
- Pla-Rabés, S.; Catalan, J. Diatom Species Variation between Lake Habitats: Implications for Interpretation of Paleolimnological Records. J. Paleolimnol. 2018, 60, 169–187. [Google Scholar] [CrossRef]
- Xu, W.; Hu, W.; Deng, J.; Zhu, J.; Zhou, N.; Liu, X. Impacts of Water Depth and Substrate Type on Vallisneria Natans at Wave-Exposed and Sheltered Sites in a Eutrophic Large Lake. Ecol. Eng. 2016, 97, 344–354. [Google Scholar] [CrossRef]
- Gu, J.; Xu, Z.; Jin, H.; Ning, X.; He, H.; Yu, J.; Jeppesen, E.; Li, K. Response of Vallisneria Natans to Increasing Nitrogen Loading Depends on Sediment Nutrient Characteristics. Water 2016, 8, 563. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, L.; Li, J.; Li, J.; Zou, Y.; Zhang, J.; Li, P.; Liu, Y.; Xu, B.; Gu, Z.; et al. Diatom Response to Climatic Warming over the Last 200years: A Record from Gonghai Lake, North China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 495, 48–59. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the Turnover and Nestedness Components of Beta Diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Wang, R.; Hu, Z.; Wang, Q.; Xu, M.; Zheng, W.; Zhang, K.; Yang, X. Discrepancy in the Responses of Diatom Diversity to Indirect and Direct Human Activities in Lakes of the Southeastern Tibetan Plateau, China. Anthropocene 2020, 30, 100243. [Google Scholar] [CrossRef]
- Li, L.; Bonser, S.P.; Lan, Z.; Xu, L.; Chen, J.; Song, Z. Water Depth Affects Reproductive Allocation and Reproductive Allometry in the Submerged Macrophyte Vallisneria Natans. Sci. Rep. 2017, 7, 16842. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Huang, S.; Yuan, J.; Du, Z.; Cao, Y.; Yuan, H.; Zhou, W.; Wu, J.; Yi, H.; Chen, B.; et al. Effect Evaluation of Remediation in Three Typical Rivers with Vallisneria Natans under Different Pollution and Spatial Characterization of Functional Microorganisms. Ecol. Eng. 2021, 171, 106353. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, L.; Liu, G.; Liu, W. Heavy Metals in Water, Sediments and Submerged Macrophytes in Ponds around the Dianchi Lake, China. Ecotoxicol. Environ. Saf. 2014, 107, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Cao, T.; Fu, H.; Ni, L.; Zhang, X.; Li, W.; Song, X.; Xie, P.; Jeppesen, E. Linking Carbon and Nitrogen Metabolism to Depth Distribution of Submersed Macrophytes Using High Ammonium Dosing Tests and a Lake Survey. Freshw. Biol. 2013, 58, 2532–2540. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-C.; Li, H.-C.; Shiau, L.-J. Impacts of Anthropogenic Disturbances on Diatom Diversity in a Shallow Spring-Fed Pool. Diversity 2022, 14, 166. https://doi.org/10.3390/d14030166
Wang L-C, Li H-C, Shiau L-J. Impacts of Anthropogenic Disturbances on Diatom Diversity in a Shallow Spring-Fed Pool. Diversity. 2022; 14(3):166. https://doi.org/10.3390/d14030166
Chicago/Turabian StyleWang, Liang-Chi, Hong-Chun Li, and Liang-Jian Shiau. 2022. "Impacts of Anthropogenic Disturbances on Diatom Diversity in a Shallow Spring-Fed Pool" Diversity 14, no. 3: 166. https://doi.org/10.3390/d14030166
APA StyleWang, L.-C., Li, H.-C., & Shiau, L.-J. (2022). Impacts of Anthropogenic Disturbances on Diatom Diversity in a Shallow Spring-Fed Pool. Diversity, 14(3), 166. https://doi.org/10.3390/d14030166






