Bauhinia (Leguminosae) Fossils from the Paleogene of Southwestern China and Its Species Accumulation in Asia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geological Setting
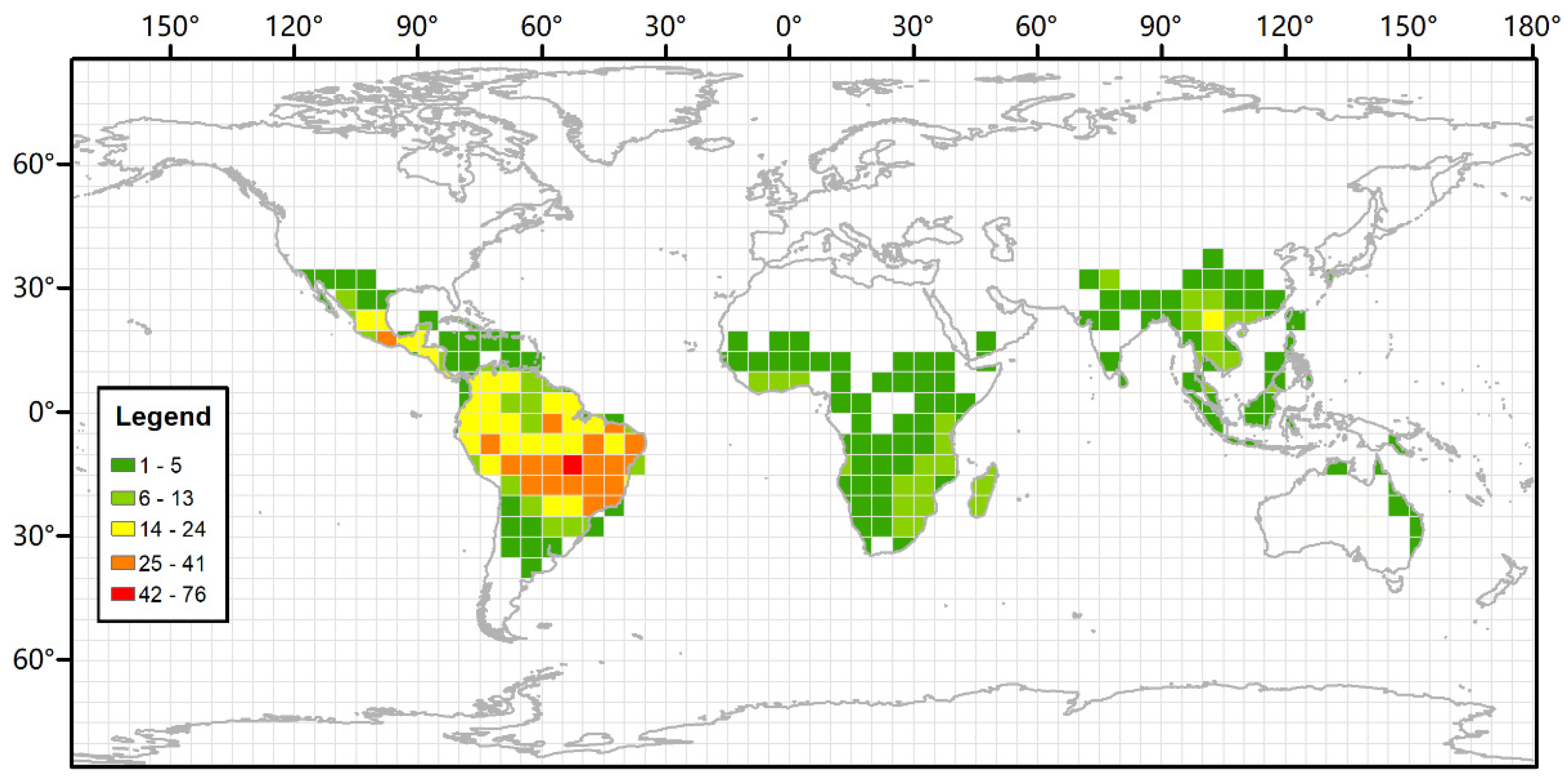
2.2. Macroscopic Feature Observations and the Modern Distribution of Bauhinia
2.3. Cuticle Preparation for Fossil and Extant Materials
3. Results
4. Discussion
4.1. Morphological Comparison
4.2. The Diversification of Bauhinia in Southeastern Asia
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Zhang, D.; Larsen, K.; Larsen, S.S. Bauhinia; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2010; pp. 6–21. [Google Scholar]
- Sinou, C.; Forest, F.; Lewis, G.P.; Bruneau, A. The genus Bauhinia s.l. (Leguminosae): A phylogeny based on the plastid trnL–trnF region. Botany 2009, 87, 947–960. [Google Scholar] [CrossRef]
- Lewis, G.; Schrire, B.; Mackinder, B.; Lock, M. Legumes of the World; Royal Botanic Gardens, Kew: London, UK, 2005. [Google Scholar]
- Zhang, R.; Wang, Y.H.; Jin, J.J.; Stull, G.W.; Bruneau, A.; Cardoso, D.; de Queiroz, L.P.; Moore, M.J.; Zhang, S.D.; Chen, S.Y.; et al. Exploration of plastid phylogenomic conflict yields new insights into the deep relationships of Leguminosae. Syst. Biol. 2020, 69, 613–622. [Google Scholar] [CrossRef]
- The Legume Phylogeny Working Group. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 2017, 66, 44–77. [Google Scholar] [CrossRef] [Green Version]
- Koenen, E.J.M.; Ojeda, D.I.; Steeves, R.; Migliore, J.; Bakker, F.T.; Wieringa, J.J.; Kidner, C.; Hardy, O.J.; Pennington, R.T.; Bruneau, A.; et al. Large-scale genomic sequence data resolve the deepest divergences in the legume phylogeny and support a near-simultaneous evolutionary origin of all six subfamilies. New Phytol. 2020, 225, 1355–1369. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhang, R.; Jiang, K.W.; Qi, J.; Hu, Y.; Guo, J.; Zhu, R.; Zhang, T.; Egan, A.N.; Yi, T.S.; et al. Nuclear phylotranscriptomics and phylogenomics support numerous polyploidization events and hypotheses for the evolution of rhizobial nitrogen-fixing symbiosis in Fabaceae. Mol. Plant 2021, 14, 748–773. [Google Scholar] [CrossRef] [PubMed]
- Mackinder, B.A.; Clark, R. A synopsis of the Asian and Australasian genus Phanera Lour. (Cercideae: Caesalpinioideae: Leguminosae) including 19 new combinations. Phytotaxa 2014, 166, 49–68. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Wong, W.O.; Shi, G.; Shen, S.; Li, Z. Bilobate leaves of Bauhinia (Leguminosae, Caesalpinioideae, Cercideae) from the middle Miocene of Fujian Province, southeastern China and their biogeographic implications. BMC Evol. Biol. 2015, 15, 252. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Song, Z.; Chen, Y.; Shen, S.; Li, Z. Leaves and fruits of Bauhinia (Leguminosae, Caesalpinioideae, Cercideae) from the Oligocene Ningming Formation of Guangxi, South China and their biogeographic implications. BMC Evol. Biol. 2014, 14, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, H.H.; Jacques, F.M.B.; Su, T.; Huang, Y.J.; Zhang, S.T.; Ma, H.J.; Zhou, Z.K. New Biogeographic insight into Bauhinia s.l. (Leguminosae): Integration from fossil records and molecular analyses. BMC Evol. Biol. 2014, 14, 181. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-F.; Zhang, D.-X. Bauhinia larsenii, a fossil legume from Guangxi, China. Bot. J. Linn. Soc. 2005, 147, 437–440. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Spicer, R.A.; Huang, J.; Zhou, Z.; Su, T.; Widdowson, M.; Jia, L.; Li, S.; Wu, W.; Xue, L.; et al. New early oligocene zircon U-Pb dates for the ‘Miocene’ Wenshan Basin, Yunnan, China: Biodiversity and paleoenvironment. Earth Planet. Sci. Lett. 2021, 565, 116929. [Google Scholar] [CrossRef]
- Calvillo-Canadell, L.; Cevallos-Ferriz, S.R.S. Bauhcis moranii gen. et sp. nov. (Cercideae, Caesalpinieae), an Oligocene plant from Tepexi de Rodríguez, Puebla, Mex., with leaf architecture similar to Bauhinia and Cercis. Rev. Palaeobot. Palynol. 2002, 122, 171–184. [Google Scholar] [CrossRef]
- Jacques, F.M.B.; Shi, G.; Su, T.; Zhou, Z. A tropical forest of the middle Miocene of Fujian (SE China) reveals Sino-Indian biogeographic affinities. Rev. Palaeobot. Palynol. 2015, 216, 76–91. [Google Scholar] [CrossRef]
- Guleria, J.; Rashmi, S.; Prasad, M. Some fossil leaves from the Kasauli Formation of Himachal Pradesh, North-West India. Himal. Geol. 2000, 21, 43–52. [Google Scholar]
- Berry, E.W. Fossil floras from southern Ecuador. Johns Hopkins Univ. Stud. Geol. 1945, 14, 93–150. [Google Scholar]
- Chaney, R.W. A tertiary flora from Uganda. J. Geol. 1933, 41, 702–709. [Google Scholar] [CrossRef]
- Bureau of Geology and Mineral Resources. Regional Geology of Yunnan Province; Geology Press: Beijing, China, 1990. [Google Scholar]
- Guo, Q.; Littke, R.; Sun, Y.; Zieger, L. Depositional history of low-mature coals from the Puyang Basin, Yunnan Province, China. Int. J. Coal Geol. 2020, 221, 103428. [Google Scholar] [CrossRef]
- Luo, X.Y.; Zhang, Y.H. Neogene coal-accumulation basin characteristics and genetic types in Yunnan Province. Coal Geol. China 2013, 25, 10–17. [Google Scholar]
- Ducrocq, S. The anthracotheriid genus Bothriogenys (Mammalia, Artiodactyla) in Africa and Asia during the Paleogene: Phylogenetical and paleobiogeographical relationships. Stuttg. Beiträge Nat. B Geol. Paläontol. 1997, 250, 1–44. [Google Scholar]
- Chow, M. Some Oligocene mammals from Lunan, Yunnan. Vertebr. PalAsiatica 1958, 2, 263–267. [Google Scholar]
- Linnemann, U.; Su, T.; Kunzmann, L.; Spicer, R.A.; Ding, W.N.; Spicer, T.E.V.; Zieger, J.; Hofmann, M.; Moraweck, K.; Gärtner, A.; et al. New U-Pb dates show a Paleogene origin for the modern Asian biodiversity hot spots. Geology 2017, 41, 3–6. [Google Scholar] [CrossRef] [Green Version]
- GBIF.org. Available online: https://www.gbif.org/ (accessed on 29 December 2021).
- Jin, J.; Yang, J. BDcleaner: A workflow for cleaning taxonomic and geographic errors in occurrence data archived in biodiversity databases. Glob. Ecol. Conserv. 2020, 21, e00852. [Google Scholar] [CrossRef]
- Kerp, H. The study of fossil gymnosperms by means of cuticular analysis. Palaios 1990, 5, 548–569. [Google Scholar] [CrossRef]
- Leng, Q. An effective method of observing fine venation from compressed angiosperm fossil leaves. Acta Palaeontol. Sin. 2000, 39, 157–158, (In Chinese with English Abstract). [Google Scholar]
- Hu, J.J.; Xing, Y.W.; Turkington, R.; Jacques, F.M.B.; Su, T.; Huang, Y.J.; Zhou, Z.K. A new positive relationship between pCO2 and stomatal frequency in Quercus guyavifolia (Fagaceae): A potential proxy for palaeo-CO2 levels. Ann. Bot. 2015, 115, 777–788. [Google Scholar] [CrossRef] [Green Version]
- Poole, I.; Kürschner, W.M. Stomatal Density and Index: The Practice. Fossil Plants and Spores: Modern Techniques. In Fossil Plants and Spores: Modern Techniques; Jones, T.P., Rowe, N.P., Eds.; Geological Society: London, UK, 1999; pp. 257–260. [Google Scholar]
- Stace, C.A. Cuticular studies as an aid to plant taxonomy. Bulletin of the British Museum (Natural History). Bot. Ser. 1965, 4, 3–78. [Google Scholar]
- Pole, M.S.; Bowman, D.M.J.S. Tertiary Plant Fossils from Australia’s ‘Top End’. Aust. Syst. Bot. 1996, 9, 113–126. [Google Scholar] [CrossRef]
- Zeng, N.; Zhang, J.R.; Chang, Z.Y. Micromorphological characteristics of leaf epidemis and systematic significance of Rosa L. from China. Guihaia 2017, 37, 169–185, (In Chinese with English Abstract). [Google Scholar]
- Yang, Z.R.; Lin, Q. Comparative morphology of the leaf epidermis in Schisandra (Schisandraceae). Bot. J. Linn. Soc. 2005, 148, 39–56. [Google Scholar] [CrossRef] [Green Version]
- Zou, P. Comparative Morphology of Cercideae (Leguminosae: Caesalpinioideae); South China Botanical Garden, Chinese Academy of Sciences: Guangzhou, China, 2006; p. 109. [Google Scholar]
- Awasthi, N. India Fossil Legumes. In Advances in Legume Systematics: Part 4 The fossil records; Herendeen, P.S., Dilcher, D.L., Eds.; Royal Botanic Gardens, Kew: London, UK, 1992; pp. 225–250. [Google Scholar]
- Awasthi, N.; Prasad, M. Siwalik plant fossils from Surai Khola area, western Nepal. Palaeobotanist 1990, 38, 298–318. [Google Scholar]
- Lakhanpal, R.; Awasthi, N. A late Tertiary Florule from near Bhikhnathoree in West Champaran District, Bihar. In Proceedings of the Symposium on Evolutionary Botany and Biostratigraphy, Current Trends in Life Sciences 10 (A. K. Ghosh Commem-oration Volume); Sharma AK Mitra, G.C., Banerjee, M., Eds.; Today and Tomorrow’s Printers & Publishers: New Delhi, India, 1984; pp. 587–596. [Google Scholar]
- Berry, E.W. Fossil plants from Bolivia and their bearing upon the age of uplift of the eastern Andes. Proc. U. S. Natl. Mus. 1917, 54, 103–164. [Google Scholar] [CrossRef] [Green Version]
- Bande, M.; Srivastava, G. Late Cenozoic plant impressions from Mahuadanr Valley, Palamu District, Bihar. Palaeobotanist 1990, 37, 331–336. [Google Scholar]
- Carpenter, R.J.; Goodwin, M.P.; Hill, R.S.; Kanold, K. Silcrete plant fossils from Lightning Ridge, New South Wales: New evidence for climate change and monsoon elements in the Australian Cenozoic. Aust. J. Bot. 2011, 59, 399–425. [Google Scholar] [CrossRef]
- Biagolini, C.H.; Bernardes-de-Oliveira, M.E.C.; Caramês, A.G. Itaquaquecetuba Formation, São Paulo basin, Brazil: New angiosperm components of Paleogene Taphoflora. Braz. J. Geol. 2013, 43, 639–652. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, K.; He, S. Study on the Scientific Survey of Wenshan National Reserve in China; Science Press: Beijing, China, 2008. [Google Scholar]
- Shui, Y.-M. The Phytogeography of Seed Plants from Dawei Mountain; Kunming Institute of Botany: Kunming, China, 2000; p. 211. [Google Scholar]









| No. | Species | Type | Age | Locality | Reference |
|---|---|---|---|---|---|
| 1 | Bauhinia wenshanensis H.H. Meng et Z.K. Zhou | Leaf | Early Oligocene | Dashidong Town, Wenshan County, Yunnan Province, China | [11] |
| 2 | Bauhinia larsenii D.X. Zhang et Y.F. Chen | Leaf and fruit | Late Oligocene | Ningming County, Guangxi, China | [10,12] |
| 3 | Bauhinia ningmingensis Q. Wang | Leaf | Late Oligocene | Ningming, Guangxi, China | [10] |
| 4 | Bauhinia cheniae Q. Wang | Leaf | Late Oligocene | Ningming, Guangxi, China | [10] |
| 5 | Bauhinia ningmingensis Q. Wang | Leaf | Late Oligocene | Ningming, Guangxi, China | [10] |
| 6 | Bauhcis moranii Calvillo-Canadell et Cevallos-Ferriz | Leaf | Oligocene | Los Ahuehuetes, Tepexi de Rodríguez, Puebla, Mexico | [14] |
| 7 | Bauhinia krishnanunnii A. K. Mathur | Leaf | Early Miocene | Unmetalled way to Babu Mohalla, Dagshai Cantonment, Solan District, Himachal Pradesh, India | [16] |
| 8 | Bauhinia fotana F. M. B. Jacques | Leaf | Middle Miocene | Zhangpu, County, Zhangzhou City, Fujian Province, Southeast China | [15] |
| 9 | Bauhinia ungulatoides Y. X. Lin, W. O. Wong, G. L. Shi, S. Shen et Z. Y. Li | Leaf | Middle Miocene | Zhangpu, Fujian, China | [9] |
| 10 | Bauhinia ecuadorensis E.W. Berry | Leaf | Miocene | Loja Basin, Ecuador | [17] |
| 11 | Bauhinia siwalika R.N. Lakh. et N. Awasthi | Leaf | Middle Miocene-Pleistocene | Bhikhnathoree, West Champaran District, Bihar, India | [36] |
| 12 | Bauhinia nepalensis N. Awasthi et N. Prasad | Leaf | Middle Miocene-Pleistocene | Bhikhnathoree, West Champaran District, Bihar, India | [36] |
| 13 | Bauhinia nepalensis N. Awasthi et N. Prasad | Leaf | Middle Miocene-Pleistocene | Surai Khola beds, near SuraiKhola bridge, Surai Khola area, India | [37] |
| 14 | Bauhinia siwalika R.N. Lakh. et N. Awasthi | Leaf | Middle Miocene-Middle Pleistocene | Bhikhnathoree, West Champaran District, Bihar, India | [38] |
| 15 | Bauhinia waylandii R.W. Chaney | Leaf | Pliocene | Busano, Bugishu, District, Eastern, Province, Uganda | [18] |
| 16 | Bauhinia potosiana E.W. Berry | Leaf | Pliocene-Early Pleistocene | Potosi, Bolivia | [39] |
| 17 | Bauhinia sp. cf. B. purpurea L. | Leaf | Late Cenozoic | Mahuadanr, Palamau District, Bihar, India | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, L.-B.; Hu, J.-J.; Zhang, S.-T.; Su, T.; Spicer, R.A.; Liu, J.; Yang, J.-C.; Zou, P.; Huang, Y.-J.; Zhou, Z.-K. Bauhinia (Leguminosae) Fossils from the Paleogene of Southwestern China and Its Species Accumulation in Asia. Diversity 2022, 14, 173. https://doi.org/10.3390/d14030173
Jia L-B, Hu J-J, Zhang S-T, Su T, Spicer RA, Liu J, Yang J-C, Zou P, Huang Y-J, Zhou Z-K. Bauhinia (Leguminosae) Fossils from the Paleogene of Southwestern China and Its Species Accumulation in Asia. Diversity. 2022; 14(3):173. https://doi.org/10.3390/d14030173
Chicago/Turabian StyleJia, Lin-Bo, Jin-Jin Hu, Shi-Tao Zhang, Tao Su, Robert A. Spicer, Jia Liu, Jiu-Cheng Yang, Pu Zou, Yong-Jiang Huang, and Zhe-Kun Zhou. 2022. "Bauhinia (Leguminosae) Fossils from the Paleogene of Southwestern China and Its Species Accumulation in Asia" Diversity 14, no. 3: 173. https://doi.org/10.3390/d14030173
APA StyleJia, L.-B., Hu, J.-J., Zhang, S.-T., Su, T., Spicer, R. A., Liu, J., Yang, J.-C., Zou, P., Huang, Y.-J., & Zhou, Z.-K. (2022). Bauhinia (Leguminosae) Fossils from the Paleogene of Southwestern China and Its Species Accumulation in Asia. Diversity, 14(3), 173. https://doi.org/10.3390/d14030173








