Interspecific Competition and Vertical Niche Partitioning in Fiji’s Forest Birds
Abstract
:1. Introduction
2. Materials and Methods
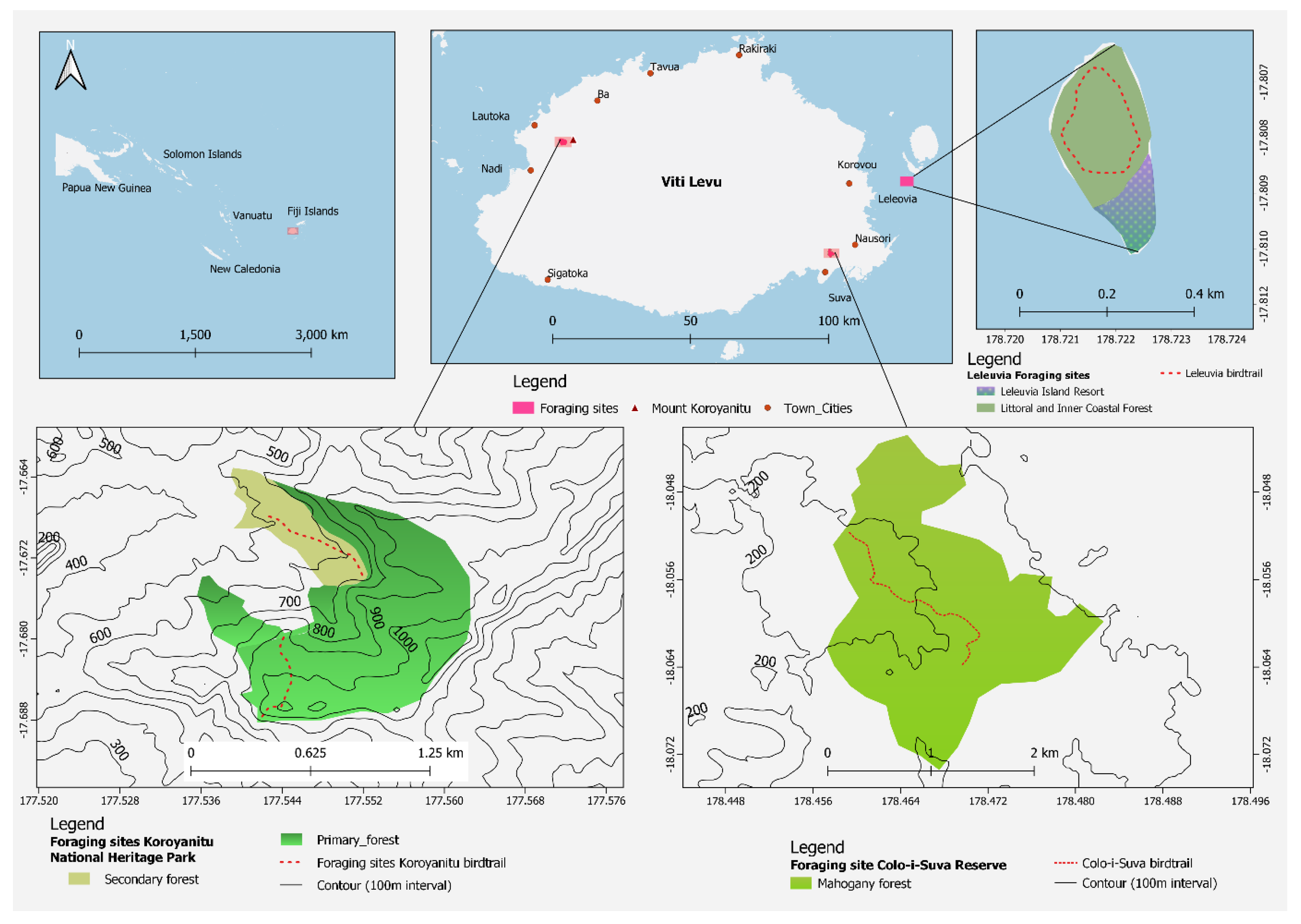
2.1. Study Sites
2.2. Foraging Behaviour Data Collection
2.3. Statistics and Data Analysis
3. Results
3.1. Foraging Location and Species Differences
3.2. Comparisons within Foraging Guilds
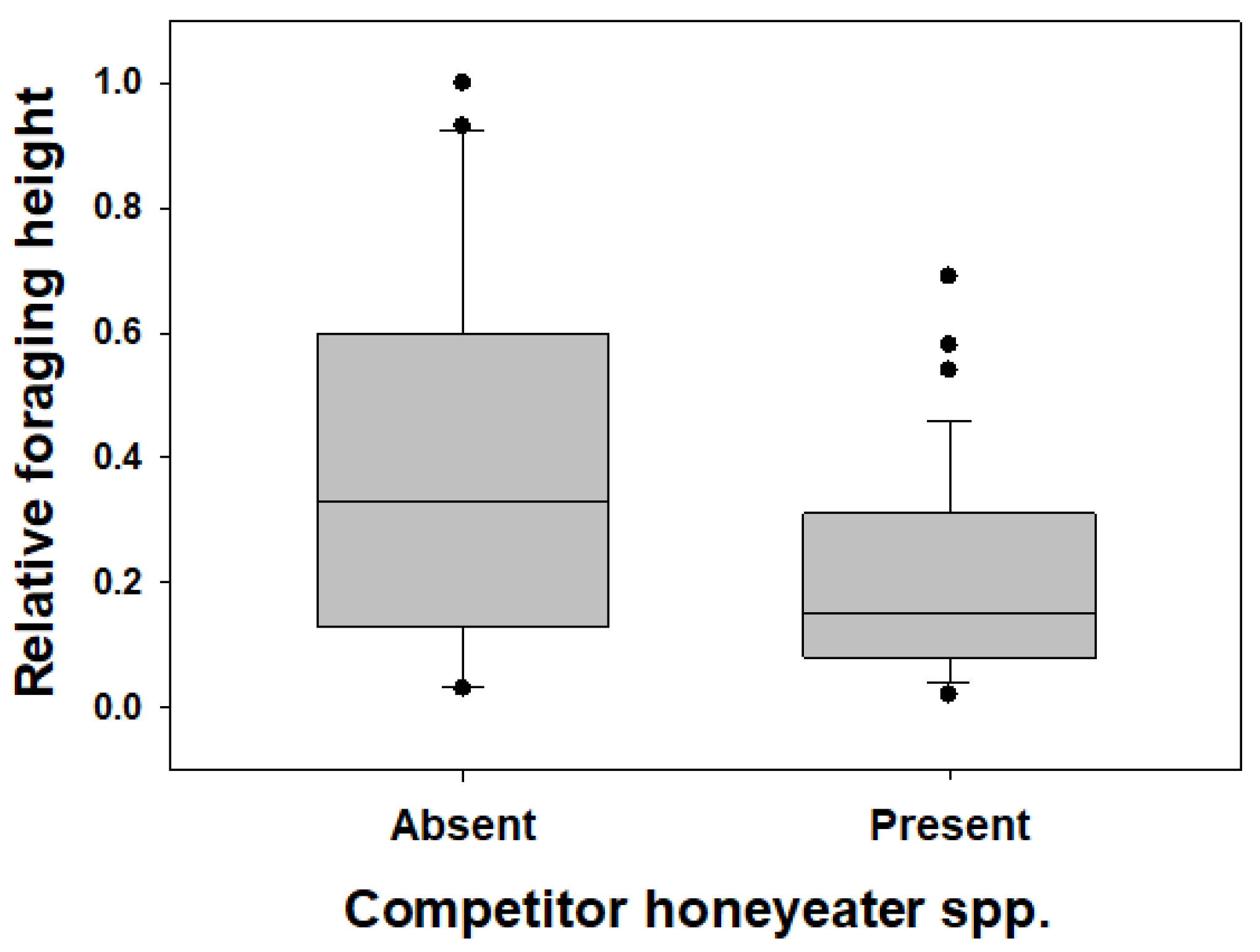
3.3. Myzomela Foraging in Areas with and without Other Honeyeater Species
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacArthur, R.H.; Wilson, E.O. An equilibrium theory of insular zoogeography. Evolution 1963, 17, 373–387. [Google Scholar] [CrossRef]
- Mayr, E. Avifauna: Turnover on islands. Science 1965, 150, 1587–1588. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.J.; Triantis, K.A.; Ladle, R.J. A general dynamic theory of oceanic island biogeography. J. Biogeogr. 2008, 35, 977–994. [Google Scholar] [CrossRef]
- Barajas-Barbosa, M.P.; Weigelt, P.; Borregaard, M.K.; Keppel, G.; Kreft, H. Environmental heterogeneity dynamics drive plant diversity on oceanic islands. J. Biogeogr. 2020, 47, 2248–2260. [Google Scholar] [CrossRef]
- Diamond, J.M.; Mayr, E. Species-area relation for birds of the Solomon Archipelago. Proc. Natl. Acad. Sci. USA 1976, 73, 262–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamond, J.M.; Gilpin, M.E.; Mayr, E. Species-distance relation for birds of the Solomon Archipelago, and the paradox of the great speciators. Proc. Natl. Acad. Sci. USA 1976, 73, 2160–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayr, E.; Diamond, J.M. Birds on islands in the sky: Origin of the montane avifauna of northern Melanesia. Proc. Natl. Acad. Sci. USA 1976, 73, 1765–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamond, J.M. Continental and insular speciation in Pacific land birds. Syst. Biol. 1977, 26, 263–268. [Google Scholar] [CrossRef]
- Kearney, M.; Simpson, S.J.; Raubenheimer, D.; Helmuth, B. Modelling the ecological niche from functional traits. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3469–3483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, R.D. Bringing the Hutchinsonian niche into the 21st century: Ecological and evolutionary perspectives. Proc. Natl. Acad. Sci. USA 2009, 106, 19659–19665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamond, J. Factors controlling species diversity: Overview and synthesis. Ann. Mo. Bot. Gard. 1988, 75, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Finke, D.L.; Snyder, W.E. Niche partitioning increases resource exploitation by diverse communities. Science 2008, 321, 1488–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kent, C.M.; Sherry, T.W. Behavioral niche partitioning re-examined: Do behavioral differences predict dietary differences in warblers? Ecology 2020, 101, e03077. [Google Scholar] [CrossRef]
- Traba, J.; Morales, M.B.; Carmona, C.P.; Delgado, M.P. Resource partitioning and niche segregation in a steppe bird assemblage. Community Ecol. 2015, 16, 178–188. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Xu, Y.; Price, M.; Yang, N.; Liu, W.; Zhu, B.; Zhong, X.; Ran, J. Niche partitioning among three montane ground-dwelling pheasant species along multiple ecological dimensions. Ibis 2021, 163, 171–182. [Google Scholar] [CrossRef]
- Mansor, M.S.; Ramli, R. Foraging niche segregation in Malaysian babblers (Family: Timaliidae). PLoS ONE 2017, 12, e0172836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansor, M.S.; Ramli, R. Niche separation in flycatcher-like species in the lowland rainforests of Malaysia. Behav. Processes 2017, 140, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.R.; Grant, B.R. How and Why Species Multiply; Princeton University Press: Princeton, NJ, USA, 2020; ISBN 9780691149998. [Google Scholar]
- Schluter, D.; Grant, P.R. Determinants of morphological patterns in communities of Darwin’s finches. Am. Nat. 1984, 123, 175–196. [Google Scholar] [CrossRef] [Green Version]
- Boag, P.T.; Grant, P.R. Intense natural selection in a population of Darwin’s finches (Geospizinae) in the Galapagos. Science 1981, 214, 82–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tebbich, S.; Taborsky, M.; Fessl, B.; Dvorak, M.; Winkler, H. Feeding behavior of four arboreal Darwin’s finches: Adaptations to spatial and seasonal variability. Condor 2004, 106, 95–105. [Google Scholar] [CrossRef]
- Oliveira, B.F.; Scheffers, B.R. Vertical stratification influences global patterns of biodiversity. Ecography 2019, 42, 249. [Google Scholar] [CrossRef] [Green Version]
- Basset, Y.; Kitching, R.; Miller, S.; Novotny, V. (Eds.) Arthropods of Tropical Forests: Spatio-Temporal Dynamics and Resource Use in the Canopy; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Bernard, E. Vertical stratification of bat communities in primary forests of Central Amazon, Brazil. J. Trop. Ecol. 2001, 17, 115–126. [Google Scholar] [CrossRef]
- Tanabe, S.I. Between-forest variation in vertical stratification of drosophilid populations. Ecol. Entomol. 2002, 27, 720–731. [Google Scholar] [CrossRef]
- Pearson, D.L. Vertical stratification of birds in a tropical dry forest. Condor 1971, 73, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Diamond, J.M. Ecological consequences of island colonization by southwest Pacific birds, I. Types of niche shifts. Proc. Natl. Acad. Sci. USA 1970, 67, 529–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birdlife Datazone. Species List: Fiji. 2017. Available online: http://datazone.birdlife.org/species/downloadcsv/C6C19152-AA6E-47F3-84BO-A12A4E4BDC7E (accessed on 18 August 2017).
- Watling, D. A Guide to the Birds of Fiji and Western Polynesia; Environmental Consultants: Suva, Fiji, 2001. [Google Scholar]
- Watling, D. The breeding biology of the red-vented bulbul Pycnonotus cafer in Fiji. Emu-Austral Ornithol. 1983, 83, 173–180. [Google Scholar] [CrossRef]
- Langham, N.P.E. The annual cycle of the Avadavat (Amandava amandava) on Fiji. Emu 1987, 87, 232–243. [Google Scholar] [CrossRef]
- England, J. Nesting behaviour of Natewa Silktail Lamprolia klinesmithi. Bull. Br. Ornithol. Club 2019, 139, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Langham, N.P.E. The stratification of passerines in Fijian forests. Notornis 1989, 36, 267–279. [Google Scholar]
- Thaman, R. The biodiversity of Koroyanitu National Park. Domodomo 1996, 10, 28–51. [Google Scholar]
- Waqaisavou, T. Parks, Reserves and Tourism in Fiji: Native Landowner Attitude and Involvement. Ph.D. Thesis, Victoria University of Technology, Melbourne, Australia, 1997. [Google Scholar]
- Malani, M. Ecotourism in Fiji. In Linking Green Productivity to Ecoutourism; Asian Productivity Organisation: Tokyo, Japan, 2002; pp. 45–55. [Google Scholar]
- Anderson, J.; Keppel, G.; Thomson, S.-M.; Randell, A.; Raituve, J.; Koroi, I.; Anisi, R.; Charlson, T.; Boehmer, H.J.; Kleindorfer, S. Changes in climate and vegetation with altitude on Mount Batilamu, Viti Levu, Fiji. J. Trop. Ecol. 2018, 34, 316–325. [Google Scholar] [CrossRef]
- Keppel, G.; Peters, S.; Taoi, J.; Raituku, N.; Thomas-Moko, N. The threat by the invasive African tulip tree, Spathodea campanulata P. Beauv., for the critically endangered Fijian tree, Pterocymbium oceanicum AC Sm.; revisiting an assessment based on expert knowledge after extensive field surveys. Pac. Conserv. Biol. 2021. [Google Scholar] [CrossRef]
- Tuiwawa, M.; Sakiti-Waqa, H.; Tuiwawa, S.; Naikatini, A.; Copeland, L.; Rashni, B. Colo-i-Suva Forest Park Wildlife; The University of the South Pacific Press: Suva, Fiji, 2018; ISBN 978-982-01-0981-0. [Google Scholar]
- Tuiwawa, S.H.; Keppel, G. Species diversity, composition and the regeneration potential of native plants at the Wainiveiota Mahoganu Plantation, Viti Levu, Fiji Islands. South Pac. J. Nat. Appl. Sci. 2012, 30, 51–57. [Google Scholar] [CrossRef]
- Reid, E.; Naikatini, A.; Keppel, G.; Kleindorfer, S. The conservation value of secondary vegetation for Fijian woodland birds. Emu 2019, 119, 286–295. [Google Scholar] [CrossRef]
- Thaman, R. Plants and Vegetation of Leleuvia Island, Tailevu Province, Fiji; The University of the South Pacific Press: Suva, Fiji, 2013; ISBN 978-982-01-0986-0. [Google Scholar]
- Westwood, N.; Pearson, M.; Mustafa, E.; Scanlon, A.T. Differences in abundance and diversity of diurnal invertebrates among three Fijian forests, and a comparison of two trapping methods for rapid assessments. Pac. Conserv. Biol. 2018, 24, 183–188. [Google Scholar] [CrossRef]
- Naikatini, A. Monitoring Comparative and Temporal Variation in the Landbirds of Vago-Savura Forest Reserve, a Native Lowland Rainforest in South East Viti Levu, Fiji. Master’s Thesis, The University of the South Pacific, Suva, Fiji, 2009. [Google Scholar]
- Schlotfeldt, B.E.; Kleindorfer, S. Adaptive divergence in the Superb Fairy-wren (Malurus cyaneus): A mainland versus island comparison of morphology and foraging behaviour. Emu-Austral Ornithol. 2006, 106, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Myers, S.; Brown, G.; Kleindorfer, S. Divergence in New Holland Honeyeaters (Phylidonyris novaehollandiae): Evidence from morphology and feeding behavior. J. Ornithol. 2010, 151, 287–296. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 25.0; IBM Corp.: Armonk, NY, USA, 2013. [Google Scholar]
- Keppel, G.; Buckley, Y.M.; Possingham, H.P. Drivers of lowland rain forest community assembly, species diversity and forest structure on islands in the tropical South Pacific. J. Ecol. 2010, 98, 87–95. [Google Scholar] [CrossRef]
- Rodda, P. Geology of Fiji. In Geology and Submarine Resources of the Tonga–Lau–Fiji Region; Stevenson, A.J., Herzer, R.H., Ballance, P.F., Eds.; Sopac Secretaria: Suva, Fiji, 1994; pp. 131–151. [Google Scholar]
- Anderson, A.; Clark, G. (Eds.) Research on the early prehistory of Fiji. In The Early Prehistory of Fiji; 31. ANU E Press, Australian National University: Canberra, Australia, 2009; ISBN 9781921666070. [Google Scholar]
- Andersen, M.J.; Naikatini, A.; Moyle, R.G. A molecular phylogeny of Pacific honeyeaters (Aves: Meliphagidae) reveals extensive paraphyly and an isolated Polynesian radiation. Mol. Phylogenet. Evol. 2014, 71, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Ash, J. Vegetation ecology of Fiji: Past, present and future perspectives. Pac. Sci. 1992, 46, 111–127. [Google Scholar]
- Faaborg, J. Further observations on ecological release in Mona Island birds. Auk 1980, 97, 624–627. [Google Scholar]
- Robert, A.; Melo, M.; Lengagne, T.; Julien, S.; Gomez, D.; Doutrelant, C. Patterns of bird song evolution on islands support the character release hypothesis in tropical but not in temperate latitudes. J. Evol. Biol. 2021, 34, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.M. Foraging ecology and niche partitioning in orb-weaving spiders. Oecologia 1981, 50, 380–385. [Google Scholar] [CrossRef]
- Andreas, M.; Reiter, A.; Cepáková, E.; Uhrin, M. Body size as an important factor determining trophic niche partitioning in three syntopic rhinolophid bat species. Biologia 2013, 68, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Eduardo, L.N.; Bertrand, A.; Mincarone, M.M.; Santos, L.V.; Frédou, T.; Assunção, R.V.; Silva, A.; Ménard, F.; Schwamborn, R.; Le Loc’h, F.; et al. Hatchetfishes (Stomiiformes: Sternoptychidae) biodiversity, trophic ecology, vertical niche partitioning and functional roles in the western Tropical Atlantic. Prog. Oceanogr. 2020, 187, 87–100. [Google Scholar] [CrossRef]



| Species | Colo-i-Suva (100 m.a.s.l.) | Koroyanitu (500–1000 m.a.s.l.) | Leleuvia (0 m.a.s.l.) |
|---|---|---|---|
| Nectarivore | |||
| Orange-breasted Myzomela (Myzomela jugularis) | 26 | 22 | 40 |
| Kikau Honeyeater (Foulehaio procerior) | 9 | 85 | |
| Giant Honeyeater (Gymnomyza brunneirostris) | 2 | 11 | |
| Insectivore | |||
| Slaty Monarch (Mayrornis lesson) | 4 | 17 | |
| Vanikoro Broadbill (Myiagra vanikorensis) | 10 | 26 | |
| Omnivore | |||
| Fiji White-eye (Zosterops explorator) | 15 | 74 | |
| Silvereye (Zosterops lateralis) | 0 | 60 |
| Substrate | Nectarivore | Insectivore | Omnivore | |||||
|---|---|---|---|---|---|---|---|---|
| Myzomela Island | Myzomela Mainland | Kikau | Giant | Slaty | Vanikoro | White-Eye | Silver-Eye | |
| Ground | 0 | 0 | 0 | 0 | 0 | 14% | 0 | 0 |
| Bark | 53% | 25% | 24% | 44% | 57% | 32% | 39% | 38% |
| Leaves | 2.5% | 21% | 20% | 6% | 39% | 27% | 45% | 56% |
| Flower | 38% | 54% | 49% | 50% | 4% | 16% | 17% | 7% |
| Air | 8% | 0 | 8% | 0 | 0 | 11% | 0 | 0 |
| Technique | Nectarivore | Insectivore | Omnivore | |||||
|---|---|---|---|---|---|---|---|---|
| Myzomela Island | Myzomela Mainland | Kikau | Giant | Slaty | Vanikoro | White-Eye | Silver-Eye | |
| Glean | 55% | 40% | 30% | 17% | 93% | 31% | 81% | 90% |
| Bite | 15% | 13% | 0 | 6% | 3% | 18% | 8% | 2% |
| Probe | 20% | 44% | 55% | 78% | 4% | 15% | 11% | 8% |
| Chip off + Pry | 0 | 0 | 5% | 0 | 0 | 3% | 0 | 0 |
| Sally + Hoverglean | 10% | 4% | 10% | 0 | 0 | 33% | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naikatini, A.N.; Keppel, G.; Brodie, G.; Kleindorfer, S. Interspecific Competition and Vertical Niche Partitioning in Fiji’s Forest Birds. Diversity 2022, 14, 223. https://doi.org/10.3390/d14030223
Naikatini AN, Keppel G, Brodie G, Kleindorfer S. Interspecific Competition and Vertical Niche Partitioning in Fiji’s Forest Birds. Diversity. 2022; 14(3):223. https://doi.org/10.3390/d14030223
Chicago/Turabian StyleNaikatini, Alivereti N., Gunnar Keppel, Gilianne Brodie, and Sonia Kleindorfer. 2022. "Interspecific Competition and Vertical Niche Partitioning in Fiji’s Forest Birds" Diversity 14, no. 3: 223. https://doi.org/10.3390/d14030223
APA StyleNaikatini, A. N., Keppel, G., Brodie, G., & Kleindorfer, S. (2022). Interspecific Competition and Vertical Niche Partitioning in Fiji’s Forest Birds. Diversity, 14(3), 223. https://doi.org/10.3390/d14030223






