Abstract
The extremely variable and widespread cetoniine species Mausoleopsis amabilis (Schaum, 1844) is reviewed here using analyses of over 1500 specimens and observations across its entire distribution range. As a result, M. a. heterospila (Gerstaecker, 1867) is reinstated as a valid subspecies and a new subspecies, M. a. interrupta ssp. nov., is also erected, in order to account for the geographically distinct populations occurring across East Africa and the eastern lowlands of southern Africa, respectively. Mausoleopsis a. ruteri Antoine, 1989 is confirmed as a valid subspecies for the West African populations, while M. lerui Antoine, 2004 is synonymised with M. a. heterospila, as it appears to represent an extreme variation of that subspecies. On the other hand, a new species, M. gariepena sp. nov., is described from the Gariep Desert and surrounding arid areas of South Africa and Namibia and compared to its sister species, M. amabilis s. l. The new species appears to be an arid habitat specialist, adapted to survive in the driest part of the subcontinent.
1. Introduction
The subfamily Cetoniinae, popularly known as fruit and/or flower chafers, is a rather diverse scarab grouping which currently consists of 12 tribes subdivided into 46 subtribes [1]. The tribe Cetoniini is particularly well-represented in the Afrotropical Region, with the subtribes Cetoniina and Leucocelina comprising some 60 genera and over 550 species [2]. With the re-demotion of Elassochiton to the rank of subgenus [3], the Leucocelina genus Mausoleopsis now includes two rather diverse subgenera, together including 21 species and three subspecies [2]. Within Mausoleopsis s. s., M. amabilis is undoubtedly the most widespread and variable species across the African continent and adjacent islands. Originally described by Schaum [4] from material collected by M. Melly in the Algoa Bay area (ZAF, EC), the species was subsequently reported from the then Natal (now ZN) and Mozambique colonial territories and eventually even in East Africa, leading to Gestaecker [5] being the first to comment on its remarkable variability, particularly as recorded in its dorsal white ornamentation.
Mausoleopsis amabilis s. l. actually exhibits an extraordinary range of patterns in its maculation, which has caused confusion and controversy throughout the literature of its description and revision (cf. [6] and references therein). Gerstaecker [5] first proposed the name heterospila for its population from East Africa, using descriptions of specimens from Mombasa (KEN) and Zanzibar (TAN). Much later, Antoine [7] proposed the name ruteri for the West African populations, using material from Cote d’Ivoire, Angola, Burkina Faso, and Nigeria. Marais and Holm [8] recognised these as proper subspecies, along with the nominal one from southern Africa. Subsequently, however, Holm and Marais [9] proposed the synonymy of M. a. heterospila with M. amabilis s. s. while avoiding any mention of M. a. ruteri. In the introduction to the genus, they also made reference to a forthcoming revision (“Marais in prep.”), which has yet to be published. Nevertheless, they designated both a lectotype and a paralectotype from the material of the Melly Collection used by Schaum in his original description and currently reposited in the MHNG. More recently, Beinhundner [2] reversed the synonymy of M. a. heterospila, albeit recognising it only at the level of variation (“var.”), as originally proposed by Gerstaecker [5] himself. Beinhundner [2] also endorsed M. a. ruteri as a valid subspecies.
With more specimens and live observations now becoming progressively more numerous, further consistent patterns are being revealed, necessitating a new, thorough revision of this species and its closest allies. In particular, isolated populations with specific adaptation to extremely dry environmental conditions have been identified in the western part of the southern African region and appear to represent a separate species. Moreover, several cases of variability in dorsal white maculation observed across the full range of M. amabilis s. l. are not as randomly distributed as earlier believed, but are actually geographically coherent and distinct, with some intermediate forms found in transitional zones where physical barriers are not sufficiently developed to keep adjacent populations separated continuously. This implies that some of its populations actually better fulfil the definition of parapatric subspecies, rather than variation.
2. Materials and Methods
A total of 1564 between preserved specimens and live observations were analysed during the course of this investigation. Specimens reposited in museum and private collections were either inspected directly or analysed using high quality photographic material provided by the various curators. Original collecting data accompanying each specimen were also obtained from key holders of material of interest. Complementary observations and direct collections of recent specimens were undertaken by the first author throughout the southern African region during the period 1993–2018. Searches were mainly conducted by direct inspection of flowering plants and sapping trees, while fruit-baited cylindrical traps were deployed in selected area, whenever the duration of the visit to a specific area was long enough to result in meaningful trapping. Specimens were often also netted in flight using standard entomological nets, or picked from trees, herbs, and shrubs on which they were perching. A few specimens were also retrieved by hand after drowning in farm troughs and dams, or even just dead on the ground. In all cases, when specimens were selected for in-depth analysis, they were immediately preserved with ethyl acetate fumes and subsequently set and dried in the laboratory or preserved in a frozen state.
Complementary data were obtained from key literature sources providing quality illustrations and collecting data of Mausoleopsis amabilis s. l. specimens, such as [2,10,11,12]. In the text, data records are accompanied by the number and sex of individuals in front of each entry only when provided by the collection owners or curators. Otherwise, such details are omitted and only a generalized reference to an unspecified number (n) of individuals (inds) is given. The holotype male and allotype female specimens of M. lerui Antoine, 2004 (“Coast Kenya, Malindi, V 2002 et XII 2001, B. Le Rü leg, in MHHN”) were studied in detail through high-resolution photographs kindly provided by Christophe Rivier (MNHN). Similarly, the lectotype and paralectotype male specimens of “M. amabilis (“Coll. Melly, amabilis, Schaum, Algoa” and “Coll. Melly, var. Port Natal” were analysed from photographic material provided by Guido Sabatinelli (MNHG).
The standard Cetoniinae terminology used by Krikken [13] and Holm and Marais [9] is followed again in this study for the description of specimen morphological characters. Total individual length (TL) and maximum width (MW) were measured using a Vernier calliper, from the anterior margin of the clypeus to the apex of the pygidium and at the widest point of the elytra, respectively. Photos of specimen dorsal and ventral habitus were taken with a Nikon CoolPix S9700 digital camera with macro setting, while photos of the male genitalia were obtained using a Nikon DigitalSight DS-Fi2 camera attached to a Nikon SMZ25 dissecting microscope. Where necessary, the background was removed from the photos using Microsoft Word 2010 (Picture Tools), in order to increase the clarity of resolution. The Combine ZP Image Stacking Software by Alan Hadley (alan@micropics.org.uk) was used to obtain z-stacking composite images.
Specimen repositories are abbreviated as follows:
- BMNH—Natural History Museum, London, United Kingdom;
- BMPC—Jonathan Ball and Andre Marais Private Collection, Cape Town, South Africa;
- DNSM—Durban Natural Science Museum, Durban, South Africa;
- GBEG—Gerhard Beinhundner Private Collection, Euerbach, Germany;
- ISAM—Iziko South African Museum, Cape Town, South Africa;
- KNEM—National Museums of Kenya, Entomology Department, Nairobi, Kenya;
- MHNG—Muséum d’Histoire Naturelle, Geneva, Switzerland;
- MRAC—Musée Royal de I’Afrique Centrale, Tervuren, Belgium;
- MZUC–Museu Zoológico da Universidade de Coimbra, Portugal;
- PLPC—Philippe Léonard Private Collection, Embourg, Belgium;
- PMPC—Petr Malec Private Collection, Brno, Czech Republic;
- RBINS—Institut Royal des Sciences Naturelles de Belgique, Bruxelles, Belgium
- RPRM—Renzo Perissinotto, Research Material, Gqeberha, South Africa;
- TGMF—Thierry Garnier Private Collection, Montpellier, France;
- TMSA—Ditsong National Museum of Natural History, Pretoria, South Africa.
Countries within the text are reported with their international ISO Alpha-3 codes (https://www.nationsonline.org/oneworld/country_code_list.htm, accessed on 18 March 2022), as follows:
- Angola—AGO; Benin—BEN; Botswana—BWA; Burkina Faso—BFA; Cameroon—CMR; Central African Republic—CAF; Congo (Kinshasa)—COD; Côte d’Ivoire—CIV; eSwatini—SWZ; Ghana—GHA; Kenya—KEN; Malawi—MWI; Mali—MLI; Mozambique—MOZ; Namibia—NAM; Nigeria—NGA; South Africa—ZAF; South Sudan—SSD; Tanzania—TZA; Uganda—UGA; Zambia—ZMB; Zimbabwe—ZWE. Within South Africa, the key area of this study, provincial codes are used as follows: Eastern Cape Province—EC; Free State Province—FS; Gauteng Province—GP; KwaZulu-Natal Province—ZN; Limpopo Province—LP; Mpumalanga Province—MP; Northern Cape Province—NC; North-West Province—NW; Western Cape Province—WC. Physical distribution codes are: N = North; S = South; E = East; W = West; C = Centre; H = Highlands/veld; L = Lowlands/veld; M = Midlands).
3. Results/Taxonomy
3.1. Mausoleopsis (Mausoleopsis) gariepena sp. nov (Figures 1–4)
3.1.1. Diagnosis
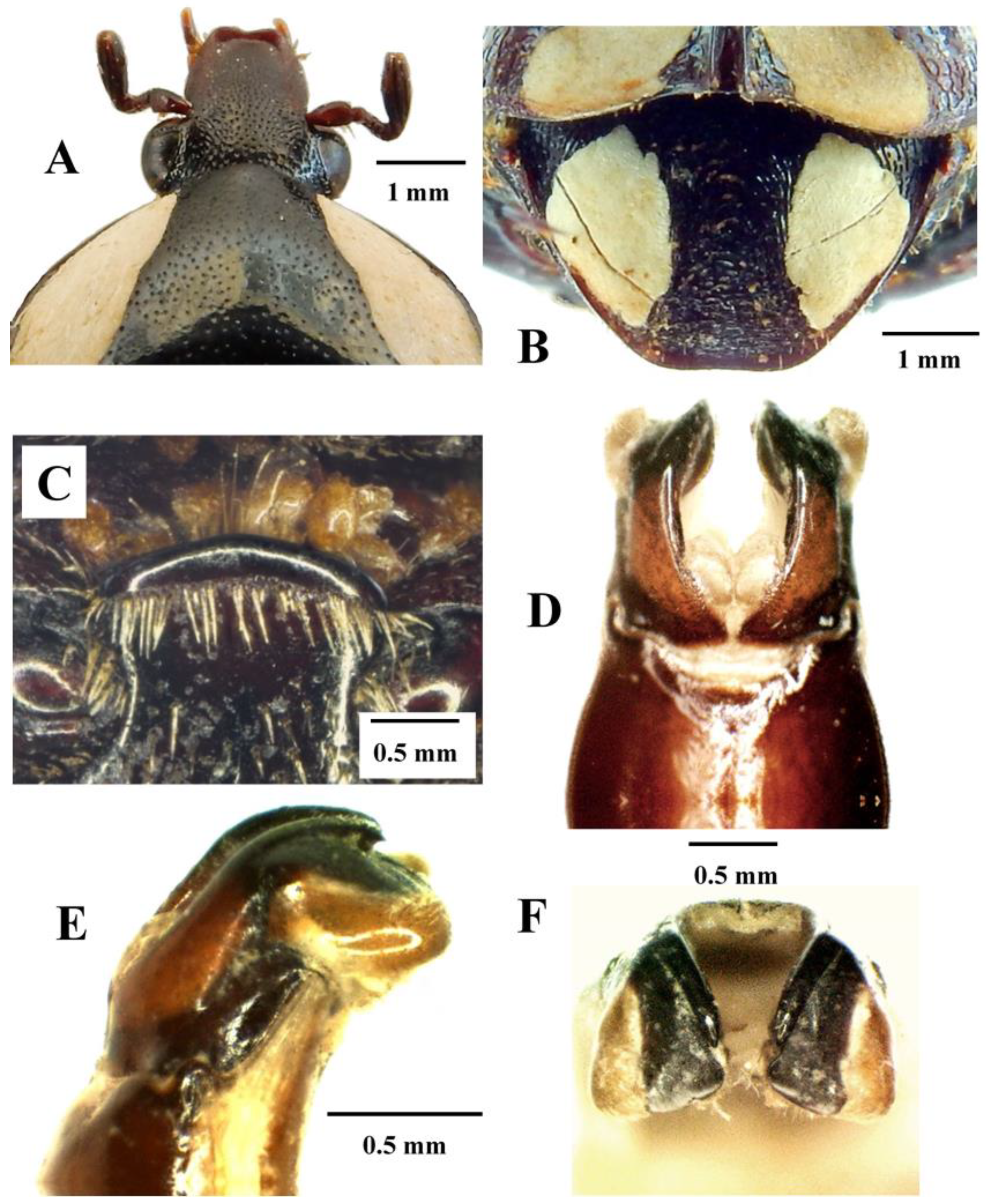
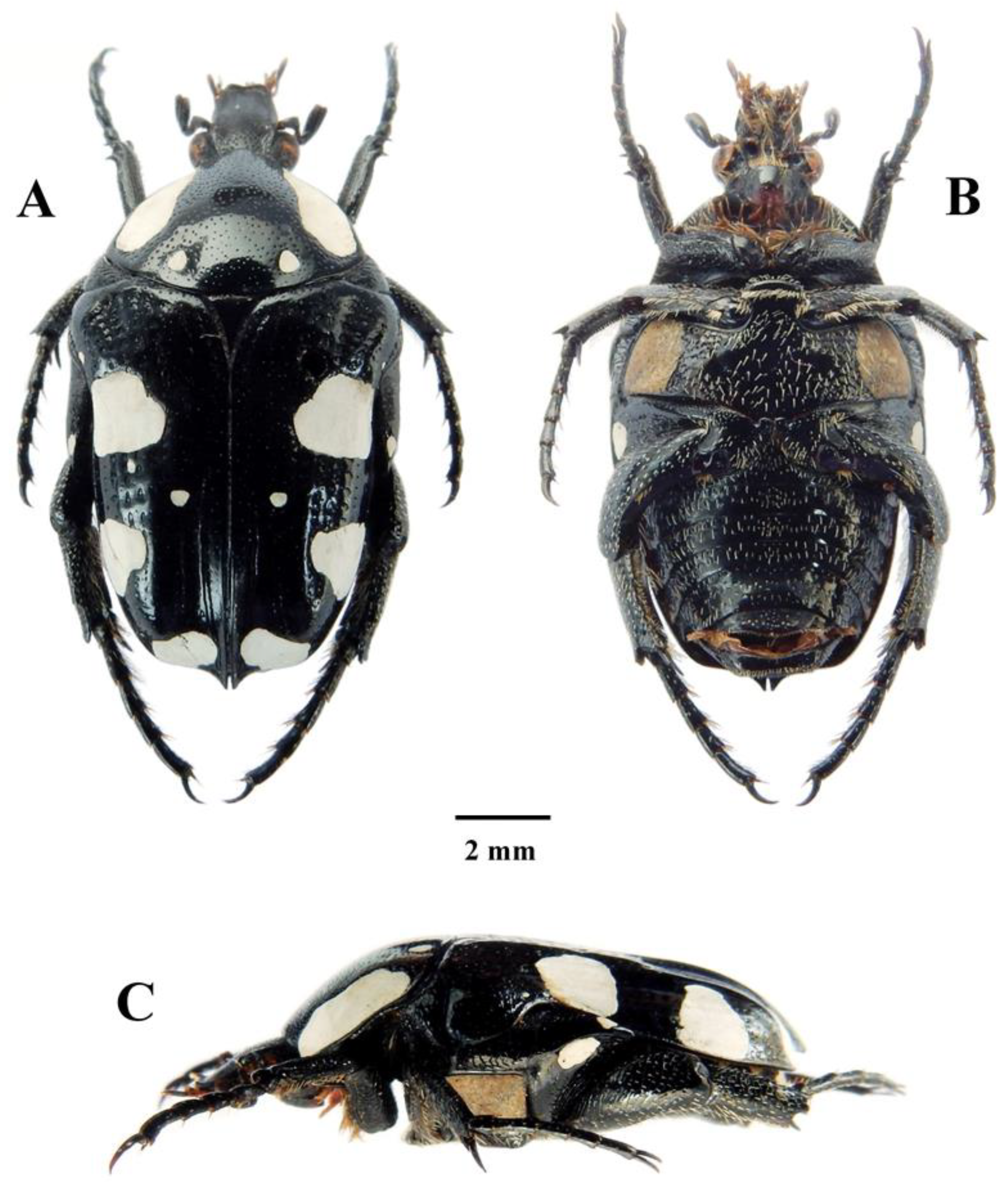
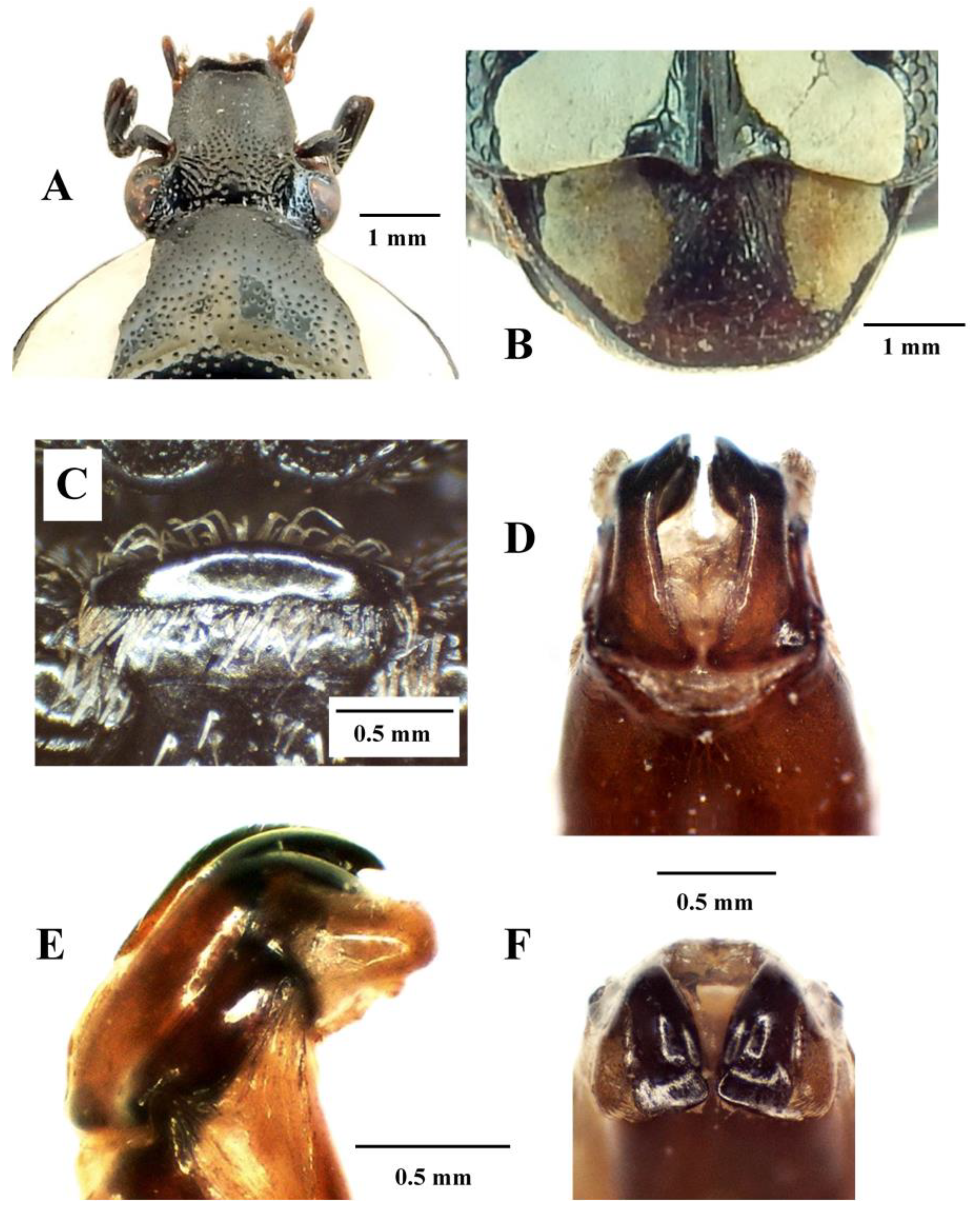
The background colour in this new species is reddish-brown to dark brown, rather than shiny black as is typical in Mausoleopsis amabilis s. l. The white cretaceous spots on its pronotal and elytral discs are consistently present and generally well-developed (Figure 1A,C), although the two pairs on the side of the scutellar apex and above the elytral apical maculae, respectively, are normally very small and even absent in some specimens. Both pronotal bands and elytral maculae are generally creamy in colour, rather than pure white as in most M. amabilis s. l. cases. Furthermore, the clypeus of M. gariepena sp. nov. is more densely sculptured and with a thicker anterior margin than in M. amabilis s. l. The posterior margin of the elytron is smoothly rounded in M. gariepena and exhibits an apical spine that is far less protruding than in M. amabilis s. l. Its mesosternal lobe exhibits a broader base and smaller and sparser punctures than in M. amabilis s. l. (Figure 2C). Finally, in the new species, the apical portion of the dorsal lobes of the aedeagal parameres are wider but shorter and bent downwards at right angles, rather than further towards the base as in M. amabilis s. l. The apical spines on its dorsal lobes are also much shorter than in M. amabilis s. l. (Figure 2D–F).
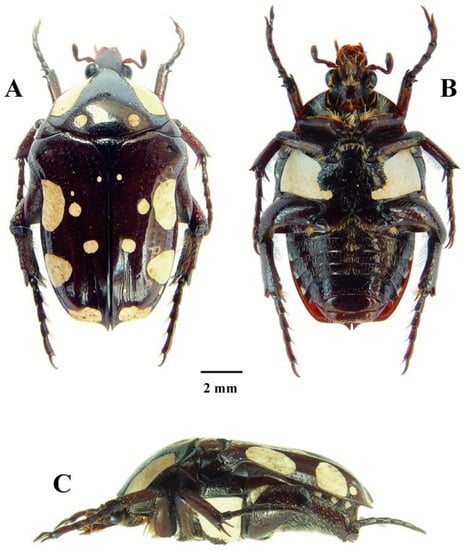
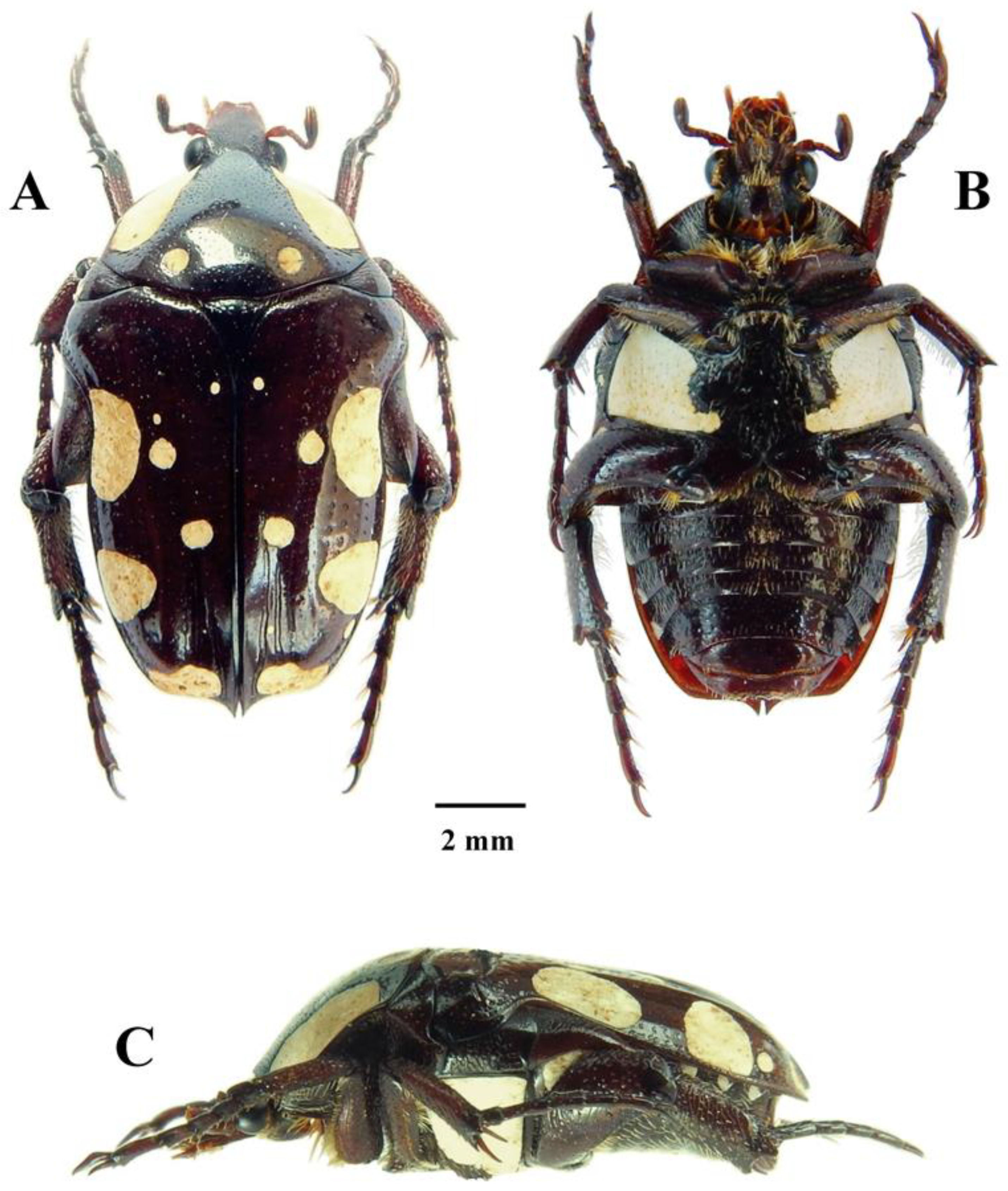
Figure 1.
Mausoleopsis (M.) gariepena sp. nov. Holotype male: (A) dorsal habitus; (B) ventral habitus; (C) lateral habitus. Photographs by Lynette Clennell.
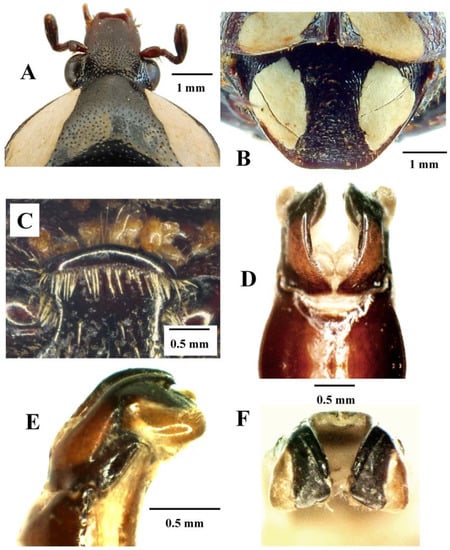
Figure 2.
Mausoleopsis (M.) gariepena sp. nov. Holotype male: (A) clypeus; (B) pygidium; (C) mesosternal lobe; (D) parameres, dorsal view; (E) parameres, lateral view; (F) parameres, frontal view. Photographs by Lynette Clennell.
3.1.2. Description of Holotype Male
Size: Total length = 14.4 mm; maximum width = 7.9 mm.
Body: Dark brown, shiny and dorsally glabrous, with creamy-coloured cretaceous bands, maculae, and spots distributed across pronotal and elytral surfaces as well as on metacoxal dorsal ridge; with dense and fine round sculpture on head, becoming scattered on pronotum and large and predominantly horseshoe to geminate-shaped along elytral striae (Figure 1A–C and Figure 2A).
Head: Dark brown to black, with deep concavity between frons and apex; clypeus with lateral and anterior margins steeply upturned, mildly sinuate at apex and with antero-lateral corners smoothly rounded; surface entirely glabrous, but covered in round, dense and regularly spaced round sculpture, becoming rugulose along margins; antenna dark brown, with club approximately as long as flagellum and pedicel combined; few thin tawny setae scattered across flagellum, but lacking completely on pedicel (Figure 2A).
Pronotum: Brown, shiny, and completely glabrous, with wide creamy band on each lateral margin uninterrupted but not reaching basal margin; pair of creamy circular spots on the disc in suprascutellar position and near base; irregularly octagonal, widening at base, with angles smoothly rounded and small tubercle in central apical area; basal margin evenly convex, with mild sinuation above scutellum and shallow depression between pair of creamy spots; small and shallow round punctures regularly spaced across entire surface, becoming larger on declivities but not visible in areas covered by cretaceous bands (Figure 1A,C).
Scutellum: Dark brown, shiny, and glabrous, with scattered but large oval to crescent sculpture in basal fourth; broadly isoscelic triangular in shape, elongate with concave lateral margins and extremely pointed at apex; lateral grooves well-defined and deep across entire length, widening progressively towards base (Figure 1A).
Elytron: Brown, shiny and glabrous, with two large creamy maculae on lateral margin, one on apical margin and four spots on disc forming symmetric pairs with opposite elytron and distributed as follows: (1) basal near scutellar apex, (2) median near first macula, (3) median near suture, (4) apical above macula; costae obsolete to weakly elevated and barely visible but humeral callus prominent and protruding outwards as widest area, leading also to extreme concavity in subhumeral arch; striae marked with lines of large horseshoe punctures, becoming geminate on medio-apical portion of disc; posterior margin smoothly rounded and apico-sutural margin sharply upturned, forming acute spinal projection at apex (Figure 1A,C and Figure 2B).
Pygidium: Dark brown to black, with pair of symmetric creamy maculae on each side not reaching apical margin; quadrilateral in shape, with bulging dome at centre and steep declivity on apical margin; with dense network of shallow and subconcentric rugulose sculpture across entire surface and short light-yellow to tawny setae scattered around apical declivity, becoming longer and finer along lateral margins and apex (Figure 2B).
Legs: Dark brown and shiny with occasional black tips or edges; tarsi of average cetoniine length and longer than tibiae, with basal segment of meso- and metatarsi much shorter than other segments; protarsal claws unequal, with inner claw approximately twice as long and thick as outer one; tibiae irregularly sculptured and covered in short and regularly spaced light-yellow setae, becoming longer and finer on inner margins; protibia narrow and bidentate, with both denticles curving outwards and apical denticle approximately twice as long as second; meso- and metatibia with supra-apical tranverse carina on outer margin and apical margin bluntly tridentate; spurs on both legs equal and poorly produced; metatibia and metafemur hypertrophic, with convex carina on inner margin and marked curvature, respectively (Figure 1A–C).
Ventral surface: Dark brown to black and shiny, with large creamy macula on metasternum reaching margin of meso-metasternal process; lesser macula also present on metacoxa and minor spots on metepisternum and on marginal end of abdominal sternites 1–5; covered in light-yellow to tawny setae emerging at centre of round to horseshoe punctures, except on femoral bases and central areas of abdominal sternites 6–7; setae short to medium sized and scattered on abdominal sternites, ventral femoral surfaces, metasternum and mesometasternal process, becoming denser and longer around mesosternal lobe, on inner femoral margins and on prosternum; mesosternal lobe crescent-shaped, very narrow but laterally oblong and smoothly rounded, not protruding either forward or downwards; metasternal lobe exhibiting deep median sulcus, with wide lateral depression tapering gradually towards apex; spiracles on 6th abdominal sternite forming typical tubercular projection on each side; abdominal sternites with marked concavity at centre (Figure 1B).
Aedeagus: Parameres much shorter than pars basalis, approximately 1/3 of total length; dorsal lobes complex with apical region subdivided into three distinct areas: (1) outer part forming light oblong protuberance with ciliate surface, (2) median part forming dark disc-like tranverse and ventrally curved projection, (3) inner part forming dark, elongate and arched spinal projection (Figure 2C–E); ventral lobes as wide as dorsal ones, but extremely short and interrupted by protrusion of outer light component of dorsal lobes (Figure 2E).
3.1.3. Derivatio Nominis
This species is named after the broad region in which it occurs, the Gariep Desert, where it is also part of an endemic community of species highly specialised to the extreme hot and dry conditions that characterize its climate (Figure 3).

Figure 3.
Typical habitat of Mausoleopsis (M.) gariepena sp. nov. in the Gariep Desert near Pofadder, Northern Cape. Photo by Lynette Clennell.
3.1.4. Distribution
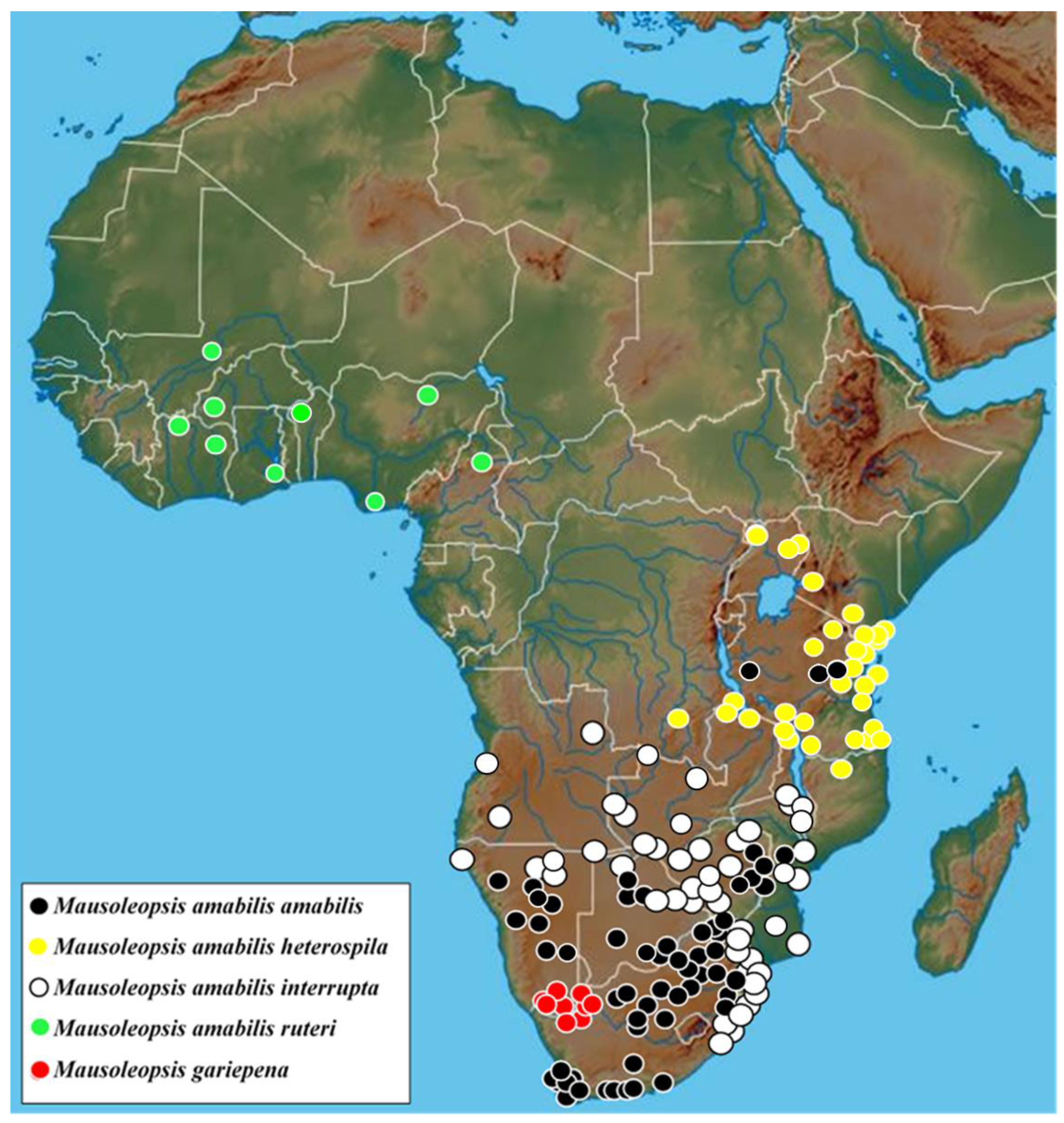
All specimens and observations currently available are either from the South African or the Namibian side of the Orange River valley, from approximately the Richtersveld—Ai-Ais Transfrontier Park to the Augrabies Falls and the western portion of Gordonia (Figure 9).
3.1.5. Type Series
Holotype ♂: South Africa NC, Pofadder, 17 Feb 2018, R Perissinotto & L Clennell (ISAM). Paratypes. NAM: 2♂, 25 km E Fish River Canyon, −27.6250 S 17.6250 E, 1984/04/08, M Macpherson (ISAM-COL-A028156, ISAM-COL-A028158). ZAF: 16♂ + 10♂ idem (RPRM); 3♂, Gordonia, Vrouenspan, 27°48′23″ S 20°25′38″ E, 13 Apr 1994, R Perissinotto & L Clennell (BMPC, RPRM); 3♂ + 1♀, NC, Dabenoris, 23 Jan 2005, R Perissinotto & T Brinkman (BMPC, RPRM); 1♂, NC, Augrabies Falls NP, 5 Jan 1997 (R Perissinotto & L Clennell (BMPC); 1♀, Upington, NC, −28.45 S 21.25 E, 20 Feb 1980, VB Whitehead (ISAM-COL-A028139); 1♂, Narugas, NC, −19.3750 S 18.8750 E, 1 Jan 1919, Lightfoot (ISAM-COL-A028148); 1♀, Oemsberg Water, Richtersveld, N Cape, 28.27 S 17.10 E, 23.9.1991, Endrody-Younga E-Y: 2790, On flowering vegetation (TMSA-CPH2930); 1♂, 10 km S of Lutzputz, 28.II.1999, Lízler & Kulhánek leg. (GBPC).
3.1.6. Other Records
NAM: 1♂, Karas, NA-KA, 13 Apr 2021 6:07, Isabelle van der Linden (https://www.inaturalist.org/observations/73922339, accessed on 10 December 2021); 1♀, ibidem 20 Apr 2020, James Jordan (https://www.inaturalist.org/observations/42674407, accessed on 10 December 2021).
3.1.7. Remarks
Specimen size lies in the range of 13.5–14.8 mm in total length and 7.3–8.0 mm in maximum width. The background colour changes substantially across specimens, within a gradient of reddish-brown to dark brown, while the white dorsal maculation shows much of the same variability observed in M. amabilis s. l. The lateral pronotal bands are continuous in virtually all the specimens currently known, with the exception of one from the extreme southwestern range (Oemsberg, Richtersveld), which exhibits bands interrupted into two portions, similar to the pattern observed in M. a. interrupta ssp. nov. described further down. The two lateral elytral maculae are wide and compact, with the anterior predominantly rectangular but the posterior rather triangular in shape. Approximately one third of the specimens known have also a small to minute white spot in subhumeral position, just ahead of the large anterior macula. The discal spots on pronotal base and elytra are always present and rather well-developed, with the exception of the periscutellar and supra-apical elytral pairs, which can be extremely reduced or even absent in some specimens. In two cases, the mid-elytral discal spots are merged with the larger lateral maculae.
Females are slightly stockier than males, have broader protibia but much shorter protarsi and identical tarsal claws. Their metatibia, on the other hand, is narrower than in the males and lack the typical convex carina on the inner surface which is so typical of their male counterparts. Their elytral apical spine is also shorter than in males, while their inner metatibial spur is almost twice as long as in males. Finally, their abdominal sternites are rather straight, by comparison with those of the males, which are markedly concave with median depression at centre.
Adult activity seems to occur mainly in the late summer and early autumn months (Jan–April), coinciding with the period of highest rainfall in the region [14]. Only one record so far reports adult activity in the spring (September). Like many other cetoniines dwelling in desert and other arid habitats, adults emerge immediately after a substantial rainfall event, cf. [15,16]. Most specimens have been observed feeding on a variety of flowers, mainly of stem-succulent shrubs such as Euphorbia gregaria or small trees like Boscia foetida, where adults also gather to form mating aggregations (Figure 4). Larvae and other early life stages are yet unknown.

Figure 4.
Small aggregation of Mausoleopsis (M.) gariepena sp. nov. on fruits of Boscia foetida near Pofadder, 18 February 2018. Photo by Lynette Clennell.
3.2. Mausoleopsis (Mausoleopsis) amabilis (Schaum, 1844) (Figures 5–11)
Oxythyrea amabilis Schaum, 1844: 408. [17]: 26; [18]: 280; [19]: 265; [20]: 239.
Leucocelis amabilis (Schaum). [21]: 557; [22]: 576.
Mausoelopsis amabilis (Schaum). [23]: 29; [24]: 39; [25]: 287; [26]: 486; [27]: 119; [28]: 270; [29]: 180; [30]: 41; [31]: 324; [32]: 104; [33]: 88; [7]: 8; [8]: 48; [9]: 253; [10]: 316; [34]: 69; [2]: 469.
Microthyrea amabilis (Schaum). [35]: 77.
Mausoelopsis lerui Antoine, 2004 (= amabilis heterospila) syn. nov.
3.2.1. Mausoleopsis amabilis interrupta ssp. nov.
As previously reported in several publications, the populations from southern Africa representing this species show a remarkable variability in the size and presence/absence of the small discal spots, both on pronotum and elytra [2,6,9,26]. However, their lateral pronotal bands and subhumeral elytral maculae are rather consistent and geographically distinct. In particular, the pronotal lateral bands are broken into anterior and posterior lobes in the populations from the eastern part of the subcontinent that exhibits a high summer rainfall rate and the central savanna regions (NAM N-E, BWA N-E, ZWE W, ZN L, MP, LP L, ZMB, MOZ, MWI)(see identification key below, under Section 4) (Figure 5 and Figure 6). Conversely, the pronotal bands are uninterrupted in the populations from the southwestern part and the central highlands of the species distribution range (WC, EC, NC, FS, ZN M, NW, GP, LP H, NAM S-W, BWA S-W, ZWE E, TAN C) (Figure 7 and Figure 8). Hence, the new subspecies name, reflects this key character. The specimen from the Melly Collection, currently housed in the MNHG and designated as M. amabilis paralectotype in Holm and Marais [9], is actually a typical example of this new subspecies and indeed originates from “Port Natal”, the old name for the city of Durban (ZN).
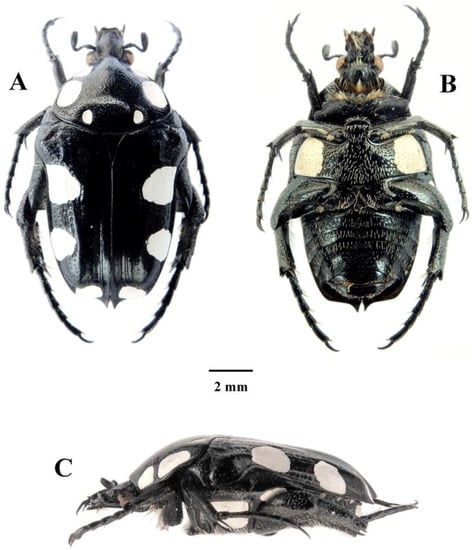
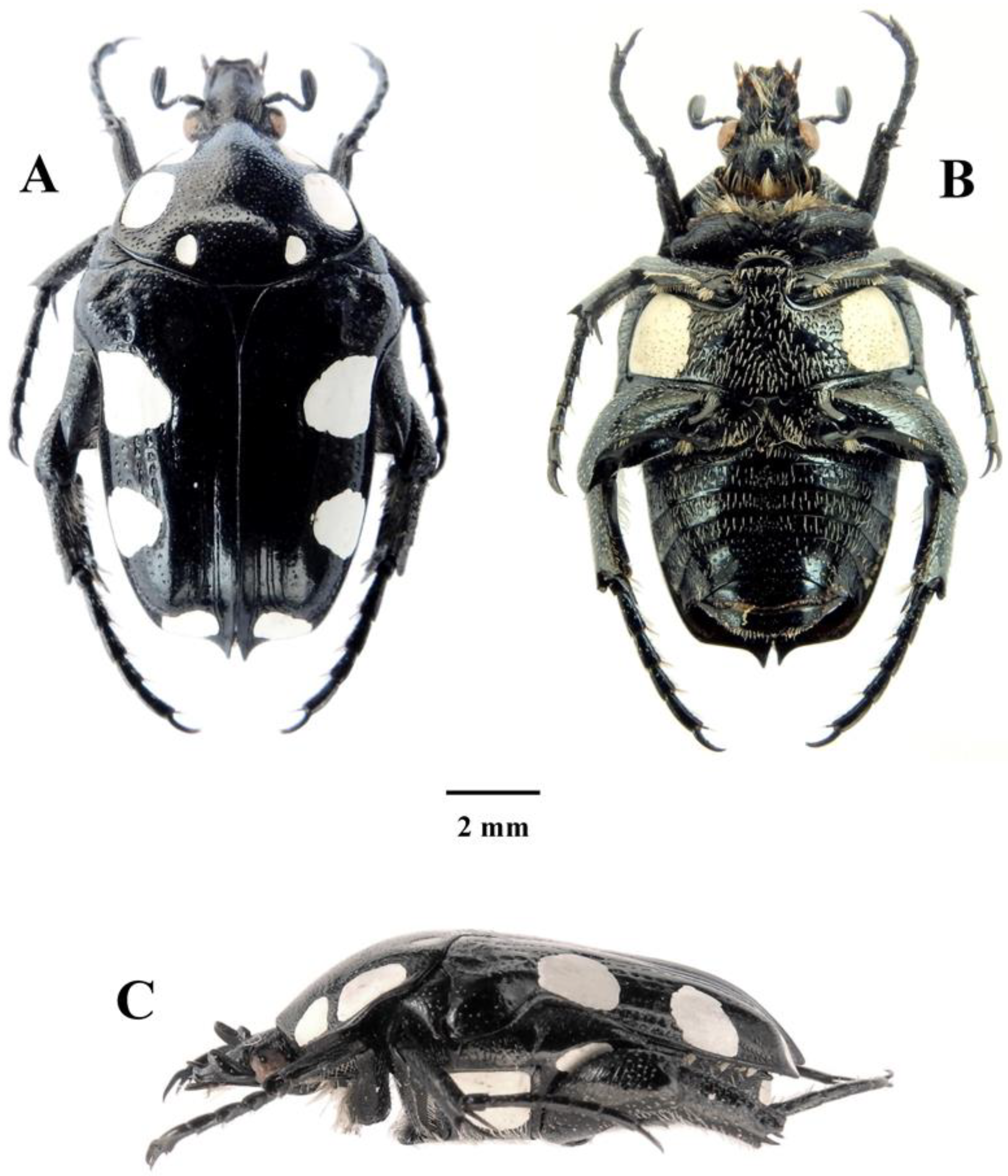
Figure 5.
Mausoleopsis (M.) amabilis interrupta ssp. nov. Holotype male: (A) dorsal habitus; (B) ventral habitus; (C) lateral habitus. Photographs by Lynette Clennell.
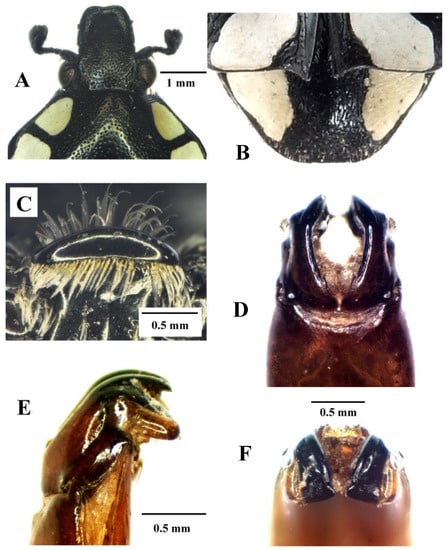
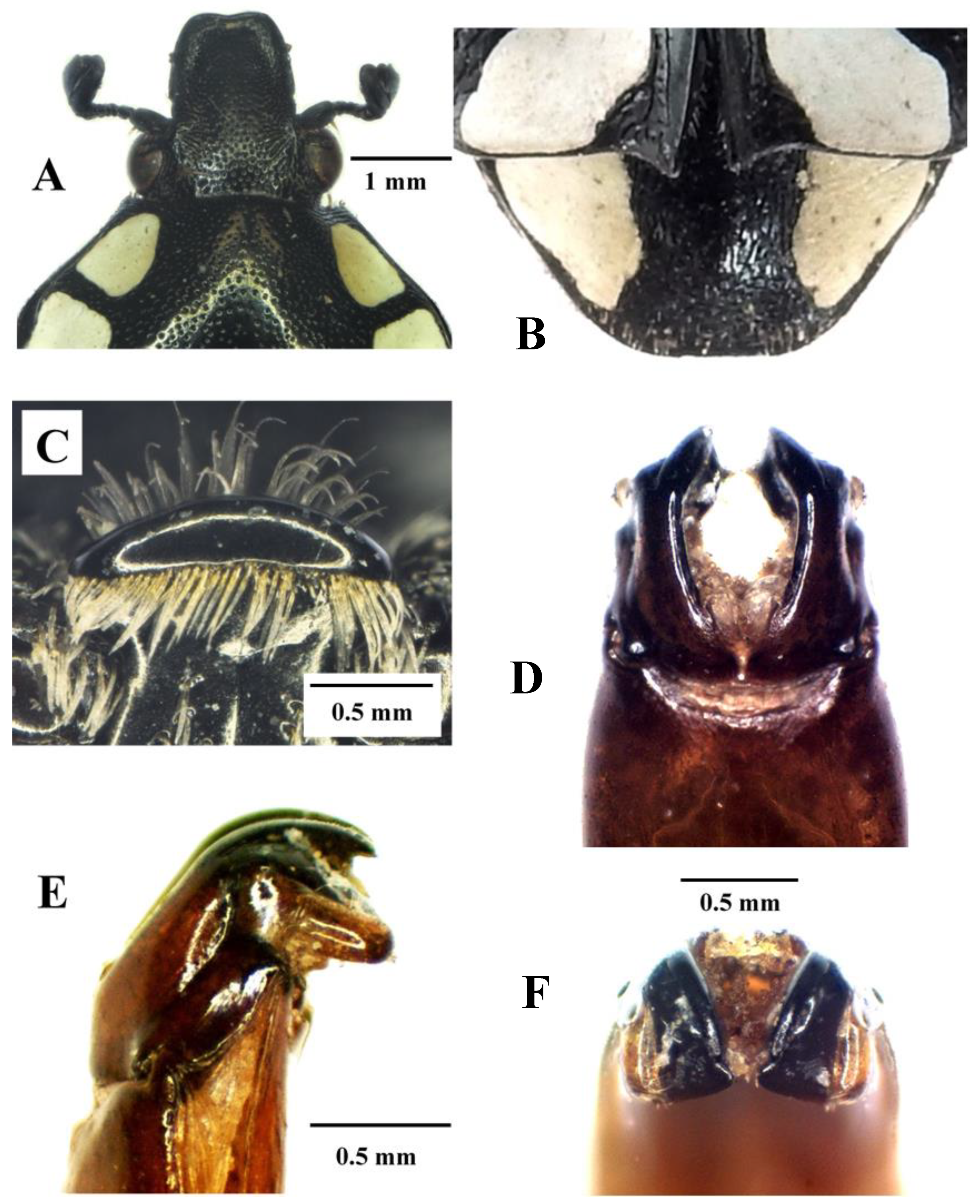
Figure 6.
Mausoleopsis (M.) amabilis interrupta ssp. nov. Holotype male: (A) clypeus; (B) pygidium; (C) mesosternal lobe; (D) parameres, dorsal view; (E) parameres, lateral view; (F) parameres, frontal view. Photographs by Lynette Clennell.
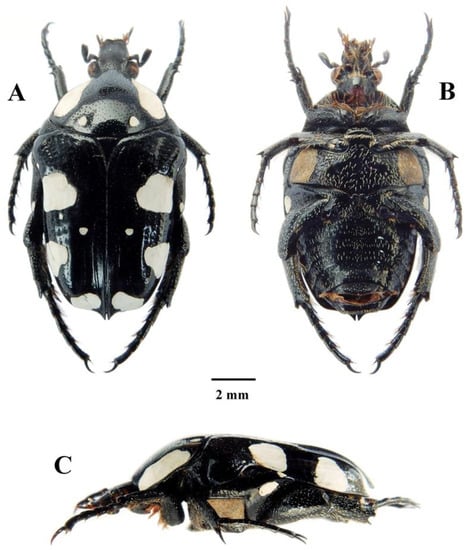
Figure 7.
Mausoleopsis (M.) amabilis amabilis (Schaum, 1884). Male: (A) dorsal habitus; (B) ventral habitus; (C) lateral habitus. Photographs by Lynette Clennell.
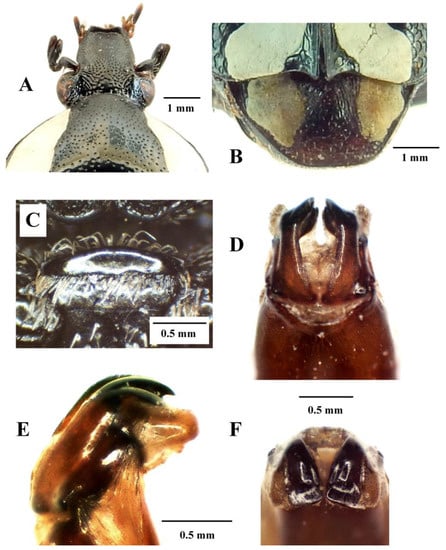
Figure 8.
Mausoleopsis (M.) amabilis amabilis (Schaum, 1884). Male: (A) clypeus; (B) pygidium; (C) mesosternal lobe; (D) parameres, dorsal view; (E) parameres, lateral view; (F) parameres, frontal view. Photographs by Lynette Clennell.
The size of the holotype male is 13.2 mm in total length and 7.3 mm in maximum width, while the range across a sample of 46 individuals is 11.9–13.9 mm and 5.5–7.5 mm, respectively, thereby exhibiting the smallest individuals among the four subspecies [2,9]. Intermediate specimens, with constrained or partially interrupted pronotal bands are known from the interface between the two populations and should be regarded as part of the nominal subspecies. Specimens with uninterrupted pronotal bands actually reach as far northeastward as the central highlands of Tanzania, through the eastern highlands of Zimbabwe and western Mozambique (Figure 9). This may indicate that some recent degree of connectivity exists between populations living in these mountainous habitats. On the other hand, from the coastal lowlands of Mozambique, M. a. interrupta penetrates into the savanna regions of the interior through the Limpopo and the Zambesi River basins, reaching northern Botswana and Namibia as well as western Zambia and Angola (Figure 9).
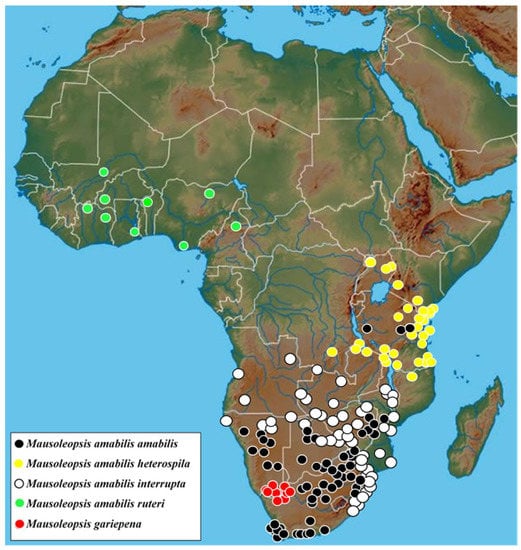
Figure 9.
Known distribution of Mausoleopsis gariepena sp. nov. and the four subspecies of Mausoleopsis amabilis s. l. (Schaum, 1844) across the Afrotropical Region (map adapted from www.geology.com, accessed on 8 October 2021).
3.2.2. Type Series
Holotype ♂: South Africa, KZN, Enseleni NR, 29 Oct 2000, R Perissinotto & L Clennell (TMSA). Paratypes: AGO. 1♀, Morro de Pundo, 21.V.1934, K. Jordan leg. [7: 8] (BMNH); 1♂, Humbe [Cunene, 16°41′ S, 14°54′ E, 1100 m alt.], Anchieta leg. [12: 52, fig. 11a] (MZUC); 1♂, N°1907 [no locality] [12: 52, fig. 11b] (MZUC); 1♂, Zumbo [Lunda Sul] [12: 52, fig. 11c] (MZUC); 1♂, Huila Prov., near Chiapepe, 10.XII.2012, P, Schüle leg. (PMPC). BWA. 1 ind, Ngoma Bridge at Chobe R., 17.55 S 24.43 E, 15–18.1.1986, Holm, Marais, Nel, Saieva (TMSA-CPH3023); 1 ind, Chobe R., 5 km E Kasane, 17.48 S 25.09 E, Holm, Marais, Nel, Saieva (TMSA-CPH3024); 1 ind, South Gate, 12 km SE Moremi Game Res., 12–14.1.1986, Holm, Marais, Nel, Saieva (TMSA-CPH3025); 1 ind, Nata, 14.1.1978, Holm, Jacobs, Kirsten, Scholtz (TMSA-CPH3028); 3 inds, ♀Okavango, Thamalakane Riv., 12.1973, P Reavel (TMSA-CPH3030); 2 inds, Okavango, Samandupi, 12–18.12.1973, P Reavel (TMSA-CPH3031); 2 inds, Boteti Riv., 12.12.1973, P Reavel (TMSA-CPH3032). MOZ. 1 ind, Masiene, P.E. Afr., 1 Jan 1924, Lawrence (ISAM-COL-A028180); 1 ind, ibidem 1923/12/01, Lawrence (ISAM-COL-A028164); 1 ind, Beira, 1905/01/01, Sheppard (ISAM-COL-A028175); 1 ind, Zambesi, Fry (ISAM-COL-A028173); 1 ind, Delagoa B. [Bay], −26.125 S 32.625 E, Monteiro (ISAM-COL-A028170);1 ind, Nyaka [Inhaca], −23.8597 S 35.3472 E, 1924/02/01, Lawrence (ISAM-COL-A028162); 1 ind, Inhambane, P.E. Africa, −23.85 S 35.383 E, 1928/02/01, Lawrence (ISAM-COL-A028161); 1 ind, Magude, 10.1918, CJ Swierstra (TMSA-CPH2954); 3 inds, Delagoa Bay, coll. E Candèze (RBINS); 2 inds, ibidem, Le Moult vend. (RBINS); 1 ind, Sandacca Moz III, JJGillet det., vend. R.M.H.N. Belg. 10,640 (RBINS); 1♂, Prov. Manica, 60 km W of Chimoio, 10–12.12.2003, A Kudrna Jr leg. (GBPC); 1♂, Inhambane Prov., 20 Km W Tesenane, 10.II.2009, G Werner leg. (GBPC). MWI. 1 ind, Central, 15 km S Salima, 13.55 S 34.28 E, 25.1.1985, CL Bellamy, S Chown, AV Evans, AS Schoeman, CH Scholtz (TMSA-CPH3062); 1 ind, 7 km West Golomoti, 2.12.1983, Dept. Entomology UP (TMSA-CPH3061); 1♂, Lake Njasa (Mc Lear), 20–30.I.1998, Mráček leg. (GBPC); 2♂, Mwanza Distr., Tambani Fst. (TGPC). NAM. 1♂, Otjo-Otavi Rd, 23 Dec 2000, R Perissinotto & L Clennell (RPRM); 2♂, Ovamboland, nr. Tsintabis, 2 Jan 2007, R Perissinotto & L Clennell (RPRM, BMPC); 1♂, Grootfontein, 21 Dec 2000, R Perissinotto & L Clennell (RPRM); 1♂, Otavi Mnts, 27 Dec 2007, R Perissinotto & L Clennell (BMPC); 1♂, Kombat, 19 Dec 2000, R Perissinotto & L Clennell (BMPC); 1♂, Kavango, Shamvura Lodge, 1 Jan 2007, R Perissinotto & L Clennell (BMPC); 1 ind, Abenab, S.W.A., −19.283 S 18.083 E, 1929/01/01, Frampton (ISAM-COL-A028146); 1 ind, Hoarusib, Otshu, S.W.A., −18.05 S 13.383 E, 1926/03/01, South African Museum Expedition (ISAM-COL-A028145); 2 inds, Kwandu River, West Caprivi, 14.11.1979, CH Scholtz (TMSA-CPH3033); 1 ind, Katima Mulilo, Caprivi, 7.12.2002, O Aschenborn (TMSA-CPH3035); 1 ind, Ghaub Valley, 3.1.1972, D Kroon (TMSA-CPH3036); 1♂, Namibia, Otjozondjupa, zw. Otjiwarongo u. Otavi, Lodge Korab, 1420 m B1, S 19°39′45.3″ E 17°19′57.4″, 2.III.2016, leg. A. Puchner (GBPC); 2♂, Mururani, I.1993, coll. RC Owen (GBPC). SWZ. 1♂, Hlane G. Reserve, Dec 1996, E & R Stronkhorst (PMPC). ZAF. 1♂, idem (RPRM); 1♂, ibidem 25 Sep 2000 (BMPC); 1♀, ibidem 14 Oct 2000 (BMPC); 3♂, LP, Nwanedi, 28 Dec 2009, R Perissinotto & L Clennell (RPRM, BMPC); 1♂, KZN, False Bay, 26 Nov 2006, R Perissinotto & L Clennell (BMPC); 1♂, ibidem 16 Dec 2006, R Perissinotto & L Clennell (RPRM); 1♂, ibidem 1 Oct 2008, R Perissinotto & L Clennell (BMPC); 4♂, KZN, Mkuzi GR, 3 Nov 1995, M Burger (RPRM); 1♂, KZN, Eland’s Lake, 19 Nov 2005, R Perissinotto & L Clennell (BMPC); 1♂, KZN, Umfolozi GR, 31 Oct 1999, R Perissinotto & L Clennell (BMPC); 1♂, KZN, Harold Johnson NR, 30 Sep 2000, R Perissinotto & L Clennell (BMPC); 1♂ + 1♀, KZN, Tugela, 26 Oct 2003, R Perissinotto & L Clennell (BMPC); 1 ind, Kloof, Natal, 29°48′ S 30°50′ E, 2930DD, Marley (DNSM-10425); 1 ind, D’Urban [Durban], −29.875 S 30.875 E, 1894/01/01, Barker (ISAM-COL-A028192); 1 ind, ibidem −29.875 S 30.875 E, Butter (ISAM-COL-A028191); 1 ind, ibidem, Barker (ISAM-COL-A028168); 1 ind, ibidem 15.12.1918, H Bell-Marley (TMSA-CPH3021); 1 ind, ibidem (TMSA-CPH3022); 1 ind, Zoulouland [Zululand], Martin (ISAM-COL-A028188); 1 ind, Pietermaritzburg (ISAM-COL-A028467); 1 ind, Impingo [Isipingo], Natal, −29.983 S 30.93 E, Barker (ISAM-COL-A028166); 1 ind, Ubombo, KZN, 1922/07/01, Marley (ISAM-COL-A028163); MP Skukuza, Kruger Nat. Park, 25.00 S 31.35 E, 6–15.12.1993, M Kruger (TMSA-CPH2906); 4 inds, idem Kruger & Dunning (TMSA-CPH2908); 1 ind, ibidem 16–24.12.1993, M. Kruger (TMSA-CPH2907); 1 ind, ibidem, 15 km NE, 24.55 S 31.41 E, 20.11.1994, Endrody & Bellamy, E-Y: 3057, Grewia etc. flowers (TMSA-CPH2909); 2 inds, ibidem, 1 km N, 24.59 S 31.36 E, 23.1.1995, CL Bellamy, E-Y: 3091, On vegetation (TMSA-CPH2910); 2 inds, ibidem, 20 km NE, 24.53 S 31.45 E, 3.12.1984, CH Scholtz (TMSA-CPH2911); 1 ind, idem (TMSA-CPH2913); 4 inds, ibidem, 10 km NW, 1.12.1984, CH Scholtz (TMSA-CPH2912); 3 inds, Satara, Kruger Nat. Park, 24.26 S 31.47 E, 16.12.1985, CH Scholtz (TMSA-CPH2914); 4 inds, Transvaal, Guernsey, NE of Klaserie, 24.49 S 31.06 E, 21–26.12.1985, CH Scholtz (TMSA-CPH2915); 1 ind, MP Stentor Estates, 3 km E Kaapmuiden, 25.32 S 31.32 E, 5–6.12.1984, CH Scholtz (TMSA-CPH2925); 5 inds, Transvaal, Manyeleti Game Res., 24.38 S 31.28 E, 19.11.1987, Th v Viegen, Cetoniid Trap (TMSA-CPH2937); 1 ind, Transvaal, Phalaborwa, 12.1956, L. van Bergen (TMSA-CPH2953); 3 inds, Mpumalanga, Lydenburg Distr., 1896, PA Krantz (TMSA-CPH2956); 1 ind, Mpumalanga, Barberton 3.12.1909, Miss de Beer (TMSA-CPH2960); 1 ind, ibidem 1906, Miss de Beer (TMSA-CPH2961); 2 inds, Natal, Hluhluwe Game Res., 28.05 S 32.04 E, 27.11.1992. Endrody-Younga E-Y: 2857, Flowering Acacia (TMSA-CPH3003); 2 inds, ibidem 14–15.10.1947, G van Son (TMSA-CPH3004); 5 inds, ibidem 28°06′ S 32°05′ E, 26–29.xi.1992, M Kruger (TMSA); 3 inds, Natal, Malvern (TMSA-CPH3007); 5 inds, Natal, Ndumu Game Res., 26.54 S 32.17 E, 1.12.1992, Endrody-Younga E-Y: 2869, Fruit Traps, vlei (TMSA-CPH3008); 1 ind, ibidem 26.51–55 S 32.12–20 E, 5–8.11.1984, CL Bellamy, H & A Howden, RG Oberprieler & CH Scholtz (TMSA-CPH3009); 2 inds, 12.1960 van Son (TMSA-CPH3010); 7 inds, Natal, Burm (TMSA-CPH3012); 3 inds, ibidem 1904, Paulus (TMSA-CPH3013); 1 ind, ibidem 1900, Paulus (TMSA-CPH3014); 4 inds, ibidem (RBINS); 1 ind, ibidem, Coll. Roelofs, R.I.Sc.N.B. 18.862 (RBINS); 2 inds, ibidem, Le Moult vend. (RBINS); 3 inds, Pongolo/Mogul, 11.1970, O Bourquin (TMSA-CPH3016); 1 ind, Pongolo R.,11.1933, HW B-M (TMSA-CPH3017); 1 ind, Tongaat, 1908–1909, HC Burnup (TMSA-CPH3018); 2 inds, ibidem 1909, HC Burnup (TMSA-CPH3019); 2 inds, Ben Lavin NR, 9 km S Louis Trichardt, 8–14.xii.2000, M Kruger (TMSA); 1 ind, Port Natal, Coll. P De Moffarts, R.I.Sc.N.B. 21.418 (RBINS); 1 ind, ibidem (RBINS); 1 ind, ibidem, Delagoga (RBINS); 1♂, Natal, 10-1996 (TGPC); 1♂, TVL, Saltpans, 12.1980, ex Collection Dr Vincent Allard received from Christophe Allard, 21.II.2015 (GBPC). ZMB. 1♂, Kalabo, 15 Nov 2003 (RPRM); 2♂, coll. near Kazungula, 13 Jan 2005, ex ovum, P. Malec breed (PMPC); 1♂, Kafue River, 21.12.1989 (GBPC); 2♂, NW Prov., Nchila, Ikelenge, 13–16.XI.2003, Werner & Smrz leg. (GBPC); 1♂, Kitwe, 01.2004 (TGPC). ZWE. 1♂, Busi Valley, Simuchembu, 17°43′ S 28°10′ E, Apr 1995, AJ Gardiner (RPRM); 1 ind, Mashunaland, Mazoe, 1894/01/01, Darling (ISAM-COL-A028193); 1 ind, Plumtree, −20.625 S 27.875 E, 1906/01/10 (ISAM-COL-A028187); 1 ind, Sebakwe, −19.125 S 30.625 E, (ISAM-COL-A028185); 1 ind, ibidem −19.116 S 30.533 E, 1903/01/01, Dodds (ISAM-COL-A028176); 1 ind, ibidem 1901/01/01, Dodds (ISAM-COL-A028171); 1 ind, Matabola, 12.1896 (TMSA-CPH3020); 1 ind, Salisbury (C), 23.10.1971, AJ Duke (TMSA-CPH3049); 2 inds, ibidem 16.10.1971, AJ Duke (TMSA-CPH3050); 1♂, idem, Duke 148, ex Collection Dr Vincent Allard received from Christophe Allard, 21.II.2015 (GBPC);1 ind, Gutsa, 27.12.1972, NJ Duke (TMSA-CPH3051); 1 ind, Bulawayo, 23.11.1911 (TMSA-CPH3055); 1 ind, Sawmills, 14.11.1924, RHR Stevenson (TMSA-CPH3058); 1♂, 60 Km N of Bulawayo, Maraposa Rd, 3.XII.1998, M Snížek leg. (GBPC); 1♂, Mushandike, W of Masvingo, 9–12.12.1998, A Kudrna Jr leg. (GBPC); 2♂, Rhodesien, 1973, ex collection Florian Lang sen, Florian Lang Jun 2008 (GBPC); 1♂, Nantwich, 19.12.1949, Coll. Mus. Congo, ex coll. G Arnold, Nat. Museum S. Rhodesia, ex Collection Dr Vincent Allard received from Christophe Allard, 21.II.2015 (GBPC).
3.2.3. Other Records
BWA: 1 ind, Ngamiland East, 13 Feb 2021 4:06, Grant Reed (https://www.inaturalist.org/observations/78219741); 1♂, Ngamiland West, BW-NC, 21 Dec 2021 13:38, Robert Taylor (https://www.inaturalist.org/observations/103490366, accessed on 10 December 2021). MOZ: 1 ind, TCT Dalmann Catapu, 23 Nov 2014 7:58, IC Riddell (https://www.inaturalist.org/observations/1120591, accessed on 10 December 2021). NAM: 1♂, S.W. Afrika, Fontein Omuramba, Sammler F. Gaerdes (Schein 1960: 104). SWZ: 1♂, Mbuluzi Game Reserve, 17 Nov 2021 15:22, Phil White (https://www.inaturalist.org/observations/101333907, accessed on 10 December 2021). ZAF: 1♂, ZN, Mahlungulu, 25 Nov 2021 16:32, Annette Gerber (https://www.inaturalist.org/observations/102594514, accessed on 10 December 2021; 1♂, LP Shingwedzi camp, Kruger NP, 23 Nov 2018 12:50, Copper (https://www.inaturalist.org/observations/19116327, accessed on 10 December 2021); 1♀, ibidem, 28 Nov 2019 10:51, Copper (https://www.inaturalist.org/observations/37087288, accessed on 10 December 2021); 1♂, LP Phiring, 23 Feb 2021 13:15, Lucius Oupa (https://www.inaturalist.org/observations/70034888, accessed on 10 December 2021); 2♂ + 1♀, iSimangaliso Wetland Park, Lake St Lucia, 18 Dec 2021 12:19, magdastlucia (https://www.inaturalist.org/observations/103537058, accessed on 10 December 2021 ). ZMB: 1 ind., Sefula Forest, Mongu, 10 Dec 2008 12:36, J Tourolt (https://www.inaturalist.org/observations/16459239, accessed on 10 December 2021). ZWE: 1♂, Chipinga, 17 Dec 1980 ([10]: 316, pl. 111, fig. 1289).
3.2.4. Records of the Other Subspecies of M. amabilis
M. a. amabilis. Lectotype ♂: Algoa, Coll. Melly, amabilis Schaum (MHNG). BWA: 1 ind., 30 km S of Kang, 23.1.1978, Holm & Jacobs & Kirsten& Scholtz (TMSA-CPH3027); 1 ind., [no locality], 2.1974, R. Boro (TMSA-CPH3029); 1♂, Maun, Island Safari Lodge, 2–15.1.1994, Lgt. M. Snizek (GBPC); 1♂, ibidem, 15–29.1.1997, Lgt. M. Snizek (GBPC); 1♂, Jwaneng, 17.11.2001, leg. O. Aschenborn (GBPC). MOZ: 1 ind., Sofala Pr., Condue, Sofala, 193m, 18.45 S 34.55 E, 5.12.2006, On ground and bark, E-Y: 3743, Gussmann & Muller (TMSA-CPH3044); 1♂, Manica Prov., 35 km SW Chimoio, 14–15.12.2005, A. Kudrna Jn. Lgt. (GBPC). NAM: 1 ind., Hochfeld Summerdon, 16.I.1999, G. Betti leg. (RBINS); 1 ind., Windhuk [Windhoek], −22.56667 S 17.10000 E, 1919/01/01 (ISAM-COL-A028181); 1 ind., ibidem, 1919/01/01 (ISAM-COL-A028151); 1 ind., S.W.A., Zesfontein [Sesfontein], −19.13333 S 13.61667 E, 1925/02/01, South African Museum Expedition (ISAM-COL-A028179); 1 ind., S.W.A., Grootfontain [Grootfontein], −19.56667 S 18.10000 E, 1947/12/31 (ISAM-COL-A028174); 1 ind., Gaub, −23.37500 S 15.62500 E, Lightfoot (ISAM-COL-A028165); 1 ind., S.W.A., Hochberg, −21.87500 S 17.62500 E, 1982/02/25, Vincent & Booth & Whitehead (ISAM-COL-A028160); 1 ind., ibidem, 1982/02/25, Vincent & Booth & Whitehead (ISAM-COL-A028143); 1 ind., S.W.A., Abenab, Grootfontein, −19.28333 S 18.08333 E, 1928/09/01–1928/11/01, Frampton (ISAM-COL-A028154); 1 ind., Outjo, S.W.A., −20.12500 S 16.12500 E, 1926/01/01, South African Museum Expedition (ISAM-COL-A028153); 1 ind., Otavi, S.W.Protect., −19.65000 S 17.33333 E, 1918/12/01, Lightfoot (ISAM-COL-A028152); 1 ind., Ombombo, −18.68333 S 13.95000 E, 1926/02/01, South African Museum Expedition (ISAM-COL-A028150); 1 ind., Kaoko, Otavi,−18.30000 S 13.65000 E, 1926/03/01, South African Museum Expedition (ISAM-COL-A028149); 1 ind., Otavi fontein, S.W.A., −19.62500 S 17.37500 E, 1979/03/08, Vincent & Booth & Whitehead (ISAM-COL-A028144); 1♂ + 1♀, Etosha, Epasha, 13.II.2004 PLPC); 1♂, 45 km S of Kalkfeld, III.1998 (PLPC); 30♂ + n♀, Epako Lodge, Omaruru Reg., 01-2001 (TGPC); 4♂, Kunene Reg., Hobatere, Dec 2008, Steve & Louise Braine (RPRM); 1♂, Hereroland, Nr Windhoek, 5 Jan 2007, R Perissinotto & L Clennell (RPRM); 7 inds, Felseneck Reserve, Naukluft, Hardap, 24.21 S 16.04 E, 11.3.1975, Endrody-Younga, E-Y: 733 (TMSA-CPH3040); 1 ind., Kunene, Kaokoveld, Sesfontein basin, 19.09 S 13.32 E, 2.2.1975, At light, Endrody-Younga, E-Y: 611 (TMSA-CPH3034); 1♂, Erongo Region, Elephant Camp, 1536 m, 21°25’53.9” S 16°30´12.7” E, S. Naumann & E.Ott & H. Sulak leg. (PMPC); 1♀, Koes to Gobabies, 3.III.1999, Lízler & Kulhánek leg. (GBPC); 1♂, Waterberg Plateau, 22–23.XI.2010, Lgt. P. Macháček (GBPC). SWZ: 1 ind., Mpaka, 20.12.1992, N.J. Duke (TMSA-CPH3041). TAN: 1♀, Morogoro, Nguru Mountains, V.2020 (PLPC); 1♀, Dodoma, Mapinduzi, IV.2018 (PLPC); 3♂ + 1♀, ibidem, XII.2015 (PLPC); 1♀, ibidem, 9–20.XII.2017 (PLPC); 1♂ + 1♀, ibidem, XII.2018 (PLPC); 1♂, Mtwara, Masasi, 2010 (PLPC); 2♂, Katavi, Mpanda Dist., Ugalla Masito Prov., 03-2017 (TGPC). ZAF: 1 ind., Transvaal, Bobbejaanberg vic., MC Roodeplat, XII.1987 (RBINS); 1 ind., Natal, Weenen, coll. b G.H. Burn (RBINS); 1♂ + 1♀ Cape Town, 13 Dec 2003, R Perissinotto & L Clennell (BMPC); 1 ind., ibidem, Constantia, −34.12500 S 18.37500 E, 2008/01/01, Hans Beele (ISAM- COL-A066552); 1 ind., ibidem, Vredehoek, −34.01667 S 8.36667 E, 2006/11/21, Margie Cochrane (ISAM-COL-A064726); 1 ind., ibidem, Diep Rivier, −34.03277 S 18.46277 E, 2000/11/14, Taylor (ISAM-COL-A043386); 1 ind., ibidem, 2009/11/23, Simon van Noort (ISAM-COL-A067764); 1 ind., ibidem, Kirstenhof, 2005/01/23, −34.23333 S 18.45250 E, Simon van Noort (ISAM-COL-A067763); 1 ind., ibidem, 19 Dec 2007, Simon van Noort (ISAM-COL-A070085); 1 ind., ibidem, 2010/01/29, Simon van Noort (ISAM-COL-A067767); 1 ind., ibidem, Lakeside, −34.08838 S 18.45430 E, 16 Dec 2010, Simon van Noort (ISAM-COL-A069640); 1 ind., ibidem, Devil’s Peak, −33.95000 S 18.43333 E, 2 Nov 2014, Dawn Larsen (ISAM-COL-A071613); 7 inds., Tamboerskloof, 24.12.2012, on rose petals, H.D. Brown (TMSA); 13 inds., Pinelands, 18.12.2012, on blue flowering shrub, H.D. Brown (TMSA); 1 ind., WC, Farm Zandrivier, 24°39′ S 28°02′ E, 13.xii.2007, T Beyers (TMSA); 8 inds, LP, Steelpoort district, Der Brochen, 1135 m, 25°04′ S 30°07′ E, 26.x.2001, M Kruger (TMSA); 3 inds, GP, Boekenhoutskloof, 25°33′ S 28°29′ E, 11.i.1993, on flowers of Acacia, M Kruger (TMSA); 6 inds, NW, Soutpan/Tswaing, 25°24′ S 28°06′ E, 11.xii.1992, Mixed bushveld, M Kruger (TMSA); 2 inds, LP, Farm Scott/Amatola, 8 km E of Vivo, 22°56′ S 29°23′ E, 26.i.1998, Mixed bushveld, M Kruger (TMSA); 5 inds, EC, Graaf-Reinet, 32°15′ S 24°33′ E, 4.xii.2005, DM Kroom (TMSA); 1 ind., LP, Ben Lavin NR, 9km S Louis Trichardt, 8–14.xii.2000, M Kruger (TMSA); 1 ind., LP, Mmabolela, 22°40′ S 28°15′ E, 20–24.xi.1991, M Kruger (TMSA); 1 ind., NC, Rooipoort, 1129 m, 28°38′ S 24°16′ E, 2.iii.2009, day, Kalahari bushveld, Macfayden & Muller (TMSA); 1 ind., GP, Henopsrivier, Vlakplaas, xii.2009, M Paulsen (TMSA); 1 ind., FS, Vredefort Dome, 1388 m, 26°50′ S 27°19′ E, 19–20.ii.2012, E-Y: 3934, R Muller (TMSA); 1 ind., LP, Lindani NR, 24°02 S 28°20′ E, 4.i.2008, E-Y: 3774, R Muller (TMSA); 4 inds., LP, R515 Thabazimbi, 1.ii.1995 (TMSA); 2 inds., NW, Rustenburg Kloof, 7.i.1996 (TMSA); 1 ind., Natal, E. Zululand, F. Toppin (TMSA-CPH3015); 1 ind., ZN, Empangeni Distr., 2.1.1980, P. Reavell (TMSA-CPH3011); 1 ind., Natal, Eshowe, 11.1904, Anderson (TMSA-CPH3005); 1 ind., Zoutpan, Pretoria, 10.12.1926, G.v. Son (TMSA-CPH3002); 3 inds, GP, Saartjiesnek, 25.45 S 27.58 E, 14.12.1985, A.V. Evans (TMSA-CPH3001); 1 ind., GP, Pretoria, 25.43 S 28.18 E, 15.2.1987, M. Erasmus (TMSA-CPH3000); 1 ind., ibidem, 6.2.1905, Swierstra (TMSA-CPH2999); 1 ind., ibidem, 1960 (TMSA-CPH2995); 1 ind., GP, Boekenhoutkloof, 15.12.1928, G.v. Son (TMSA-CPH2998); 1 ind., GP, Pretoria Distr., Kalkheuvel, 10.1908, Swierstra (TMSA-CPH2996); 6 ind., ibidem, Waterkloof, 11.1903, F. Noome (TMSA-CPH2994); 1 ind., GP, Onderstepoort, 25.39 S 28.11 E, 3.12.1992, A.M. Schwan (TMSA-CPH2989); 12 inds., GP, Saartjiesnek, 20km W Pretoria, 25.46 S 27.54 E,1.1980, on flowers of Girsium vulgare, E. Holm (TMSA-CPH2984); 3 inds, ibidem, 12.1979, on Protea caffra flowers, E. Holm (TMSA-CPH2985); 1 ind., Transvaal, Brits, 25.35 S 27.47 E, 2.12.1987, T.v. Viegen (TMSA-CPH2980); 1♂, ibidem, Kleinfontein, 4.11.2002, leg. T. Beyers (GBPC); 1 ind., Rietfontein, 25.45S 27.51E, 1.11.1987, K.S. Coles (TMSA-CPH2979); 1 ind., Blyde River, 3.12.1987, A.E. Gildenhuys (TMSA-CPH2978); 2 inds., NW, Kroondal, 25.45 S 27.20 E, 11.1963, L. Schulze (TMSA-CPH2976); 1 ind., LP, Ellisras 23.40 S 27.14 E, 20.2.1988, E. Swanepoel (TMSA-CPH2974); 1 ind., LP, Roosenekal/Steelpoort, 25.03 S 29.52 E, 22.11.2002, M. Burger (TMSA-CPH2973); 1 ind., Orkney, 19.11.1982, J.C. Bekker (TMSA-CPH2972); 2 inds, NW, Potchefstroom, 26.38 S 26.52 E, 26.12.1986, Grassnetting, Th.v. Viegen (TMSA-CPH2971); 1 ind., LP, Nylstroom 11.1961, B.T.B.(TMSA-CPH2966); 1 ind., LP, Warmbaths, 30.11.1987, R. Stals (TMSA-CPH2965); 1 ind., NW, Schoemansville, 11.1928, G. Kobrow (TMSA-CPH2963); 2 inds, LP, Pienaars River, 10–11.1900, v. Jutrzencka (TMSA-CPH2958); 1 ind., LP, Waterberg Distr.,1898–1899, v. Jutrzencka (TMSA-CPH2957); 4 inds, MP, Lydenburg Distr.,1896, P.A. Krantz (TMSA-CPH2956); 1 ind., LP, Potgietersrus, 10.1912, G. Kobrow (TMSA-CPH2955); 2 inds, LP, Grootdraai, 10.1927, H. Lang (TMSA-CPH2952); 2 inds, LP, Zoutpansberg Distr., 11.1924, H.J. Heske (TMSA-CPH2951); 1 ind., NW, Marico, 5.1921, Dr. Brauns (TMSA-CPH2950); 1 ind., LP, Naboomspruit, Mosdene, Nylsvley, 27.11.1969 (TMSA-CPH2948); 1 ind., NW, Swartruggens, 6.1988, H.P. Terblanche (TMSA-CPH2947); 1 ind., E Transvaal, Penge, 13–17.11.1972, A. Strydom (TMSA-CPH2944); 1 ind., LP, Baltimore, 26.12.1986 (TMSA-CPH2943); 1 ind., NW, Marikana, 1.1988, H.P. Terblanche (TMSA-CPH2942); 1 ind., NW, Buffelspoort, 20.12.1986, J.v.d. Berg (TMSA-CPH2941); 1 ind., NW, Rustenburg, 15.3.1982 (TMSA-CPH2939); 3 inds., NW, Soupan, Tswaing, 25.24 S 26.06 E, 6.11.1996, Kruger & Dombrowsky (TMSA-CPH2936); 1 ind., LP, Soutpansberg N, 22.54 S 29.41 E, 17.3.1973, Beating, Endrody-Younga E-Y: 64 (TMSA-CPH2935); 1 ind., GP, Silkaatsneck, 25.40 S 27.55 E, 30.11.1995, Beating, E-Y: 3163, C.L. Bellamy (TMSA-CPH2932); 1 ind., Transvaal, Magaliesberg, 8.1.1985, Endrody-Younga (TMSA-CPH2931); 1 ind., MP, Groblersdal, Blaauwbank, 25.15 S 29.35 E, 19–20.1.2004, singled, near camp TMSA Staff, E-Y: 3601 (TMSA-CPH2929); 2 inds., MP, Mareesburg, 25.01 S 30.08 E, 22.10.2000, general collecting, TMSA Staff, E-Y: 3422 (TMSA-CPH2928); 7 inds., LP, Geelhoutbush Farm, Waterberg, 24.22 S 27.33 E, 3.10.1995, Fruit Traps, Endrody & Bellamy, E-Y: 3145 (TMSA-CPH2927); 1 ind., LP, Zoutpan Nature Res., 25.24 S 28.04 E, 16–17.2.1985, A.V. Evans & S. Chown (TMSA-CPH2926); 1 ind., Farm “Sericea”, Nylsvley, 17–18.12.1984, C.H. Scholtz (TMSA-CPH2924); 1 ind., LP, Thabazimbi, 13.11.1983, E. Holm (TMSA-CPH2922); 2 inds, LP, 22 km SE Thabazimbi, 900 m, 24.43 S 27.28 E, 8.11.1985, A.V. Evans & C.L. Bellamy (TMSA-CPH2919); 1 ind., LP, Wileyspoort, 20 km N of Louis Trichardt, 22.56 S 29.55 E, 23–24.1.1986, Holm & Marais & Nel & Saieva (TMSA-CPH2918); 2 inds, NW, 12 km NE Assen, 25.05 S 27.39 E, 8.11.1985, 950 m, A.V. Evans & C.L. Bellamy (TMSA-CPH2917); 3 inds, Transvaal, Sand River Mt., 24.32 S 27.41 E, 1100 m, 2–3.11.1985, A.V. Evans (TMSA-CPH2916); 1 ind., Potchefstroom Dist., −26.6250 S 27.12500 E, 1879/01/01, Ayres (ISAM-COL-A028190); 1 ind., Pietersburg, −23.75000 S 29.4667 E, 1903/01/01, Janse (ISAM- COL-A028172); 1 ind., Sandfontein (NW), 1921/01/01, Drury (ISAM-COL-A028155); 1 ind., KZN, Mfongosi, Jones (ISAM-COL-A028147); 1 ind., Somerset West, Helderberg Village, 2006/12/01–2006/01/01, Leonard McLeod (ISAM-COL-A067779); 1♀, ibidem, Helderberg Hills, 5 Apr 1999, G Gerber & D du Randt (BMPC); 1 ind., ibidem, 100 m asl, −4.08333 S 18.85000 E, 10.ii.2013, Patrick Reavell (ISAM-COL-A070083); 1 ind., ibidem, 18.xii.2015, Patrick Reavell (ISAM-COL-A071611); 2♂, Natal, Ulundi, XII 1984 (PLPC); 1♂, Johanesbourg, 1969 (PLPC); 1♂, Lindani Nature Res., nr. Vaalwater, 1190 m, 4–6.1.2008, P. Schule lgt (PMPC); 1♂, WC Prov., SW Klapmuts, Babylon Storen, 33°49′32″ S 18°55′32″ E, 184 m, 17.XII.2018, S. Naumann leg. (PMPC); 1♀, KZN, 20 km N Hluhluwe, 100 m, 1–3.1.2009, P. Schüle leg (PMPC); 1♂, NW, Hartbeespoort, 1.1999 (GBPC); 1♂ + 1♀, NC, Hayfield, 4 Jan 2006, R Perissinotto & L Clennell (RPRM, BMPC); 2♂, LP, Ben Lavin, 4 Jan 2002, R Perissinotto & L Clennell (RPRM, BMPC); 1♂, LP, Bergpan, 1 Jan 2002, R Perissinotto & L Clennell (RPRM); 2♂ + 1♀, NC, Postmastburg, 9 Jan 1997, R Perissinotto & L Clennell (RPRM, BMPC); 4♂, Transvaal, Warmbad, 5.11.1994, P Stobbia (RPRM, BMPC); 2♂, FS, Bloemhof, 9 Dec 2000, R Perissinotto & L Clennell (RPRM); 1♂ + 1♀, NC, Witsand NR, 08.01.1997, R Perissinotto & L Clennell (BMPC); 1♀, NC, Kimberley, 17.11.1995, M Burger (BMPC); 1♂, ibidem, 12.12.1993 (BMPC); 1♂, NC, Vaalbos N Park, 10 Jan 1997, R Perissinotto & L Clennell (BMPC); 1♂, FS, Nr Parys, 16 Dec 2009, R Perissinotto & L Clennell (BMPC); 1♂, EC, Graaff Reinet, 16 Dec 2004, R Perissinotto & LClennell (BMPC); 1♂ + 1♀, WC, E Paarl, Mountain Shadow Lodge, 33°44’26” S 19°02’39” E, 262 m, 16–18.XII.2018, leg. S. Naumann (PMPC). ZWE: 1♂, Chipinga, 17.XII.1980 [10: 316, no. 1289]; 1 ind., betwn Limpopo and Zambezi Rvs., 1879/01/01, Ayres (ISAM-COL-A028169); 2♂, Lake Kyle, 15.12.1993, [R Perissinotto & L Clennell] (BMPC); 1 ind., Birchenough Bridge, 7.1.1947, K.T. Coates-Palgrave (TMSA-CPH3057); 3 inds, ibidem, 1.1938, G.v. Son (TMSA-CPH3054); 1 ind., Hillside, 1.12.1922, Swinburne & Stevenson (TMSA-CPH3056); 2 inds, Umtali, 11–12.1931, P.A. Sheppard (TMSA-CPH3053); 1 ind., Beit Bridge, 3.5.1971, A.J. Duke (TMSA-CPH3052); 2 inds, Salisbury (C), 3.10.1971, A.J. Duke (TMSA-CPH3048); 2 inds, Hotsprings, 15.11.1972, A.J. Duke (TMSA-CPH3047); 1 ind., ibidem, 16.11.1972, A.J. Duke (TMSA-CPH3046); 1♂, ibidem, 11.1973, Ex collection Dr Vincent Allard1 (GBPC); 1♂, Mushandike Sanct. (Masvingo env.), 9–11.XII.1998, lgt S. Bečvář (GBPC); 1♀, S Masvingo, 25.XI.2006, 20.35 S 30.40 E, P. Schüle leg (PMPC).
M. a. heterospila. Holotype ♀: Mombas v.d. Decken, var. heterospila Gerst. (ZMHB). Holotype ♂ + Allotype ♀ (M. lerui): Coast Kenya, Malindi, V 2002 et XII 2001, B. Le Rü leg. (MNHN). Paratype ♂ (M. lerui): Coast Kenya, Diani Fst, IV 1984, J.-Ph. Legrand leg., in coll. J.-Ph. Legrand [6: 24]. COD: 1 ind., Moliro, J. Duvivier, M. amabilis var. heterospila G., det. Moser, 1908 (RBINS); 1♀, Upemba, Riv Lufira, 6/10.X.2002 (PLPC). KEN: 1 ind., Mamboia, E. Africa, Le Moult vend. (RBINS); 1 ind., Mombasa, Péringuey (ISAM-COL-A028159); 2♂, ibidem, 06-2012 (TGPC); 1♂, ibidem, Shimo la Tewa (GBPC); 5♂ + 3♀, Shimba Hills, VI 2007 (PLPC); 2♂ + 5♀, ibidem, 04-1993 (TGPC); 1♀, Bungule Kasigau, 1 XII 2005 (PLPC); 1♂ + 1♀, Arabuko Fst., 100 km. N. Mombasa, 05-1996 (TGPC); 1♂, Diani, Robinson-Baobab, 27.III.1977, ca. 0 m, leg. M. Blech (GBPC); 1♂, Malindi, 21.5.1978 (GBPC); 1♂ + ♀, ibidem, Sokoke-Gede Forest, 50–70 m, IX.2006, local people (GBPC); 1♀, Marenje Forest, X.1999, Ex Collection Richard Lang (GBPC); 1♂, Kibwezi, March 1990, Ex collection Dr Vincent Allard (GBPC); 1♂, Sagala Hills, bei Voi, 15–30.1.1993, leg. G. Beinhundner (GBPC). MOZ: 1♀, Nyassa, 01-2008 (TGPC); 1♀, Niasa Park, Jan 2008, via Thierry Garnier (RPRM). MWI: 1 ind., 10 km N Chilumba, 10.12.1986, T. Beyers (TMSA-CPH3059); 1 ind., Chilumba, 10.12.1986, T. Beyers (TMSA-CPH3060). TAN: 1 ind., Bagamoyo, H. Schaedle, coll. E. Candèze, M. amabilis Schaum, det. Moser, 1908 (RBINS); 2 inds., ibidem Le Moult vend. (RBINS); 2 inds, Mhonda, D. Ost. Africa, H. Stichel (RBINS); 10 inds, Nguela, Usambara, M. amabilis var. heterospila G., det. Moser, 1908 (RBINS); 1 ind., Dar-es-Salaam, ex coll. Fruhstorfer, M. amabilis var. heterospila G., det. Moser, 1908 (RBINS); 1 ind., ibidem, Le Moult vend. (RBINS); 1 ind., ibidem, Htld., D.O.Afr., Emmerling (ISAM-COL-A028157); 5♂ + 1♀, ibidem, VII 1991 (PLPC); 1♂, ibidem, 07-1991 (TGPC); 2♂ + 1♀, ibidem, Oct 2000, DC Moore leg (RPRM, BMPC); 1♀, ibidem, May 2001, DC Moore leg (RPRM); 1♀, ibidem, Dec 2004,Via Thierry Bouyer (BMPC); 1♂ + 1♀, ibidem, 7.1973 (GBPC); 1♂, ibidem, 4.1981, Dr V. Allard (GBPC); 1♀, Bulwa, Amani, 8.2003, tea plantage, coll. S. Skenjewala (GBPC); 4♂ + 1♀, Unguu Mtns, 11.2004, leg. V. Kayombo (GBPC); 1♀, Njombe Forest, 03.2005, leg. H. Ntangeki (GBPC); 2♀, [no locality], 2004 (GBPC); 11♂ + 3♀, [no locality], 2003, leg. native collectors (GBPC); 1♂, Masasi, 07.2005, leg. H. Ntangeki (GBPC); 5♂ + 2♀, ibidem, 01.2007, leg. H. Ntangeki (GBPC); 31♂ + 8♀, ibidem, XI.2009, leg. H. Ntangeki (GBPC); 3♂ + 3♀, Kondoa, 05.2007, leg. H. Ntangeki (GBPC); 2♂ + 2♀, Newala, 01.2007, leg. V. Kayombo (GBPC); 2♂ + 1♀, 03.2007, Lindi, leg H. Ntangeki (GBPC); 1♂, Pugu, 12.2004, leg. H. Ntangeki (GBPC); 2♂, Kilimajaro, 05.2007, leg. V. Kayombo (GBPC); 1♂, Nambuju village, Mtwara, X-XII.2009, leg. S. Husein (GBPC); 4♂ + 1♀, Uluguru Mtns, 12.2006, leg. H. Ntangeki (GBPC); 1♀, Ruvuma Pr., near Songea, 9–13.12.1997, Werner & Lizler leg. (GBPC); 1 ind., Tanganyika, Le Moult vend. (RBINS); 1 ind., Zanzibar, Raffray (RBINS); 3 inds, ibidem (RBINS); 1 ind., ibidem v. Heterospila Gers, ex coll. Bonneuil (RBINS); 1 ind., ibidem (“Zanguebar”), Oxythyrea amabilis Schaum, Coll. H. d’Udekem d’Acoz, R. Mus. Hist. Nat. Belg. I.G.10.095 (RBINS); 1 ind., ibidem (RBINS); 1♀, ibidem, Kiwengwa, 07-2004, R. Mourglia leg.(TGPC); 1 ind., N Nyassa-See, Unyika-Senga, S. Rukwa-see, 2–4.XI. [18] 99, Goetze S., Microthyrea amabilis Schaum, det. Dr. Ondrej (RBINS); 1 ind., Mkomazi Game Reserve, Peak of Maji Kununua, −3.8833 S 37.8167 E, 7/12/1995, Simon van Noort (ISAM-COL-A063003); 2♂, Tanga, Mbeza, V.2013 (PLPC); 5♂ + 2♀, Morogoro Prov., Mikumi, 12–14.I.2011, ex ovum F1, P. Malec leg. & bred (PMPC); 1♂, ibidem, I.1993 (PLPC); 2♂, Pangani, DC Moore leg (DMPC); 1♂, Tanganyika, Kigonsera, leg. P.C. Hartl (GBPC). UGA: 2♂ + 3♀, Napak Mt, Moroto Prov., 05-2008 (TGPC); 1♂, Moroto Mt, Moroto Dist., 05-2008 (TGPC); 2♂ + 1♀, Nebbi Distr., Padyere Subcty, Pamora, April 2002/2004, DC Moore leg (DMPC, BMPC); 1 ind., Kotido, IV-1950 (KNEM). ZMB: 1 ind., Rhodésie du Nord, Kaputa, 3.II.1944, H. J. Bredo, R. I. Sc. Nat. Belg. 15.333, Mausoleopsis amabilis Schaum, G. Ruter det., 1952 (RBINS); 1 ind., Rhodésie du Nord, Abercorn, II.1944, H. J. Bredo, R. I. Sc. Nat. Belg. 15.333, Mausoleopsis amabilis Schaum v., G. Ruter det., 1969 (RBINS).
M. a. ruteri. Holotype ♂: Cote-d’Ivoire, Niangbo, V.1962, J. Decelle leg. (MRAC). Paratypes: 1♂ + 1♀, Burkina Fasso, Bobo Dioulasso, 11.Vl.1975, (coll. Ph. Antoine)[7: 8]; 1♂ + 5♀, Nigeria: S.E. Kano, Azare, 1925, Dr Ll. Llyod (BMNH, MNHN, coll. Ph. Antoine)[7: 8]. BEN: 1♀, Tanguieta, 06-2006 (TGPC). BFA: 1♂, Nasso, Bobo-Dioulasso, 06-1996 (TGPC). CMR: 2♂, Wacka, 15 km sud de Ngaoundere, VI.2008 (PLPC); 2♂ + 2♀, ibidem, 05-2010 (TGPC); 1♂, Ngaoundere, VII.2008 (DMPC); 1♂, ibidem, VIII.2006, Coll. Th. Garnier (GBPC). CIV: 2♀, Gansé, 1991, leg. V. Allard (GBPC). GHA: 2 inds, Mt Gemi, collect. Duvivier, M. amabilis Schaum, det. Moser, 1908 (RBINS). MLI: 1 ind., N’Gomi, Le Moult vend. (RBINS). NGA: 1 ind., Buguma, Nov. Calabar, M. amabilis var. heterospila G., det. Moser, 1908 (RBINS).
3.2.5. Remarks
The dorsal ornamentation in this subspecies is rather stable as far as the large white pronotal band and three latero-apical maculae are concerned. However, there is a whole range of variability in the number and size of white spots marking the pronotal base and the elytral disc. Although the basal pronotal spots are always present in the specimens analysed, they are extremely reduced in size in some cases, particularly from ZN and southern MOZ. On the other hand, these spots, are often lacking completely or obsolete in the nominal subspecies from the EC and WC. Concerning the elytral disc pairs, the most consistent and generally present is the one positioned towards the apical third. However, there is occasionally another pair positioned at mid elytral length, next to the large marginal maculae, and even a third albeit smaller pair just anteriad of the apical maculae. This latter pattern appears to be most frequent in notheastern NAM.
As expected, M. a. interrupta females are stockier than males, have shorter protarsi (approx. same length as protibia), shorter but symmetric protarsal claws, and longer metatibial spurs than males. Their metatibiae are also narrower than the male’s and lack the prominent carina on inner surface. Their abdominal sternites are straight to slightly convex at centre, rather than markedly concave with median depression like in their male counterparts. Adults of this subspecies are active from early Austral spring (September) through the summer to mid-autumn (May). They are attracted to fermenting fruits, sap flows, and especially flowers. Among the flowering plants most commonly visited by M. amabilis s.l. are Bauhinia sp., Catophractes alexandri, Cirsium vulgare, Grewia sp., Protea caffra, Rosa spp., Terminalia sericea, Vachellia spp., Zantedeschia aethiopica, and various Asteraceae [9,36] (pers. obs.).
Larvae of M. amabilis s. l. have been reported as breeding in cattle, goat, and horse dung [9] and have also been found in nursery potting soil in Cape Town [36]. Holm and Marais [9] have further reported that adult individuals have been retrieved from birds’ nests, indicating that these may also serve as breeding ground for their larvae. A concise but sufficiently diagnostic description of the larva has been reported by Donaldson [37]. Given that the larvae used in this description were reared in the laboratory from adults trapped at the Pretoria Horticultural Research Institute [37], it can safely be assumed that they represented the nominal subspecies. According to Malec and Ŝípek [11], populations from Zambia (presumably M. a. interrupta) and Tanzania (presumably M. a. heterospila) have been successfully reproduced in captivity. Apparently, larvae from both populations do not undertake diapause in any phase of their development and normally form a cocoon within a period of 3–4 months from hatching. They prosper in rather dry substrates containing a mixture of rotten wood, leaf litter, and soil [11].
4. Discussion
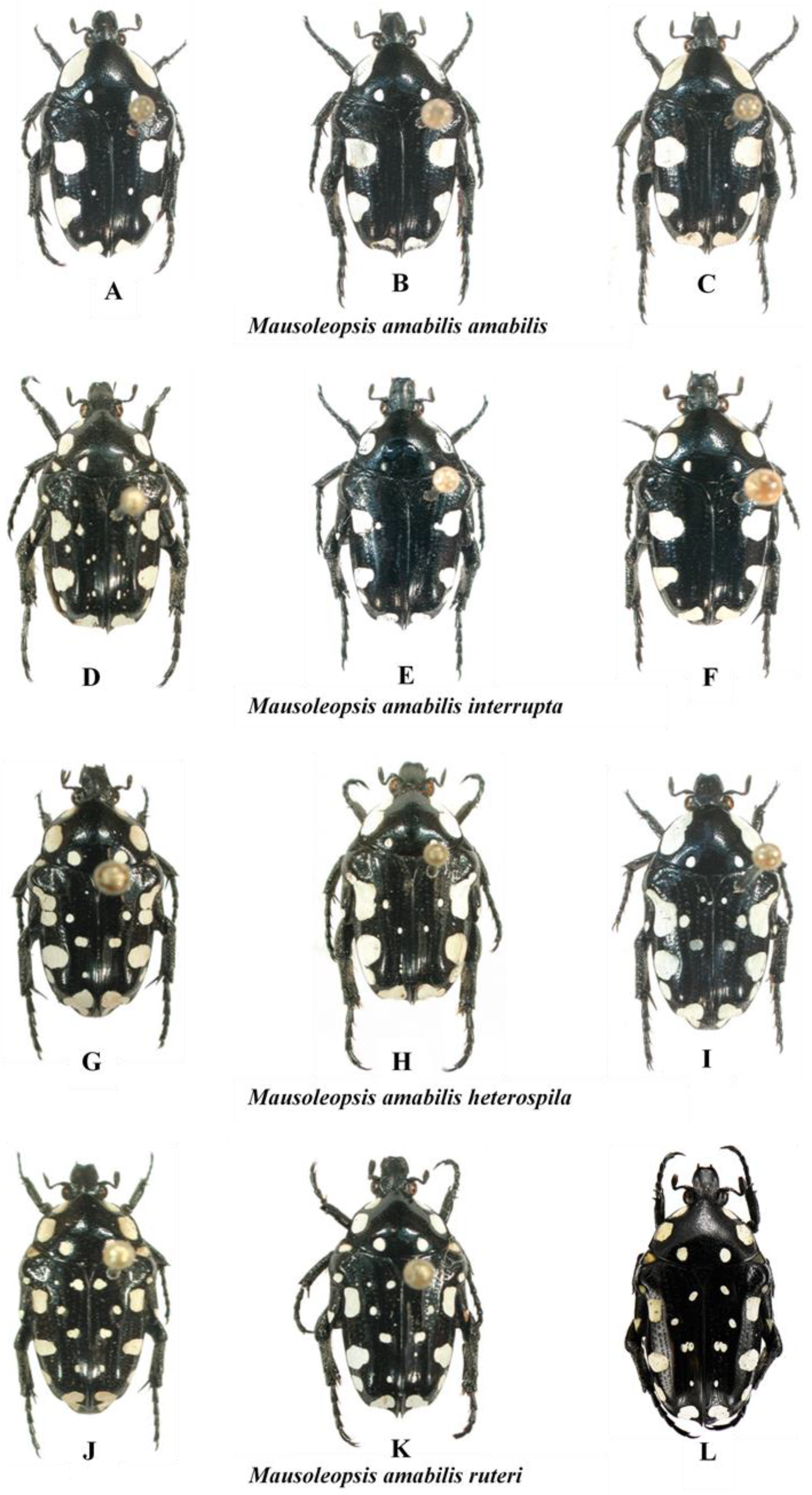
Even though what was previously regarded as a desert population of the nominal subspecies is now recognised as a separate species (i.e., Mausoleopsis gariepena sp. nov.), M. amabilis s. l. exhibits an extraordinary variability in its dorsal ornamentation and habitat characteristics, which has caused remarkable confusion and controversy throughout the literature of its description and revisions (cf. [6] and references therein). The basic structure of its dorsal white maculation consists of one broad band on each pronotal lateral margin, a pair of maculae on each elytral margin and a single macula on each elytral apex. In addition to these, there is a pair of smaller spots in the suprascutellar area of the pronotal posterior margin, as well as 1–3 pairs of similar spots of variable size on the elytral disc. Specimens from East and West Africa, however, also exhibit two extra smaller spots on the elytral lateral margins, below the humeral and apical calluses, respectively. In specimens from the eastern range, the first one can take the shape of an elongate projection of the main macula (Figure 10H,I). Finally, specimens from both East and West Africa also exhibit mesepimeral and metepisternal spots, but these are only fully developed and entirely coalesced only in the extreme western range (Figure 10J–L).
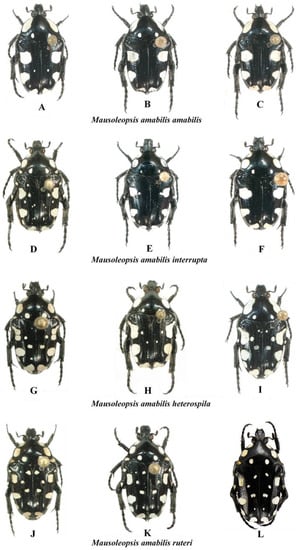
Figure 10.
Mausoleopsis amabilis s. l.. Gradient of variations observed in the four subspecies across their respective distribution ranges. (A): ZAF-NW, Voëlkop; (B): ZWE, Mushandike; (C): ZAF-LP, Warmbad; (D): NAM, Otjozondjupa; (E): MWI, Cape Maclear; (F): ZWE, Masvingo env.; (G): TAN, Njombe Forest; (H): TAN, [no locality]; (I): TAN, Dar es Salaam; (J): RCI, Gansé; (K): CMR, Ngaoundere; (L): BFA, Bobo Dioulasso. Photos: A-K by Gerhard Beinhundner; L by Thierry Garnier.
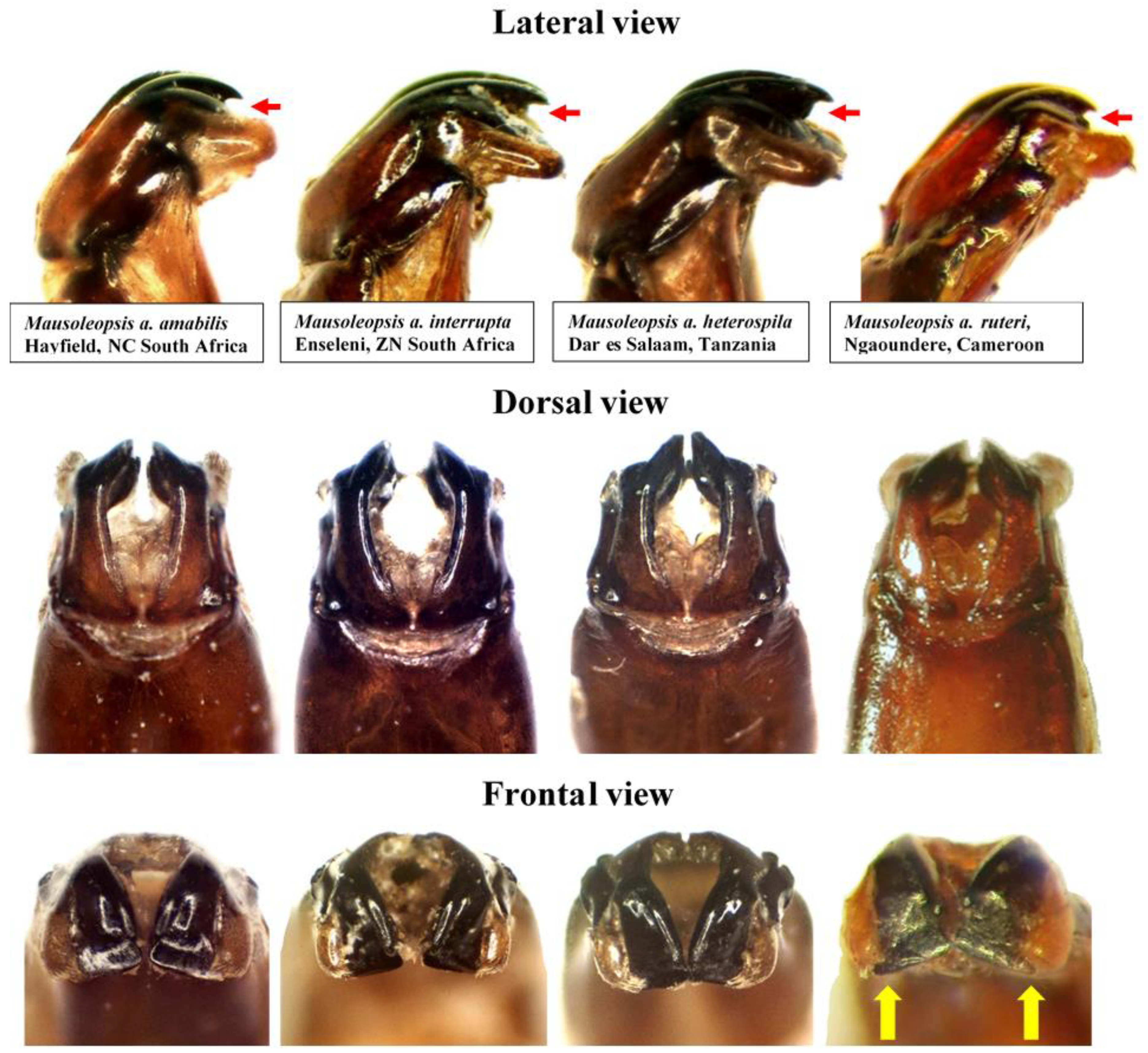
Thus, what is observed in essence is a progressive fragmentation and proliferation of white cretaceous maculation on the dorsum of the species, with the most coalesced configuration seen in the south-western populations (M. a. amabilis) and the most dispersed in the north-western populations (M. a. ruteri) (Figure 10). Although the configuration of the aedeagal parameres is very conservative across the various populations, a clear pattern of progressive decrease in the elevation of the apical projection of the dorsal lobe (with corresponding increase in distance between inner and central projections) can be seen moving from the south- and northwestern populations (M. a. amabilis and M. a. ruteri) to the southeast (M. a. interrupta) and then to the northeast (M. a. heterospila), particularly in lateral view (Figure 11). Mausoleopsis a. ruteri from the west also shows some typical lateral expansions on the central lobe towards the external one, best visible in frontal view (Figure 11).
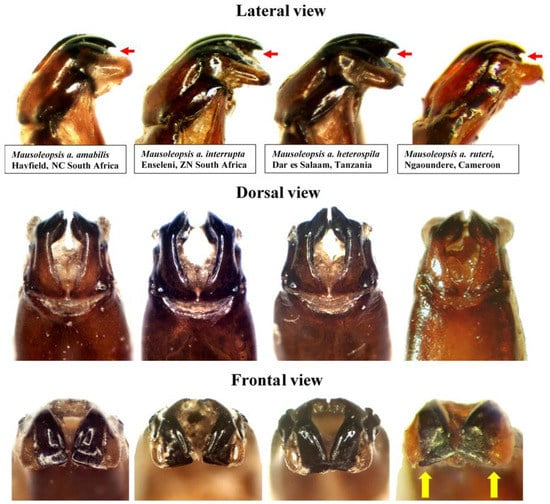
Figure 11.
Mausoleopsis amabilis s. l. Variability in the shape of aedeagal parameres observed in the four subspecies in lateral (top), dorsal (middle) and frontal (bottom) views. Red arrows indicate elevation of the apical projection, while yellow arrows indicate lateral projections on central lobe in M. a. ruteri. Photos by Renzo Perissinotto and Gerhard Beinhundner.
Specimens from coastal East Africa, including the broad region between Mombasa, Zanzibar, and Dar es Salaam as well as neighbouring areas, show the consistent pattern described by Gerstaecker [5], with oblong white stripes on pronotal margins uninterrupted and the large subhumeral maculae exhibiting a narrow anterior lobe projected forward along the margin. Thus, it is proposed here that M. a. heterospila be reinstated as valid parapatric subspecies, along with M. a. ruteri. Their status as subspecies is further supported by observations that intermediate forms are found along the transitional zone between different populations (“overlapping sites” cf. [11]), as expected, rather than randomly across the entire distribution range of the species. Their distribution range is only apparently disjunct (Figure 9), and this is probably because there have been no proper investigations carried out in the countries that lie in between (CAF, SSD, COD-N), due to the persistent inaccessibility of these areas. On the other hand, M. lerui Antoine, 2004, described on the basis of only three types from Malindi (about 100 km north of Mombasa) and Diani Forest (35 Km south of Mombasa), does not show any key diagnostic character separating it from its sympatric M. a. heterospila. The characters considered by Antoine [6] were of relatively minor diagnostic value and are actually quite variable in the numerous material that has become available since the original description. The structure of the aedeagal parameres and the rest of the morphology are also not different to those of the variational gradient observed in M. a. heterospila (Figure 10 and Figure 11). Thus, the most logical conclusion that can be drawn from this is that M. lerui actually represents an extreme variation of M. a. heterospila, and therefore it is here synonymised with it.
In summary, the following identification key can be proposed at this stage to systematically clarify the variability observed within the species. The potential addition of a molecular DNA analysis in the future would certainly be beneficial, in order to conclusively resolve the complex taxonomy of this species. In particular, this may help to better understand some apparent outliers of M. a. amabilis found in the central highlands of Tanzania, which may possibly represent a different taxon.
Mausoleopsis amabilis: Key to Subspecies
- 1 .
- Lateral margin of elytron with two simple, large white maculae, squarish or triangular in shape; mesepimeral and metepisternal white spots absent, or rarely present but then small and fragmented…..………………………………………...................................................2
- –
- Lateral margin of elytron with anterior macula rectangularly elongate and both maculae forming anterior or posterior extensions, respectively, often separated from main macula to form distinct spots; mesepimeral and metepisternal white spots absent or present...............................................................................................................................................3
- 2 .
- Pronotal lateral bands continuous or only partially interrupted along entire margins; aedeagal parameres with depressed elevation of apical projection above central lobe; distribution: WC, NC, EC, FS, GP, NW, LP(H), MOZ(C-W), NAM(S-W), BWA(S-W), SWZ(W), TAN(C), ZWE (E) (Figure 9, Figure 10 and Figure 11)....................M. amabilis amabilis (Schaum, 1844)
- –
- Pronotal lateral bands neatly interrupted at anterior third to form two distinct maculae on each margin; aedeagal parameres with moderate elevation of apical projection above central lobe; distribution: ZN, MP, LP(L), SWZ(E), MOZ(S-C), MWI(S), NAM(N-E), BWA(N-E), ZWE(W), ZMB(S-W), AGO (Figure 9, Figure 10 and Figure 11)........M. amabilis interrupta ssp. nov.
- 3 .
- Lateral pronotal bands and elytral maculae coalesced, or with various degrees of disjunction; mesepimeral and metepisternal spots absent or small and fragmented, respectively; basal pronotal spots of small to moderate size; elytral disc with 1-4 pairs of white spots; aedeagal parameres with elevated apical projection above central lobe; distribution: COD, KEN, MOZ(N), MWI(N), TAN, UGA, ZMB(N) (Figure 9, Figure 10 and Figure 11).............. ......................................................................................M. amabilis heterospila (Gerstaecker, 1867)
- –
- Lateral pronotal bands and elytral maculae completely disjuncted; mesepimeral and metepisternal spots wide and coalesced; basal pronotal spots pair consistently large; elytral disc with 4 pairs of white spots; aedeagal parameres with depressed elevation of apical projection above central lobe; distribution: BEN, BFA, CMR, CIV, GHA, MLI, NGA (Figure 9, Figure 10 and Figure 11)……....................................................................M. amabilis ruteri Antoine, 1989
Mausoleopsis amabilis s. l. is a widespread, opportunistic species occurring in most African biomes (mainly Grassland and Savanna), with the exception of the densest tropical rain forests of central Africa, the Succulent and Nama Karoo of southern Africa, and historically the Fynbos of the southwestern Cape [36]. It has recently expanded its distribution range remarkably across the western part of South Africa. Despite Algoa Bay representing its type locality in the original description by Schaum [4], the species was mentioned by Péringuey [26] as occurring throughout South Africa with “the exception of the Cape Colony”. Holm and Marais [9] reported the species as widely distributed from southern to western Africa, skirting the Congo basin. The distribution map presented in the latter work seems to confirm its historical absence from the present day three Cape provinces [9: 253, fig. 142i]. However, the numerous observations and data that have recently become available show that now the species occurs widely throughout the three Cape provinces, with the exception of the coastal Namaqualand and the driest part of the Karoo interior (Figure 9).
Its arrival in the coastal region of the Western Cape appears to be part of a southward range expansion, possibly related to artificial transport of nursery plants with colonised potting soil [36], or to the phenomenon of poleward migration in response to global warming [38,39]. It seems likely that both factors are actually involved in this process, given the migration pattern similarities observed with other insect species in the region [36,40]. Within the Cape Town metropolitan area, it was first recorded near Somerset West in 1999 and has since been found in great abundance throughout the city itself and across the entire Cape Peninsula, at least since 2002. Currently, it is regarded as one of the most common cetoniine in the gardens of the whole Cape Town metropolitan area and has also spread to the southern coastal areas, such as Hermanus and Franskraal, as well as the Cape Garden Route, especially in and around the town of George [36].
Author Contributions
Conceptualization, R.P.; methodology, R.P. and G.B.; software, R.P.; validation, R.P. and G.B.; formal analysis, R.P. and G.B.; investigation, R.P. and G.B.; resources, R.P. and G.B.; data curation, R.P. and G.B.; writing—original draft preparation, R.P.; writing—review and editing, G.B.; visualization, R.P.; supervision, R.P.; project administration, R.P.; funding acquisition, R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received partial funding from the Nelson Mandela University (Gqeberha, South Africa) in the form of productivity grants.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All the data are reported here in this manuscript.
Acknowledgments
Thanks to the South African National Parks, the Northern Cape Department of Environment and Nature Conservation, Cape Nature (Western Cape), the Eastern Cape Department of Economic Development, Environmental Affairs and Tourism as well as KwaZulu-Natal Wildlife for providing permits and logistical assistance towards specimen and data collections. We are especially grateful to Lynette Clennell (Macau SAR, China) for taking most of the photos included in the manuscript and to Guido Sabatinelli (MNHG), Christophe Rivier, Antoine Mantilleri, and Olivier Montreuil (MNHN) for providing images of the type specimens of M. amabilis s. l. and M. lerui, respectively. The following curators, researchers, and collectors are also thanked for submitting photos, specimens and data towards the completion of this work: Werner Strümpher (TMSA), Aisha Mayekiso (ISAM), Tanza Crouch (ex DNSM), Alain Drumont (RBINS), Thierry Garnier (TGMF), Philippe Léonard (PLPC), Petr Malec (PMBC), and Jonathan Ball (BMCS).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bouchard, P.; Bousquet, Y.; Davies, A.E.; Alonso-Zarazaga, M.A.; Lawrence, J.F.; Lyal, C.H.C.; Newton, A.F.; Reid, C.A.M.; Schmitt, M.; Ślipiński, S.A.; et al. Family-group names in Coleoptera (Insecta). ZooKeys 2011, 88, 1–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beinhundner, G. The Cetoniinae of Africa; Gerhard Beinhundner: Euerbach, Germany, 2017; pp. 1–1199. [Google Scholar]
- Antoine, P. Notes sur la systématique des genres voisins d’Oxythyrea Mulsant et Leucocelis Burmeister (Coleoptera, Cetoniidae). Coléoptères 1997, 3, 175–208. [Google Scholar]
- Schaum, H.R. Observationes critiques sur la famille des Lamellicornes mélitophiles. Ann. Société Entomol. Fr. 1844, 2, 333–426. [Google Scholar]
- Gerstaecker, A. Beitrag zur Insekten-fauna von Zanzibar, nach dem während der Expedition des Baron v.d. Decken gesammelten Material zusammengestellt. Arch. Nat. 1867, 33, 1–49. [Google Scholar]
- Antoine, P. Contribution à la connaissance des Cetoniidae du Kenya (Coleoptera, Scarabaeoidea). Coléoptères 2004, 10, 13–28. [Google Scholar]
- Antoine, P. Quelques especes nouvelles ou peu connues de la famille des Cetoniidae (Coleoptera, Scarabaeoidea). Bull. Société Sci. Nat. Compiegne 1989, 64, 3–13. [Google Scholar]
- Marais, E.; Holm, E. Type catalogue and bibliography of the Cetoniinae of the Sub-Saharan Africa. C. Mem. Ser. 1992, 8, 1–117. [Google Scholar]
- Holm, E.; Marais, E. Fruit Chafers of Southern Africa (Scarabaeidae: Cetoniini); Ekogilde: Hartbeespoort, South Africa, 1992; pp. 1–326. [Google Scholar]
- Sakai, K.; Nagai, S. The Cetoniine Beetles of the World; Mushi-Sha’s Iconographic Series of Insects 3; Mushi-Sha: Tokyo, Japan, 1998; pp. 1–421. [Google Scholar]
- Malec, P.; Šípek, P. Additional notes on the biology and ecology of the Cetoniinae fauna of Eastern Cape (EC) and KwaZulu-Natal (KZN) and remarks on captive breeding of these beetles (Coleoptera, Scarabaeidae, Cetoniinae). Cetoniimania NS 2017, 12, 35–75. [Google Scholar]
- Serrano, A.R.M.; Capela, R.A.; Nunes, T.; Van-Dúnem Neto Santos, C. The rose chafers (Coleoptera: Scarabaeidae: Cetoniinae) of Angola: A descriptive checklist with new records and synonymic notes. Zootaxa Monogr. 2020, 4776, 1–130. [Google Scholar] [CrossRef]
- Krikken, J. A new key to the suprageneric taxa in the beetle family Cetoniidae with annotated lists of the known genera. Zool. Verh. 1984, 210, 1–75. [Google Scholar]
- Mucina, L.; Rutherford, M.C. The Vegetation of South Africa, Lesotho and Swaziland. Strelitzia 19; South African National Biodiversity Institute: Pretoria, South Africa, 2006; pp. 1–807. [Google Scholar]
- Perissinotto, R.; Smith, T.J.; Stobbia, P. Description of adult and larva of Ichnestoma pringlei n. sp. (Coleoptera Scarabaeidae Cetoniinae), with notes on its biology and ecology. Trop. Zool. 1999, 12, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Perissinotto, R. Systematics and biology of the Ichnestomina, including new genera and species (Coleoptera: Scarabaeidae, Cetoniinae). Fragm. Entomol. 2020, 52, 217–320. [Google Scholar] [CrossRef]
- Boheman, C.H. Insecta Caffrariae Annis 1838-1845 A J.A. Wahlberg collecta. II. Coleoptera (Scarabaeides); Officina Norstedtiana: Holmiae [Stockholm], Sweden, 1857; pp. 1–395. [Google Scholar]
- Coquerel, C. Note sur lꞌ Oxythirea amabilis, Schaum, et sur la Leucocelis eustalacta. Burm. Ann. Société Entomol. Fr. 1848, 6, 280–282. [Google Scholar]
- Klug, J.C.F. Coleoptera, Käfer. In Naturwissenschaftliche Reise nach Mossambique auf Befehl seiner Majestät des Königs Friedrich Wilhelm IV in den Jahren 1842 bis 1848 ausgeführt. Zoologie V. Insecten und Myriapoden; Peters, W.C.H., Ed.; Georg Reiner: Berlin, Germany, 1862; pp. 1–566. [Google Scholar]
- Dohrn, C.A. Exotisches. Stettin. Entomol. Ztg. Stettin. 1868, 29, 229–243. [Google Scholar]
- Burmeister, H.C.C. Coleoptera Lamellicornia, Xylophila et Pectinicornia. Handbuch der Entomologie 5; Enslin: Berlin, Germany, 1847; pp. 1–584. [Google Scholar]
- Distant, W.L. Coleoptera collected in the Transvaal. Ann. Mag. Nat. Hist. Incl. Zool. Bot. Geol. Lond. 1897, 6, 575–579. [Google Scholar] [CrossRef]
- van Lansberge, J.W. Enumération des Scarabaeides. Rapportes du Pays de Çomalis (Afrique équatoriale) par M. Révoil. Avec diagnoses des espèces nouvelles (1). Bull. Comptes-Rendus Des. Séances Société Entomol. Belg. 1882, 26, 21–31. [Google Scholar]
- van Lansberge, J.W. Coléoptères recueillis par M. G. Révoil chez les Çomalis, Scarabaeidae. In Faune et Flore de Pays Çomalis (Afrique orientale); Revoil, G., Ed.; Challamel Ainé: Paris, France, 1882; pp. 12–44. [Google Scholar]
- Kolbe, H.J. Beiträge zur Kenntniss der melitophilen Lamellicornier. Stettin. Entomol. Ztg. Stettin. 1895, 56, 271–293. [Google Scholar]
- Péringuey, L. Descriptive catalogue of the Coleoptera of South Africa (Lucanidae and Scarabaeidae). Trans. S. Afr. Philos. Society 1907, 13, 1–546. [Google Scholar]
- Heyne, A.; Taschenberg, O. Die Exotischen Käfer in Wort und Bild; G. Reusche: Leipzig, Germany, 1907; pp. 1–262. [Google Scholar]
- Distant, W.L. Insecta Transvaaliensa. A contribution to a knowledge of the entomology of South Africa; W.L. Distant: London, UK, 1911; Volume 1, pp. 253–299. [Google Scholar]
- Bourgoin, A. Scarabaeidae: Trichiini et Cetoniini. In Voyage de Ch. Alluaud et R. Jeannel en Afrique orientale (1911-1912), Résultats scientifiques, Insectes Coléoptères; Librairie des Sciences Naturelles, Léon L’Homme: Paris, France, 1919; Volume 14, pp. 121–192. [Google Scholar]
- Bourgoin, A. Cetoniini. In Voyage de Guy Babault en Afrique Orientale (1912-1913), Résultats scientifiques: Insectes Coléoptères; Babault, G., Ed.; Lahure: Paris, France, 1921; Volume 2, pp. 1–46. [Google Scholar]
- Schenkling, S. Scarabaeidae: Cetoniinae. In Coleopterorum Catalogus; Junk, W., Ed.; W Junk: Berlin, Germany, 1921; Volume 72, pp. 1–431. [Google Scholar]
- Schein, H. Coleoptera (Scarabaeidae): Cetoniinae and Trichiinae. In South African Animal Life; Hanstrom, B., Brinck, P., Rudebeck, G., Eds.; Almquist & Wiksell: Uppsala, Sweden, 1961; Volume 7, pp. 83–112. [Google Scholar]
- Ruter, G. Contribution à l’étude des Cétonides africains. Bull. De L’institut Fondam. D’afrique Noire Dakar 1969, 31, 899–919. [Google Scholar]
- Krajcik, M. Cetoniidae of the World. Catalogue Part I; Typos Studio: Most, Czech Republic, 1998; p. 132. [Google Scholar]
- Kraatz, G. Die africanischen Leucoceliden und die ihnen zunächst verwandten Gattungen der Cetoniden. Dtsch. Entomol. Z. Berl. 1882, 26, 65–78. [Google Scholar] [CrossRef]
- Roets, F.; Allison, J.D.; Basson, R.J. Recent records of fruit chafers (Scarabaeidae: Cetoniinae: Cetoniini) in the southwestern Cape region of South Africa suggest that range expansions were facilitated by human-mediated jump-dispersal and pre-adaptation to transformed landscapes. Afr. Entomol. 2019, 27, 135–145. [Google Scholar] [CrossRef]
- Donaldson, J.M.I. Description of, and a key to larvae of some South African Cetoniinae (Coleoptera, Scarabaeidae). Entomol. Mon. Mag. 1987, 123, 1–13. [Google Scholar]
- Parmesan, C.; Ryrholm, N.; Stefanescu, C.; Hill, J.K.; Thomas, C.D.; Descimon, H.; Huntly, B.; Kaila, L.; Kullberg, J.; Tammaru, T.; et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 1999, 399, 579–583. [Google Scholar] [CrossRef]
- Perissinotto, R.; Pringle, E.L.; Giliomee, J.H. Southward expansion in beetle and butterfly ranges in South Africa. Afr. Entomol. 2011, 19, 61–69. [Google Scholar] [CrossRef]
- Geertsema, H. Range expansion, distribution records and abundance of some Western Cape insects. S. Afr. J. Sci. 2000, 96, 396–398. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).