Taxonomy and Distribution of the Gomphid Dragonfly Orientogomphus minor (Laidlaw, 1931) (Odonata: Gomphidae) in Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Rearing and Identification
2.3. Data Analysis
3. Results
3.1. Taxonomy
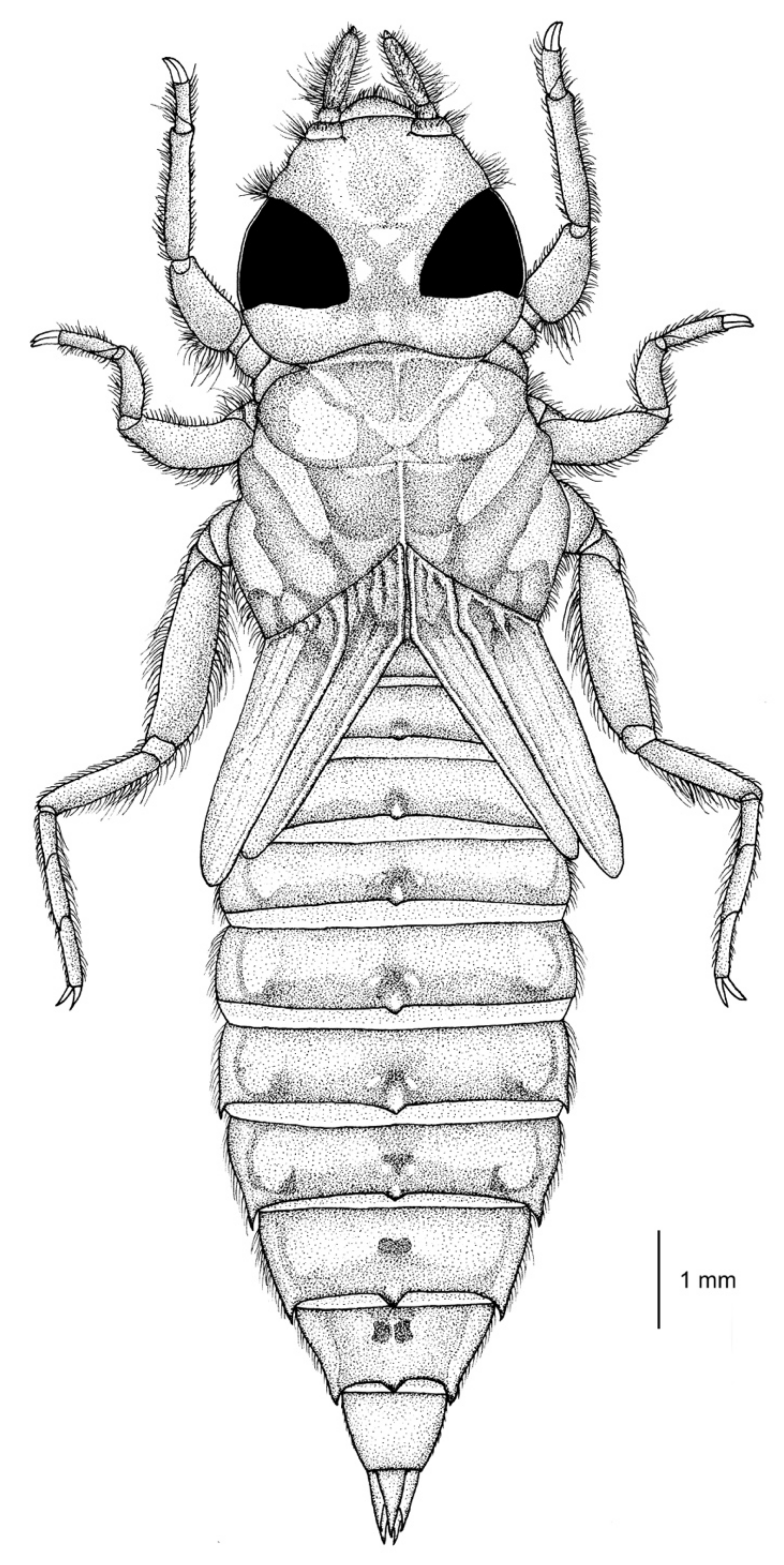
3.1.1. Description of the Last Stadium Nymph
3.1.2. Taxonomy of the Adult
3.2. Distribution
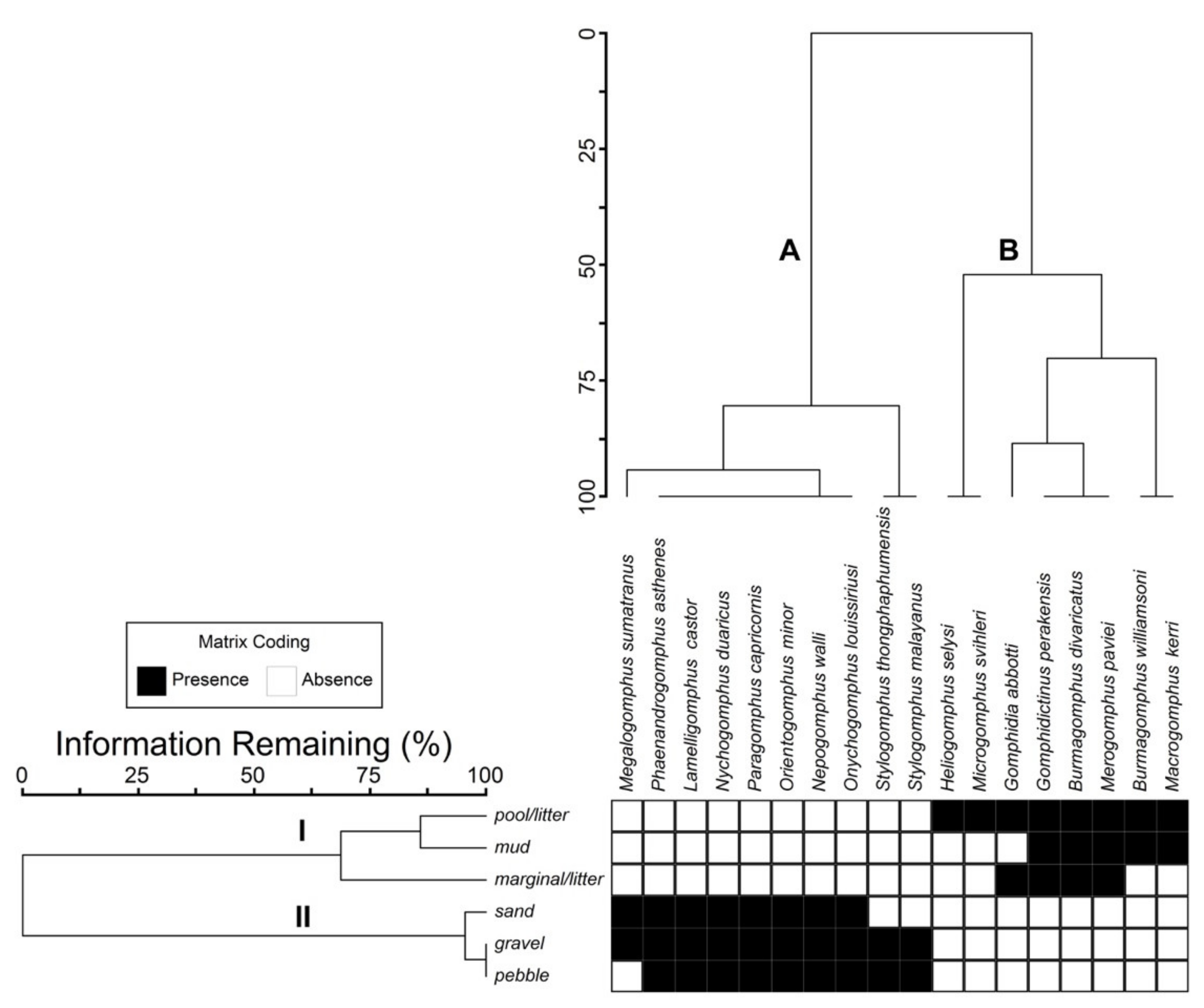
3.2.1. Spatial Distribution of Gomphidae Species
3.2.2. Substrate Preference of O. minor Nymphs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suhling, F.; Sahlén, G.; Gorb, S.; Kalkman, V.J.; Dijkstra, K.D.B.; van Tol, J. Oder Odonata. In Ecology and General Biology: Thorp and Covich’ Freshwater Invertebrates, 4th ed.; Thorp, J.H., Rogers, D.C., Eds.; Academic Press: New York, NY, USA, 2015; pp. 894–932. [Google Scholar]
- Novelo-Gutiérrez, R.; Ramírez, A.; González-Soriano, E. Superfamily Gomphidae. In Keys to Neotropical Hexapoda: Thorp and Covich’ Freshwater Invertebrates, 4th ed.; Hamada, N., Thorp, J.H., Rogers, D.C., Eds.; Academic Press: New York, NY, USA, 2018; pp. 367–375. [Google Scholar]
- Carle, F.L.; Kjer, K.M.; May, M.L. A molecular phylogeny and classification of Anisoptera (Odonata). Arthropod Syst. Phylog. 2015, 73, 281–301. [Google Scholar]
- Kalkman, V.J.; Clausnitzer, V.; Dijkstra, K.D.B.; Orr, A.G.; Paulson, D.R.; van Tol, J. Global diversity of dragonflies (Odonata) in freshwater. Hydrobiologia 2008, 595, 351–363. [Google Scholar] [CrossRef]
- May, M.L. Odonata: Who They Are and What They Have Done for Us Lately: Classification and Ecosystem Services of Dragonflies. Insects 2019, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Dudgeon, D.D. Tropical Asian Streams Zoobenthos, Ecology and Conservation. Hong Kong University Press: Hong Kong, China, 1999. [Google Scholar]
- Sasamoto, A. Anisogomphus yanagisawai sp. nov., a new gomphid dragonfly from northern Thailand (Odonata: Anisoptera: Gomphidae). Zootaxa 2015, 3904, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Makbun, N. Anisogomphus yingsaki (Odonata: Gomphidae) sp. nov., a new gomphid species from Thailand. Zootaxa 2017, 4306, 437–443. [Google Scholar] [CrossRef]
- Makbun, N. A new species of the genus Burmagomphus Williamson (Odonata: Gomphidae) from Northern Thailand. Zootaxa 2017, 4269, 133–136. [Google Scholar] [CrossRef]
- Chainthong, D.; Sartori, M.; Boonsoong, B. Stylogomphus thongphaphumensis (Odonata: Anisoptera: Gomphidae), a new gomphid dragonfly and the first record of S. malayanus Sasamoto, 2001 from Thailand. Zootaxa 2020, 4763, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Fleck, G. Onychogomphus (Siriusonychogomphus) louissiriusi, a new species and new subgenus from Thailand (Odonata: Anisoptera: Gomphidae). Faunitaxys 2021, 8, 1–9. [Google Scholar]
- Makbun, N.; Fleck, G. Description of Microgomphus farrelli sp. nov. (Odonata: Anisoptera: Gomphidae) based on adults of both sexes and larvae from Northern Thailand. Zootaxa 2018, 4422, 442–450. [Google Scholar] [CrossRef]
- Ferro, M.L.; Sites, R.W. Description of the Gomphidictinus perakensis (Laidlaw) (Odonata: Gomphidae) with distributional notes. Proc. Entomol. Soc. Wash. 2006, 108, 76–81. [Google Scholar]
- Boonsoong, B.; Chainthong, D. Description of the final-instar larva of Heliogomphus selysi Fraser (Odonata: Gomphidae). Zootaxa 2014, 3764, 482–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonsoong, B.; Chainthong, D. Description of the last stadium larva and female of Microgomphus thailandica Asahina, 1981 (Odonata: Gomphidae). Zootaxa 2014, 3811, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chainthong, D.; Boonsoong, B. Description of two final stadium Onychogomphus larvae from Thailand (Odonata: Gomphidae). Zootaxa 2016, 4066, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Novelo-Gutiérrez, R.; Sites, R.W. The larvae of Phaenandrogomphus Lieftinck, 1964 in Thailand, including the description of P. tonkinicus (Fraser, 1926) with a larval diagnosis and new province records of P. asthenes Lieftinck, 1964 (Odonata: Gomphidae). Zootaxa 2019, 4700, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Novelo-Gutiérrez, R.; Sites, R.W. The larva of Amphigomphus somnuki Hämäläinen, 1996 and the first records of the genus Stylogomphus Fraser, 1922 for Thailand (Odonata: Gomphidae). Zootaxa 2019, 4555, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.F.; Xu, J.F. Descriptions of a new genus and species of gomphid dragonfly reared from nymph in Fujian Province, with notes on allied species (Gomphidae: Onychogomphinae). J. Fujian Agric. Coll. 1987, 16, 259–266. [Google Scholar]
- World Odonata List. Available online: https://www2.pugetsound.edu/academics/academic-resources/slater-museum/biodiversity-resources/dragonflies/world-odonata-list2/ (accessed on 9 March 2022).
- Wilson, K.D.P. Crepuscular activity in Orientogomphus minor (Laidlaw) comb. nov. from Thailand and clarification of the taxonomic status of closely related species. Agrion 2008, 12, 15–21. [Google Scholar]
- Chao, H.F. The Gomphid Dragonflies of China (Odonata: Gomphidae); The Science and Technology Publishing House: Fuzhou, China, 1990. [Google Scholar]
- Lieftinck, M.A. Synonymic notes on East Asiatic Gomphidae with descriptions of two new species (Odonata). Zool. Meded. 1964, 39, 89–110. [Google Scholar]
- Shorthouse, D.P. SimpleMappr, an Online Tool to Produce Publication-Quality Point Maps. Available online: https://www.simplemappr.net (accessed on 4 March 2022).
- Asahina, S.A. list of the Odonata from Thailand. Thailand. Part XIV. Gomphidae-2. Tombo 1986, 29, 7–53. [Google Scholar]
- Watson, M.C. The utilization of mandibular armature in taxonomic studies of anisopterous nymphs. Trans. Am. Entomol. Soc. 1956, 81, 155–202. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data; MjM Software Design: Gleneden Beach, OR, USA, 2011. [Google Scholar]
- Hämäläinen, M.; Pinratana, A. Distribution maps by provinces. In Atlas of the Dragonflies of Thailand; Gabriel in Thailand: Bangkok, Thailand, 1999. [Google Scholar]
- Kosterin, O.E. Notes on intraspecific variation of some Gomphidae (Odonata) species in Cambodia. Int. Dragonfly Fund 2014, 68, 1–16. [Google Scholar]
- Xu, Q.H. Description of the last instar larva of Amphigomphus hansoni Chao, with notes on the systematic status of the genus Amphigomphus chao (Anisoptera: Gomphidae). Odonatologica 2012, 41, 55–59. [Google Scholar]
- Rith-Najarian, J. The influence of forest vegetation variables on the distribution and diversity of dragonflies in a northern Minnesota forest landscape: A preliminary study (Anisoptera). Odonatologica 1998, 27, 335–351. [Google Scholar]
- Suhling, F. Spatial distribution of the larvae of Gomphus pulchellus Selys (Anisoptera: Gomphidae). Advan. Odonatol. 1994, 6, 101–111. [Google Scholar]
- da Silva Monteiro Júnior, C.; Couceiro, S.R.M.; Hamada, N.; Juen, L. Effect of vegetation removal for road building on richness and composition of Odonata communities in Amazonia, Brazil. Int. J. Odonatol. 2013, 16, 135–144. [Google Scholar] [CrossRef]
- Kositpon, T.; Phalaraksh, C. Effects of check dams on water quality and macroinvertebrate diversity of Hom Jom Stream, Lamphun Province, Thailand. Suranaree J. Sci. Technol. 2012, 19, 113–123. [Google Scholar]






| Characters/Species | O. minor | O. armatus | A. somnuki Hämäläinen, 1996 | A. hansoni |
|---|---|---|---|---|
| Third antennal segment of nymph | slender, stick-shaped, longer than antennal S1 + 2 | spindle-shaped, shorter than antennal S1 + 2 | cylindrical, parallel-sided, longer than antennal S1 + 2 | stick-shaped, longer than antennal S1 + 2 |
| Prementum length-to-width ratio | about 3:2 | about 3:2 | about 1:0.83 | about 3:2 |
| Front margin of median lobe | furnished with about 50 finger-shaped serrations | furnished with about 60 finger-shaped serrations | furnished with about 27 or 28 small teeth | furnished with about 40 finger-shaped serrations |
| Wing pads length | strongly divergent, reaching middle of S4 | strongly divergent, reaching middle of S4 | reaching basal half and posterior margin of S4, | strongly divergent, reaching middle of S4 |
| Mid-dorsal spines on abdomen | present on S2–9 | present on S2–9 | present on S2–9 | present on S2–9 |
| Lateral spines on abdomen | present on S6–9 | present on S7–9 | present on S7–9 | present on S7–9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chainthong, D.; Boonsoong, B. Taxonomy and Distribution of the Gomphid Dragonfly Orientogomphus minor (Laidlaw, 1931) (Odonata: Gomphidae) in Thailand. Diversity 2022, 14, 291. https://doi.org/10.3390/d14040291
Chainthong D, Boonsoong B. Taxonomy and Distribution of the Gomphid Dragonfly Orientogomphus minor (Laidlaw, 1931) (Odonata: Gomphidae) in Thailand. Diversity. 2022; 14(4):291. https://doi.org/10.3390/d14040291
Chicago/Turabian StyleChainthong, Damrong, and Boonsatien Boonsoong. 2022. "Taxonomy and Distribution of the Gomphid Dragonfly Orientogomphus minor (Laidlaw, 1931) (Odonata: Gomphidae) in Thailand" Diversity 14, no. 4: 291. https://doi.org/10.3390/d14040291
APA StyleChainthong, D., & Boonsoong, B. (2022). Taxonomy and Distribution of the Gomphid Dragonfly Orientogomphus minor (Laidlaw, 1931) (Odonata: Gomphidae) in Thailand. Diversity, 14(4), 291. https://doi.org/10.3390/d14040291






