The Interplay of Environment and Biota in Assessing the Freshwater Quality in Karst
Abstract
:1. Introduction
2. Materials and Methods
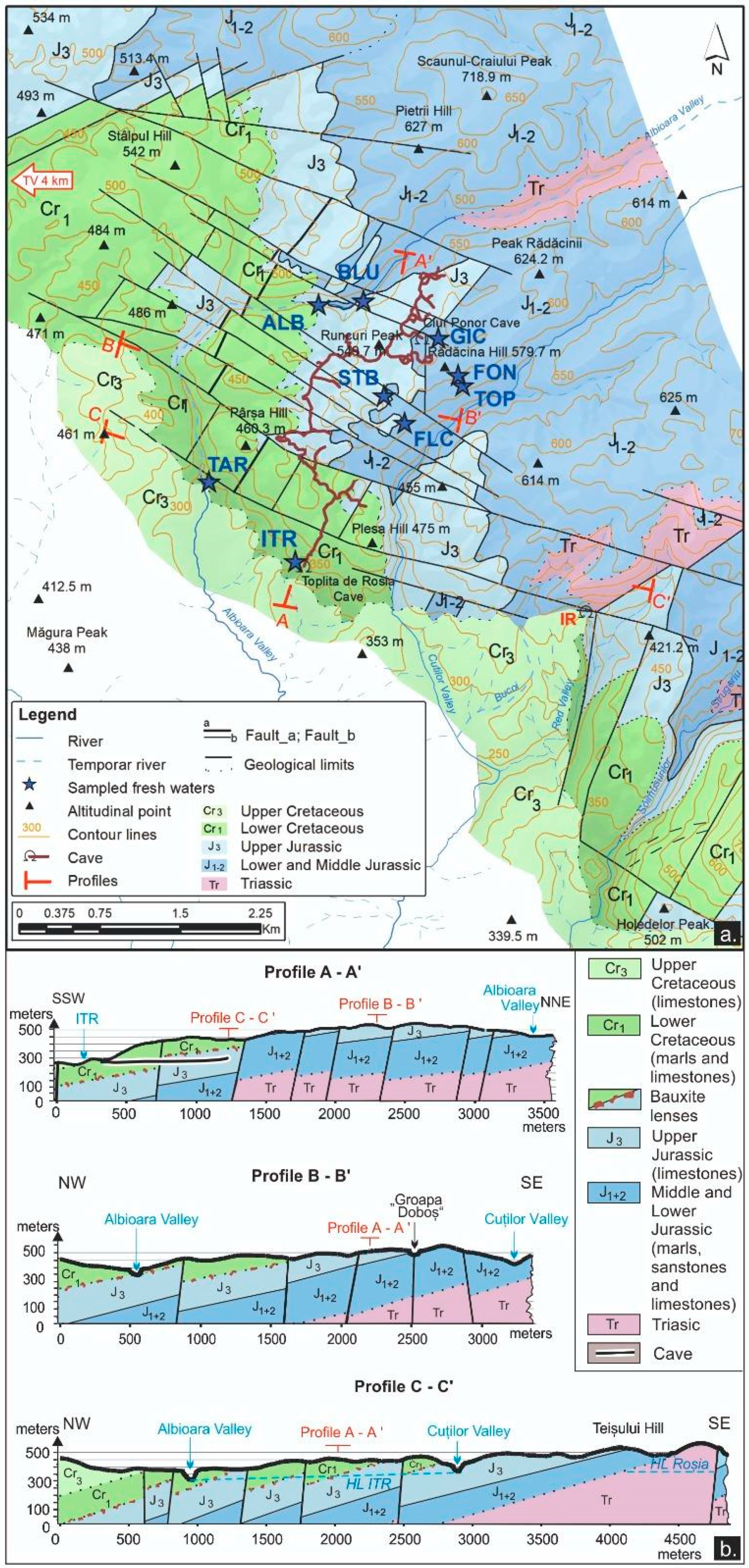
2.1. Study Area and Sampling Sites
2.2. Sampling Strategy
2.3. Environmental Variables
2.3.1. Geological Methods
2.3.2. Land Use, Normalized Difference Vegetation Index, Precipitations, and Air Temperature
2.3.3. Physicochemical Parameters
2.4. Microbial Contamination of the Water Sources
2.5. Meiofauna
2.6. Data Analyses
3. Results
3.1. Abiotic Features of the Studied Freshwater Sources of the KSRP
3.1.1. Geology and Tectonics
3.1.2. Environmental Features
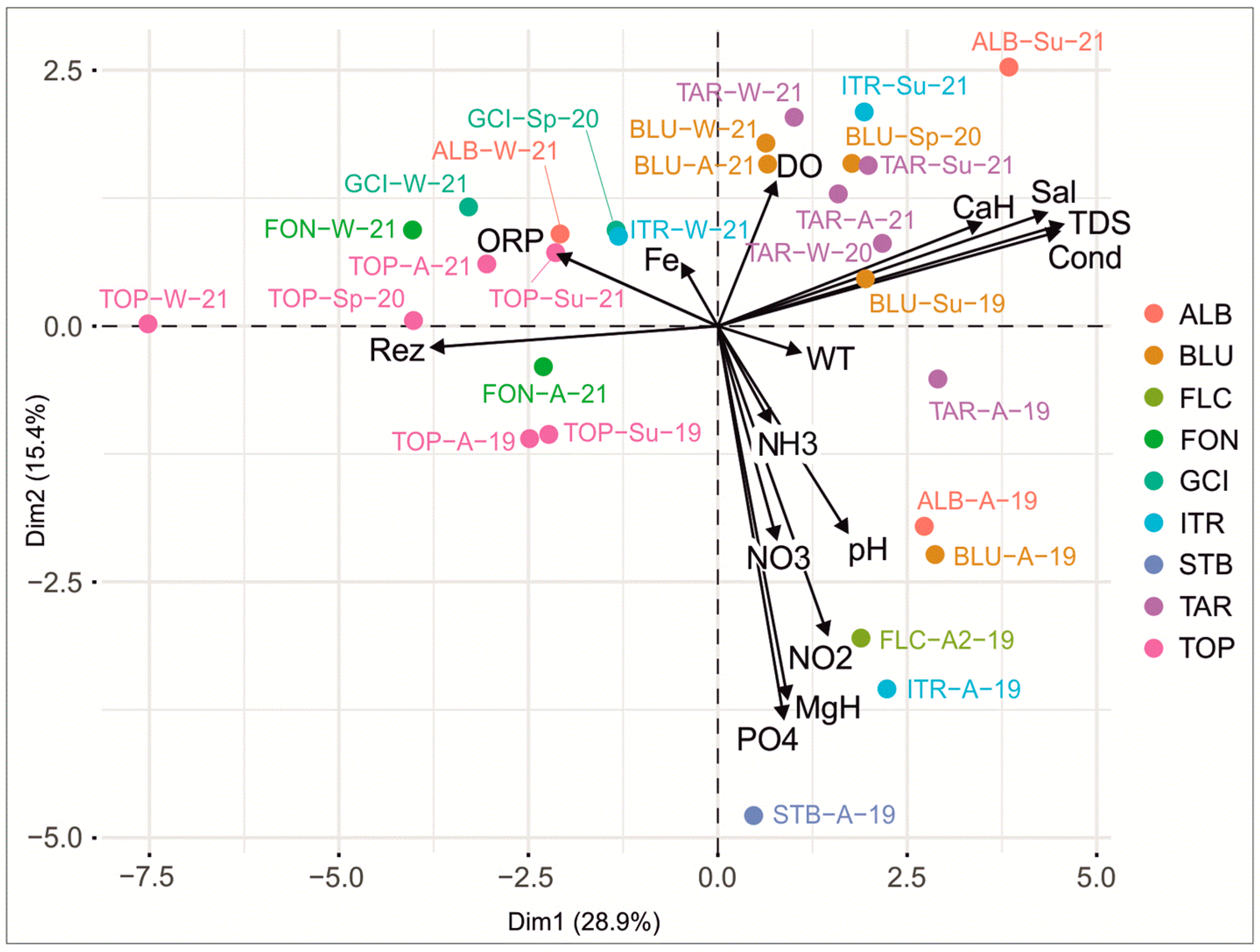
3.1.3. Physicochemical Parameters
3.2. Biotic Features of the Studied Freshwater Sources of the KSRP
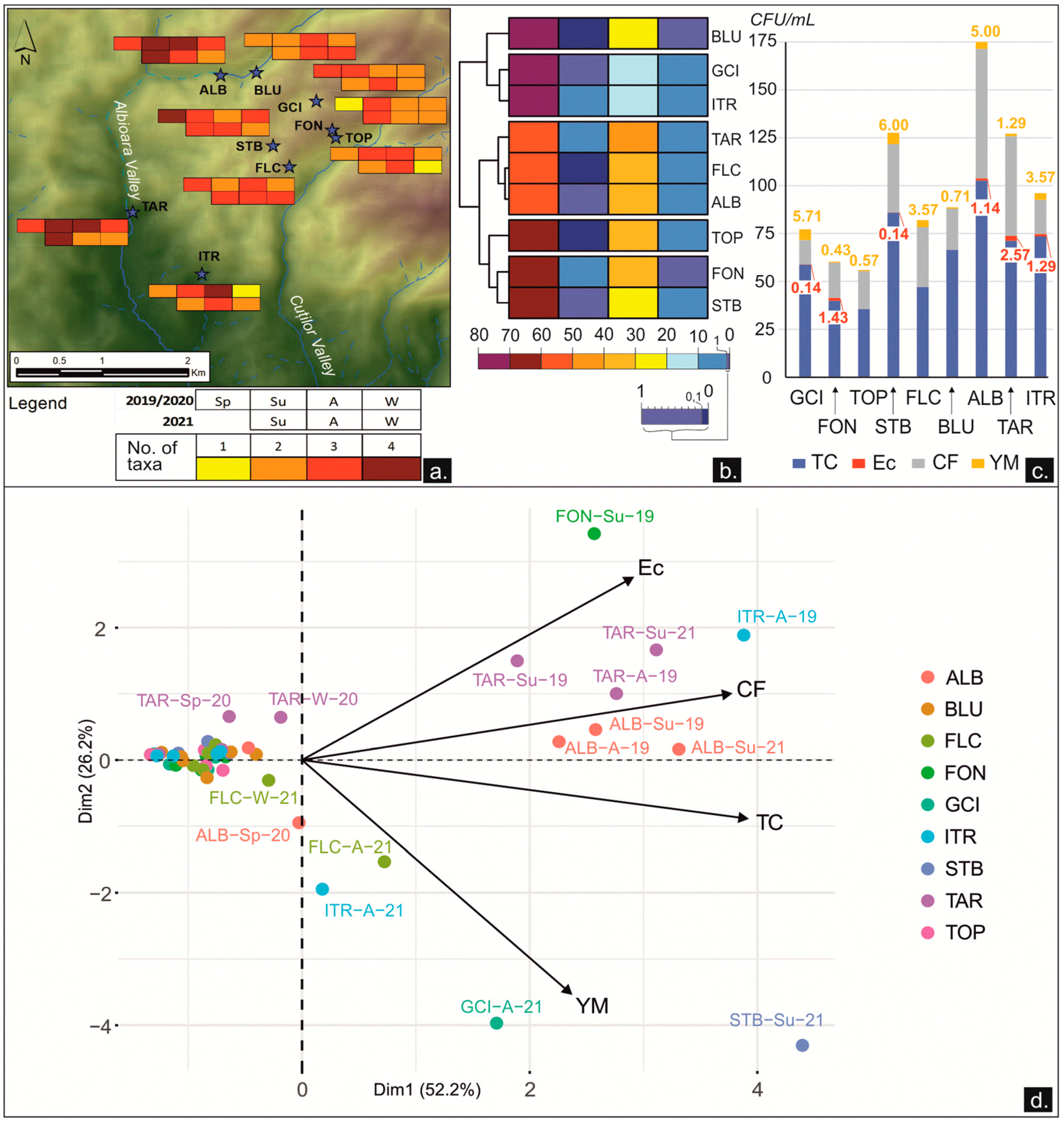
3.2.1. Microbial Content
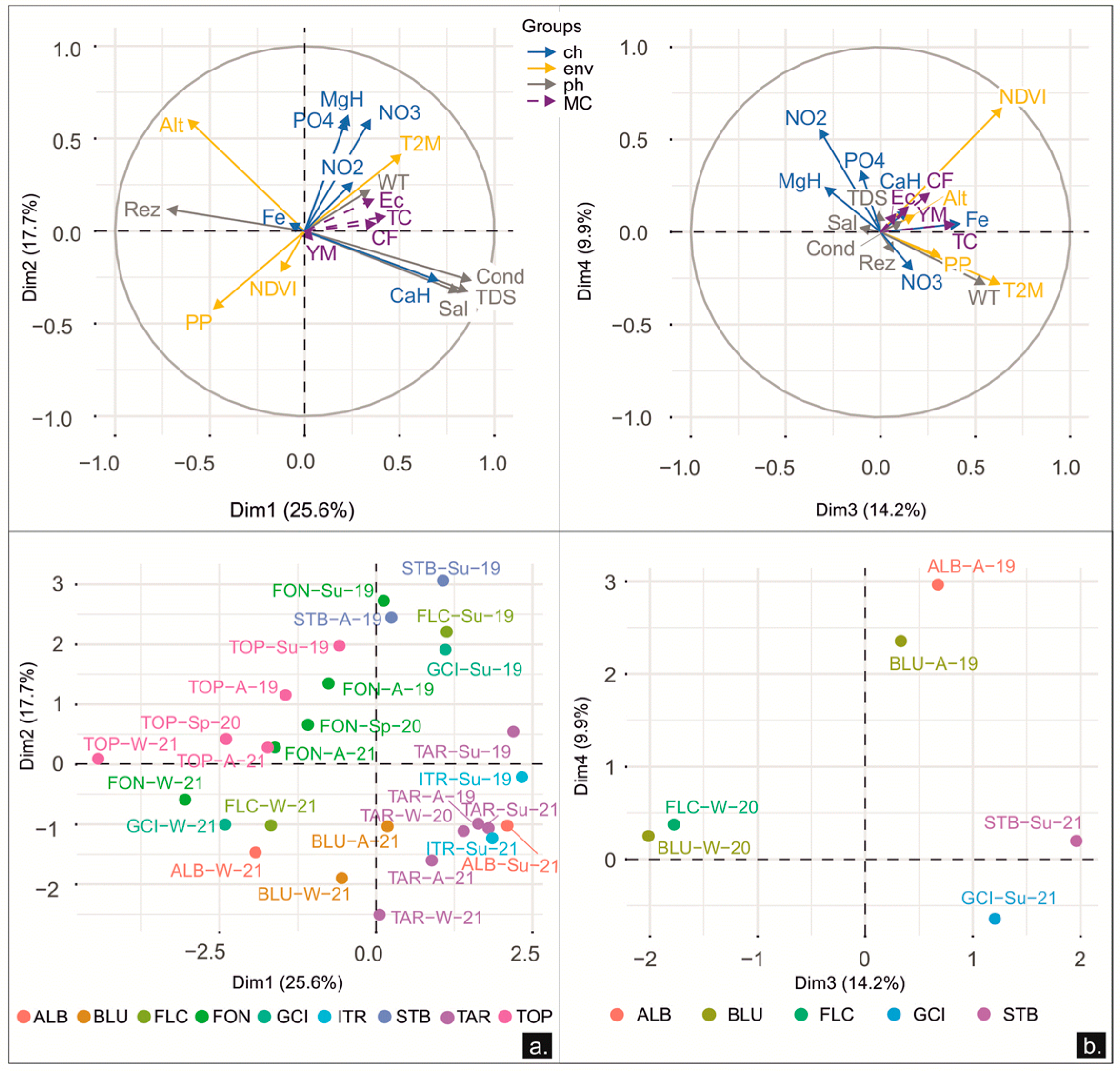
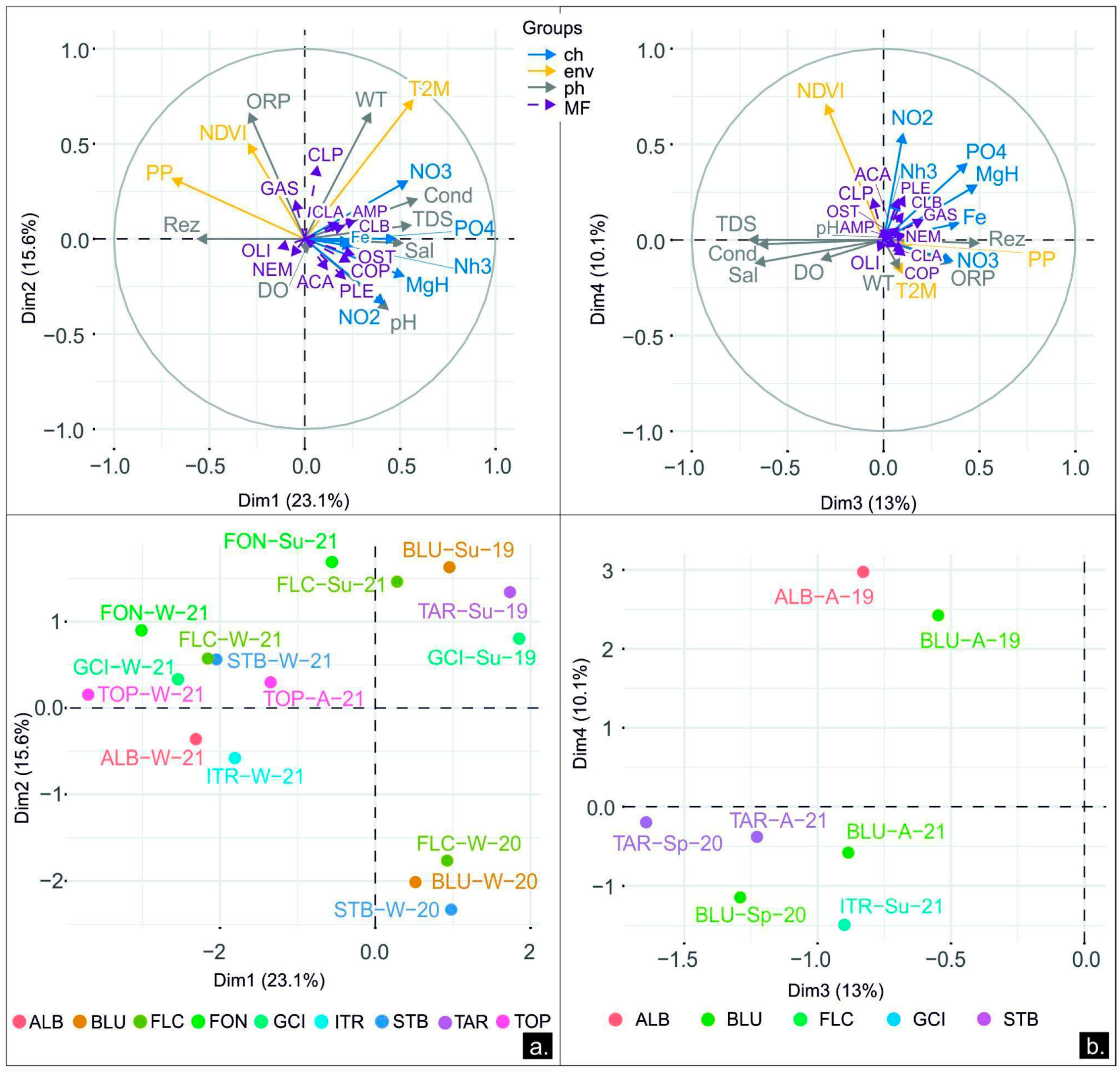
3.2.2. Dynamics between the Microbial Content and the Physicochemical and Environmental Parameters
3.2.3. Meiofauna
3.2.4. Dynamics between Meiofauna, Physicochemical, Environmental, and Microbiological Parameters
3.3. Differences between the Inputs and Outputs of the Freshwater Sources from the KSRP
4. Discussion
4.1. Relevance of the Geological Structure of the KSRP and the Anthropogenic Impact on the Physicochemical Features of the Freshwater Sources
4.1.1. Geological Characteristics and Tectonic Setting of the KSRP and Their Impacts on the Physicochemical Features
4.1.2. Nutrients and the Anthropogenic Impact on the Freshwater Sources of the KSRP
4.2. Interplay of the Micro-Organisms and Meiofauna with the Abiotic Features of Freshwater Sources in KSRP
4.2.1. Micro-Organisms
4.2.2. Meiofauna
4.3. Assessing the Self-Purification Process in the KSRP in Regard to the Chemical and Microbial Pollutants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costanza, R.; d’Arge, R.; de Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Bakalowicz, M. Karst, a Renewable Water Resource in Limestone Rocks, Encyclopédie de l’Environnement. 2021. Available online: https://www.encyclopedie-environnement.org/?p=6816 (accessed on 2 March 2022).
- Hartmann, A.; Goldscheider, N.; Wagener, T.; Lange, J.; Weiler, M. Karst water resources in a changing world: Review of hydrological modeling approaches. Rev. Geophys. 2014, 52, 218–242. [Google Scholar] [CrossRef]
- Stevanović, Z. Karst waters in potable water supply: A global scale overview. Environ. Earth Sci. 2019, 78, 662. [Google Scholar] [CrossRef]
- Sokolova, E.; Lindström, G.; Pers, C.; Strömqvist, J.; Lewerin, S.S.; Wahlström, H.; Sörén, K. Water quality modelling: Microbial risks associated with manure on pasture and arable land. J. Water Health 2018, 16, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Ashjari, J.; Raeisi, E. Lithological control on water chemistry in karst aquifers of the Zagros range, Iran. J. Cave Karst Stud. 2006, 33, 111–118. [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry Groundwater and Pollution, 2nd ed.; CRC Press: London, UK, 2005; p. 649. [Google Scholar]
- Gerič, B.; Pipan, T.; Mulec, J. Diversity of culturable bacteria and meiofauna in the epikarst of Škocjanske jame Caves (Slovenia). Acta Carsologica 2004, 33, 301–309. [Google Scholar] [CrossRef]
- Hershey, O.S.; Kallmeyer, J.; Wallace, A.; Barton, M.D.; Barton, H.A. High Microbial Diversity Despite Extremely Low Biomass in a Deep Karst Aquifer. Front. Microbiol. 2018, 9, 2823. [Google Scholar] [CrossRef] [Green Version]
- Slabe, M.O.; Danevčič, T.; Hug, K.; Fillinger, L.; Mandić-Mulec, I.; Griebler, C.; Brancelj, A. Key drivers of microbial abundance, activity, and diversity in karst spring waters across an altitudinal gradient in Slovenia. Aquat. Microb. Ecol. 2021, 86, 99–114. [Google Scholar] [CrossRef]
- Notenboom, J.; Hendrix, W.; Folkerts, A.J. Meiofauna assemblages discharged by springs from a phreatic aquifer system in the Netherlands. Neth. J. Aquat. Ecol. 1996, 30, 1–13. [Google Scholar] [CrossRef]
- Danielopol, D.L.; Pospisil, P.; Rouch, R. Biodiversity in groundwater: A large-scale view. Trends Ecol. Evol. 2000, 15, 223–224. [Google Scholar] [CrossRef]
- Fiasca, B.; Stoch, F.; Olivier, M.J.; Maazouzi, C.; Petitta, M.; Di Cioccio, A.; Galassi, D.M. The dark side of springs: What drives small-scale spatial patterns of subsurface meiofaunal assemblages? J. Limnol. 2014, 73, 71–80. [Google Scholar] [CrossRef]
- Iepure, S.; Feurdean, A.; Bădăluţă, C.; Nagavciuc, V.; Perşoiu, A. Pattern of richness and distribution of groundwater Copepoda (Cyclopoida: Harpacticoida) and Ostracoda in Romania: An evolutionary perspective. Biol. J. Linn. Soc. 2016, 119, 593–608. [Google Scholar] [CrossRef]
- Saxena, G.; Bharagava, R.N.; Kaithwas, G.; Raj, A. Microbial indicators, pathogens and methods for their monitoring in water environment. J. Water Health 2015, 13, 319–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanzler, D.; Buzina, W.; Paulitsch, A.; Haas, D.; Platzer, S.; Marth, E.; Mascher, F. Occurrence and hygienic relevance of fungi in drinking water. Mycoses 2007, 51, 165–169. [Google Scholar] [CrossRef]
- Pereira, V.J.; Basílio, M.C.; Fernandes, D.; Domingues, M.; Paiva, J.M.; Benoliel, M.J.; Crespo, M.T.; San Romão, M.V. Occurrence of filamentous fungi and yeasts in three different drinking water sources. Water Res. 2009, 43, 3813–3819. [Google Scholar] [CrossRef] [PubMed]
- Novak Babič, M.; Gunde-Cimerman, N.; Vargha, M.; Tischner, Z.; Magyar, D.; Veríssimo, C.; Sabino, R.; Viegas, C.; Meyer, W.; Brandão, J. Fungal Contaminants in Drinking Water Regulation? A Tale of Ecology, Exposure, Purification and Clinical Relevance. Int. J. Environ. Res. Public Health 2017, 14, 636. [Google Scholar] [CrossRef] [Green Version]
- Novak Babič, M.; Zalar, P.; Ženko, B.; Džeroski, S.; Gunde-Cimerman, N. Yeasts and yeast-like fungi in tap water and groundwater, and their transmission to household appliances. Fungal Ecol. 2016, 20, 30–39. [Google Scholar] [CrossRef]
- Revised Drinking Water Directive. (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj (accessed on 12 April 2022).
- Lege nr.458 din 8 iulie 2002 Privind Calitatea Apei Potabile, Republicată cu Modificările și Completarile Aduse de Legile Ulterioare (Law 458/2002 Regarding the Drinking Water Quality, Republished with Adjustments and Completions Added by the Subsequent Laws). Available online: https://legislatie.just.ro/Public/DetaliiDocument/37723 (accessed on 7 March 2022).
- Palmer, M.A.; Straver, D.L.; Rundle, S.D. Meiofauna. In Methods in Stream Ecology, 2nd ed.; Hauer, F.R., Lamberti, G.A., Eds.; Academic Press: Amsterdam, Holland, 2007; Volume 1, pp. 415–433. [Google Scholar] [CrossRef]
- Manenti, R.; Barzaghi, B.; Lana, E.; Stocchino, G.A.; Manconi, R.; Lunghi, E. The stenoendemic cave-dwelling planarians (Platyhelminthes, Tricladida) of the Italian Alps and Apennines: Conservation issues. J. Nat. Conserv. 2018, 45, 90–97. [Google Scholar] [CrossRef]
- Galassi, D.; Huys, R.; Reid, J. Diversity, ecology and evolution of groundwater Copepods. Freshw. Biol. 2009, 54, 691–708. [Google Scholar] [CrossRef] [Green Version]
- Pacioglu, O. Ecology of the hyporheic zone: A review. Cave Karst Sci. 2009, 36, 69–76. [Google Scholar]
- Yusal, M.S.; Marfai, M.A.; Hadisusanto, S.; Khakhim, N. Abundance and diversity of meiofauna as water quality bioindicator in Losari Coast, Makassar, Indonesia. Ecol. Environ. Conserv. 2019, 25, 589–598. [Google Scholar]
- Odonkor, S.T.; Ampofo, J.K. Escherichia coli as an indicator of bacteriological quality of water: An overview. Microbiol. Res. 2013, 4, e2. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.M.; Gupta, R. Escherichia coli (E. coli) as an Indicator of Fecal Contamination in Groundwater: A Review. In Sustainable Development of Water and Environment; Jeon, H.Y., Ed.; ICSDWE 2020. Environmental Science and Engineering; Springer: Cham, Switzerland, 2020; pp. 225–235. [Google Scholar] [CrossRef]
- Varol, S.; Şekerci, M. Hydrogeochemistry, water quality and health risk assessment of water resources contaminated by agricultural activities in Korkuteli (Antalya, Turkey) district center. J. Water Health 2018, 16, 574–599. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Wu, X.; Chang, L.; Ham, B.; Song, L.; Groves, C. Nitrate sources and biogeochemical processes in karst underground rivers impacted by different anthropogenic input characteristics. Environ. Pollut. 2020, 265, 114835. [Google Scholar] [CrossRef]
- Moldovan, A.; Hoaghia, M.-A.; Kovacs, E.; Mirea, I.C.; Kenesz, M.; Arghir, R.A.; Petculescu, A.; Levei, E.A.; Moldovan, O.T. Quality and Health Risk Assessment Associated with Water Consumption—A Case Study on Karstic Springs. Water 2020, 12, 3510. [Google Scholar] [CrossRef]
- Hoaghia, M.-A.; Moldovan, A.; Kovacs, E.; Mirea, I.C.; Kenesz, M.; Brad, T.; Cadar, O.; Micle, V.; Levei, E.A.; Moldovan, O.T. Water Quality and Hydrogeochemical Characteristics of Some Karst Water Sources in Apuseni Mountains, Romania. Water 2021, 13, 857. [Google Scholar] [CrossRef]
- Smitha, S.; Ajay, D.; Shivashankar, P. Physico Chemical Analysis of the Freshwater at River Kapila, Nanjangud Industrial Area, Mysore, India. Int. Res. J. Environ. Sci. 2013, 2, 59–65. [Google Scholar]
- Guo, F.; Jiang, G.; Zhao, H.; Polk, J.; Liu, S. Physicochemical parameters and phytoplankton as indicators of the aquatic environment in karstic springs of South China. Sci. Total Environ. 2019, 659, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Pronk, M.; Goldscheider, N.; Zopfi, J. Microbial communities in karst groundwater and their potential use for biomonitoring. Hydrogeology 2009, 17, 37–48. [Google Scholar] [CrossRef]
- Ibekwe, A.M.; Murinda, S.E. Linking Microbial Community Composition in Treated Wastewater with Water Quality in Distribution Systems and Subsequent Health Effects. Microorganisms 2019, 7, 660. [Google Scholar] [CrossRef] [Green Version]
- Zeppilli, D.; Sarrazin, J.; Leduc, D.; Arbizu, P.M.; Fontaneto, D.; Fontanier, C.; Gooday, A.J.; Kristensen, R.M.; Ivanenko, V.N.; Sørensen, M.V.; et al. Is the meiofauna a good indicator for climate change and anthropogenic impacts? Mar. Biodiv. 2015, 45, 505–535. [Google Scholar] [CrossRef] [Green Version]
- Veronna, M.; Johnson, L. Microbial indicators and environmental relationships in the Umhlangane River, Durban, South Africa. Open Life Sci. 2018, 13, 385–395. [Google Scholar] [CrossRef]
- Borda, D.; Epure, L. Groundwater contamination and the relationship between water chemistry and biotic components in a karst system (bihor mountains, romania). Trav. Inst. Spéol. Emil Racovitza 2014, 53, 69–84. [Google Scholar]
- Borda, D.; Epure, L.; Meleg, I.N.; Cociuba, I. Preliminary results on the quality of drinking water sources in the Runcuri Plateau. Trav. Inst. Spéol. Emil Racovitza 2019, 58, 19–46. [Google Scholar]
- Cai, L.; Fu, S.; Yang, J.; Zhou, X. Distribution of meiofaunal abundance in relation to environmental factors in Beibu Gulf, South China Sea. Acta Oceanol. Sin. 2012, 31, 92–103. [Google Scholar] [CrossRef]
- Frontalini, F.; Semprucci, F.; Du, E.; Francescangeli, F.; Margaritelli, G.; Rettori, R.; Spagnoli, F.; Balsamo, M.; Coccioni, R. Biodiversity trends of the meiofaunal and foraminiferal assemblages of Lake Varano (southern Italy). Proc. Biol. Soc. Wash. 2014, 127, 7–22. [Google Scholar] [CrossRef]
- Murdock, S.A.; Tunnicliffe, V.; Boschen-Rose, R.E.; Juniper, S.K. Emergent “core communities” of microbes, meiofauna and macrofauna at hydrothermal vents. ISME Commun. 2021, 1, 27. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T. Conservation and protection of shallow subterranean habitats. In Shallow Subterranean Habitats, 1st ed.; OxfordUniversity Press: Oxford, UK, 2014; pp. 211–224. [Google Scholar] [CrossRef]
- Orăşeanu, I.; Jurkiewicz, A. Hydrological Karst Systems in Pădurea Craiului Mountains. Theor. Appl. Karstol. 1987, 3, 215–222. [Google Scholar]
- Orăşeanu, I. Hydrogeological map of the Pădurea Craiului Mountains (România). Theor. Appl. Karstol. 1991, 4, 97–127. [Google Scholar]
- Orăşeanu, I. Pădurea Craiului Mountains. In Karst Hydrogeology of Romania; Orăşeanu, I., Yurkiewicz, A., Eds.; Belvedere: Oradea, România, 2010; pp. 199–217. Available online: http://www.ahgr.ro/media/227251/42.-2010-karst-hydrogeology-of-romania-table-of-contents-.pdf (accessed on 30 March 2022).
- Bucur, I.I.; Cociuba, I. La plate-forme carbonatée du Crétacé inférieur des Monts Pădurea Craiului (Monts Apuseni, Roumanie). Biostratigraphy et configuration. Studia UBB Geol. 1998, 43, 89–100. [Google Scholar]
- Cociuba, I. Stratigraphical study of the Mesozoic deposits from the South-Western part of Pădurea Craiului. Ph.D Thesis, University Babeş-Bolyai, Cluj-Napoca, Romania, 1999. [Google Scholar]
- Cociuba, I. Upper Jurassic and Lower Cretaceous deposit in the south-western part of Pădurea Craiului. Formal lithostratigraphic units. Studia UBB Geol. 2000, 45, 33–61. [Google Scholar] [CrossRef] [Green Version]
- Copernicus Land Monitoring Service. Available online: https://land.copernicus.eu (accessed on 24 February 2022).
- Collection 1 Level-2A-Sentinel Online. Available online: https://sentinels.copernicus.eu/web/sentinel/sentinel-data-access/sentinel-products/sentinel-2-data-products/collection-1-level-2a (accessed on 24 February 2022).
- Learn Geomatics. Available online: https://learngeom.com/ (accessed on 4 March 2022).
- NASA Power. Available online: https://power.larc.nasa.gov/ (accessed on 4 March 2022).
- NASA Power. Available online: https://gmao.gsfc.nasa.gov/reanalysis/MERRA/data_access/ (accessed on 4 March 2022).
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. Available online: https://www.R-project.org/ (accessed on 30 March 2022).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kolde, R. Pheatmap: Pretty Heatmaps. R Package Version 1.0.12. Cran—Package Pheatmap. 2019. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 30 March 2022).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. Cran—Package Factoextra. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 30 March 2022).
- Peterson, B.G.; Carl, P. Performance Analytics: Econometric Tools for Performance and Risk Analysis (R Package Version 2.0.4). 2020. Available online: https://CRAN.R-project.org/package=PerformanceAnalytics (accessed on 30 March 2020).
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Bayley, H.; Smith, S.; Medeiros, K. Water Temperature as a Limiting Factor in the Colonization of a Partially-Restored Coastal Lagoon: Case Study of a Gastropod Herbivore and Control of Macroalgae. Ecol. Restor. 2011, 29, 243–251. [Google Scholar] [CrossRef]
- Bonacci, O. Water Temperature in Karst. In Karst Hydrology, Springer Series in Physical Environment, 2; Springer: Berlin/Heidelberg, Germany, 1987; pp. 141–149. [Google Scholar] [CrossRef]
- Winter, T.C.; Harvey, J.W.; Franke, O.L.; William, A.M. Ground Water and Surface Water: A Single Resource. U.S. Geol. Surv. Circ. 1139; U.S. Government Printing Office: Denver, CO, USA, 1998; pp. 19–32.
- Racoviţǎ, G. The underground climatology. In The karst of Pădurea Craiului Mountains. Monographic Study; Racoviţǎ, G., Moldovan, O., Onac, B., Eds.; Presa Universitarǎ Clujeanǎ: Cluj-Napoca, România, 2002; pp. 93–115. [Google Scholar]
- Cociuba, I.; Silvestru, E. Hypothesis on a Genetic Relation between the Actual Karst and the Bauxite-Bearing Paleokarst at the Jurassic/Cretaceous Boundary in the Piatra Craiului Mountains (Romania). Trav. Inst. Spéol. Emil Racovitza 1989, XXVIII, 87–90. [Google Scholar]
- Ozyurt, N.N.; Lutz, H.O.; Hunjak, T.; Mance, D.; Roller-Lutz, Z. Characterization of the Gacka River basin karst aquifer (Croatia): Hydrochemistry, stable isotopes and tritium-based mean residence times. Sci.Total Environ. 2014, 487, 245–254. [Google Scholar] [CrossRef]
- Lecomte, K.L.; Bicalho, C.C.; Silva-Filho, E.V. Geochemical characterization in karst basin tributaries of the San Franciscan depression: The Corrente River, western Bahia, NE-Brazil. J. South Am. Earth Sci. 2016, 69, 119–130. [Google Scholar] [CrossRef]
- Ianovici, V.; Borcoş, M.; Bleahu, M.; Patrulius, D.; Lupu, M.; Dimitrescu, R.; Savu, H. Geologia Munţilor Apuseni, 1st ed.; Edit. Acad.: București, Romania, 1976; p. 631. [Google Scholar]
- Fonollá, C.; Sanz, E.; Menéndez-Pidal, I. Lateral ferruginous groundwater transfer as the origin of the iron crusts in caves: A case study. J. Cave Karst Stud. 2020, 82, 183–197. [Google Scholar] [CrossRef]
- Gomes, P.P.; Ferreira, V.; Tonin, A.M.; Medeiros, A.O.; Júnior, J.F.G. Combined Effects of Dissolved Nutrients and Oxygen on Plant Litter Decomposition and Associated Fungal Communities. Microb. Ecol. 2018, 75, 854–862. [Google Scholar] [CrossRef]
- Ferreira, V.; Elosegi, A.D.; Tiegs, S.; von Schiller, D.; Young, R. Organic Matter Decomposition and Ecosystem Metabolism as Tools to Assess the Functional Integrity of Streams and Rivers—A Systematic Review. Water 2020, 12, 3523. [Google Scholar] [CrossRef]
- Horne, A.J.; Goldman, C.R. Limnology, 2nd ed.; McGraw-Hill, Inc.: New York, NY, USA, 1994; p. 576. [Google Scholar]
- Copeland, A.; Lytle, D.A. Measuring the Oxidation—Reduction Potential of Important Oxidants in Drinking Water. J. Am. Water Work. Assoc. 2014, 106, E10–E20. [Google Scholar] [CrossRef]
- Schullehner, J.; Stayner, L.; Hansen, B. Nitrate, nitrite, and ammonium variability in drinking water distribution systems. Int. J. Environ. Res. Public Health 2017, 14, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, A.N.; Lehmann, M.F.; Rügner, H.; D’Affonseca, F.M.; Grathwohl, P.; Blackwell, N.; Kappler, A.; Osenbrück, K. Fate of nitrate during groundwater recharge in a fractured karst aquifer in Southwest Germany. Hydrogeol. J. 2021, 29, 1153–1171. [Google Scholar] [CrossRef]
- Boyer, D.G.; Pasquarell, G.C. Nitrate concentrations in karst springs in an extensively grazed area 1995. J. Am. Water Resour. Assoc. 1995, 31, 729–736. [Google Scholar] [CrossRef]
- Opsahl, S.P.; Musgrove, M.; Slattery, R.N. New insights into nitrate dynamics in a karst groundwater system gained from in situ high-frequency optical sensor measurements. J. Hydrol. 2017, 546, 179–188. [Google Scholar] [CrossRef]
- Bartram, J.; Thyssen, N.; Gowers, A.; Pond, K.; Lack, T. (Eds.) Water and Health in Europe; European Series, No. 93; WHO Regional Publications: Copenhagen, Denmark, 2002; pp. 7–47. Available online: https://www.researchgate.net/publication/253953045_Water_and_Health_in_Europe_A_Joint_Report_from_the_European_Environment_Agency_and_the_WHO_Regional_Office_for_Europe (accessed on 4 March 2022).
- Manu, E.; Afrifa, G.Y.; Ansah-Narh, T.; Sam, F.; Loh, Y.S.A. Estimation of natural background and source identification of nitrate-nitrogen in groundwater in parts of the Bono, Ahafo and Bono East regions of Ghana. Groundw. Sustain. Dev. 2022, 16, 100696. [Google Scholar] [CrossRef]
- Rahman, A.; Mondal, N.C.; Tiwari, K.K. Anthropogenic nitrate in groundwater and its health risks in the view of background concentration in a semi arid area of Rajasthan, India. Sci. Rep. 2021, 11, 9279. [Google Scholar] [CrossRef]
- Nakić, Z.; Kovač, Z.; Parlov, J.; Perković, D. Ambient Background Values of Selected Chemical Substances in Four Groundwater Bodies in the Pannonian Region of Croatia. Water 2020, 12, 2671. [Google Scholar] [CrossRef]
- European Environment Agency (EEA) Groundwater Nitrate. Available online: https://www.eea.europa.eu/data-and-maps/daviz/groundwater-nitrate-4#tab-chart_2_filters=%7B%22rowFilters%22%3A%7B%7D%3B%22columnFilters%22%3A%7B%22pre_config_country%22%3A%5B%22Europe%22%5D%7D%7D (accessed on 5 April 2022).
- Radcliff, C.; Ford, W.I.; Nazari, S.; Shepard, C. Impact of water source dynamics on dissolved reactive phosphorus loadings in heterogeneous karst agroecosystems with phosphatic limestones. Hydrol. Processes 2021, 35, e14422. [Google Scholar] [CrossRef]
- Karathanasis, A.D. Phosphate mineralogy and equilibria in two Kentucky Alfisols derived from Ordovician limestones. Soil Sci. Soc. Am. J. 1991, 55, 1774–1782. [Google Scholar] [CrossRef]
- Einsiedl, F.; Mayer, B. Hydrodynamic and microbial processes controlling nitrate in a fissured-porous karst aquifer of the Franconian Alb, southern Germany. Environ. Sci. Technol. 2006, 40, 6697–6702. [Google Scholar] [CrossRef]
- Figueras, M.J.; Borrego, J.J. New perspectives in monitoring drinking water microbial quality. Int. J. Environ. Res. Public Health 2010, 7, 4179–4202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, P.R. Climate change and waterborne and vector-borne disease. J. Appl. Microbiol 2003, 94, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, R.A.; Pachepsky, Y.; Hill, R.L.; Shelton, D.R.; Whelan, G. Escherichia coli survival in waters: Temperature dependence. Water Res. 2013, 47, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Bogosian, G.; Sammons, L.E.; Morris, P.J.L.; O’Neil, J.P.; Heitkamp, M.A.; Weber, D.B. Death of the Escherichia coli K-12 strain W3110 in soil and water. Appl. Environ. Microbiol. 1996, 62, 4114–4120. [Google Scholar] [CrossRef] [Green Version]
- Sampson, R.W.; Swiatnicki, S.A.; Osinga, V.L.; Supita, J.L.; McDermott, C.M.; Kleinheinz, G.T. Effects of temperature and sand on E. coli survival in a northern lake water microcosm. J. Water Health 2006, 4, 389–393. [Google Scholar] [CrossRef]
- Hinzelin, F.; Block, J.C. Yeasts and filamentous fungi in drinking water. Environ. Technol. Lett. 1985, 6, 101–106. [Google Scholar] [CrossRef]
- Ormerod, K.S. Heterotrophic microorganisms in distribution systems for drinking water. Vatten 1987, 43, 262–268. [Google Scholar]
- Chowdhury, S. Heterotrophic bacteria in drinking water distribution system: A review. Environ. Monit. Assess. 2012, 184, 6087–6137. [Google Scholar] [CrossRef]
- Warris, A.; Gaustad, P.; Meis, J.F.G.M.; Voss, A.; Verweij, P.E.; Abrahamsen, T.G. Recovery of filamentous fungi from water in a pediatric bone marrow transplantation unit. J. Hosp. Infect. 2001, 47, 143–148. [Google Scholar] [CrossRef]
- Hageskal, G.; Knutsen, A.K.; Gaustad, P.; de Hoog, G.S.; Skaar, I. Diversity and significance of mold species in Norwegian drinking water. Appl. Environ. Microbiol. 2006, 72, 7586–7593. [Google Scholar] [CrossRef] [Green Version]
- Nagy, L.A.; Olson, B.H. The occurrence of filamentous fungi in drinking water distribution systems. Can. J. Microbiol. 1982, 28, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.S.; Morsy, E.M.; Soliman, S.A.; Hussein, A.E. Yeast and Filamentous Fungi which contaminate of Surface and Groundwater in Sohag Governorate, Egypt. J. Basic Appl. Mycol. 2019, 10, 22–38. Available online: https://www.researchgate.net/publication/356597931_Yeast_and_Filamentous_Fungi_which_contaminate_of_Surface_and_Groundwater_in_Sohag_Governorate_Egypt (accessed on 30 March 2022).
- Calero Preciado, C.; Boxall, J.; Soria-Carrasco, V.; Martínez, S.; Douterelo, I. Implications of Climate Change: How Does Increased Water Temperature Influence Biofilm and Water Quality of Chlorinated Drinking Water Distribution Systems? Front. Microbiol. 2021, 12, 1361. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Dou, C.; Lian, B.; Dong, H. The interaction of fungus with calcite and the effect on aqueous geochemistry in karst systems. Carbonate. Evaporite. 2013, 28, 413–418. [Google Scholar] [CrossRef]
- Swan, C.M.; Palmer, M.A. What drives small-scale spatial patterns in lotic meiofauna communities? Freshw. Biol. 2000, 44, 109–121. [Google Scholar] [CrossRef]
- Baia, E.; Rollnic, M.; Venekey, V. Seasonality of pluviosity and saline intrusion drive meiofauna and nematodes on an Amazon freshwater-oligohaline beach. J. Sea Res. 2021, 170, 102022. [Google Scholar] [CrossRef]
- Todaro, M.A.; Leasi, F.; Bizzarri, N. Meiofauna densities and gastrotrich community composition in a Mediterranean Sea cave. Mar. Biol. 2006, 149, 1079–1091. [Google Scholar] [CrossRef]
- Brankovits, D.; Little, S.; Winkler, T.; Tamalavage, A.; Mejía-Ortíz, L.; Maupin, C.; Yáñez-Mendoza, G.; van Hengstum, P. Changes in Organic Matter Deposition Can Impact Benthic Marine Meiofauna in Karst Subterranean Estuaries. Front. Environ. Sci. 2021, 9, 157. [Google Scholar] [CrossRef]
- Meleg, I.N.; Fiers, F.; Robu, M.; Moldovan, O.T. Distribution patterns of subsurface copepods and the impact of environmental parameters. Limnologica 2012, 42, 156–164. [Google Scholar] [CrossRef]
- Gaudes, A.; Munoz, I.; Moens, T. Bottom-up effects on freshwater bacterivorous nematode populations: A microcosm approach. Hydrobiologia 2013, 70, 159–172. [Google Scholar] [CrossRef]
- Karaouzas, I.; Smeti, E.; Vourka, A.; Vardakas, L.; Mentzafou, A.; Torn’es, E.; Sabater, S.; Muñoz, I.; Skoulikidis, N.T.; Kalogianni, E. Assessing the ecological effects of water stress and pollution in a temporary river—Implications for water management. Sci. Total Environ. 2018, 618, 1591–2160. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, T.; Fiasca, B.; Di Cicco, M.; Cifoni, M.; Galassi, D.M. Taxonomic and functional trait variation along a gradient of ammonium contamination in the hyporheic zone of a Mediterranean stream. Ecol. Indic. 2021, 132, 108268. [Google Scholar] [CrossRef]
- Iburg, S.; Izabel-Shen, D.; Austin, Å.N.; Hansen, J.P.; Eklöf, J.S.; Nascimento, F. Effects of Recreational Boating on Microbial and Meiofauna Diversity in Coastal Shallow Ecosystems of the Baltic Sea. mSphere 2021, 6, e00127-21. [Google Scholar] [CrossRef] [PubMed]
- Tanentzap, A.J.; Fitch, A.; Orland, C.; Emilson, E.J.S.; Yakimovich, K.M.; Osterholz, H.; Dittmar, T. Chemical and microbial diversity covary in fresh water to influence ecosystem functioning. Proc. Natl. Acad. Sci. USA 2019, 116, 24689–24695. [Google Scholar] [CrossRef]
- Hakenkamp, C.C.; Morin, A. The importance of meiofauna to lotic ecosystem functioning. Freshw. Biol. 2001, 44, 165–175. [Google Scholar] [CrossRef]
- Mulec, J.; Oarga, A. Ecological evaluation of air and water habitats in the Great Cavern of SantoTomás, Cuba. Rev. Mex. De Biodivers. 2014, 85, 910–917. [Google Scholar] [CrossRef] [Green Version]
- Iacurto, S.; Grelle, G.; Filippi, F.M.D.; Sappa, G. Karst spring recharge areas and discharge relationship by oxygen-18 and deuterium isotopes analyses: A case study in southern latium region, Italy. Appl. Sci. 2020, 10, 1882. [Google Scholar] [CrossRef] [Green Version]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: San Diego, CA, USA, 2001; pp. 151–168. [Google Scholar]
- Kalvāns, A.; Popovs, K.; Priede, A.; Koit, O.; Retiķe, I.; Bikše, J.; Dēliņa, A.; Babre, A. Nitrate vulnerability of karst aquifers and associated groundwater-dependent ecosystems in the Baltic region. Environ. Earth Sci. 2021, 80, 628. [Google Scholar] [CrossRef]
- De Mandal, S.; Laskar, F.; Kumari Panda, A.; Mishra, R. Chapter 12—Microbial diversity and functional potential in wetland ecosystems. In Recent Advancements in Microbial Diversity; De Mandal, A., Bhatt, P., Eds.; Academic Press: San Diego, CA, USA, 2020; pp. 289–314. [Google Scholar] [CrossRef]
- Herrmann, M.; Opitz, S.; Harzer, R.; Totsche, K.U.; Küsel, K. Attached and Suspended Denitrifier Communities in Pristine Limestone Aquifers Harbor High Fractions of Potential Autotrophs Oxidizing Reduced Iron and Sulfur Compounds. Microb. Ecol. 2017, 74, 264–277. [Google Scholar] [CrossRef]
- Holmes, D.E.; Dang, Y.; Smith, J.A. Nitrogen cycling during wastewater treatment. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press: San Diego, CA, USA, 2019; Volume 106, pp. 113–192. [Google Scholar] [CrossRef]
- Li, E.; Saleem, F.; Edge, T.A.; Schellhorn, H.E. Biological Indicators for Fecal Pollution Detection and Source Tracking: A Review. Processes 2021, 9, 2058. [Google Scholar] [CrossRef]
- Einsiedl, F.; Maloszewski, P.; Stichler, W. Estimation of denitrification potential in a karst aquifer using the 15N and 18O isotopes of NO3−. Biogeochemistry 2005, 72, 67–86. [Google Scholar] [CrossRef]
- Guo, F.; Wang, W.; Jiang, G.; Ma, Z. Contaminant transport behavior in a karst subterranean river and its capacity of self-purification: A case study of Lihu, Guangxi. Adv. Water Sci. 2014, 25, 414–419. [Google Scholar]
- Ravbar, N.; Petrič, M.; Blatnik, M.; Švara, A. A multi-methodological approach to create improved indicators for the adequate karst water source protection. Ecol. Indic. 2021, 126, 107693. [Google Scholar] [CrossRef]




| Sampling Sites/Drinking Purpose | Water Type | Substrate | Hydrological Type | Age/ Lithology | Land Use | NDVI (Year/Index Value) | Altitude (m a.s.l.) |
|---|---|---|---|---|---|---|---|
| Groapa Ciur Izbuc (GCI)/occasionally used | Open-air Spring | Mud + Leaf | Input | UJ1/Lm | Bf | 2019/0.63 2020/0.66 2021/0.66 | 516 |
| La Foncea (FON)/ frequently used | Wood-covered Spring | Concrete dam + Ma | Input | UJ1/Ma | Cc, Pa, Bf | 2019/0.6 2020/0.66 2021/0.74 | 595 |
| La Topitori (TOP)/permanently used | Captured Spring | Concrete dam + Ss | Input | UJ1/Ss | Cc, Pa, Bf | 2019/0.61 2020/0.67 2021/0.66 | 580 |
| Știubei (STB)/permanently used | Open Well | Concrete tube + Mud | Input | J2-J3/Lm, Ma | Pa, Ws | 2019/0.56 2020/0.57 2021/0.75 | 531 |
| Fântâna lui Ciocan (FLC)/occasionally used | Wood-covered Spring | Lm + Mud | Input | UJ1/ Lm | Pa, Bf | 2019/0.54 2020/0.6 2021/0.67 | 503 |
| Izvorul Albastru (BLU)/occasionally used | Captured Spring | Concrete basin + Mud | Input | J2-J3/Lm, Ma | Bf | 2019/0.75 2020/0.59 2021/0.64 | 445 |
| Albioara (ALB)/ not used | Surface Stream | Mud + Gravel | Input | J1, J2, J3/ Ss, Lm, Ma | Bf | 2019/0.81 2020/0.66 2021/0.66 | 432 |
| Izvorul Țarina (TAR)/occasionally used | Open-air Spring | Sand + Lm | Independent output | C1/Lm | Bf, Pa, Rf | 2019/0.69 2020/0.66 2021/0.66 | 304 |
| Izbucul Toplița de Roșia (ITR)/permanently used | Cave stream resurgence | Gravel + Lm | Output | C1/Lm | Ws, Ps, Pa | 2019/0.62 2020/0.6 2021/0.59 | 290 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borda, D.R.; Cociuba, I.; Epure, L.; Cruceru, N.; Meleg, I.N. The Interplay of Environment and Biota in Assessing the Freshwater Quality in Karst. Diversity 2022, 14, 475. https://doi.org/10.3390/d14060475
Borda DR, Cociuba I, Epure L, Cruceru N, Meleg IN. The Interplay of Environment and Biota in Assessing the Freshwater Quality in Karst. Diversity. 2022; 14(6):475. https://doi.org/10.3390/d14060475
Chicago/Turabian StyleBorda, Daniela R., Ioan Cociuba, Laura Epure, Nicolae Cruceru, and Ioana N. Meleg. 2022. "The Interplay of Environment and Biota in Assessing the Freshwater Quality in Karst" Diversity 14, no. 6: 475. https://doi.org/10.3390/d14060475
APA StyleBorda, D. R., Cociuba, I., Epure, L., Cruceru, N., & Meleg, I. N. (2022). The Interplay of Environment and Biota in Assessing the Freshwater Quality in Karst. Diversity, 14(6), 475. https://doi.org/10.3390/d14060475