Abstract
In Indonesia, Pinus merkusii, the Sumatran pine, is the most important forest tree in the industry. This study aimed to determine the effects of pine forest types and sites on the abundance of ambrosia beetles in four pine forest types and sites, i.e., the protected pine forest (PF1 & PF2), the pine forests-based agroforestry (PA1 & PA2), the tapped-pine forests (TP1 & TP2), and the non-tapped pine forests (NP1 & NP2). The environmental variables and the stand-ages were also studied related to the number of ambrosia beetle individuals and species. Twenty ethanol baited traps were installed to attract and collect the ambrosia beetles in each pine forest site. The descriptive analysis, the nested analysis of variance, and correlation analysis were applied to determine the differences in ambrosia beetle abundance between the pine forest sites, the relationship between the stand-age, humidity, elevation, and temperature to the number of individuals and species of ambrosia beetle, and species diversity of ambrosia beetles in each pine forest type. The Jaccard distance was calculated to investigate the dissimilarity between each pine forest site based on the ambrosia beetle species composition and abundance. The 999 ambrosia beetles (15 species) were reported in this study. Two were Platypodinae, and 13 were Scolytinae subfamilies. Xyleborinus andrewesi was the most abundant ambrosia beetle. The abundance of ambrosia beetles was significantly different between pine forest types (F = 89.23, p < 0.001). The population of ambrosia beetles was the highest in the protected pine forest, and the lowest one was identified in the non-tapped pine forest and the pine forest-based agroforestry. Based on the pine forest types, the highest number of ambrosia beetle species was in the protected pine forest (13 species), and the lowest one was in the pine forest-based agroforestry (7 species). The highest and the lowest numbers of ambrosia beetles were in the protected pine forest site 2 (11 species) and in the pine forest–based agroforestry site 2 & the non-tapped pine forest site 1 (3 species). The highest dissimilarity was shown by the highest distances between the pine forest-based agroforestry site 1 and the non-tapped pine forest site 1 based on the Jaccard distance. The stand-age, humidity, and temperature potentially effected the number of ambrosia beetle individuals and species. The elevation also indicated to influence the number of ambrosia beetle individuals however it isn’t happened on the number of ambrosia beetle species. This study contributes to identify the suitable pine forest types related to the ambrosia beetle management.
1. Introduction
Pinus is a conifer tree and the largest genus of the Pinaceae family, including 110 species worldwide [1]. Pinus merkusii Junghuhn et de Vriese, 1845 also known as the Sumatran pine, is the only pine that grows naturally south of the equator. It is found in Indonesia, Thailand, Myanmar, Philippines, Vietnam, Laos, and Cambodia [2]. Sumatran pine shows good growth performance at high and low altitudes, and this is the reason why this species has become widely planted in Java and Sulawesi [2].
Java is the largest Sumatran pine plantation, covering 900,000 ha in area. It is widespread from the eastern to the western part of the island, with 570,000 ha of production forests and 330,000 ha of protected forests [3]. Sumatran pine, the second commodity after teak (Tectona grandis Linn. F., 1974), has been cultivated in agroforestry systems to produce resin in Java [2]. In addition, this pine species comprises more than 30% of the total plantation area on the island [2], and it’s commonly chosen as a tree for reforestation and rehabilitation purposes in many degraded areas [4].
Ambrosia beetle is a group of adult beetles that bore into wood and culture the enlarged nutritious spores of their fungi (ambrosial fungi) by horizontal movements. Scolytinae is a dominant group, and Platypodinae is a minor group in the ambrosia beetles [5]. Ambrosia beetles belong to the Scolytinae and Platypodinae subfamilies, while bark beetles belong to the Scolytinae subfamily of Curculionidae [5]. Currently, more than 6000 species of Scolytinae and 1400 species of Platypodinae are described, and the majority of them are found in tropical or subtropical areas [5]. Bark beetles mostly live in phloem tissues, whereas ambrosia beetles live in xylem tissues and are associated with nutritional symbiotic fungi [6]. Both bark and ambrosia beetle species can attack healthy trees and cause damage, especially when introduced outside their native range [7,8]. However, ambrosia beetles species, most often exotic ones, are known to attack healthy living trees growing in managed and unmanaged systems [9]. Some invasive and native ambrosia beetles are among the most damaging forest pests and can cause ecological damage and economic losses to the forest ecosystems. Several species of ambrosia beetles are quarantine pests [10,11].
Pines are potential hosts for a number of bark beetles and some ambrosia beetle species [12]. In Uruguay, beetles from the Scolytinae subfamily (bark beetles) reportedly cause severe damage in pine and eucalyptus commercial plantations such as Hylurgus ligniperda (Fabricius, 1787) (red-haired pine bark beetle), Orthotomicus erosus (Mediterranean pine beetle) and Cyrtogenius luteus (Wollaston, 1857) [13,14]. In Brazil, at least 80 species from the Scolytinae subfamily have been detected in relation to the increase in damage in pine tree plantations including two species of ambrosia beetles Xyleborus ferrugineus (Fabricius, 1801) and Xyleborus affinis Eichhoff, 1868 which were considered to be two of the most aggressive ambrosia beetle species in Brazil and other tropical regions [12]. Despite the importance of the ambrosia beetle, their habitat and host species preferences in forest ecosystems are not well understood. This is especially true of the role and existence of native species in the forest because most research on ambrosia beetles are carried out in urban or nursery environments [15,16]. A number of studies also investigated how communities of ambrosia beetles change depending on forest type [17] and host species [18], especially in broadleaf forests.
Invasive forest insects pose severe threats to natural and managed forested ecosystems [19]. An integrated management program’s primary components include the investigation of composition of insects in natural forests, production forests, and plantations and their temporal and spatial distribution [20]. Currently, traps baited with attractants are among the best tools to survey and monitor insects [21]. Specifically, ethanol-baited traps are used to monitor native and non-native ambrosia beetles worldwide [22,23]. Several studies have shown for example that simple bottle traps baited with ethanol can collect various ambrosia beetle species [24,25,26,27].
The influence of forest management in the forest ecosystem or structure can be investigated by using biological indicator species [28]. Several management practices impact insect richness either directly by affecting resources or indirectly by creating microclimatic changes and changes in species assemblages [29]. However, studies about forest management practices’ impact on ambrosia beetle diversity have been carried out in broadleaf forests but not conifer forests [30,31]. This study aimed to determine the effects of pine forest types and sites on the diversities and the species composition of ambrosia beetles. The environmental variables and stand-ages were also investigated related to the number of ambrosia beetle individuals and species.
2. Materials and Methods
2.1. Study Site
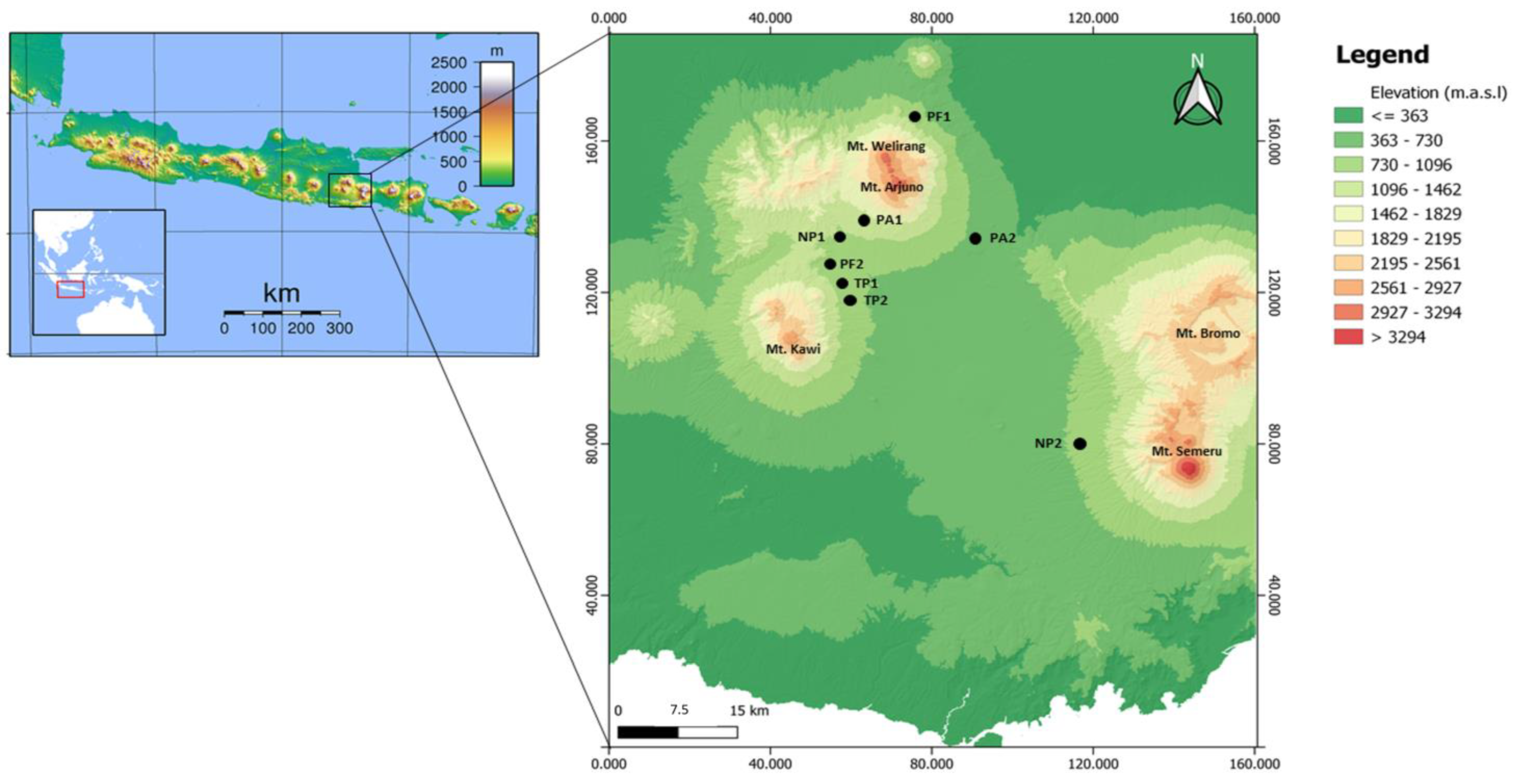
The study was conducted in Sumatran pine forests, East Java, Indonesia (Figure 1). We analyzed four pine forest types, including the protected pine forest (PF), the non-tapped pine forest (NP), the tapped-pine forest (TP), and the pine forest-based agroforestry (PA), please see from Figure 2. The PFs in this study are very homogenous, with an understory of grasses and shrubs that lack proper management, such as thinning and pruning. The NPs are also very homogenous, with an understory of grasses. Intensive sap-tapping activities are carried out once every three days in the TPs. At the same time, PAs are cultivated with various vegetable crops such as cabbage and intensive pesticide application.
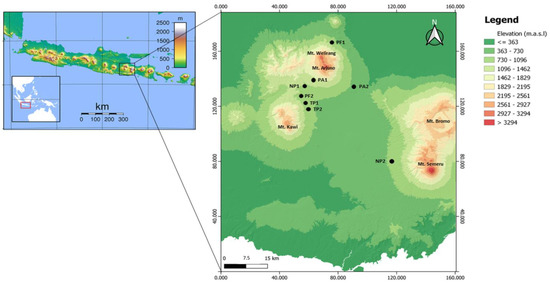
Figure 1.
Distribution of collection sites for the ambrosia beetles in the pine forest types, East Java, Indonesia. PA = pine forest-based agroforestry, NP = non-tapped pine forest, PF = protected pine forest, TP = tapped-pine forest.

Figure 2.
Views of the pine forests. The protected pine forest (PF) in Mojokerto District, the pine forest-based agroforestry (PA) in Batu City, the non-tapped pine forest (NP) in Batu City and the tapped-pine forest (TP) in Batu City.
The pine trees included in this study ranged from 10 to 40 years. We sampled beetles from two sites for each forest type or eight sites in total (Figure 1 and Table 1), and the selected sites had no current pest and disease symptoms. The pine forests in this study were at altitudes between 438 and 1350 masl. Orographically, the study plots are located on high land, at the base of Mt. Arjuno, Mt. Welirang, Mt. Kawi, and Mt. Semeru. We also measured the site characteristics at each location, including temperature and humidity.

Table 1.
Characteristics of the studied pine forests in East Java, Indonesia.
2.2. Trapping and Identifications of Ambrosia Beetles
At each site, 20 transparent polyethylene terephthalate (PET) bottle traps with 95% ethanol were used as bait as deployed along a line transecting the pine forest edges and separated at about 20 m. Based on a volume and an average release rate for ethanol as bait was 3.8 g per day at 25 °C, we replaced baits every 5 days [32]. The bottle (with a diameter of 7 cm, a height of 30 cm, and a volume of 1.5 L) was modified into one window cut on the side and a specimen container (water containing soap solution) in the below part [24]. The traps were attached to trees sampled 1.5 m above the ground [33]. Ambrosia beetles were collected ten times at 5-day intervals, after which the bait was replenished. This study was conducted during the rainy and dry seasons (Table 1).
All trapped ambrosia beetles that immersed in a specimen container were collected and preserved in 95% ethanol. The identification of ambrosia beetles was performed based on morphological characters using the Olympus SZ51 stereomicroscope (Olympus Optical Co., Ltd., Beijing, China). Ambrosia beetles were identified at the Plant Pest Laboratory, Department of Plant Pests and Diseases, Faculty of Agriculture, Universitas Brawijaya, using ambrosia beetle identification keys Southeast Asian Ambrosia Beetle ID by Smith et al. [34] and several identification references [35,36].
2.3. Data Analysis
Descriptive analysis was adopted to describe the species composition of ambrosia beetle in each pine forest type and site. The nested analysis of variance (ANOVA) and Tukey HSD were applied to analyze the abundance of ambrosia beetles between the pine forest types and sites. The Jaccard distance [37] was calculated to evaluate the distances between each pine forest. Heatmap was also provided to visualize the Jaccard distance between the pine forest sites. In addition, the relationships between stand-age, elevation, temperature, and humidity to the number of individuals and species of ambrosia beetles were also analyzed via the multiple regression analysis. All analyses were performed using RStudio statistical software [38]. The tidyverse, rshape2, ggplot2, multcompview, dplyr, lessR, and vegan were applied to run all analyses.
3. Results
3.1. Ambrosia Beetle Abundance, Diversities and Species Composition in Different Pine Forest Management
During the sampling period, 999 specimens (15 species) were collected. Among the 15 species, 2 were Platypodinae, and 13 were Scolytinae. Xyleborinus andrewesi (Blandford, 1896) was the most abundant ambrosia beetle, with 245 individuals (24.52% of the trapped beetles in this study). Xylosandrus crassiusculus (Motschulsky, 1866) was the second most abundant species with 209 individuals (20.92%), followed X. affinis (17.02%), Premnobius cavipennis Eichhoff, 1878 (10.71%), Cryphalus sp. (8.61%), Xylosandrus germanus (Blandford, 1894) (4.60%), Dendrocanulus sp. (4.40%), Xylosandrus morigerus (Blandford, 1894) (4.40%), Euplatypus parallelus (Fabricius, 1801) (1.50%), Diuncus haberkorni (Eggers, 1920) (1.00%), and Premnobius sp. (1.00%). Treptoplatypus micrurus (Schedl., 1968), Xylosandrus discolor (Blandford, 1898), Scolytoplatypus sp., and Beaverium sp. were less than 1.00% (Table 2).

Table 2.
Total number of ambrosia beetles and their relative abundance (%) collected from different pine forest types in East Java, Indonesia.
Among the 15 ambrosia beetle species, five were found in all pine forest types i.e., X. crassiusculus, X. morigerus, X. affinis, X. andrewesi, and Cryphalus sp., while the other species were found only at one or some of the studied forest types (Table 2). The highest number of ambrosia beetle species was found in the PFs, which was also the only forest type hosting Scolytoplatypus sp., and X. discolor. However, two other species, i.e., T. micrurus, and Beaverium sp., was not recorded in PFs (Table 2). The highest and the lowest numbers of ambrosia beetles were the PFs (500 individuals) and the NPs (93 individuals).
Based on the pine forest sites, among the 15-ambrosia beetle species, X. crassiusculus was found in all pine forest sites. The highest number of ambrosia beetle species was the PF2 (about 11 species) and the lowest one was reported in the PA2 & NP1 (3 species) (Table 2). The highest and the lowest populations of ambrosia beetles were the PF2 (301 individuals) and the NP2 (41 individuals).
3.2. Comparison and Distances between Pine Forest Management Based on Abundance of Ambrosia Beetles
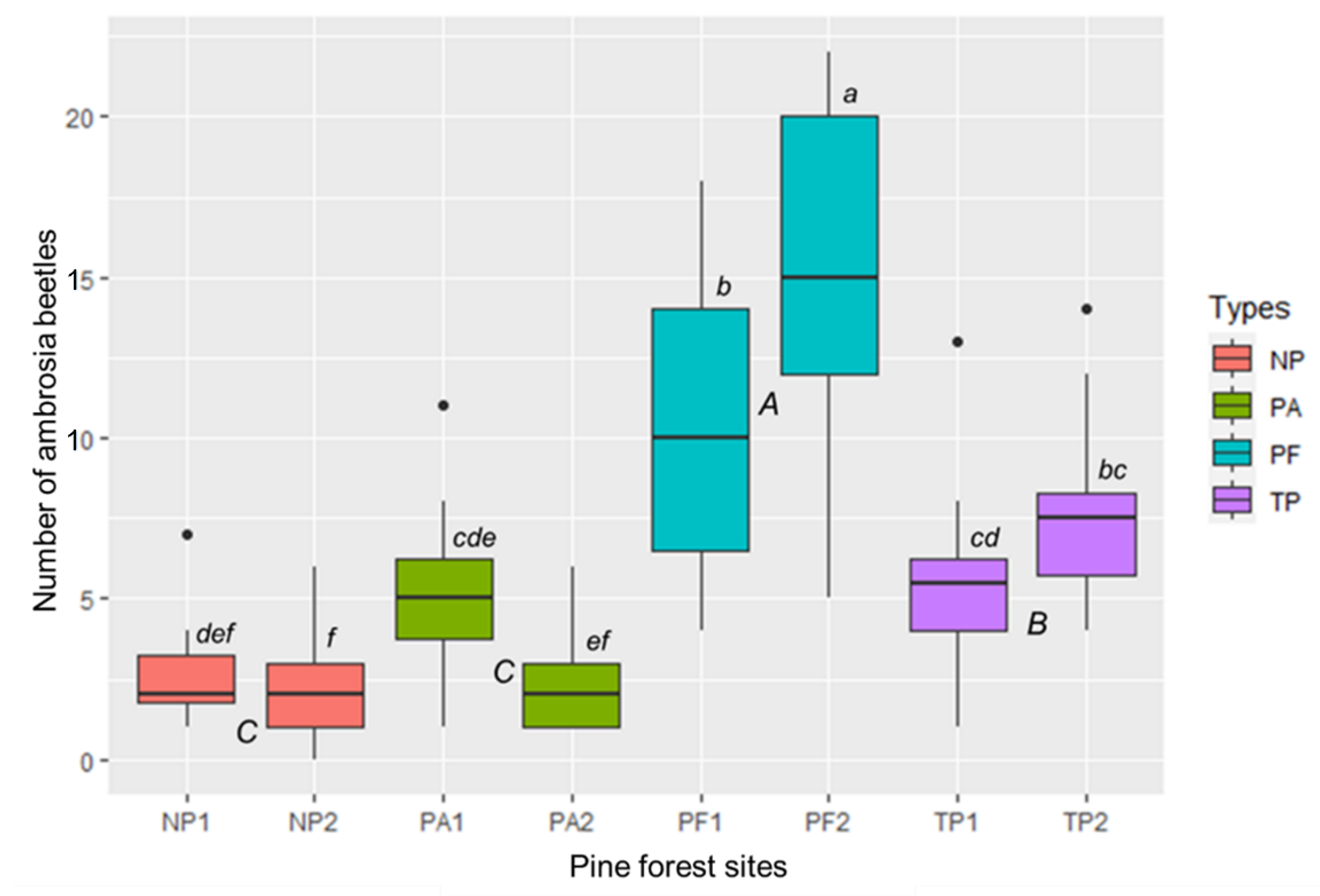
The abundance of ambrosia beetles was significantly different between the pine forest types (F = 89.23, p < 0.001) (Figure 3). Based on the pine forest types, the population of ambrosia beetles was highest in PFs. The lowest population was shown in NPs and PAs. Comparison between all pine forest sites described that PF2 was a significant difference from others i.e., PF1, TP1, TP2, PA1, PA2, NP1, and NP2 (F = 10.73, p < 0.001) (Figure 3).
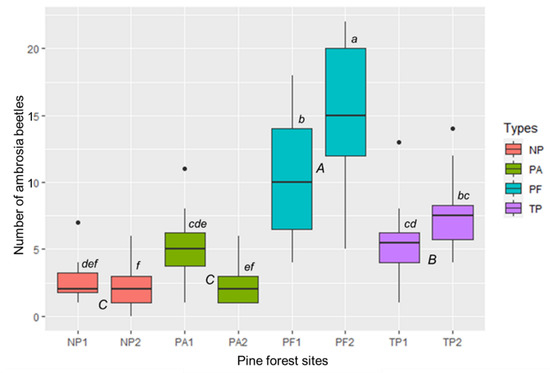
Figure 3.
Difference of the ambrosia beetle’s population between the pine forest types or sites in East Java, Indonesia. PA = pine forest-based agroforestry, NP = non-tapped pine forest, PF = protected pine forest, TP = tapped-pine forest. Boxplots with different capital and small letters were significantly different between the pine forest types and sites at p < 0.05, according to Tukey HSD. Bars represent the interquartile range with the median value. Circles indicate outliers. Vertical solid lines indicate minimum and maximum values.
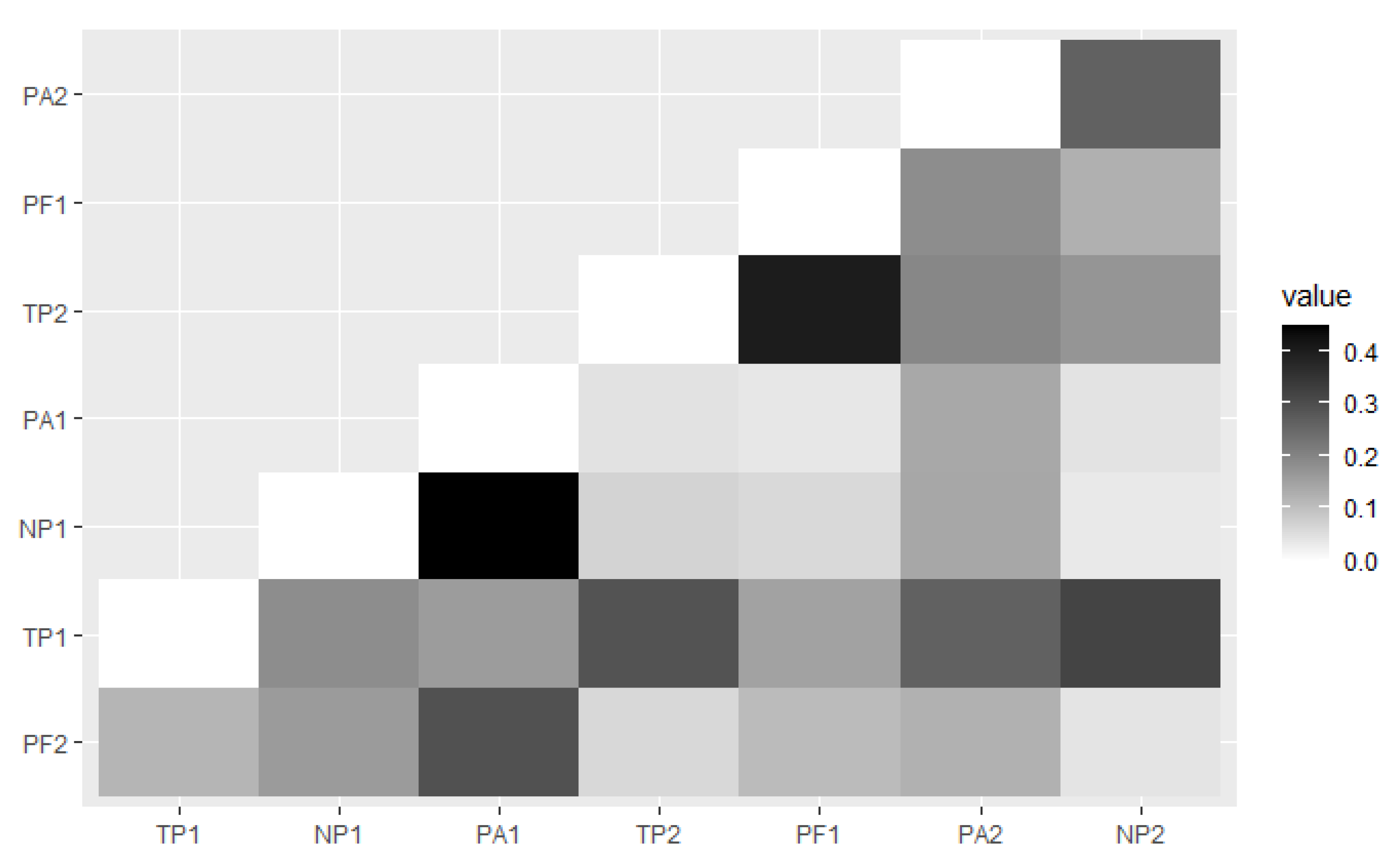
The distances between the pine forest sites describe that the PA1 provide higher coefficients (about 0.449) when paired with NP1. The coefficient value of the PF1 that paired with TP2 were 0.402 (Figure 4). Both paired sites were recorded as the highest coefficient that showed the highest dissimilarity between each paired site.
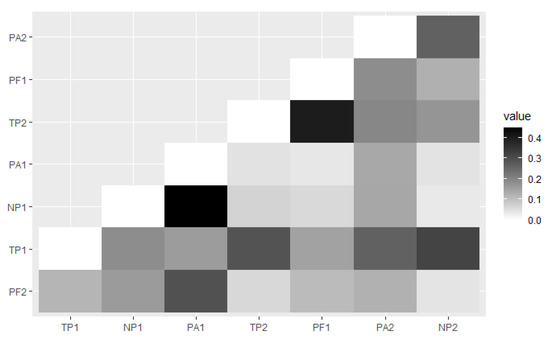
Figure 4.
The heatmap of the Jaccard distance is based on the number of ambrosia beetle abundance and species between each pine forest site.
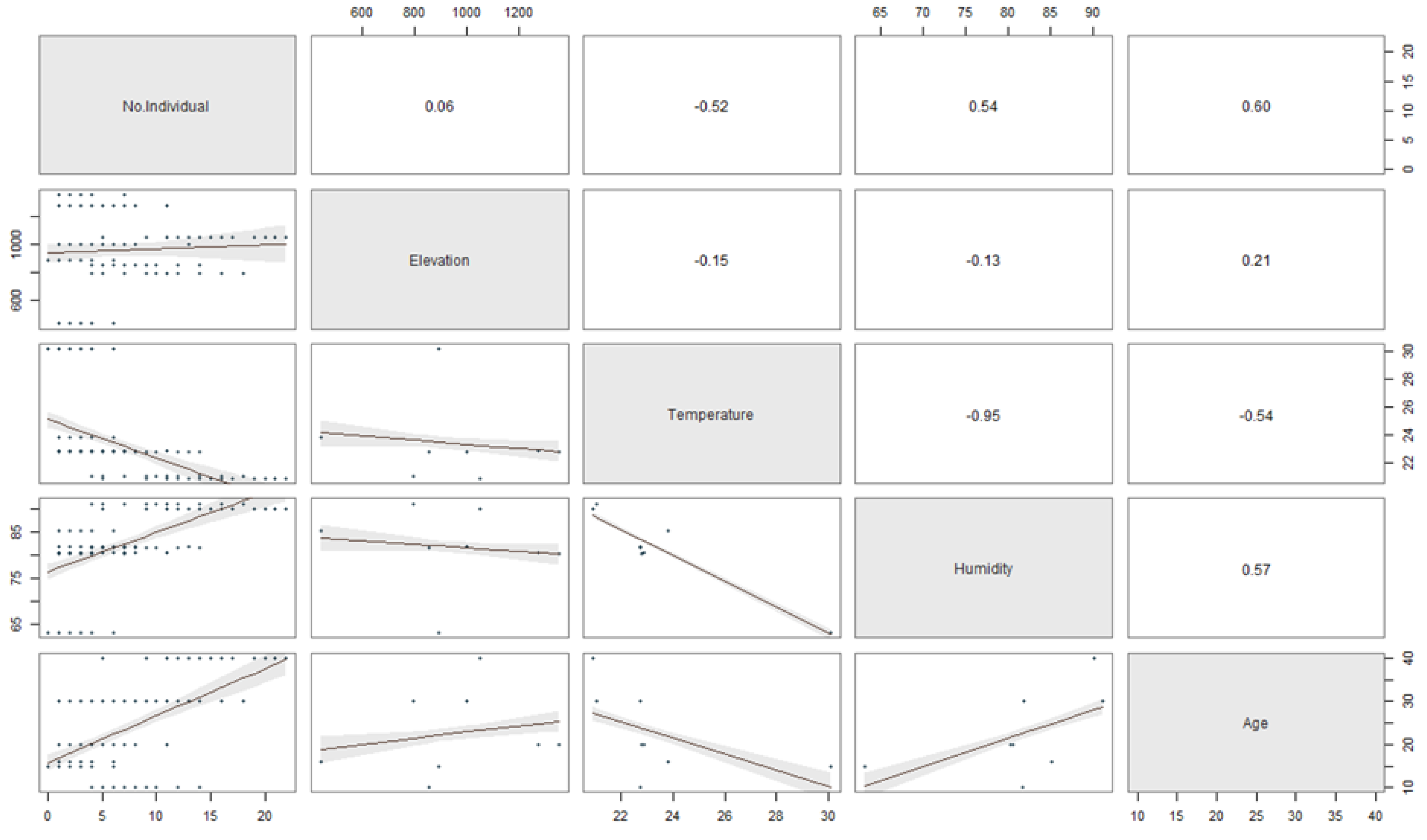
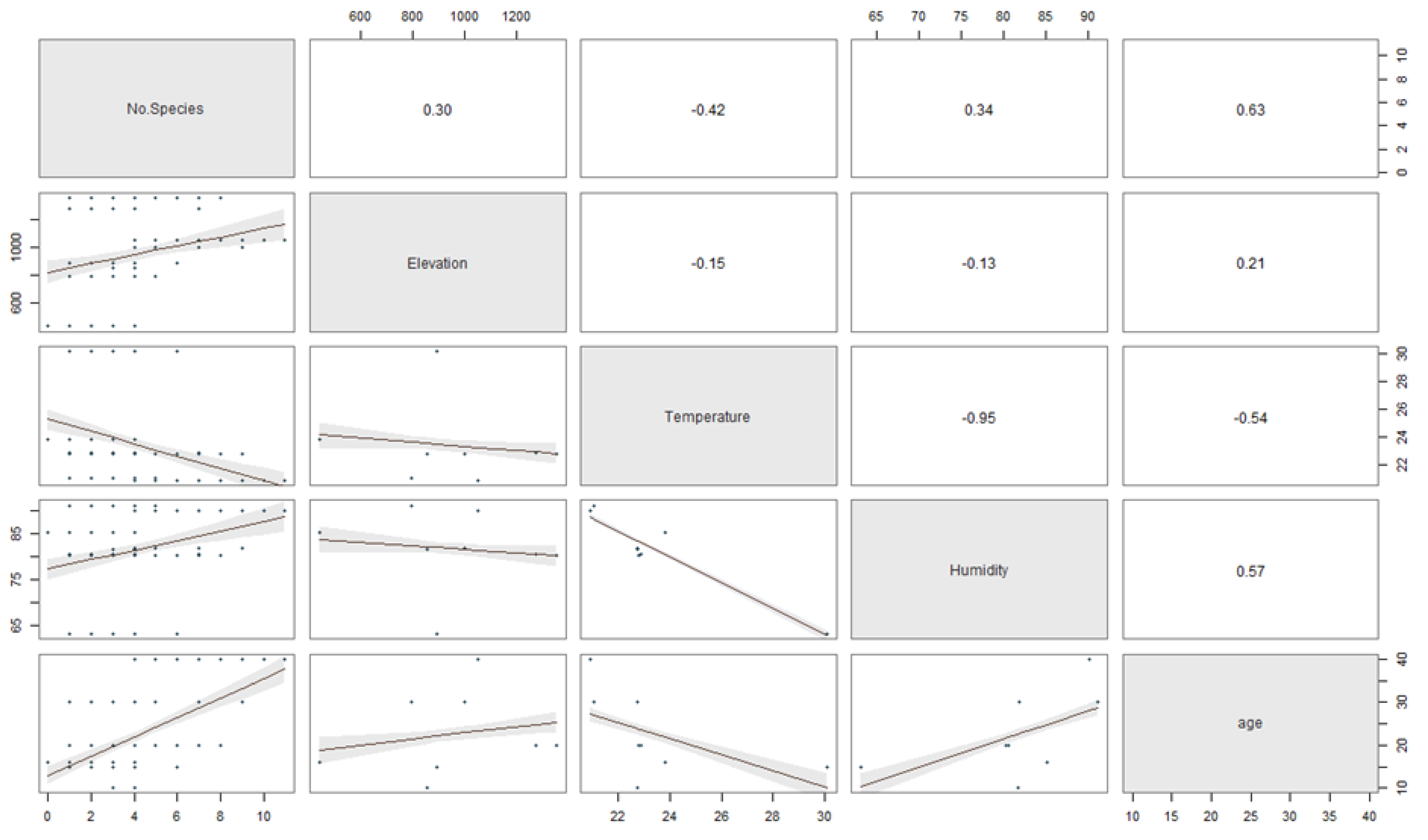
The relationship between the stand-age, humidity, elevation, and temperature to the number of ambrosia beetle individuals and species was described in Figure 5 and 6. The regression coefficients between the stand-age, humidity, elevation, temperature and the number of ambrosia beetle individuals were 0.60, 0.54, 0.06, and −0.52 (Figure 5). The regression coefficient between the stand-age, humidity, elevation, temperature, and the number of ambrosia beetle species were 0.63, 0.34, 0.30, and −0.42 (Figure 6).
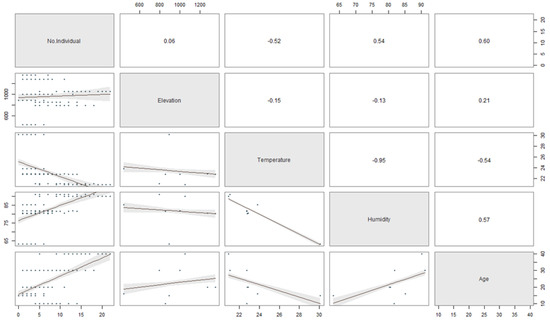
Figure 5.
The scatter plot matrix describes the multiple regression between the stand-age, elevation, temperature, and humidity to the number of ambrosia beetle individuals.
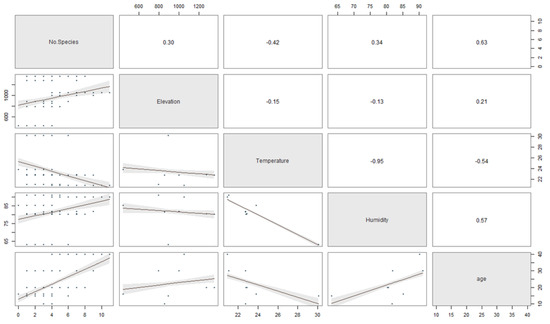
Figure 6.
The scatter plot matrix describes the multiple regression between the stand-age, elevation, temperature, and humidity to the number of ambrosia beetle species.
4. Discussion
Effect of the pine forest types on the abundance of ambrosia beetle were clear in this research. The protected pine forest had a higher abundance and species richness of ambrosia beetles than the other forest types. This result can be attributed to the stability of protected forests over time. Undisturbed ecosystems with a long-term lack of management had an increased abundance of native and exotic ambrosia beetles, most likely because of increased deadwood [39]. Primary forests are generally considered to support high species richness [40], because there are some dead trees in the primary forests that can be used to support the growth and development of ambrosia beetles. We also found that the pine forest-based agroforestry had the lowest species richness. An increase in land use disturbance decreases insect diversity and completely changes species composition [29].
In this study, X. andrewesi and X. crassiusculus were the most abundant ambrosia beetles detected in East Java, Indonesia, similar to reports in other countries [41]. Prior to this study, X. andrewesi has been recorded in Indonesia with no host specification report [42]. Previously, X. andrewesi has been associated with T. grandis (teak) and Paraserianthes falcataria (L.) (albizia) [24,43]. Our results confirmed that this species has an established population in Indonesia, especially in East Java. Xyleborinus andrewesi is not host-specific and has been recorded in 59 hosts across 29 families worldwide [42]. Xyleborinus andrewesi was also introduced and established in the USA [44]. Xyleborinus andrewesi was reported associated with plant pathogenic fungus. These species caused substantial damage to their host plant and maintained symbiotic plant pathogenic fungi, Raffaelea sp. [45].
On the other hand, X. crassiusculus is the only species documented in all pine forest types. Related to this result, X. crassiusculus distribution in temperate forests is strongly affected by climatic variables but not forest types [17]. Xylosandrus crassiusculus is polyphagous species that colonized on most warm and humid regions [46]. This species, most likely native to Asia’s tropical and subtropical regions, has become one of the most widespread ambrosia beetles [47]. Xyleborus affinis was the third most abundant species. This species is also widespread and common among ambrosia beetles globally, but confirmed records of X. affinis attacking healthy trees are rare [48]. In this study, the species of Scolytinae were more abundant than those from the Platypodinae subfamily. Furthermore, in Southern Brazil, Xyleborini species were more common than species in other tribes in the pine stand [12,49], which agrees with our study.
Xylosandrus discolor was only found in the protected pine forest; this species has been recorded in Indonesia and is native to Java [50]. In this study, protected forests have various types of shrubs which may be the host of this species. Xylosandrus discolor has been recorded in several other host tress [51]. This species is a secondary pest but sometimes attacks living twigs and small branches of crop and plantation trees [52]. We also collected Scolytoplatypus sp. only in managed pine forests. Gebhardt et al. [53] reported that Scolytoplatypus sp. was collected in many locations in different forest types and at different altitudes in Yunnan but did not mention the forest type or management. Kim et al. [54] also reported some species of Scolytoplatypus sp. (i.e., Scolytoplatypus tycon Blandford, 1893) collected in pine forests with different management in Korea. Moreover, Scolytoplatypus has been reported in diverse climatic zones from Sakhalin-island, Russia, to the tropical rainforests of Indonesia, with a few species found in temperate areas. Scolytoplatypus is represented mainly by oligophagous or polyphagous species, and its host plants are unknown [55]. Generally, the species are of little or no economic importance, and they do not infest healthy trees [51]. Scolytoplatypus also carries fungi, enabling its reliable transmission from one to the next generation [56].
In this study, we collected only two species and a few individuals of Platypodinae (i.e., E. parallelus and T. micrurus), where E. parallelus was found in the protected pine forests and the non-tapped pine forests. Li et al. [57], collected E. parallelus at 10 locations on the Hainan island, including natural old-growth forests, planted forests, and most rubber tree plantations. Interestingly, E. parallelus recently spread in East Java and attacked Pterocarpus indicus Willd., 1802 (Fabaceae) [15,58,59]. It was the dominant species attacking P. indicus in the cities of Malang and Batu, East Java [15]. The species was first collected in forest ecosystems, especially in East Java pine forests, not only in urban areas. It also caused economic damage by attacking large diameter stems and living trees. It attacks more than 80 species of conifers and broadleaf trees and bores deep galleries into the wood, inoculating fungal symbionts inside [60]. Euplatypus parallelus and X. affinis were responsible for most of the damage caused on 18 tree species of timber in Amazonia, Brazil [61].
Treptoplatypus micrurus was also only found in the tapped-pine forest in this study. Treptoplatypus micrurus was reported as pest of P. indicus trees in Batu City since 2015 [59]. Our results indicate that this species has also attacked on several host trees in Batu City, specifically in the tapped-pine forest. Moreover, in Indonesia, this species has never been found in other host trees and has only been reported to attack P. indicus in East Java.
The higher coefficient of the Jaccard distance index described that the dissimilarity between the pine forest-based agroforestry site 1 (PA1) and the non-tapped pine forest site 1 (NP1) was higher than others. Dissimilarity is shown by the abundance, and the overlap number of species of ambrosia beetle. The PA1 provided couple time higher than NP1 based on the ambrosia beetle abundance, and few overlaps number of species. The vegetation composition under pine forest is one of the contributed factors to provide the higher dissimilarity between the PA1 and NP1. Within landscapes, system of agroforestry increases functional and overall biodiversity [62]. In agroforestry system, farmer tends to increase plant diversity and soil quality that can promote the biodiversity [63]. The high coefficient of the Jaccard distance index between the protected pine forest site 1 (PF1) and the tapped pine forest site 2 (TP2) was also caused by the abundance, and the number of species of ambrosia beetle. The high diversity of vegetation in the protected pine forest increases functional biodiversity [62], the provision of ecosystem services [64], and stimulates tree resistance to insect pest damage [65].
In this research, we confirmed that the stand-age, humidity, and temperature related to the number of individuals and species of ambrosia beetles. In case of the elevation, regression coefficient was 0.06 to the number of individuals but it was higher to the number of species. The beetle diversity described the multiple alpha and beta patterns according to elevation and mountain ecosystem type [66]. There was relationship between the proportion of infested trees and average temperature [67]. These accounts are considerably higher than the maximum altitudes described by others in Western Europe [68]. Insects reach their highest diversity in old-growth forests because of their stable moderate temperature and relative humidity and the rich variety of resources represented by high plant species richness and structural complexity [69].
5. Conclusions
The protected pine forests were determined as a pine forest type that provided the highest number of ambrosia beetle species, and X. discolor (Blandford) and Scolytoplatypus sp. were found only in PFs. From 15 species of the collected ambrosia beetles, five species were found in all pine forest types i.e., X. crassiusculus, X. morigerus, Xyleborus affinis, X. andrewesi, and Cryphalus sp. The most abundant species was X. andrewesi, representing 24.52% of all beetles collected in the study. The abundance of ambrosia beetles was significantly different between pine forest types, where the highest abundances was in the protected pine forest. Based on the pine forest types, the highest number of ambrosia beetle species was in the protected pine forest (13 species). The ambrosia beetle species for each forest site was the highest in the PF2 (about 11 species). The distances between the pine forest sites described that the PA1 provide higher coefficients (about 0.449) when paired with NP1. The regression coefficients between the stand-age, humidity, elevation, temperature and the number of ambrosia beetle individuals were 0.60, 0.54, 0.06, and −0.52. In case of the number of ambrosia beetle species that paired to the stand-age, humidity, elevation, and temperature, resulted 0.63, 0.34, 0.30, and −0.42 of regression coefficient respectively.
Our study is preliminary research regarding the abundance and the species composition of the ambrosia beetle associated with the pine forest types. The stand-age, temperature, and humidity indicated to effect on the abundance and species composition of the ambrosia beetles. The elevation indicates to influence the number of ambrosia beetle individuals but it isn’t happened on the number of ambrosia beetle species. This study contributes to identification of the suitable pine forest types related to the ambrosia beetle management.
Author Contributions
Conceptualization, H.T. and Y.S.; methodology, H.T., Y.S. and J.W.; software, Y.S. and H.T.; validation, H.T. and J.W.; formal analysis, H.T. and Y.S.; investigation, R.A.A.P., A.N., F.G.T. and J.A.; resources, H.T.; data curation, Y.S., H.T. and J.W.; writing—original draft preparation, Y.S. and H.T.; writing—review and editing, H.T. and J.W.; visualization, Y.S.; supervision, H.T. and J.W.; funding acquisition, H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Acknowledgments
We thank the Department of Plant Pests and Diseases, Faculty of Agriculture, University of Brawijaya for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Richardson, D.M.; Rundel, P.W.; Jackson, S.T.; Teskey, R.O.; Aronson, J.; Bytnerowicz, A.; Wingfield, M.J.; Procheş, Ş. Human Impacts in Pine Forests: Past, Present, and Future. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 275–297. [Google Scholar] [CrossRef]
- Imanuddin, R.; Hidayat, A.; Rachmat, H.H.; Turjaman, M.; Pratiwi; Nurfatriani, F.; Indrajaya, Y.; Susilowati, A. Reforestation and Sustainable Management of Pinus merkusii Forest Plantation in Indonesia: A Review. Forests 2020, 11, 1235. [Google Scholar] [CrossRef]
- Hadiyane, A.; Sulistyawati, E.; Asharina, W.P.; Dungani, R. A Study on Production of Resin from Pinus merkusii Jungh. Et De Vriese in the Bosscha Observatory Area, West Java-Indonesia. Asian J. Plant Sci. 2015, 14, 89–93. [Google Scholar] [CrossRef]
- Suryatmojo, H. Rainfall-Runoff Investigation of Pine Forest Plantation in the Upstream Area of Gajah Mungkur Reservoir. Procedia Environ. Sci. 2015, 28, 307–314. [Google Scholar] [CrossRef]
- Kirkendall, L.R.; Biedermann, P.H.W.; Jordal, B.H. Evolution and Diversity of Bark and Ambrosia Beetles; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Six, D.L. Ecological and Evolutionary Determinants of Bark Beetle—Fungus Symbioses. Insects 2012, 3, 339–366. [Google Scholar] [CrossRef]
- Morris, J.L.; Cottrell, S.; Fettig, C.J.; Hansen, W.D.; Sherriff, R.L.; Carter, V.A.; Clear, J.L.; Clement, J.; DeRose, R.J.; Hicke, J.A.; et al. Managing Bark Beetle Impacts on Ecosystems and Society: Priority Questions to Motivate Future Research. J. Appl. Ecol. 2017, 54, 750–760. [Google Scholar] [CrossRef]
- Ranger, C.M.; Schultz, P.B.; Frank, S.D.; Chong, J.H.; Reding, M.E. Non-Native Ambrosia Beetles as Opportunistic Exploiters of Living but Weakened Trees. PLoS ONE 2015, 10, e0131496. [Google Scholar] [CrossRef]
- Hulcr, J.; Dunn, R.R. The Sudden Emergence of Pathogenicity in Insect-Fungus Symbioses Threatens Naive Forest Ecosystems. Proc. R. Soc. B Biol. Sci. 2011, 278, 2866–2873. [Google Scholar] [CrossRef]
- Aukema, J.E.; Leung, B.; Kovacs, K.; Chivers, C.; Britton, K.O.; Englin, J.; Frankel, S.J.; Haight, R.G.; Holmes, T.P.; Liebhold, A.M.; et al. Economic Impacts of Non-Native Forest Insects in the Continental United States. PLoS ONE 2011, 6, e24587. [Google Scholar] [CrossRef]
- Grousset, F.; Grégoire, J.C.; Jactel, H.; Battisti, A.; Beloglavec, A.B.; Hrašovec, B.; Hulcr, J.; Inward, D.; Orlinski, A.; Petter, F. The Risk of Bark and Ambrosia Beetles Associated with Imported Non-Coniferous Wood and Potential Horizontal Phytosanitary Measures. Forests 2020, 11, 342. [Google Scholar] [CrossRef]
- Flechtmann, C.A.H.; Ottati, A.L.T.; Berisford, C.W. Ambrosia and Bark Beetles (Scolytidae: Coleoptera) in Pine and Eucalypt Stands in Southern Brazil. For. Ecol. Manag. 2001, 142, 183–191. [Google Scholar] [CrossRef]
- Gómez, D.; Martínez, G. Bark Beetles in Pine Tree Plantations in Uruguay: First Record of Orthotomicus erosus Wollaston (Coleoptera: Curculionidae: Scolytinae). Coleopt. Bull. 2013, 67, 470. [Google Scholar] [CrossRef]
- Gómez, D.; Hirigoyen, A.; Balmelli, G.; Viera, C.; Martínez, G. Estacionalidad de Vuelo de Escarabajos de Corteza (Coleoptera: Scolytinae) En Plantaciones Comerciales de Pino En Uruguay. Bosque 2017, 38, 47–53. [Google Scholar] [CrossRef]
- Tarno, H.; Setiawan, Y.; Tri Rahardjo, B.; Wang, J. Evaluation of the Ambrosia Beetles Traps on Pterocarpus indicus in Indonesia. Biodiversitas 2021, 22, 1332–1339. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.E.; Schultz, P.B.; Oliver, J.B.; Frank, S.D.; Addesso, K.M.; Chong, J.H.; Sampson, B.; Werle, C.; Gill, S.; et al. Biology, Ecology, and Management of Nonnative Ambrosia Beetles (Coleoptera: Curculionidae: Scolytinae) in Ornamental Plant Nurseries. J. Integr. Pest Manag. 2016, 7, 1. [Google Scholar] [CrossRef]
- Rassati, D.; Faccoli, M.; Battisti, A.; Marini, L. Habitat and Climatic Preferences Drive Invasions of Non-Native Ambrosia Beetles in Deciduous Temperate Forests. Biol. Invasions 2016, 18, 2809–2821. [Google Scholar] [CrossRef]
- Hulcr, J.; Mogia, M.; Isua, B.; Novotny, V. Host Specificity of Ambrosia and Bark Beetles (Col., Curculionidae: Scolytinae and Platypodinae) in a New Guinea Rainforest. Ecol. Entomol. 2007, 32, 762–772. [Google Scholar] [CrossRef]
- Bonello, P.; Campbell, F.T.; Cipollini, D.; Conrad, A.O.; Farinas, C.; Gandhi, K.J.K.; Hain, F.P.; Parry, D.; Showalter, D.N.; Villari, C.; et al. Invasive Tree Pests Devastate Ecosystems—A Proposed New Response Framework. Front. For. Glob. Chang. 2020, 3, 2. [Google Scholar] [CrossRef]
- Carnus, J.; Parrotta, J.; Brockerhoff, E.; Arbez, M.; Jactel, H.; Kremer, A.; Lamb, D.; O’Hara, K.; Walters, B. Planted Forests and Biodiversity. J. For. 2006, 104, 65–77. [Google Scholar]
- Poland, T.M.; Rassati, D. Improved Biosecurity Surveillance of Non-Native Forest Insects: A Review of Current Methods. J. Pest Sci. 2019, 92, 37–49. [Google Scholar] [CrossRef]
- Steininger, M.S.; Hulcr, J.; Šigut, M.; Lucky, A. Simple and Efficient Trap for Bark and Ambrosia Beetles (Coleoptera: Curculionidae) to Facilitate Invasive Species Monitoring and Citizen Involvement. J. Econ. Entomol. 2015, 108, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Hauptman, T.; Pavlin, R.; Groselj, P.; Jurc, M. Distribution and Abundance of the Alien Xylosandrus germanus and Other Ambrosia Beetles (Coleoptera: Curculionidae, Scolytinae) in Different Forest Stands in Central Slovenia. iForest-Biogeosci. For. 2019, 12, 451–458. [Google Scholar] [CrossRef]
- Tarno, H.; Setiawan, Y.; Kusuma, C.B.; Fitriyah, M.; Hudan, A.N.; Yawandika, A.P.; Nasution, H.A.; Saragih, R.; Bagasta, A.P.Y.; Wang, Z.; et al. Diversity and Species Composition of Bark and Ambrosia Beetles Captured Using Ethanol Baited Traps on Different Hosts in East Java, Indonesia. Zool. Stud. 2021, 60, e55. [Google Scholar] [CrossRef] [PubMed]
- Galko, J.; Nikolov, C.; Kimoto, T.; Kunca, A.; Gubka, A.; Vakula, J.; Zúbrik, M.; Ostrihoň, M. Attraction of Ambrosia Beetles to Ethanol Baited Traps in a Slovakian Oak Forest. Biologia 2014, 69, 1376–1383. [Google Scholar] [CrossRef]
- Gugliuzzo, A.; Biedermann, P.H.W.; Carrillo, D.; Castrillo, L.A.; Egonyu, J.P.; Gallego, D.; Haddi, K.; Hulcr, J.; Jactel, H.; Kajimura, H.; et al. Recent Advances toward the Sustainable Management of Invasive Xylosandrus Ambrosia Beetles. J. Pest Sci. 2021, 94, 615–637. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.E.; Addesso, K.; Ginzel, M.; Rassati, D. Semiochemical-Mediated Host Selection by Xylosandrus spp. Ambrosia Beetles (Coleoptera: Curculionidae) Attacking Horticultural Tree Crops: A Review of Basic and Applied Science. Can. Entomol. 2021, 153, 103–120. [Google Scholar] [CrossRef]
- Lee, C.M.; Kwon, T.-S.; Park, Y.K.; Kim, S.-S.; Sung, J.H.; Lee, Y.G. Diversity of Beetles in Gariwangsan Mountain, South Korea: Influence of Forest Management and Sampling Efficiency of Collecting Method. J. Asia-Pac. Biodivers. 2014, 7, 319–346. [Google Scholar] [CrossRef]
- Perry, J.; Lojka, B.; Quinones Ruiz, L.G.; Van Damme, P.; Houška, J.; Fernandez Cusimamani, E. How Natural Forest Conversion Affects Insect Biodiversity in the Peruvian Amazon: Can Agroforestry Help? Forests 2016, 7, 82. [Google Scholar] [CrossRef]
- Gossner, M.M.; Falck, K.; Weisser, W.W. Effects of Management on Ambrosia Beetles and Their Antagonists in European Beech Forests. For. Ecol. Manag. 2019, 437, 126–133. [Google Scholar] [CrossRef]
- Marchioro, M.; Rassati, D.; Faccoli, M.; Van Rooyen, K.; Kostanowicz, C.; Webster, V.; Mayo, P.; Sweeney, J. Maximizing Bark and Ambrosia Beetle (Coleoptera: Curculionidae) Catches in Trapping Surveys for Longhorn and Jewel Beetles. J. Econ. Entomol. 2020, 113, 2745–2757. [Google Scholar] [CrossRef]
- Gorzlancyk, A.M.; Held, D.W.; Ranger, C.M.; Barwary, Z.; Kim, D.J. Capture of Cnestus mutilatus, Xylosandrus crassiusculus, and Other Scolytinae (Coleoptera: Curculionidae) in Response to Green Light Emitting Diodes, Ethanol, and Conophthorin. Fla. Entomol. 2014, 97, 301–303. [Google Scholar] [CrossRef]
- Reding, M.; Oliver, J.; Schultz, P.; Ranger, C. Monitoring Flight Activity of Ambrosia Beetles in Ornamental Nurseries with Ethanol-Baited Traps: Influence of Trap Height on Captures. J. Environ. Hortic. 2010, 28, 85–90. [Google Scholar] [CrossRef]
- Smith, S.M.; Beaver, R.A.; Cognato, A.I.; Hulcr, J.; Redford, A.J. Southeast Asian Ambrosia Beetle ID. USDA. Available online: http://idtools.org/id/wbb/sea-ambrosia/about_index.php (accessed on 5 March 2022).
- Rabaglia, R.J.; Dole, S.A.; Cognato, A.I. Review of American Xyleborina (Coleoptera: Curculionidae: Scolytinae) Occurring North of Mexico, with an Illustrated Key. Ann. Entomol. Soc. Am. 2006, 99, 1034–1056. [Google Scholar] [CrossRef]
- Mandelsham, M.Y.S.L. Wood, Bark and Ambrosia Beetles of South America (Coleoptera: Scolytidae) (Monte L. Bean Life Science Museum, Brigham Young University, Provo, 2007), 900 P. Entomol. Rev. 2009, 89, 245–246. [Google Scholar] [CrossRef]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T.J. Abundance-Based Similarity Indices and Their Estimation When There Are Unseen Species in Samples. Biometrics 2006, 62, 361–371. [Google Scholar] [CrossRef]
- R Core Development Team. R: A Language and Environment for Statistical Computing. 2019, Volume 2. Available online: https://www.R-project.org/ (accessed on 10 January 2022).
- Reed, S.; Muzika, R.-M. The Influence of Forest Stand and Site Characteristics on the Composition of Exotic Dominated Ambrosia Beetle Communities (Coleoptera: Curculionidae: Scolytinae). Environ. Entomol. 2010, 39, 1482–1491. [Google Scholar] [CrossRef]
- Mackey, B.; DellaSala, D.A.; Kormos, C.; Lindenmayer, D.; Kumpel, N.; Zimmerman, B.; Hugh, S.; Young, V.; Foley, S.; Arsenis, K.; et al. Policy Options for the World’s Primary Forests in Multilateral Environmental Agreements. Conserv. Lett. 2015, 8, 139–147. [Google Scholar] [CrossRef]
- Roy, K.; Jaenecke, K.A.; Peck, R.W. Ambrosia Beetle (Coleoptera: Curculionidae) Communities and Frass Production in ʻŌhiʻa (Myrtales: Myrtaceae) Infected With Ceratocystis (Microascales: Ceratocystidaceae) Fungi Responsible for Rapid ʻŌhiʻa Death. Environ. Entomol. 2020, 49, 1345–1354. [Google Scholar] [CrossRef]
- Okins, K.; Services, C.; Thomas, M.C.; Services, C. New North American Record for Xyleborinus andrewesi (Coleoptera: Curculionidae: Scolytinae). Fla. Entomol. 2010, 93, 133–134. [Google Scholar] [CrossRef]
- Setiawan, Y.; Rachmawati, R.; Tarno, H. Diversity of Ambrosia Beetles (Coleoptera: Scolytidae) on Teak Forest in Malang District, East Java, Indonesia. Biodiversitas 2018, 19, 1791–1797. [Google Scholar] [CrossRef]
- Gomez, D.F.; Rabaglia, R.J.; Fairbanks, K.E.O.; Hulcr, J. North American Xyleborini North of Mexico: A Review and Key to Genera and Species (Coleoptera, Curculionidae, Scolytinae). Zookeys 2018, 2018, 19–68. [Google Scholar] [CrossRef] [PubMed]
- Bateman, C.; Kendra, P.E.; Rabaglia, R.; Hulcr, J. Fungal Symbionts in Three Exotic Ambrosia Beetles, Xylosandrus amputatus, Xyleborinus andrewesi, and Dryoxylon onoharaense (Coleoptera: Curculionidae: Scolytinae: Xyleborini) in Florida. Symbiosis 2015, 66, 141–148. [Google Scholar] [CrossRef]
- Kirkendall, L.; Faccoli, M. Bark Beetles and Pinhole Borers (Curculionidae, Scolytinae, Platypodinae) Alien to Europe Bark Beetles and Pinhole Borers (Curculionidae, Scolytinae, Platypodinae) Alien to Europe. Zookeys 2010, 56, 227–251. [Google Scholar] [CrossRef] [PubMed]
- Urvois, T.; Auger-Rozenberg, M.A.; Roques, A.; Rossi, J.P.; Kerdelhue, C. Climate Change Impact on the Potential Geographical Distribution of Two Invading Xylosandrus Ambrosia Beetles. Sci. Rep. 2021, 11, 1339. [Google Scholar] [CrossRef]
- Sobel, L.; Lucky, A.; Hulcr, J. An Ambrosia Beetle Xyleborus affinis Eichhoff (1868) (Insecta: Coleoptera: Curculionidae: Scolytinae). IFAS Extention 2021, 1868, 1–4. Available online: https://edis.ifas.ufl.edu/pdf/IN/IN1094/IN1094-8420079.pdf (accessed on 5 March 2022).
- Wood, S.L. Bark and Ambrosia Beetles of South America; Bean Life Science Museum: Provo, UT, USA, 2007. [Google Scholar]
- Wood, S.L.; Bright, D.E. A Catalog of Scolytidae and Platypodidae (Coleoptera), Part 2: Taxonomic Index. Volume B. Gt. Basin Nat. Mem. 1992, 13, 835–1557. [Google Scholar]
- Browne, F.B. The Biology of Malayan Scolytidae and Platypodidae. Malay. For. Rec. 1961, 22, 1–225. [Google Scholar]
- Browne, F.G. Pests and Diseases of Forest Plantation Trees: An Annotated List of the Principal Species Occurring in the British Commonwealth; Clarendon Press: Oxford, UK; Oxford University Press: Oxford, UK, 1968. [Google Scholar]
- Gebhardt, H.; Beaver, R.A.; Allgaier, C. Three New Species of Scolytoplatypus Schaufuss from China, and Notes on the Movement and Functions of the Prosternal Processes (Coleoptera: Curculionidae: Scolytinae). Zootaxa 2021, 5082, 485–493. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, S.W.; Roh, S.J.; Jeon, J.H.; Yoo, T.H.; Yoon, H.K.; Kim, H.S.; Byun, B.K. Responses of Bark Beetle (Coleoptera: Curculionidae: Scolytinae) Community Structure to Green-Tree Retention in Pine Tree Forest from Korea. J. Asia-Pac. Biodivers. 2016, 9, 443–447. [Google Scholar] [CrossRef]
- Beaver, R.A.; Gebhardt, H. A Review of the Oriental Species of Scolytoplatypus Schaufuss (Coleoptera, Curculionidae, Scolytinae). Dtsch. Entomol. Z. 2006, 53, 155–178. [Google Scholar] [CrossRef]
- Mayers, C.G.; Harrington, T.C.; Masuya, H.; Jordal, B.H.; McNew, D.L.; Shih, H.H.; Roets, F.; Kietzka, G.J. Patterns of Coevolution between Ambrosia Beetle Mycangia and the Ceratocystidaceae, with Five New Fungal Genera and Seven New Species. Pers. Mol. Phylogeny Evol. Fungi 2020, 44, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, X.; Lai, S.; Yin, T.; Ji, Y.; Wang, S.; Wang, J.; Hulcr, J. First Record of Euplatypus parallelus (Coleoptera: Curculionidae) in China. Fla. Entomol. 2018, 101, 141–143. [Google Scholar] [CrossRef]
- Tarno, H.; Suprapto, H.; Himawan, T. First Record of Ambrosia Beetle (Euplatypus paralellus Fabricius) Infestation on Sonokembang (Pterocarpus indicus Willd.) from Malang Indonesia. Agrivita 2014, 36, 189–200. [Google Scholar] [CrossRef]
- Tarno, H.; Suprapto, H.; Himawan, T. New Record of the Ambrosia Beetle, Treptoplatypus micrurus Schedl. Attack on Sonokembang (Pterocarpus indicus Willd.) in Batu, Indonesia. Agrivita 2015, 37, 220–225. [Google Scholar] [CrossRef]
- Beaver, R. The Invasive Neotropical Ambrosia Beetle Euplatypus parallelus (Fabricus, 1801) in the Oriental Region and Its Pest Status (Coleoptera: Curculionidae, Platypodinae). Entomol. Mon. Mag. 2013, 149, 143–154. [Google Scholar]
- EPPO. EPPO Technical Document No. 1081, EPPO Study on the Risk of Bark and Ambrosia Beetles Associated with Imported Non-Coniferous Wood. EPPO Publ. 2020, 1081, 1–218. Available online: https://www.eppo.int/media/uploaded_images/RESOURCES/eppo_publications/TD-1081_EPPO_Study_bark_ambrosia.pdf (accessed on 3 March 2022).
- Santos, M.; Cajaiba, R.L.; Bastos, R.; Gonzalez, D.; Petrescu Bakış, A.L.; Ferreira, D.; Leote, P.; Barreto da Silva, W.; Cabral, J.A.; Gonçalves, B.; et al. Why Do Agroforestry Systems Enhance Biodiversity? Evidence From Habitat Amount Hypothesis Predictions. Front. Ecol. Evol. 2022, 9, 630151. [Google Scholar] [CrossRef]
- Sistla, S.A.; Roddy, A.B.; Williams, N.E.; Kramer, D.B.; Stevens, K.; Allison, S.D. Agroforestry Practices Promote Biodiversity and Natural Resource Diversity in Atlantic Nicaragua. PLoS ONE 2016, 11, e0162529. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Barbaro, L.; Castagneyrol, B.; Forrester, D.I.; Gardiner, B.; González-Olabarria, J.R.; Lyver, P.O.B.; Meurisse, N.; Oxbrough, A.; Taki, H.; et al. Forest Biodiversity, Ecosystem Functioning and the Provision of Ecosystem Services. Biodivers. Conserv. 2017, 26, 3005–3035. [Google Scholar] [CrossRef]
- Jactel, H.; Moreira, X.; Castagneyrol, B. Tree Diversity and Forest Resistance to Insect Pests: Patterns, Mechanisms, and Prospects. Annu. Rev. Entomol. 2021, 66, 277–296. [Google Scholar] [CrossRef]
- Musthafa, M.M.; Abdullah, F.; Martínez-Falcón, A.P.; de Bruyn, M. How Mountains and Elevations Shape the Spatial Distribution of Beetles in Peninsular Malaysia. Sci. Rep. 2021, 11, 5791. [Google Scholar] [CrossRef] [PubMed]
- Bellahirech, A.; Branco, M.; Catry, F.X.; Bonifácio, L.; Sousa, E.; Ben Jamâa, M.L. Site- and Tree-Related Factors Affecting Colonization of Cork Oaks Quercus suber L. by Ambrosia Beetles in Tunisia. Ann. For. Sci. 2019, 76, 45. [Google Scholar] [CrossRef]
- Galko, J.; Dzurenko, M.; Ranger, C.M.; Kulfan, J.; Kula, E.; Nikolov, C.; Zúbrik, M.; Zach, P. Distribution, Habitat Preference, and Management of the Invasive Ambrosia Beetle Xylosandrus germanus (Coleoptera: Curculionidae, Scolytinae) in European Forests with an Emphasis on the West Carpathians. Forests 2019, 10, 10. [Google Scholar] [CrossRef]
- Schowalter, T. Arthropod Diversity and Functional Importance in Old-Growth Forests of North America. Forests 2017, 8, 97. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).