Diversity Loss of Epigeic Collembola after Grassland Conversion into Eucalyptus Forestry in Brazilian Pampa Domain
Abstract
:1. Introduction
- (i)
- How is the epigeic Collembola diversity affected after changes in land-use/land-cover (LULC) from native grassland to Eucalyptus plantation?
- (ii)
- Is the species composition of epigeic Collembola communities in the Eucalyptus plantation a subgroup of the community present in the original native vegetation? Or is it originated from species replacement?
- (iii)
- What were the most important environmental factors affecting the structure and composition of the epigeic Collembola community?
2. Materials and Methods
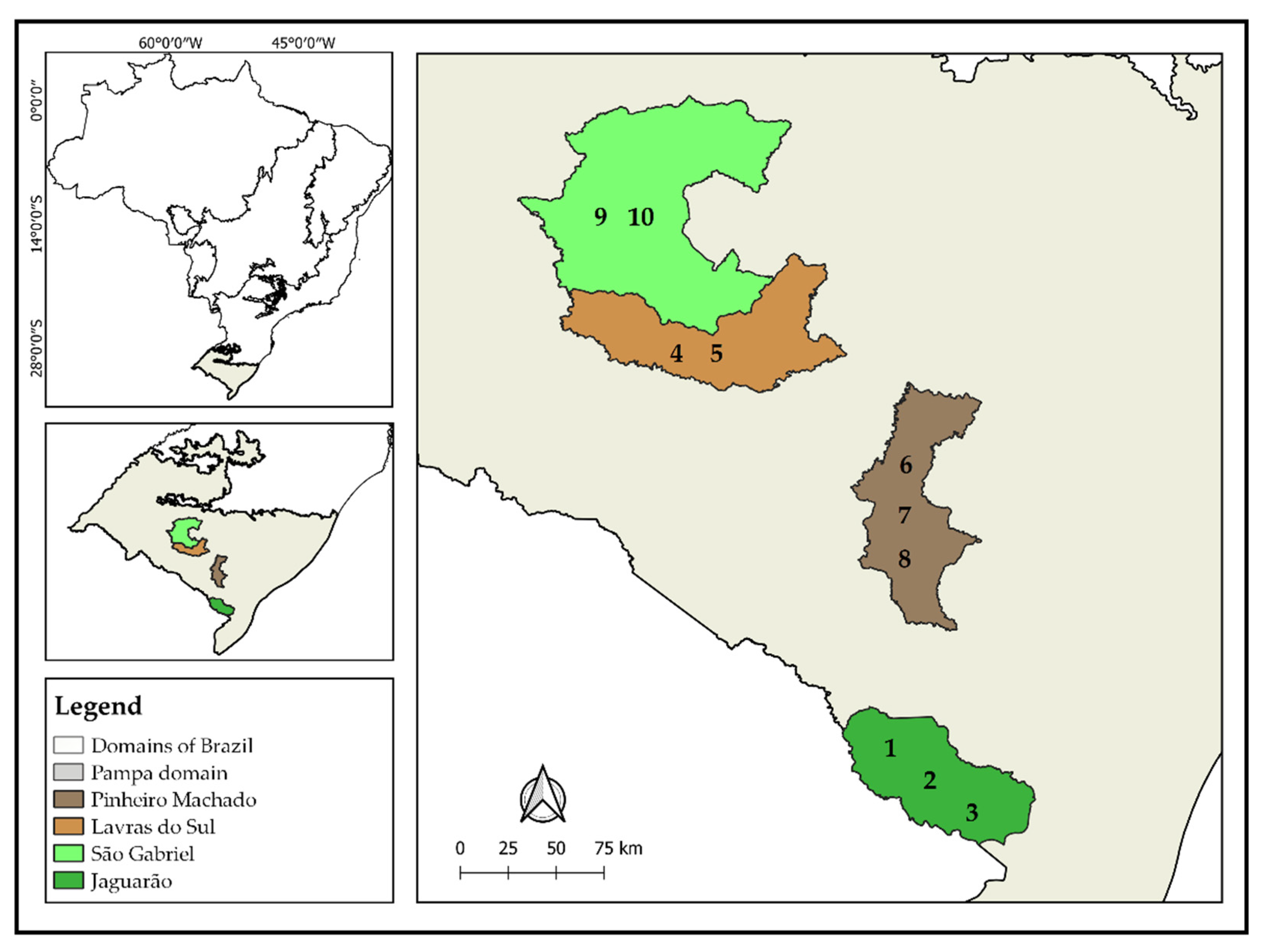
2.1. Sites Description and Sampling Design
2.2. Collembola Sampling and Taxonomic Identification
2.3. Ecological Data and Statistical Analysis
2.3.1. Species Composition
2.3.2. Beta Diversity
2.3.3. Alpha Diversity
2.3.4. Effects of the Environmental Factors on the Epigeic Collembola Community
3. Results
3.1. Epigeic Collembola Community in the Two Land-Use Types
3.2. Effects after Grassland Conversion into Forestry
4. Discussion
4.1. Taxa Occurrence in the Brazilian Pampa
4.2. Effects of LULC Changes on Epigeic Collembola Community
4.3. Conservation Perspectives of Soil Fauna in the Brazilian Pampa
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Site | Par | Lulc | Parcel | Geographic Coordinates | |
|---|---|---|---|---|---|
| Longitude | Latitude | ||||
| JAGUARÃO (JAG) | 1 | Gras | GE_JAG_Gras_1 | −53,397,067 | −32,239,946 |
| Euc | GE_JAG_Euc_1 | −53,365,464 | −32,239,994 | ||
| 2 | Gras | GE_JAG_Gras_2 | −53,319,039 | −32,210,709 | |
| Euc | GE_JAG_Euc_2 | −53,349,856 | −32,240,435 | ||
| 3 | Gras | GE_JAG_Gras_3 | −53,304,145 | −32,227,443 | |
| Euc | GE_JAG_Euc_3 | −53,301,572 | −32,245,974 | ||
| LAVRAS DO SUL (LAV) | 4 | Gras | GE_LAV_Gras_2 | −54,263,648 | −30,885,178 |
| Euc | GE_LAV_Euc_2 | −5,431,076 | −30,860,155 | ||
| 5 | Gras | GE_LAV_Gras_3 | −54,267,129 | −30,962,529 | |
| Euc | GE_LAV_Euc_3 | −54,252,869 | −309,676 | ||
| PINHEIRO MACHADO (PIM) | 6 | Gras | GE_PIM_Gras_1 | −53,602,698 | −31,339,271 |
| Euc | GE_PIM_Euc_1 | −5,359,796 | −31,343,839 | ||
| 7 | Gras | GE_PIM_Gras_2 | −53,577,106 | −31,385,678 | |
| Euc | GE_PIM_Euc_2 | −53,571,586 | −31,391,762 | ||
| 8 | Gras | GE_PIM_Gras_3 | −53,499,849 | −31,402,764 | |
| Euc | GE_PIM_Euc_3 | −53,509,913 | −31,412,028 | ||
| SÃO GABRIEL (SAG) | 9 | Gras | GE_SAG_Gras_1 | −54,324,136 | −30,059,119 |
| Euc | GE_SAG_Euc_1 | −54,320,563 | −30,049,282 | ||
| 10 | Gras | GE_SAG_Gras_2 | −54,321,307 | −30,083,604 | |
| Euc | GE_SAG_Euc_2 | −54,327,398 | −30,069,851 | ||
| LULC Pairs | βSIM | βSNE | βSOR |
|---|---|---|---|
| GE_JAG_1 | 0.71 | 0.04 | 0.75 |
| GE_JAG_2 | 1 | 0 | 1 |
| GE_JAG_3 | 0.75 | 0 | 0.75 |
| GE_LAV_2 | 0.5 | 0.1 | 0.6 |
| GE_LAV_3 | 0.5 | 0.25 | 0.75 |
| GE_PIM_1 | 0.67 | 0.2 | 0.87 |
| GE_PIM_2 | 0.67 | 0.21 | 0.88 |
| GE_PIM_3 | 1 | 0 | 1 |
| GE_SAG_1 | 0 | 0.5 | 0.5 |
| GE_SAG_2 | 1 | 0 | 1 |
References
- Behling, H.; Jeruske-Pieruschka, V.; Schüler, L.; Pillar, V.P. Dinâmica dos campos no sul do Brasil durante o Quaternário Tardio. In Campos Sulinos, Conservação e uso Sustentável da Biodiversidade, 1st ed.; Pillar, V.P., Müller, S.C., Castilhos, Z.M.S., Jacques, A.V.A., Eds.; Ministério do Meio Ambiente: Brasília, Brazil, 2009; pp. 13–25. [Google Scholar]
- Overbeck, G.E.; Müller, S.C.; Fidelis, A.; Pfadenhauer, J.; Pillar, V.D.; Blanco, C.C.; Boldrini, I.I.; Both, R.; Forneck, E.D. Brazil’s neglected biome: The South Brazilian Campos. Perspect. Plant Ecol. Evol. Syst. 2007, 9, 101–116. [Google Scholar] [CrossRef]
- Cordeiro, J.L.P.; Hasenack, H. Cobertura vegetal atual do Rio Grande do Sul. In Campos Sulinos: Conservação e uso Sustentável da Biodiversidade, 1st ed.; Pillar, V.P., Müller, S.C., Castilhos, Z.M.S., Jacques, A.V.A., Eds.; Ministério do Meio Ambiente: Brasilia, Brazil, 2009; pp. 285–299. [Google Scholar]
- Bencke, G.A.; Chomenko, L.; Sant’ana, D.M. O que é o Pampa? In Nosso Pampa Desconhecido, 1st ed.; Chomenko, L., Bencke, G.A., Eds.; Fundação Zoobotânica do Rio Grande do Sul: Porto Alegre, Brazil, 2016; pp. 1–9. [Google Scholar]
- PROBIO. Cobertura Vegetal do Bioma Pampa–Relatório Técnico, 1st ed.; Ministério do Meio Ambiente: Brasília, Brazil, 2007; p. 31. [Google Scholar]
- Menezes, L.S.; Vogel, C.E.; Lucas, D.B.; Minervini, G.H.S.; Boldrini, I.I.; Overbeck, G.E. Plant species richness record in Brazilian Pampa grasslands and implications. Braz. J. Bot. 2018, 41, 817–823. [Google Scholar] [CrossRef]
- Winck, B.R.; Sá, E.L.S.; Rigotti, V.M.; Chauvat, M. Relationship between land-use types and functional diversity of epigeic Collembola in Southern Brazil. Appl. Soil Ecol. 2017, 109, 49–59. [Google Scholar] [CrossRef]
- Winck, B.R.; Rigotti, V.M.; Sá, E.L.S. Effects of different grazing intensities on the composition and diversity of Collembola communities in southern Brazilian grassland. Appl. Soil Ecol. 2019, 144, 98–106. [Google Scholar] [CrossRef]
- Silva, P.G.D.; Audino, L.D.; Nogueira, J.M.; Moraes, L.P.D.; Vaz-de-Mello, F.Z. Escarabeíneos (Coleoptera: Scarabaeidae: Scarabaeinae) de uma área de campo nativo no bioma Pampa, Rio Grande do Sul, Brasil. Biota Neotrop. 2012, 12, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Garcia, L.E.; Moraes, R.M.; Vianna, E.S. Levantamento de besouros copro-necrófagos (Coleoptera: Scarabaeidae: Scarabaeinae) do Bioma Pampa. Revista de Ciências Agroveterinárias 2016, 15, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.; Mendonça, M.S., Jr.; Rosado, J.L.O.; Loeck, A.E. Soil spiders in differing environments: Eucalyptus plantations and grasslands in the Pampa biome, southern Brazil. Rev. Colomb. Entomol. 2010, 36, 277–284. [Google Scholar] [CrossRef]
- Podgaiski, L.R.; Joner, F.; Lavorel, S.; Moretti, M.; Ibanez, S.; Mendonça, M.D.S., Jr.; Pillar, V.D. Spider trait assembly patterns and resilience under fire-induced vegetation change in South Brazilian grasslands. PLoS ONE 2013, 8, e60207. [Google Scholar] [CrossRef] [Green Version]
- Silva, G.O.; Ott, R. Short-term spider community monitoring after cattle removal in grazed grassland. Iheringia Ser. Zool. 2017, 107, e2017033. [Google Scholar] [CrossRef] [Green Version]
- Hooper, D.U.; Chapin III, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodivesity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 1, 3–35. [Google Scholar] [CrossRef]
- Vandewalle, M.; Bello, F.; Berg, M.P.; Bolger, T.; Dolédec, S.; Dubs, F.; Feld, C.K.; Harrington, R.; Harrison, P.A.; Lavorel, S.; et al. Functional traits as indicators of biodiversity response to land use changes across ecosystems and organisms. Biodivers. Conserv. 2010, 19, 2921–2947. [Google Scholar] [CrossRef] [Green Version]
- Keestra, S.; Bouma, J.; Wallinga, J.; Tittonell, P.; Smith, P.; Cerda, A.; Montanarella, L.; Quinton, J.; Pachepsky, Y.; Van Der Putten, W.; et al. The significance of soils and soil science towards realization of the United Nations Sustainable Development Goals. Soil 2016, 2, 111–128. [Google Scholar] [CrossRef] [Green Version]
- MapBiomas Project. Coleções Mapbiomas. Available online: https://mapbiomas.org/download (accessed on 15 April 2022).
- Roesch, L.F.W.; Vieira, F.C.B.; Pereira, V.A.; Schünemann, A.L.; Teixeira, I.F.; Senna, A.J.T.; Stefenon, V.M. The Brazilian Pampa: A fragile biome. Diversity 2009, 1, 182–198. [Google Scholar] [CrossRef]
- Van Vliet, J.; Groot, H.L.F.; Rietveld, P.; Verburg, P.H. Manifestations and underlying drivers of agricultural land use change in Europe Landsc. Urban Plan. 2015, 133, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Tóth, G.; Hermann, T.; Silva, M.R.; Montanarella, L. Monitoring soil for sustainable development and land degradation neutrality Environ. Monit.Assess. 2018, 190, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.S.C.; Correia, M.E.F.; Silva, E.M.R.; Maddock, J.E.L.; Pereira, M.G.; Silva, C.F. Soil Fauna Communities and Soil Attributes in the Agroforests of Paraty. Floresta Ambient. 2016, 23, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Coyle, D.R.; Nagendra, U.J.; Taylor, M.K.; Campbell, J.H.; Cunard, C.E.; Joslin, A.H.; Mundepi, A.; Phillips, C.A.; Callaham, M.A., Jr. Soil fauna responses to natural disturbances, invasive species, and global climate change: Current state of the science and a call to action. Soil Biol. Biochem. 2017, 110, 116–133. [Google Scholar] [CrossRef]
- Martins da Silva, P.; Berg, M.P.; Silva, A.A.; Dias, S.; Leitão, P.J.; Chamberlain, D.; Niemelä, J.; Serrano, A.M.F.; Sousa, J.P. Soil fauna through the landscape window: Factors shaping surface-and soil-dwelling communities across spatial scales in cork-oak mosaics. Landsc. Ecol. 2015, 30, 1511–1526. [Google Scholar] [CrossRef]
- Demetrio, W.; Assis, O.; Niva, C.C.; Bartz, M.L.C.; Paes, L.; Cardoso, G.; Santos, E.; Marzagão, M.; Nadolny, H.; Sautter, K.D.; et al. Comparison of soil invertebrate communities in organic and conventional production systems in Southern Brazil. Soil Org. 2020, 92, 143–156. [Google Scholar] [CrossRef]
- Adhikari, K.; Hartemink, A.E. Linking soils to ecosystem services—A global review. Geoderma 2016, 262, 101–111. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van der Putten, W. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Loranger, G.; Ponge, J.F.; Blanchart, E.; Lavelle, P. Influence of agricultural practices on arthropod communities in a vertisol (Martinique). Eur. J. Soil Biol. 1998, 34, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Lavelle, P.; Decaëns, T.; Aubert, B.; Barot, S.; Blouin, M.; Bureau, F.; Margeri, E.P.; Mora, J.; Rossic, P. Soil invertebrates and ecosystem services. Eur. J. Soil Biol. 2006, 42, S3–S15. [Google Scholar] [CrossRef]
- Santos, R.N.D.; Cabreira, W.V.; Pereira, M.G.; Souza, R.C.D.; Lima, S.S.D.; Louzada, M.; Santos, G.L.; Silva, A.C.R.D. Community Ecology of Soil Fauna Under Periodically Flooded Forest and Anthropic Fields. Floresta Ambient. 2021, 28, e20200052. [Google Scholar] [CrossRef]
- Hopkin, S.P. Biology of Springtails (Insecta: Collembola); Oxford University Press: Oxford, MI, USA, 1997; p. 344. [Google Scholar]
- Chamberlain, P.; Mcnamara, N.; Chaplow, J.; Stott, A.; Black, H. Translocation of surface litter carbon into soil by Collembola. Soil Biol. Biochem. 2006, 38, 2655–2664. [Google Scholar] [CrossRef]
- Salmon, S.; Ponge, J.F.; Gachet, S.; Deharveng, L.; Lefebvre, N.; Delabrosse, F. Linking species, traits and habitat characteristics of Collembola at European scale. Soil Biol. Biochem. 2014, 75, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 21, 259–263. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Moraes, G.; Leonardo, J.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Boldrini, I.I.; Ferreira, P.M.A.; Andrade, B.O.; Schneider, A.A.; Setubal, R.B.; Trevisan, R.; Freitas, E.M. Bioma Pampa: Diversidade Florística e Fisionômica, 1st ed.; Pallotti: Porto Alegre, Brazil, 2010; p. 330. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2017; p. 573. [Google Scholar]
- Ferreira, P.M.A.; Andrade, B.O.; Podgaiski, L.R.; Dias, A.C.; Pillar, V.D.; Overbeck, G.E.; Mendonça, M.S., Jr.; Boldrini, I.I. Long-term ecological research in southern Brazil grasslands: Effects of grazing exclusion and deferred grazing on plant and arthropod communities. PLoS ONE 2020, 15, e0227706. [Google Scholar] [CrossRef]
- WorldClim. Global Climate and Weather Data. Available online: https://www.worldclim.org/data/index.html# (accessed on 28 April 2022).
- MODIS—Moderate Resolution Imaging Spectroradiometer. Available online: https://modis.gsfc.nasa.gov/ (accessed on 28 April 2022).
- STRM DEM–Shuttle Radar Topographic Mission, Digital Elevation Models. Available online: https://srtm.csi.cgiar.org/ (accessed on 17 April 2022).
- Arlé, R.; Mendonça, M.C. Estudo preliminar das espécies de Dicranocentrus Schött, 1893, ocorrentes no Parque Nacional da Tijuca, Rio de Janeiro (Collembola). Rev. Bras. Biol. 1982, 42, 41–49. [Google Scholar]
- Jordana, R.; Arbea, J.I.; Simón, C.; Luciàñez, M.J. Fauna Iberica. Vol. 8. Collembola Poduromorpha; Consejo Superior de Investigaciones Cientificas: Madrid, Spain, 1997; p. 807. [Google Scholar]
- Massoud, Z. Monographie des Neanuridae, Collemboles Poduromorphes apiéces buccales modifiées. In Biologie de l’Amerique Australe, 1st ed.; Delamare Deboutteville, C., Rapoport, E.H., Eds.; Éditions du CNRS: Paris, France, 1967; Volume 3, pp. 7–399. [Google Scholar]
- Betsch, J.M. Éléments pour une monographie des Collemboles Symplyplêones (Hexapodes, Aptérygotes). Mém. Mus. Natl. Hist. Nat. Sér. A Zool. 1980, 116, 1–227. [Google Scholar]
- Christiansen, K.; Bellinger, P. The Collembola of North America North of Rio Grande, A Taxonomy Analysis, 2nd ed.; Grinnell College: Grinnell, IA, USA, 1998; pp. 1–1520. [Google Scholar]
- Bretfeld, G. Synopses on Palaearctic Collembola Volume 2: Symphypleona. Abh. Ber. Nat. Görlitz 1999, 71, 1–318. [Google Scholar]
- Potapov, M. Synopses on Palaeartic Collembola Volume 3: Isotomidae; Staaliches Museum für Naturkunde Görlitz: Görlitz, Germany, 2001; p. 603. [Google Scholar]
- Bellini, B.C.; Godeiro, N.N. Novos registros de Collembola (Arthropoda, Hexapoda) para áreas úmidas do semiárido do Brasil. In Artrópodes do Semiárido II: Biodiversidade e Conservação, 1st ed.; Bravo, F., Ed.; Métis Produção Editorial: São Paulo, Brazil, 2017; pp. 28–53. [Google Scholar]
- Cipola, N.G.; Dias, D.D.; Bellini, B.C. Chapter 2–Class Collembola. In Thorp and Covich's Freshwater Invertebrates, 4th ed.; Hamada, N., Thorp, J., Rogers, D.C., Eds.; Elsevier: New York, NY, USA, 2018; pp. 11–55. [Google Scholar]
- Bellinger, P.F.; Christiansen, K.A.; Janssens, F. Checklist of the Collembola of the World. Available online: http://www.collembola.org (accessed on 1 February 2022).
- Oksanen, A.J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Acglinn, D.; Minchin, P.R.; Hara, R.B.O.; Simpson, G.L.; Solymos, P.; et al. Vegan: Ecological Diversity. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 1 February 2022).
- Baselga, A.; Orme, C.D.L. Betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Sorensen, T.A. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content, and its application to analyses of the vegetation on Danish commons. Biol. Skr. 1948, 5, 1–34. [Google Scholar]
- Simpson, G.G. Mammals and the nature of continents. Am. J. Sci. 1943, 241, 1–31. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R Package for Rarefaction and Extrapolation of Species Diversity (Hill Numbers). Methods in Ecology and Evolution (in Revision). Available online: https://cran.r-project.org/web/packages/iNEXT/index.html (accessed on 1 February 2022).
- Magurran, A.E. Medindo a Diversidade Biológica, 1st ed.; Editora UFPR: Paraná, Brazil, 2013; p. 235. [Google Scholar]
- Gotelli, N.J.; Ellison, A.M. Princípios de Estatística em Ecologia, 2nd ed.; Artmed Editora: Porto Alegre, Brazil, 2016; p. 528. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. NLME: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-117. Available online: http://CRAN.Rproject.org/package=nlme (accessed on 1 February 2022).
- Acher, E. rfPermute. Version 2.5. Estimate Permutation p-Values for Random Forest Importance Metrics. Available online: https://cran.r-project.org/web/packages/rfPermute/index.html (accessed on 1 February 2022).
- Breiman, L.; Cutler, A.; Liaw, A.; Wiener, M. RandomForest, Version 4.7. Breiman and Cutler's Random Forests for Classification and Regression. Available online: https://cran.r-project.org/web/packages/randomForest/index.html (accessed on 1 February 2022).
- Rieff, G.G.; Machado, R.G.; Stroschein, M.R.D.; Sá, E.L.S. Diversidade de famílias de ácaros e colêmbolos edáficos em cultivo de eucalipto e áreas nativas. J. Agric. Sci. Technol. 2010, 16, 1–4. [Google Scholar] [CrossRef]
- Zeppelini, D.; Queiroz, G.C.; Bellini, B.C. Collembola in Catálogo Taxonômico da Fauna do Brasil. PNUD. Available online: http://fauna.jbrj.gov.br/fauna/faunadobrasil/379/ (accessed on 28 April 2022).
- Bellini, B.C.; Zeppelini, D. Registros da fauna de Collembola (Arthropoda, Hexapoda) no estado da Paraíba, Brasil. Rev. Bras. Entomol. 2009, 53, 386–390. [Google Scholar] [CrossRef]
- Querner, P.; Bruckner, A. Combining pitfall traps and soil samples to collect Collembola for site scale biodiversity assessments. Appl. Soil Ecol. 2010, 45, 293–297. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Bellini, B.C.; Vasconcellos, A. Temporal variations of Collembola (Arthropoda: Hexapoda) in the semiarid Caatinga in northeastern Brazil. Zool 2013, 30, 639–644. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Rocha, I.M.D.S.; Bellini, B.C.; Vasconcellos, A. Effects of habitat heterogeneity on epiedaphic Collembola (Arthropoda: Hexapoda) in a semiarid ecosystem in Northeast Brazil. Zool 2018, 35, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Nunes, L.A.P.L.; Araújo-Filho, J.A.; Meneses, R.I.Q. Recolonização da fauna edáfica em áreas de Caatinga submetidas a queimadas. Ver. Caat. 2008, 21, 214–220. [Google Scholar]
- Staude, I.R.; Vélez-Martin, E.; Andrade, B.O.; Podgaiski, L.R.; Boldrini, I.I.; Mendonca, M., Jr.; Pillar, V.D.; Overbeck, G.E. Local biodiversity erosion in south Brazilian grasslands under moderate levels of landscape habitat loss. J. Appl. Ecol. 2018, 55, 1241–1251. [Google Scholar] [CrossRef]
- Jorge, B.C.S.; Fischer, F.M.; Debastiani, V.J.; Hoss, D.; Pillar, V.D.; Winck, B. Effects of defoliation frequencies on above- and belowground biodiversity and ecosystem processes in subtropical grasslands of southern Brazil. Pedobiologia 2020, 90, 150786. [Google Scholar] [CrossRef]
- Góes, Q.R.D.; Freitas, L.D.R.; Lorentz, L.H.; Vieira, F.C.B.; Weber, M.A. Análise da fauna edáfica em diferentes usos do solo no Bioma Pampa. Ciência Florest. 2021, 31, 123–144. [Google Scholar] [CrossRef]
- Fidelis, A.; Overbeck, G.; Pillar, V.P.; Pfadenhauer, J. Effects of disturbance on population biology of the rosette species Eryngium horridum Malme in grasslands in southern Brazil. Plant Ecol. 2008, 195, 55–67. [Google Scholar] [CrossRef]
- Bomfim, L.-S.; Bitencourt, J.A.G.; Rodrigues, E.N.L.; Podigaiski, L.R. The role of a rosette-shaped plant (Eryngium horridum, Apiaceae) on grassland spiders along a grazing intensity gradient. Insect Conserv. Divers. 2021, 4, 492–503. [Google Scholar] [CrossRef]
- Malcicka, M.; Berg, M.P.; Ellers, J. Ecomorphological adaptations in Collembola in relation to feeding strategies and microhabitat. Eur. J. Soil Biol. 2016, 78, 82–91. [Google Scholar] [CrossRef]
- Kolasa, J.; Manne, L.L.; Pandit, S.N. Species–area relationships arise from interaction of habitat heterogeneity and species pool. Hydrobiologia 2012, 685, 135–144. [Google Scholar] [CrossRef]
- Relyea, R.; Ricklefs, R.E. A Economia da Natureza, 8th ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2021; p. 402. [Google Scholar]
- Castaño-Meneses, G.; Palacios-Vargas, J.G.; Cutz-Pool, L.Q. Feeding habits of Collembola and their ecological niche. Anales Inst. Biol. Univ. Nac. Autón. 2004, 75, 135–142. [Google Scholar]
- Milchunas, D.G.; Noy-Meir, I. Grazing refuges, external avoidance of herbivory and plant diversity. Oikos 2002, 99, 113–130. [Google Scholar] [CrossRef]
- Silva, P.M.; Carvalho, F.; Dirilgen, T.; Stone, D.; Creamer, R.; Bolger, T.; Sousa, J.P. Traits of collembolan life-form indicate land use types and soil properties across an European transect. Appl. Soil Ecol. 2016, 97, 69–77. [Google Scholar] [CrossRef]
- Calder, I.R.; Rosier, P.T.; Prasanna, K.T.; Parameswarappa, S. Eucalyptus water use greater than rainfall input-possible explanation from southern India. Hydrol. Earth Syst. Sci. 1997, 1, 249–256. [Google Scholar] [CrossRef]
- Leite, F.P.; Silva, I.R.; Novais, R.F.; Barros, N.F.; Neves, J.C.L. Alterations of soil chemical properties by eucalyptus cultivation in five regions in the Rio Doce Valley. Rev. Bras. Cienc. Sol. 2010, 34, 821–831. [Google Scholar] [CrossRef] [Green Version]
- Rzeszowski, K.; Zadrożny, P.; Nicia, P. The effect of soil nutrient gradients on Collembola communities inhabiting typical urban green spaces. Pedobiologia 2017, 64, 15–24. [Google Scholar] [CrossRef]
- Christiansen, K. Bionomics of Collembola. Annu. Rev. Entomolol. 1964, 9, 147–178. [Google Scholar] [CrossRef]
- Van Straalen, N.M.; Verhoef, H.A. The development of a bioindicator system for soil acidity based on arthropod pH preferences. J. Appl. Ecol. 1997, 34, 217–232. [Google Scholar] [CrossRef]
- Fountain, M.T.; Hopkin, S.P. Folsomia candida (Collembola): A “standard” soil arthropod. Annu. Rev. Entomol. 2005, 50, 201–222. [Google Scholar] [CrossRef] [Green Version]
- De Boer, T.E.; Holmstrup, M.; van Straalen, N.M.; Roelofs, D. The effect of soil pH and temperature on Folsomia candida transcriptional regulation. J. Insect Physiol. 2010, 56, 350–355. [Google Scholar] [CrossRef]
- Ponge, J.F.; Gillet, S.; Fedoroff, E.; Haese, L.; Souza, J.P.; Lavelle, P. Collembolan communities as bioindicators of land use intensification. Soil. Biol. Biochem. 2003, 35, 813–826. [Google Scholar] [CrossRef] [Green Version]
- ICMBio. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção; Ministério do Meio Ambiente: Brasília, Brazil, 2018; Volume I, p. 492. [Google Scholar]



| Family/Morphospecies | Native Grassland | Eucalyptus | |||
|---|---|---|---|---|---|
| Total Abundance | Relative Abundance (%) | Total Abundance | Relative Abundance (%) | ||
| Bourletiellidae | Rastriopes sp.1 | 01 | 0.10 | 00 | 0.00 |
| Prorastriopes sp.1 | 14 | 1.43 | 00 | 0.00 | |
| Prorastriopes sp.2 | 03 | 0.30 | 00 | 0.00 | |
| Brachystomellidae | Brachystomella sp.1 | 04 | 0.40 | 00 | 0.00 |
| Dicyrtomidae | Ptenothrix sp.1 | 00 | 0.00 | 04 | 1.48 |
| Entomobryidae | Entomobrya sp.1 | 192 | 19.61 | 02 | 0.74 |
| Entomobrya sp.2 | 62 | 6.33 | 00 | 0.00 | |
| Entomobrya sp.3 | 174 | 17.77 | 06 | 2.22 | |
| Entomobrya sp.4 | 10 | 1.02 | 53 | 19.63 | |
| Entomobrya sp.5 | 10 | 1.02 | 00 | 0.00 | |
| Lepidocyrtus sp.1 | 111 | 11.33 | 99 | 36.67 | |
| Seira sp.1 | 06 | 0.61 | 00 | 0.00 | |
| Hypogastruridae | Hypogastrura sp.1 | 14 | 1.43 | 00 | 0.00 |
| Xenylla sp.1 | 70 | 7.15 | 19 | 7.04 | |
| Isotomidae | Desoria sp.1 | 80 | 8.17 | 12 | 4.44 |
| Desoria sp.2 | 65 | 6.63 | 00 | 0.00 | |
| Folsomia sp.1 | 22 | 2.24 | 00 | 0.00 | |
| Isotomurus sp.1 | 18 | 1.83 | 00 | 0.00 | |
| Proisotoma sp.1 | 26 | 2.65 | 00 | 0.00 | |
| Katiannidae | Katianna sp.1 | 13 | 1.32 | 00 | 0.00 |
| Katianna sp.2 | 03 | 0.30 | 00 | 0.00 | |
| Sminthurinus sp.1 | 02 | 0.20 | 00 | 0.00 | |
| Neanuridae | Pseudachorutes sp.1 | 16 | 1.63 | 07 | 2.59 |
| Orchesellidae | Dicranocentrus sp.1 | 00 | 0.00 | 31 | 11.49 |
| Paronellidae | Trogolaphysa sp.1 | 00 | 0.00 | 37 | 13.70 |
| Sminthurididae | Sphaeridia sp.1 | 63 | 6.43 | 00 | 0.00 |
| Linear Mixed Model | |||||
| Land-Use Types | Total Abundance | F Value | p Value | ||
| Grassland | 979 | 12.86 | 0.0059 | ||
| Eucalyptus | 270 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, C.D.D.; Bellini, B.C.; Rigotti, V.M.; Nunes, R.C.; Menezes, L.d.S.; Winck, B.R. Diversity Loss of Epigeic Collembola after Grassland Conversion into Eucalyptus Forestry in Brazilian Pampa Domain. Diversity 2022, 14, 490. https://doi.org/10.3390/d14060490
da Silva CDD, Bellini BC, Rigotti VM, Nunes RC, Menezes LdS, Winck BR. Diversity Loss of Epigeic Collembola after Grassland Conversion into Eucalyptus Forestry in Brazilian Pampa Domain. Diversity. 2022; 14(6):490. https://doi.org/10.3390/d14060490
Chicago/Turabian Styleda Silva, Clécio Danilo Dias, Bruno Cavalcante Bellini, Vitor Mateus Rigotti, Rudy Camilo Nunes, Luciana da Silva Menezes, and Bruna Raquel Winck. 2022. "Diversity Loss of Epigeic Collembola after Grassland Conversion into Eucalyptus Forestry in Brazilian Pampa Domain" Diversity 14, no. 6: 490. https://doi.org/10.3390/d14060490






