Assessment Potential of Zooplankton to Establish Reference Conditions in Lowland Temperate Lakes
Abstract
:1. Introduction
2. Materials and Methods
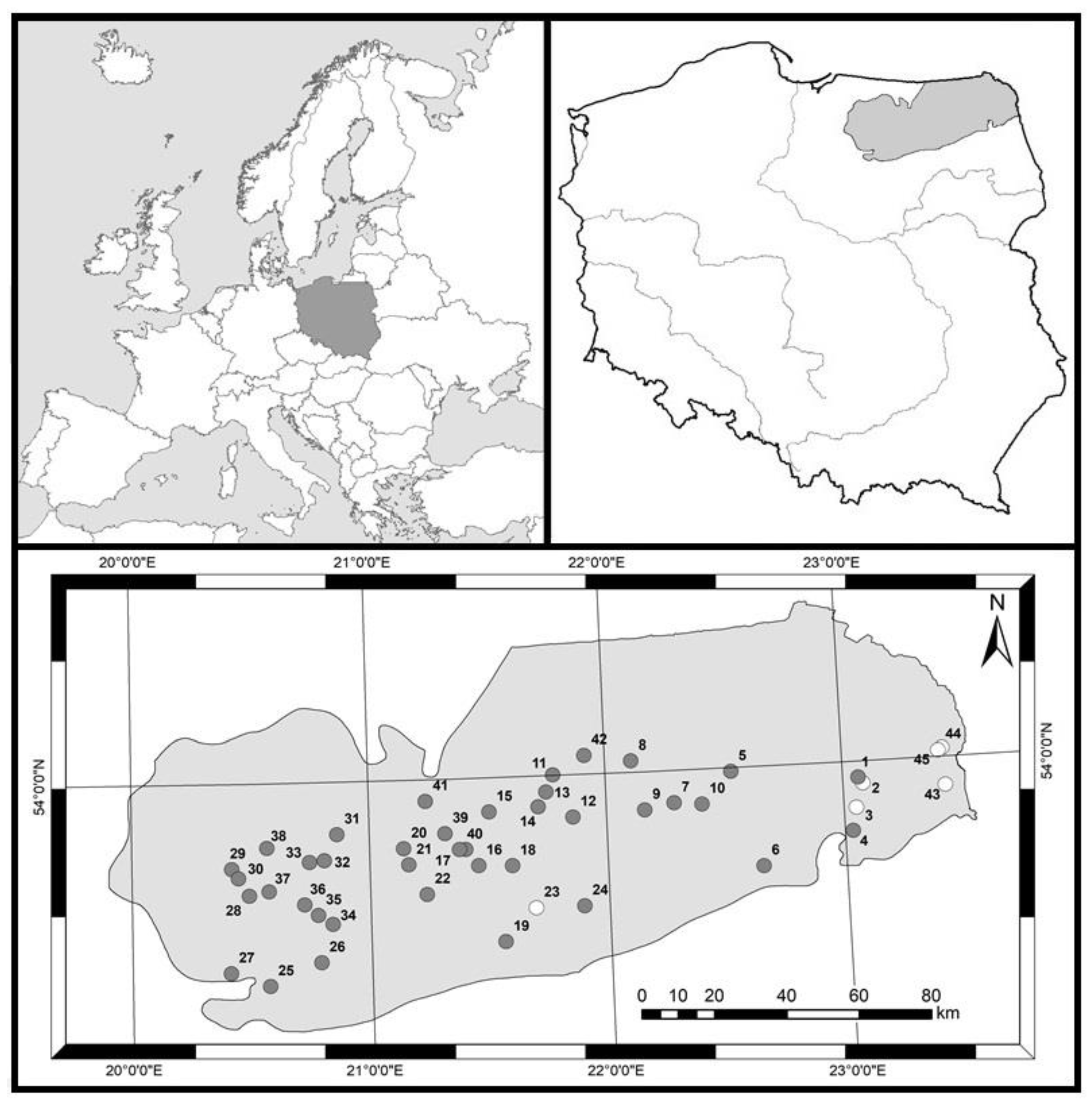
2.1. Sampling Sites and Data Collection
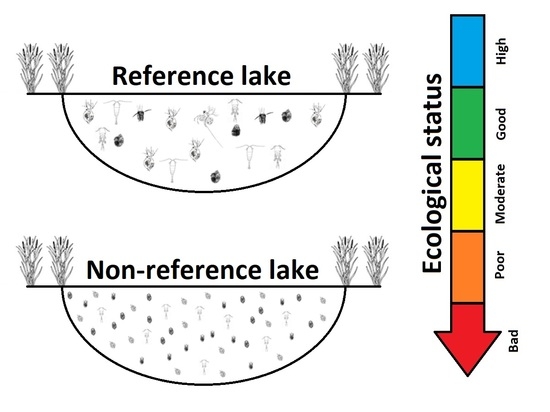
2.2. Selection of Reference Lakes
2.3. Statistical Methods
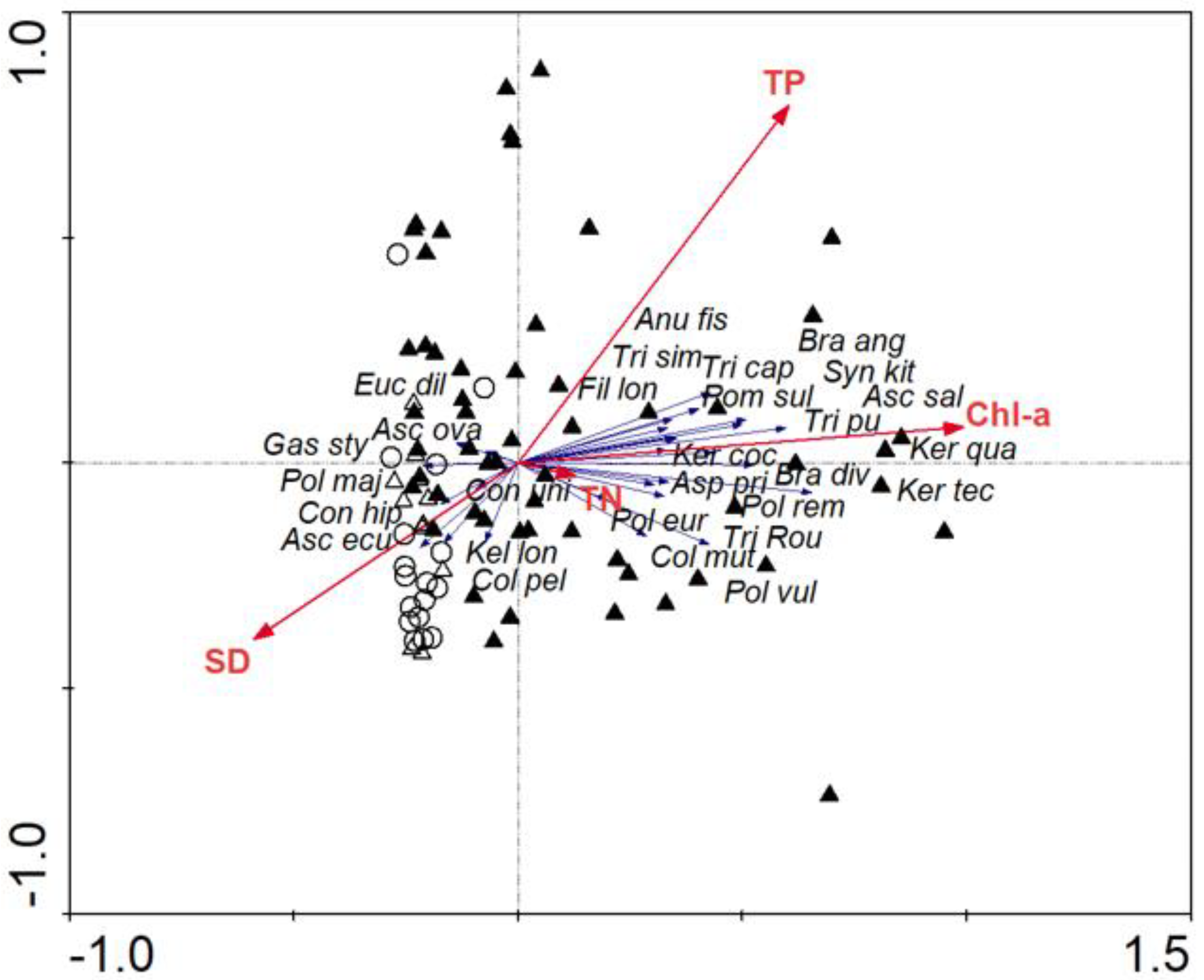
3. Results
3.1. Physicochemical Characteristics and Trophic Variables
3.2. Zooplankton Assemblage Characteristic
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haberman, J.; Haldna, M. Indices of zooplankton community as valuable tools in assessing the trophic state and water quality of eutrophic lakes: Long term study of Lake Vőrtsjärv. J. Limnol. 2014, 73, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Andronikova, I. Zooplankton characteristics in monitoring of Lake Ladoga. Hydrobiologia 1996, 322, 173–179. [Google Scholar] [CrossRef]
- Ochocka, A.; Pasztaleniec, A. Sensitivity of plankton indices to lake trophic conditions. Environ. Monit. Assess. 2016, 188, 622. [Google Scholar] [CrossRef] [Green Version]
- Karpowicz, M.; Sługocki, Ł.; Kozłowska, J.; Ochocka, A.; Lopez, C. Body size of Daphnia cucullata as an indicator of the ecological status of temperate lakes. Ecol. Indic. 2020, 117, 106585. [Google Scholar] [CrossRef]
- Gannon, J.E.; Stemberger, R.S. Zooplankton (especially Crustacean and Rotifers) as indicators of water quality. Trans. Am. Microsc. Soc. 1978, 97, 16–35. [Google Scholar] [CrossRef]
- Gliwicz, Z.M. The food sources of lake zooplankton. Ekol. Pol. 1969, 15, 205–223. [Google Scholar]
- Hakkari, L. Zooplankton species as indicators of environment. Aqua Fenn. 1972, 1, 46–54. [Google Scholar]
- Sládeček, V. Rotifers as indicators of water quality. Hydrobiologia 1983, 100, 169–201. [Google Scholar] [CrossRef]
- Gulati, R.D. Zooplankton and its grazing as indicator of trophic status in Dutch lakes. Environ. Monit. Assess. 1983, 3, 343–354. [Google Scholar] [CrossRef]
- Karabin, A. Pelagic zooplankton (Rotatoria + Crustacea) variation in the process of Lake Eutrophication. I. Structural and Quantitative Features. Ekol. Pol. 1985, 33, 567–616. [Google Scholar]
- Ejsmont-Karabin, J. The usefulness of zooplankton as lake ecosystem indicators: Rotifer trophic state index. Pol. J. Ecol. 2012, 60, 339–350. [Google Scholar]
- Ejsmont-Karabin, J.; Karabin, A. The suitability of zooplankton as lake ecosystem indicators: Crustacean trophic state index. Pol. J. Ecol. 2013, 61, 561–573. [Google Scholar]
- García-Chicote, J.; Armengol, X.; Rojo, C. Zooplankton species as indicators of trophic state in reservoirs from Mediterranean river basins. Inland Waters 2019, 9, 113–123. [Google Scholar] [CrossRef]
- Stamou, G.; Katsiapi, M.; Moustaka-Gouni, M.; Michaloudi, E. Trophic state assessment based on zooplankton communities in Mediterranean lakes. Hydrobiologia 2019, 844, 83–103. [Google Scholar] [CrossRef]
- Muñoz-Colmenares, M.E.; Soria, J.M.; Vicente, E. Can zooplankton species be used as indicators of trophic status and ecological potential of reservoirs? Aquat. Ecol. 2021, 55, 1143–1156. [Google Scholar] [CrossRef]
- European Commission (EC). Directive 2000/60 of the European Parliament and the Council of 23 October 2000 Establishing a Framework for Community actionin the field of water policy. Off. J. Eur. Union. 2000, 5, L327. [Google Scholar]
- Moss, B. Shallow lakes, the water framework directive and life. What should it all be about? Hydrobiologia 2007, 584, 381–394. [Google Scholar] [CrossRef]
- Caroni, R.; Irvine, K. The potential of zooplankton communities for ecological assessment of lakes: Redundant concept or political oversight? Biol. Environ. 2010, 110, 35–53. [Google Scholar] [CrossRef]
- Jeppesen, E.; Noges, P.; Davidson, T.A.; Haberman, J.; Noges, T.; Blank, K.; Lauridsen, T.; Sondergaard, M.; Sayer, C.; Laugaste, R.; et al. Zooplankton as indicators in lakes: A scientifcbased plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia 2011, 676, 279–297. [Google Scholar] [CrossRef]
- Ochocka, A. ZIPLAs: Zooplankton Index for Polish Lakes’ Assessment: A new method to assess the ecological status of stratified lakes. Environ. Monit. Assess. 2021, 193, 664. [Google Scholar] [CrossRef]
- Stamou, G.; Mazaris, A.D.; Moustaka-Gouni, M.; Špoljar, M.; Ternjej, I.; Dražina, T.; Zeynep, D.; Michaloudia, E. Introducing a zooplanktonic index for assessing water quality of natural lakes in the Mediterranean region. Ecol. Inform. 2022, 69, 101616. [Google Scholar] [CrossRef]
- Reynoldson, T.B.; Norris, R.H.; Resh, V.H.; Day, K.E.; Rosenberg, D.M. The reference condition: A comparation of multimetric and multivariate approaches to assess water-quality impairment using benthic invertebrates. J. N. Am. Benthol. Soc. 1997, 16, 833–852. [Google Scholar] [CrossRef]
- Nielsen, K.; Sømod, B.; Ellegaard, C.; Krause-Jensen, D. Assessing reference conditions according to the European Water Framework Directive using model-ling and analysis of historical data: An example from Randers Fjord, Denmark. Ambio 2003, 32, 287–294. [Google Scholar] [CrossRef]
- Cardoso, A.C.; Solimini, A.; Premazzi, G.; Carvalho, L.; Lyche-Solcheim, A.; Rekolainen, S. Phosphorus reference concentrations in European lakes. Hydrobiologia 2007, 584, 3–12. [Google Scholar] [CrossRef]
- CIS. Monitoring under the Water Framework Directive. Common Implementation Strategy for the Water Framework Directive (2000/60/EC). Guidance Document No 7. Working Group 2.7–Monitoring; European Communities: Luxembourg, 2003. [Google Scholar]
- Kolada, A.; Soszka, H.; Kutyła, S.; Pasztaleniec, A. The typology of Polish lakes after a decade of its use: A critical review and verification. Limnologica 2017, 67, 20–26. [Google Scholar] [CrossRef]
- Górniak, A.; Kajak, Z. Hydrobiologia. Limnologia; PWN: Warszawa, Poland, 2020; p. 452. [Google Scholar]
- Choiński, A.; Ptak, M.; Skowron, R. Tendencje zmian zjawisk lodowych jezior Polski w latach 1951–2010. Prz. Geogr. 2020, 86, 23–40. [Google Scholar] [CrossRef]
- Hermanowicz, W.; Dojlido, J.; Dożańska, W.; Koziorowski, B.; Zerbe, J. Physical-Chemical Examination of Water and Wastewater; Arkady: Warszawa, Poland, 1999; p. 556. (In Polish) [Google Scholar]
- Nusch, E.A. Comparison of diferent methods for chlorophyll and pheopigment determination. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1980, 14, 14–36. [Google Scholar]
- Carlson, R.E. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Kratzer, C.R.; Brezonik, P.L. A Carlson-type trophic state index for nitrogen in Florida lakes. Water Res. Bull. 1981, 17, 713–715. [Google Scholar] [CrossRef]
- Balushkina, E.V.; Vinberg, G.G. Dependences between mass and length of the body of plankton Crustacea. In General Basics of Research in Aquatic Ecosystems; Vinberg, G.G., Ed.; Nauka: Leningrad, Russia, 1979; pp. 169–172. (In Russian) [Google Scholar]
- Ejsmont-Karabin, J. Empirical equations for biomass calculation of planktonic rotifers. Pol. J. Ecol. 1998, 45, 513–522. [Google Scholar]
- Birk, S.; Bonne, W.; Borja, A.; Brucet, S.; Courrat, A.; Poikane, S.; Solimini, A.; van de Bund, W.; Zampoukas, N.; Hering, D. Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- Lyche-Solheim, A. (Ed.). Reference Conditions of European Lakes. Indicators and Methods for the Water Framework Directive; Assessment of Reference Conditions. REBECCA Project Deliverable 7. 2005, p. 105. Available online: http://www.rbm-toolbox.net/docstore/docs/3.1713.D7-uusi.pdf (accessed on 19 July 2012).
- Soszka, H.; Kolada, A.; Gołub, M.; Cydzik, D. Establishing reference conditions for Polish lakes–preliminary results. Oceanol. Hydrobiol. Stud. 2008, 37, 105–110. [Google Scholar] [CrossRef]
- Büttner, G.; Kosztra, B. CLC2018 Technical Guidelines; European Environment Agency: Wien, Austria, 2017; Available online: https://land.copernicus.eu/user-corner/technical-library/clc2018technicalguidelines_final.pdf (accessed on 5 January 2022).
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Biometris: Wageningen, The Netherlands, 2002. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Dufrene, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Cáceres, M.D.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Roberts, D.W. Labdsv: Ordination and Multivariate Analysis for Ecology: R Package. 2019. Available online: https://CRAN.R-project.org/package=labdsv (accessed on 10 February 2022).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2022; Available online: http://www.rstudio.com/ (accessed on 10 February 2022).
- Perbiche-Neves, G.; Saito, V.S.; Previattelli, D.; da Rocha, C.E.F.; Nogueira, M.G. Cyclopoid copepods as bioindicators of eutrophication in reservoirs:Do patterns hold for large spatial extents? Ecol. Indic. 2016, 70, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Santos-Wisniewski, M.J.; Rocha, O. Spatial distribution and secondary production of Copepoda in a tropical reservoir: Barra Bonita, SP, Brazil. Braz. J. Biol. 2007, 67, 223–233. [Google Scholar] [CrossRef]
- Kerfoot, W.C.; DeMott, W.R. Food web dynamics: Dependent chains and vaulting. In Trophic Interactions within Aquatic Ecosystems; Meyers, D.G., Strickler, J.R., Eds.; Westview press: Boulder, CO, USA, 1984; pp. 347–382. [Google Scholar]
- Walz, N.; Elster, H.J.; Mezger, M. The development of the rotifer community structure in Lake Constance during its eutrophication. Arch. Hydrobiol. Suplement 1987, 74, 452–487. [Google Scholar]
- Matveeva, L.K. Can pelagic rotifers be used as indicators of lake trophic state? Verh. Int. Ver. Limnol. 1991, 24, 2761–2763. [Google Scholar] [CrossRef]
- Yoshida, T.; Urabe, J.; Elser, J.J. Assessment of ‘top-down’ and ‘bottom-up’ forces as determinants of rotifer distribution among lakes in Ontario, Canada. Ecol. Res. 2003, 18, 639–650. [Google Scholar] [CrossRef]
- May, L.; O’Hare, M. Changes in rotifer species composition and abundance along a trophic gradient in loch Lomond, Scotland, UK. Hydrobiologia 2005, 54, 397–404. [Google Scholar]
- Karpowicz, M.; Ejsmont-Karabin, J.; Więcko, A.; Górniak, A.; Cudowski, A. A place in space-the horizontal vs vertical factors that influence zooplankton (Rotifera, Crustacea) communities in a mesotrophic lake. J. Limnol. 2019, 78, 243–258. [Google Scholar] [CrossRef]
- Karpowicz, M.; Ejsmont-Karabin, J.; Kozłowska, J.; Feniova, I.; Dzialowski, A.R. Zooplankton Community Responses to Oxygen Stress. Water 2020, 12, 706. [Google Scholar] [CrossRef] [Green Version]
- Bandara, K.; Varpe, Ø.; Wijewardene, L.; Tverberg, V.; Eiane, K. Two hundred years of zooplankton vertical migration research. Biol. Rev. 2021, 96, 1547–1589. [Google Scholar] [CrossRef]
- Lampert, W. Ultimate causes of diel vertical migration of zooplankton: New evidence for the predator-avoidance hypothesis. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1993, 39, 79–88. [Google Scholar]
- Gliwicz, Z.M.; Pijanowska, J. The role of predation in zooplankton succesion-W: Plankton ecology. In Succession in Plankton Communities; Sommer, U., Ed.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 253–296. [Google Scholar]
- Maemets, A. Rotifers as indicators of lake types in Estonia. Hydrobiologia 1983, 104, 357–361. [Google Scholar] [CrossRef]
- Karpowicz, M.; Ejsmont-Karabin, J. Diversity and structure of pelagic zooplankton (Crustacea, Rotifera) in NE Poland. Water 2021, 13, 456. [Google Scholar] [CrossRef]
- Błędzki, L.A.; Rybak, J.I. Freshwater Crustacean Zooplankton of Europe; Springer: Cham, Switzerland, 2016; pp. 1–918. [Google Scholar]
- Pejler, B. Zooplanktic indicators of trophy and their food. Hydrobiologia 1983, 101, 111–114. [Google Scholar] [CrossRef]
- Čeirāns, A. Zooplankton indicators of trophy in Latvian lakes. Acta Univ. Latv. 2007, 723, 61–69. [Google Scholar]
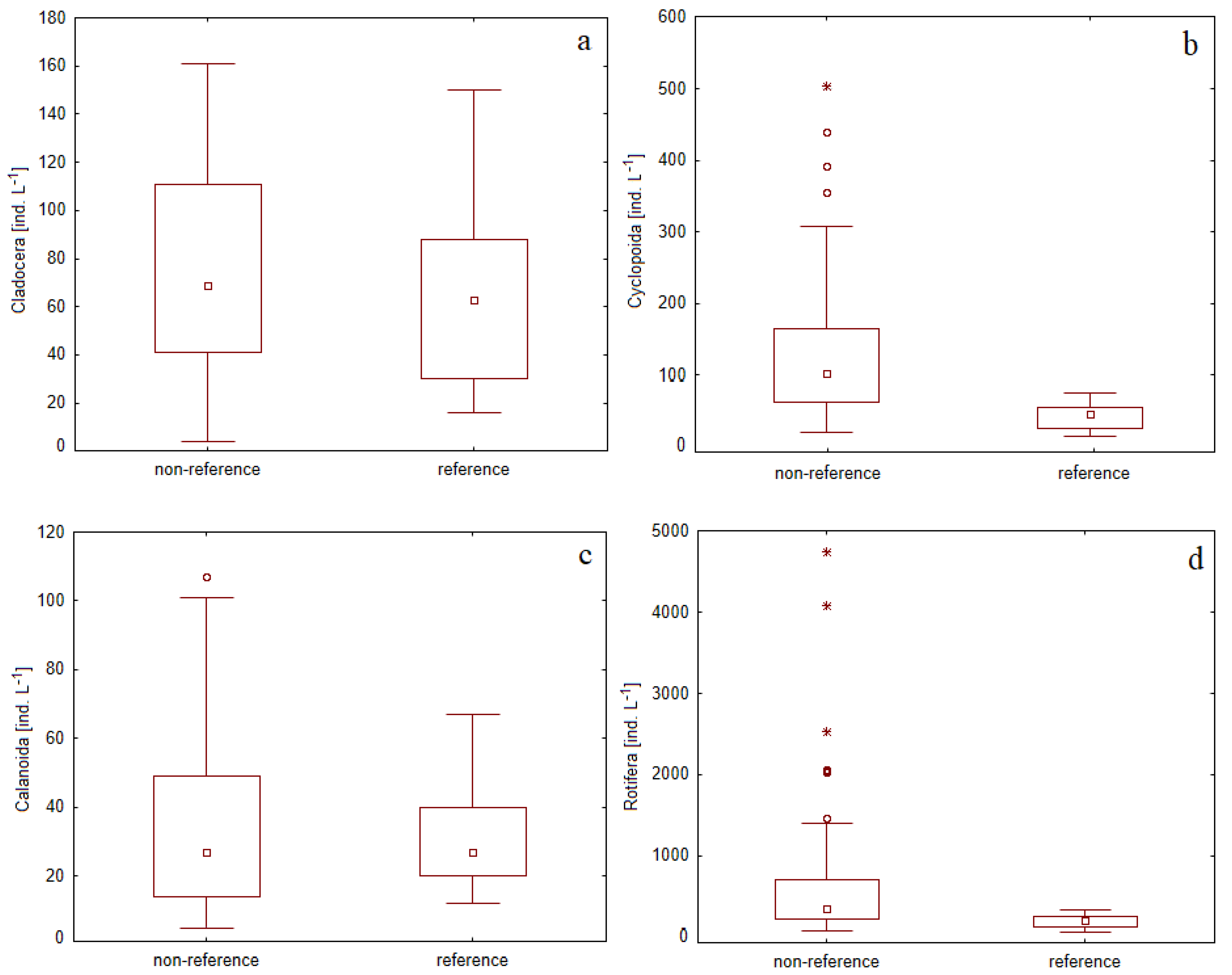
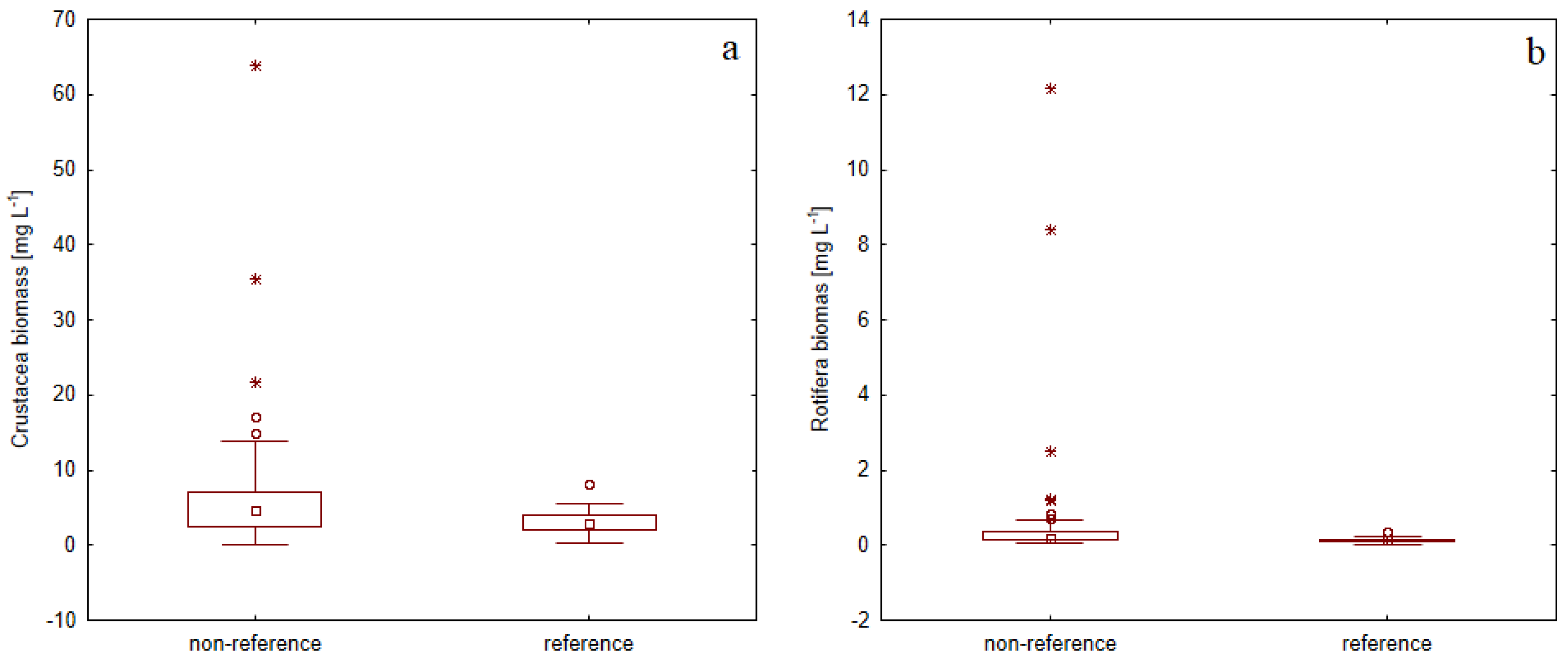

| Parameters | Reference | Non-Reference | One-Way ANOVA Results | |
|---|---|---|---|---|
| n = 17 Lake-Years | n = 69 Lake-Years | F | p | |
| TP [mg L−1] | 0.022 (0.010–0.054) | 0.043 (0.012–0.093) | 17.4 | <0.001 |
| TN [mg L−1] | 0.64 (0.15–0.94) | 0.92 (0.41–1.65) | 18.5 | <0.001 |
| Chl-a [µg L−1] | 3.4 (1.2–6.2) | 18.5 (1.3–64.2) | 13.3 | 0.001 |
| SD [m] | 4.6 (2.0–7.0) | 2.1 (0.5–7.0) | 51.3 | <0.001 |
| Natural land use (forests, wetlands and other waterbodies) | 96.6 | 47.7 | 119.2 | <0.001 |
| Continuous urban fabric | 0 | 0.5 | 4.5 | 0.037 |
| Agricultural areas and discontinous urban fabric | 1.5 | 40.8 | 89.4 | <0.001 |
| Pastures | 1.5 | 5.7 | 18.0 | <0.001 |
| Land principally occupied by agriculture with significant areas of natural vegetation | 0.4 | 5.3 | 19.0 | <0.001 |
| Reference Versus Non-Reference Lakes | ||
|---|---|---|
| Species | Contrib.% | Cumulative % |
| Keratella tecta | 4.96 | 4.96 |
| Pompholyx sulcata | 4.67 | 9.63 |
| Collotheca pelagica | 4.57 | 14.20 |
| Ascomorpha ecaudis | 3.91 | 18.11 |
| Ascomorpha saltans | 3.76 | 21.87 |
| Species | IndVal | p Value | Status |
|---|---|---|---|
| Pompholyx sulcata | 0.854 | 0.001 | Non-reference |
| Keratella tecta | 0.725 | 0.001 | Non-reference |
| Mesocyclops leuckarti | 0.693 | 0.003 | Non-reference |
| Thermocyclops oithonoides | 0.688 | 0.002 | Non-reference |
| Daphnia cucullata | 0.628 | 0.014 | Non-reference |
| Ascomorpha saltans | 0.591 | 0.009 | Non-reference |
| Trichocerca pusilla | 0.463 | 0.007 | Non-reference |
| Synchaeta kitina | 0.439 | 0.036 | Non-reference |
| Keratella quadrata | 0.417 | 0.016 | Non-reference |
| Bosmina (Eubosmina) coregoni var. thersites | 0.362 | 0.023 | Non-reference |
| Bosmina (Eubosmina) coregoni | 0.723 | 0.001 | Reference |
| Ascomorpha ecaudis | 0.634 | 0.001 | Reference |
| Collotheca pelagica | 0.583 | 0.001 | Reference |
| Gastropus stylifer | 0.564 | 0.021 | Reference |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochocka, A.; Karpowicz, M. Assessment Potential of Zooplankton to Establish Reference Conditions in Lowland Temperate Lakes. Diversity 2022, 14, 501. https://doi.org/10.3390/d14060501
Ochocka A, Karpowicz M. Assessment Potential of Zooplankton to Establish Reference Conditions in Lowland Temperate Lakes. Diversity. 2022; 14(6):501. https://doi.org/10.3390/d14060501
Chicago/Turabian StyleOchocka, Agnieszka, and Maciej Karpowicz. 2022. "Assessment Potential of Zooplankton to Establish Reference Conditions in Lowland Temperate Lakes" Diversity 14, no. 6: 501. https://doi.org/10.3390/d14060501
APA StyleOchocka, A., & Karpowicz, M. (2022). Assessment Potential of Zooplankton to Establish Reference Conditions in Lowland Temperate Lakes. Diversity, 14(6), 501. https://doi.org/10.3390/d14060501