Ventilation Systems in Wetland Plant Species
Abstract
:1. Introduction
2. Ventilation Mechanisms in Various Wetland Plant Groups
2.1. Submerged Species
2.2. Species with Natant Leaves
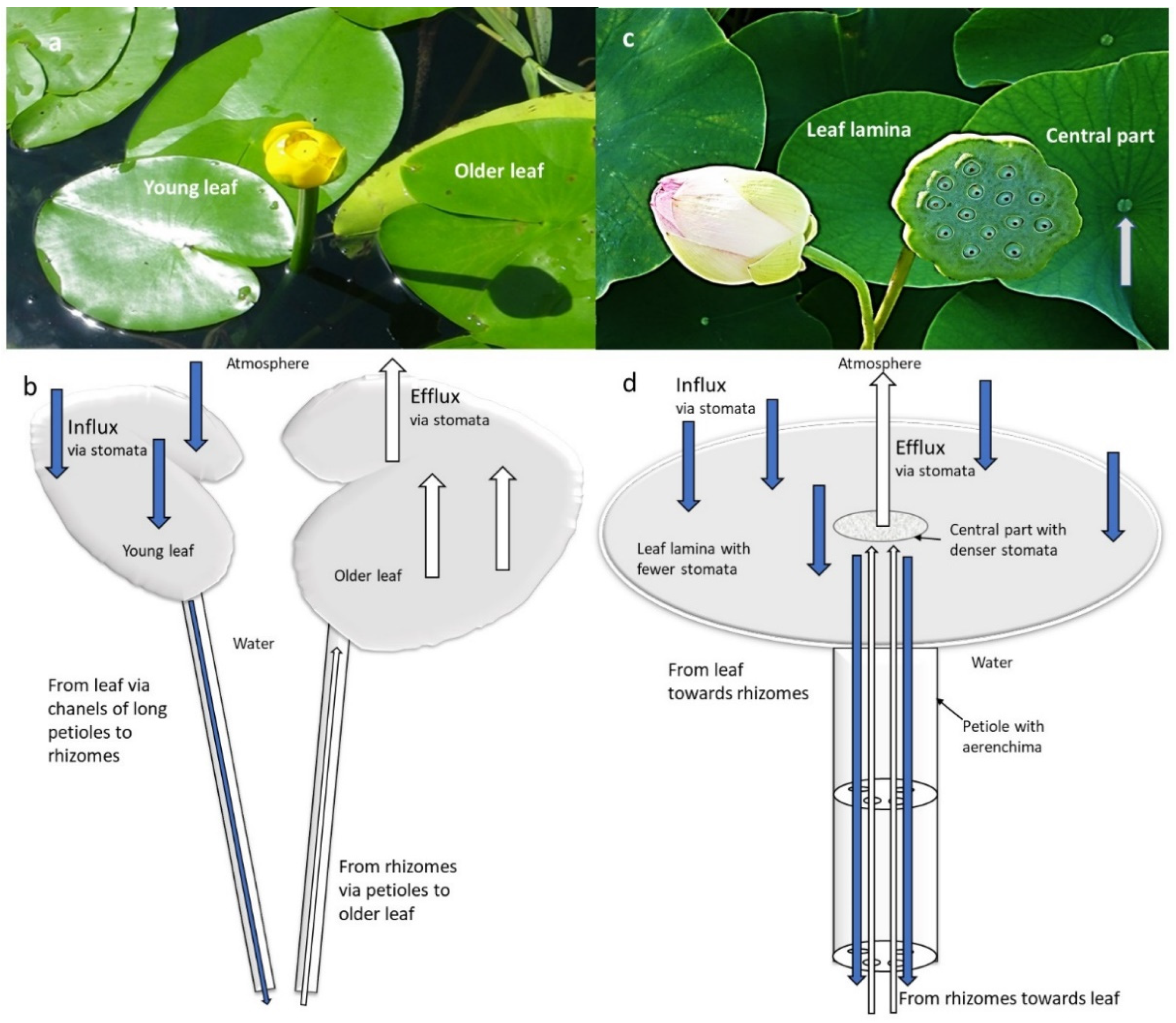
2.3. Helophytes
2.4. Mangrove Forest
2.5. Other Wetland Species
3. Diversity of Ventilation Systems
4. Plant Role in Emission of Greenhouse Gases
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jackson, M.B. Ethylene and Responses of Plants to Soil Waterlogging and Submergence. Annu. Rev. Plant Physiol. 1985, 36, 145–174. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Colmer, T.D.; Pierik, R.; Millenaar, F.F.; Peeters, A.J.M. How Plants Cope with Complete Submergence. New Phytol. 2006, 170, 213–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janauer, G.; Germ, M.; Kuhar, U.; Gaberščik, A. Ecology of Aquatic Macrophytes and Their Role in Running Waters. In Macrophytes of the River Danube Basin; Janauer, G., Gaberščik, A., Květ, J., Germ, M., Exler, N., Eds.; Academia, Živá Příroda: Prague, Czech Republic, 2018; pp. 13–33. ISBN 9788020027436. [Google Scholar]
- Große, W.; Schröder, P. Oxygen Supply of Roots by Gas Transport in Alder-Trees. Z. Für Nat. C 1984, 39, 1186–1188. [Google Scholar] [CrossRef] [Green Version]
- Colmer, T.D. Long-Distance Transport of Gases in Plants: A Perspective on Internal Aeration and Radial Oxygen Loss from Roots. Plant Cell Environ. 2003, 26, 17–36. [Google Scholar] [CrossRef] [Green Version]
- Mitsch, W.J.; Gosselink, J.G. Wetlands: Human History, Use, and Science, 4th ed.; Wiley: Hoboken, NJ, USA, 2007; ISBN 9780471699675. [Google Scholar]
- Jackson, M.B.; Armstrong, W. Formation of Aerenchyma and the Processes of Plant Ventilation in Relation to Soil Flooding and Submergence. Plant Biol. 1999, 1, 274–287. [Google Scholar] [CrossRef]
- Justin, S.H.F.W.; Armstrong, W. The Anatomical Characteristics of Roots and Plant Response to Soil Flooding. New Phytol. 1987, 106, 465–495. [Google Scholar] [CrossRef]
- Evans, D.E. Aerenchyma Formation. New Phytol. 2004, 161, 35–49. [Google Scholar] [CrossRef]
- Laing, H.E. The Composition of the Internal Atmosphere of Nuphar Advenum and Other Water Plants. Am. J. Bot. 1940, 27, 861–868. [Google Scholar] [CrossRef]
- Jung, J.; Lee, S.C.; Choi, H.-K. Anatomical Patterns of Aerenchyma in Aquatic and Wetland Plants. J. Plant Biol. 2008, 51, 428–439. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A. Life in the Balance: A Signaling Network Controlling Survival of Flooding. Curr. Opin. Plant Biol. 2010, 13, 489–494. [Google Scholar] [CrossRef]
- Takahashi, H.; Yamauchi, T.; Colmer, T.D.; Nakazono, M. Aerenchyma Formation in Plants. In Plant Cell Monographs; Springer: Berlin/Heidelberg, Germany, 2014; Volume 21, pp. 247–265. [Google Scholar]
- Doležal, J.; Kučerová, A.; Jandová, V.; Klimeš, A.; Říha, P.; Adamec, L.; Schweingruber, F.H. Anatomical Adaptations in Aquatic and Wetland Dicot Plants: Disentangling the Environmental, Morphological and Evolutionary Signals. Environ. Exp. Bot. 2021, 187, 104495. [Google Scholar] [CrossRef]
- Laan, P.; Berrevoets, M.J.; Lythe, S.; Armstrong, W.; Blom, C.W.P.M. Root Morphology and Aerenchyma Formation as Indicators of the Flood-Tolerance of Rumex Species. J. Ecol. 1989, 77, 693–703. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups, 4th ed.; Springer: Berlin, Germany, 2003. [Google Scholar]
- Jia, Y.; Huang, H.; Chen, Z.; Zhu, Y.-G. Arsenic Uptake by Rice Is Influenced by Microbe-Mediated Arsenic Redox Changes in the Rhizosphere. Environ. Sci. Technol. 2014, 48, 1001–1007. [Google Scholar] [CrossRef]
- Smith, K.E.; Luna, T.O. Radial Oxygen Loss in Wetland Plants: Potential Impacts on Remediation of Contaminated Sediments. J. Environ. Eng. 2013, 139, 496–501. [Google Scholar] [CrossRef]
- Riya, S.; Sun, H.; Furuhata, H.; Shimamura, M.; Nasu, H.; Imano, R.; Zhou, S.; Hosomi, M. Role of Leaves in Methane Emission from Sacred Lotus (Nelumbo nucifera). Aquat. Bot. 2020, 163, 103203. [Google Scholar] [CrossRef]
- Villa, J.A.; Ju, Y.; Stephen, T.; Rey-Sanchez, C.; Wrighton, K.C.; Bohrer, G. Plant-mediated Methane Transport in Emergent and Floating-leaved Species of a Temperate Freshwater Mineral-soil Wetland. Limnol. Oceanogr. 2020, 65, 1635–1650. [Google Scholar] [CrossRef]
- Caraco, N.F.; Cole, J.J. Contrasting Impacts of a Native and Alien Macrophyte on Dissolved Oxygen in a Large River. Ecol. Appl. 2002, 12, 1496. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Lodge, D.M. Effects of Submersed Macrophytes on Ecosystem Processes. Aquat. Bot. 1986, 26, 341–370. [Google Scholar] [CrossRef]
- Goodwin, K.; Caraco, N.; Cole, J. Temporal Dynamics of Dissolved Oxygen in a Floatingleaved Macrophyte Bed. Freshw. Biol. 2008, 53, 1632–1641. [Google Scholar] [CrossRef]
- Horvat, B.; Urbanc-Berčič, O.; Gaberščik, A. Water Quality and Macrophyte Community Changes in the Komarnik Accumulation Lake (Slovenia). In Wastewater Treatment, Plant Dynamics and Management in Constructed and Natural Wetlands; Springer: Dordrecht, The Netherlands, 2008; pp. 13–22. [Google Scholar]
- Raven, J.A. Into the Voids: The Distribution, Function, Development and Maintenance of Gas Spaces in Plants. Ann. Bot. Co. 1996, 78, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Rascio, N. The Underwater Life of Secondarily Aquatic Plants: Some Problems and Solutions. Crit. Rev. Plant Sci. 2002, 21, 401–427. [Google Scholar] [CrossRef]
- Sculthorpe, C.D. The Biology of Aquatic Vascular Plants; American Association for the Advancement of Science: London, UK, 1967. [Google Scholar]
- Hartman, R.T.; Brown, D.L. Changes in Internal Atmosphere of Submersed Vascular Hydrophytes in Relation to Photosynthesis. Ecology 1967, 48, 252–258. [Google Scholar] [CrossRef]
- Winkel, A.; Borum, J. Use of Sediment CO2 by Submersed Rooted Plants. Ann. Bot. 2009, 103, 1015–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, T.v.; Olesen, B.; Bagger, J. Carbon Acquisition and Carbon Dynamics by Aquatic Isoetids. Aquat. Bot. 2002, 73, 351–371. [Google Scholar] [CrossRef]
- Borum, J.; Pedersen, O.; Greve, T.M.; Frankovich, T.A.; Zieman, J.C.; Fourqurean, J.W.; Madden, C.J. The Potential Role of Plant Oxygen and Sulphide Dynamics in Die-off Events of the Tropical Seagrass, Thalassia Testudinum. J. Ecol. 2005, 93, 148–158. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Pedersen, O.; Binzer, T.; Borum, J. Contrasting Oxygen Dynamics in the Freshwater Isoetid Lobelia Dortmanna and the Marine Seagrass Zostera Marina. Ann. Bot. 2005, 96, 613–623. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, O.; Rich, S.M.; Pulido, C.; Cawthray, G.R.; Colmer, T.D. Crassulacean Acid Metabolism Enhances Underwater Photosynthesis and Diminishes Photorespiration in the Aquatic Plant Isoetes Australis. New Phytol. 2011, 190, 332–339. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Prahl, C.; Stokholm, H. Oxygen Release from Roots of Submerged Aquatic Macrophytes. Oikos 1982, 38, 349–354. [Google Scholar] [CrossRef]
- Keeley, J.E. CAM Photosynthesis in Submerged Aquatic Plants. Bot. Rev. 1998, 64, 121–175. [Google Scholar] [CrossRef]
- Břízová, E. Quillwort (Isoëtes), a Mysterious Plant from the Czech Republic. Acta Mus. Nat. Pragae Ser. B Hist. Nat. 2011, 67, 25–34. [Google Scholar]
- Green, W.A. The Parichnos Problem and the Function of Aerenchyma in the Lycopsida. Bull. Peabody Mus. Nat. Hist. 2014, 55, 191–200. [Google Scholar] [CrossRef]
- Green, W.A. The Function of the Aerenchyma in Arborescent Lycopsids: Evidence of an Unfamiliar Metabolic Strategy. Proc. R. Soc. B Biol. Sci. 2010, 277, 2257–2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breecker, D.O.; Royer, D.L. The Pedogenic Formation of Coal Balls by CO2 Degassing through the Rootlets of Arborescent Lycopsids. Am. J. Sci. 2019, 319, 529–548. [Google Scholar] [CrossRef]
- Pedersen, O.; Sand-Jensen, K.; Revsbech, N.P. Diel Pulses of O2 and CO2 in Sandy Lake Sediments Inhabited by Lobelia Dortmanna. Ecology 1995, 76, 1536–1545. [Google Scholar] [CrossRef]
- Smits, A.J.M.; Laan, P.; Thier, R.H.; van der Velde, G. Root Aerenchyma, Oxygen Leakage Patterns and Alcoholic Fermentation Ability of the Roots of Some Nymphaeid and Isoetid Macrophytes in Relation to the Sediment Type of Their Habitat. Aquat. Bot. 1990, 38, 3–17. [Google Scholar] [CrossRef]
- Smolders, A.J.P.; Lucassen, E.C.H.E.T.; Roelofs, J.G.M. The Isoetid Environment: Biogeochemistry and Threats. Aquat. Bot. 2002, 73, 325–350. [Google Scholar] [CrossRef]
- Pedersen, O.; Pulido, C.; Rich, S.M.; Colmer, T.D. In Situ O2 Dynamics in Submerged Isoetes Australis: Varied Leaf Gas Permeability Influences Underwater Photosynthesis and Internal O2. J. Exp. Bot. 2011, 62, 4691–4700. [Google Scholar] [CrossRef] [PubMed]
- Winkel, A.; Colmer, T.D.; Pedersen, O. Leaf Gas Films of Spartina Anglica Enhance Rhizome and Root Oxygen during Tidal Submergence. Plant Cell Environ. 2011, 34, 2083–2092. [Google Scholar] [CrossRef]
- Richards, J.H.; Kuhn, D.N.; Bishop, K. Interrelationships of Petiolar Air Canal Architecture, Water Depth, and Convective Air Flow in Nymphaea Odorata (Nymphaeaceae). Am. J. Bot. 2012, 99, 1903–1909. [Google Scholar] [CrossRef]
- Dacey, J.W.H. Internal Winds in Water Lilies: An Adaptation for Life in Anaerobic Sediments. Science 1980, 210, 1017–1019. [Google Scholar] [CrossRef]
- Grosse, W.; Bernhard Büchel, H.; Tiebel, H. Pressurized Ventilation in Wetland Plants. Aquat. Bot. 1991, 39, 89–98. [Google Scholar] [CrossRef]
- Dacey, J.W.H.; Klug, M.J. Ventilation by Floating Leaves in Nuphar. Am. J. Bot. 1982, 69, 999. [Google Scholar] [CrossRef]
- Grosse, W.; Jovy, K.; Tiebel, H. Influence of Plants on Redox Potential and Methane Production in Water-Saturated Soil. In Management and Ecology of Freshwater Plants; Springer: Dordrecht, The Netherlands, 1996; pp. 93–99. [Google Scholar]
- Grosse, W.; Schröder, P. Pflanzenleben Unter Anaeroben Umweltbedingungen, Die Physikalischen Grundlagen Und Anatomischen Voraussetzungen. Ber. Der Dtsch. Bot. Ges. 1986, 99, 367–381. [Google Scholar] [CrossRef]
- Schröder, P. Characterization of a Thermo-Osmotic Gas Transport Mechanism in Alnus glutinosa (L.) Gaertn. Trees 1989, 3, 38–44. [Google Scholar] [CrossRef]
- Konnerup, D.; Sorrell, B.K.; Brix, H. Do Tropical Wetland Plants Possess Convective Gas Flow Mechanisms? New Phytol. 2011, 190, 379–386. [Google Scholar] [CrossRef]
- Mevi-Scutz, J.; Grosse, W. A Two-Way Gas Transport System in Nelumbo nucifera. Plant Cell Environ. 1988, 11, 27–34. [Google Scholar] [CrossRef]
- Matthews, P.G.D.; Seymour, R.S. Stomata Actively Regulate Internal Aeration of the Sacred Lotus Nelumbo Nucifera. Plant Cell Environ. 2014, 37, 402–413. [Google Scholar] [CrossRef]
- Vogel, S. Contributions to the Functional Anatomy and Biology of Nelumbo Nucifera (Nelumbonaceae) I. Pathways of Air Circulation. Plant Syst. Evol. 2004, 249, 9–25. [Google Scholar] [CrossRef]
- Matthews, P.G.D.; Seymour, R.S. Anatomy of the Gas Canal System of Nelumbo nucifera. Aquat. Bot. 2006, 85, 147–154. [Google Scholar] [CrossRef]
- Gaberščik, A. Measurements of Apparent CO2 Flux in Amphibious Plant Polygonum amphibium L. Growing over Environmental Gradient. Photosynthetica 1993, 29, 473–476. [Google Scholar]
- Colmer, T.D.; Pedersen, O. Underwater Photosynthesis and Respiration in Leaves of Submerged Wetland Plants: Gas Films Improve CO2 and O2 Exchange. New Phytol. 2008, 177, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Hyvönen, T.; Ojala, A.; Kankaala, P.; Martikainen, P.J. Methane Release from Stands of Water Horsetail (Equisetum Fluviatile) in a Boreal Lake. Freshw. Biol. 1998, 40, 275–284. [Google Scholar] [CrossRef]
- Armstrong, J.; Armstrong, W. Reasons for the Presence or Absence of Convective (Pressurized) Ventilation in the Genus Equisetum. New Phytol. 2011, 190, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.; Armstrong, W. Record Rates of Pressurized Gas-flow in the Great Horsetail, Equisetum Telmateia. Were Carboniferous Calamites Similarly Aerated? New Phytol. 2009, 184, 202–215. [Google Scholar] [CrossRef]
- Strand, V.V. The Influence of Ventilation Systems on Water Depth Penetration of Emergent Macrophytes. Freshw. Biol. 2002, 47, 1097–1105. [Google Scholar] [CrossRef]
- Vretare Strand, V.; Weisner, S.E.B. Interactive Effects of Pressurized Ventilation, Water Depth and Substrate Conditions on Phragmites Australis. Oecologia 2002, 131, 490–497. [Google Scholar] [CrossRef]
- Vretare, V.; Weisner, S.E.B. Influence of Pressurized Ventilation on Performance of an Emergent Macrophyte (Phragmites australis). J. Ecol. 2000, 88, 978–987. [Google Scholar] [CrossRef]
- Armstrong, J.; Armstrong, W.; Beckett, P.M. Phragmites Australis: Venturi- and Humidity-Induced Pressure Flows Enhance Rhizome Aeration and Rhizosphere Oxidation. New Phytol. 1992, 120, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Faußer, A.C.; Dušek, J.; Čížková, H.; Kazda, M. Diurnal Dynamics of Oxygen and Carbon Dioxide Concentrations in Shoots and Rhizomes of a Perennial in a Constructed Wetland Indicate Down-Regulation of below Ground Oxygen Consumption. AoB Plants 2016, 8, plw025. [Google Scholar] [CrossRef] [Green Version]
- Kludze, H.K.; DeLaune, R.D.; Patrick, W.H. Aerenchyma Formation and Methane and Oxygen Exchange in Rice. Soil Sci. Soc. Am. J. 1993, 57, 386–391. [Google Scholar] [CrossRef]
- Bart, D.; Hartman, J.M. Environmental Determinants of Phragmites Australis Expansion in a New Jersey Salt Marsh: An Experimental Approach. Oikos 2000, 89, 59–69. [Google Scholar] [CrossRef]
- Visser, E.J.W.; Colmer, T.D.; Blom, C.W.P.M.; Voesenek, L.A.C.J. Changes in Growth, Porosity, and Radial Oxygen Loss from Adventitious Roots of Selected Mono- and Dicotyledonous Wetland Species with Contrasting Types of Aerenchyma. Plant Cell Environ. 2000, 23, 1237–1245. [Google Scholar] [CrossRef] [Green Version]
- Singer, A.; Eshel, A.; Agami, M.; Beer, S. The Contribution of Aerenchymal CO2 to the Photosynthesis of Emergent and Submerged Culms of Scirpus Lacustris and Cyperus Papyrus. Aquat. Bot. 1994, 49, 107–116. [Google Scholar] [CrossRef]
- Jermy, A.C.; Anthony, C.; Simpson, D.; Oswald, P.H. Botanical Society of the British Isles. “Schoenoplectus lacustris (L.) Palla”. In Sedges of the British Isles. BSBI Handbook, 3rd ed.; Botanical Society of the British Isles: London, UK, 2007; Volume 1, pp. 105–107. ISBN 9780901158352. [Google Scholar]
- Tulbure, M.G.; Ghioca-Robrecht, D.M.; Johnston, C.A.; Whigham, D.F. Inventory and Ventilation Efficiency of Nonnative and Native Phragmites Australis (Common Reed) in Tidal Wetlands of the Chesapeake Bay. Estuaries Coasts 2012, 35, 1353–1359. [Google Scholar] [CrossRef]
- Tornberg, T.; Bendix, M.; Brix, H. Internal Gas Transport in Typha latifolia L. and Typha angustifolia L. 2. Convective Throughflow Pathways and Ecological Significance. Aquat. Bot. 1994, 49, 91–105. [Google Scholar] [CrossRef]
- White, S.D.; Ganf, G.G. Influence of Stomatal Conductance on the Efficiency of Internal Pressurisation in Typha Domingensis. Aquat. Bot. 2000, 67, 1–11. [Google Scholar] [CrossRef]
- Bansal, S.; Lishawa, S.C.; Newman, S.; Tangen, B.A.; Wilcox, D.; Albert, D.; Anteau, M.J.; Chimney, M.J.; Cressey, R.L.; DeKeyser, E.; et al. Typha (Cattail) Invasion in North American Wetlands: Biology, Regional Problems, Impacts, Ecosystem Services, and Management. Wetlands 2019, 39, 645–684. [Google Scholar] [CrossRef] [Green Version]
- Duarte, V.P.; Pereira, M.P.; Corrêa, F.F.; de Castro, E.M.; Pereira, F.J. Aerenchyma, Gas Diffusion, and Catalase Activity in Typha Domingensis: A Complementary Model for Radial Oxygen Loss. Protoplasma 2021, 258, 765–777. [Google Scholar] [CrossRef]
- Matsui, T.; Tsuchiya, T. Root Aerobic Respiration and Growth Characteristics of Three Typha Species in Response to Hypoxia. Ecol. Res. 2006, 21, 470–475. [Google Scholar] [CrossRef]
- Raskin, I.; Kende, H. Mechanism of Aeration in Rice. Science 1985, 228, 327–329. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Fukao, T.; Ronald, P.; Ismail, A.; Heuer, S.; Mackill, D. Submergence Tolerant Rice: SUB1’s Journey from Landrace to Modern Cultivar. Rice 2010, 3, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Nishiuchi, S.; Yamauchi, T.; Takahashi, H.; Kotula, L.; Nakazono, M. Mechanisms for Coping with Submergence and Waterlogging in Rice. Rice 2012, 5, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzog, M.; Konnerup, D.; Pedersen, O.; Winkel, A.; Colmer, T.D. Leaf Gas Films Contribute to Rice (Oryza Sativa) Submergence Tolerance during Saline Floods. Plant Cell Environ. 2018, 41, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Konnerup, D.; Pedersen, O. Flood Tolerance of Glyceria Fluitans: The Importance of Cuticle Hydrophobicity, Permeability and Leaf Gas Films for Underwater Gas Exchange. Ann. Bot. 2017, 120, 521–528. [Google Scholar] [CrossRef] [Green Version]
- Groot, T.T.; van Bodegom, P.M.; Meijer, H.A.J.; Harren, F.J.M. Gas Transport through the Root–Shoot Transition Zone of Rice Tillers. Plant Soil 2005, 277, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Tanaka, A.; Mori, H.; Takamure, I.; Kato, K.; Nakazono, M. Ethylene-Dependent Aerenchyma Formation in Adventitious Roots Is Regulated Differently in Rice and Maize. Plant Cell Environ. 2016, 39, 2145–2157. [Google Scholar] [CrossRef]
- Steffens, B.; Geske, T.; Sauter, M. Aerenchyma Formation in the Rice Stem and Its Promotion by H2O2. New Phytol. 2011, 190, 369–378. [Google Scholar] [CrossRef]
- Ni, X.-L.; Gui, M.-Y.; Tan, L.-L.; Zhu, Q.; Liu, W.-Z.; Li, C.-X. Programmed Cell Death and Aerenchyma Formation in Water-Logged Sunflower Stems and Its Promotion by Ethylene and ROS. Front. Plant Sci. 2019, 9, 1928. [Google Scholar] [CrossRef]
- Parlanti, S.; Kudahettige, N.P.; Lombardi, L.; Mensuali-Sodi, A.; Alpi, A.; Perata, P.; Pucciariello, C. Distinct Mechanisms for Aerenchyma Formation in Leaf Sheaths of Rice Genotypes Displaying a Quiescence or Escape Strategy for Flooding Tolerance. Ann. Bot. 2011, 107, 1335–1343. [Google Scholar] [CrossRef] [Green Version]
- Nagai, K.; Kurokawa, Y.; Mori, Y.; Minami, A.; Reuscher, S.; Wu, J.; Matsumoto, T.; Ashikari, M. SNORKEL Genes Relating to Flood Tolerance Were Pseudogenized in Normal Cultivated Rice. Plants 2022, 11, 376. [Google Scholar] [CrossRef]
- Colmer, T.D.; Cox, M.C.H.; Voesenek, L.A.C.J. Root Aeration in Rice (Oryza sativa): Evaluation of Oxygen, Carbon Dioxide, and Ethylene as Possible Regulators of Root Acclimatizations. New Phytol. 2006, 170, 767–778. [Google Scholar] [CrossRef]
- Colmer, T.D.; Pedersen, O. Oxygen Dynamics in Submerged Rice (Oryza sativa). New Phytol. 2008, 178, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Seago, J.L.; Peterson, C.A.; Enstone, D.E. Cortical Development in Roots of the Aquatic Plant Pontederia Cordata (Pontederiaceae). Am. J. Bot. 2000, 87, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.; Pedersen, O. Partial versus Complete Submergence: Snorkelling Aids Root Aeration in R Umex Palustris but Not in R. Acetosa. Plant Cell Environ. 2014, 37, 2381–2390. [Google Scholar] [CrossRef] [PubMed]
- Ting, T.M.; Poulsen, A.D. Understorey Vegetation at Two Mud Volcanoes in North-East Borneo. J. Trop. For. Sci. 2009, 21, 198–209. [Google Scholar]
- Bonde, S.D.; Kumaran, K.P.N. A Permineralized Species of Mangrove Fern Acrostichum L. from Deccan Intertrappean Beds of India. Rev. Palaeobot. Palynol. 2002, 120, 285–299. [Google Scholar] [CrossRef]
- Fonini, A.M.; Barufi, J.B.; Schmidt, É.C.; Rodrigues, A.C.; Randi, Á.M. Leaf Anatomy and Photosynthetic Efficiency of Acrostichum Danaeifolium after UV Radiation. Photosynthetica 2017, 55, 401–410. [Google Scholar] [CrossRef]
- Chomicki, G.; Bidel, L.P.R.; Baker, W.J.; Jay-Allemand, C. Palm Snorkelling: Leaf Bases as Aeration Structures in the Mangrove Palm (Nypa fruticans). Bot. J. Linn. Soc. 2014, 174, 257–270. [Google Scholar] [CrossRef] [Green Version]
- Rozainah, M.Z.; Aslezaeim, N. A Demographic Study of a Mangrove Palm, Nypa Fruticans. Sci. Res. Essays 2010, 5, 3896–3902. [Google Scholar]
- Srikanth, S.; Lum, S.K.Y.; Chen, Z. Mangrove Root: Adaptations and Ecological Importance. Trees 2016, 30, 451–465. [Google Scholar] [CrossRef]
- McKee, K.L. Soil Physicochemical Patterns and Mangrove Species Distribution-Reciprocal Effects? J. Ecol. 1993, 81, 477–487. [Google Scholar] [CrossRef]
- Scholander, P.F.; van Dam, L.; Scholander, S.I. Gas Exchange in the Roots of Mangroves. Am. J. Bot. 1955, 42, 92. [Google Scholar] [CrossRef]
- Purnobasuki, H.; Suzuki, M. Aerenchyma Formation and Porosity in Root of a Mangrove Plant, Sonneratia Alba (Lythraceae). J. Plant Res. 2004, 117, 465–472. [Google Scholar] [CrossRef]
- Purnobasuk, H. Primary Structure and Intercellular Space Formation of Feeding Root in Sonneratia alba J. Smith. Int. J. Bot. 2012, 9, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ding, Y.; Wang, W.; Li, Y.; Wang, M. Distribution of Fish among Avicennia and Sonneratia Microhabitats in a Tropical Mangrove Ecosystem in South China. Ecosphere 2019, 10, e02759. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Wu, M.-L.; Li, C.-D.; Sun, F.-L.; Sun, C.-C.; Wang, Y.-S. Dynamics of Radial Oxygen Loss in Mangroves Subjected to Waterlogging. Ecotoxicology 2020, 29, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, Y.-S.; Fei, J.; Jiang, Z.-Y.; Ye, Z.-H. Differences in Root Aeration, Iron Plaque Formation and Waterlogging Tolerance in Six Mangroves along a Continues Tidal Gradient. Ecotoxicology 2015, 24, 1659–1667. [Google Scholar] [CrossRef]
- Curran, M.; Cole, M.; Allaway, W.G. Root Aeration and Respiration in Young Mangrove Plants (Avicennia marina (Forsk.) Vierh.). J. Exp. Bot. 1986, 37, 1225–1233. [Google Scholar] [CrossRef]
- Hovenden, M. Horizontal Structures on Pneumatophores of Avicennia marina (Forsk.) Vierh.—A New Site of Oxygen Conductance. Ann. Bot. 1994, 73, 377–383. [Google Scholar] [CrossRef]
- Curran, M.; James, P.; Allaway, W.G. The Measurement of Gas Spaces in the Roots of Aquatic Plants—Archimedes Revisited. Aquat. Bot. 1996, 54, 255–261. [Google Scholar] [CrossRef]
- Rogers, G.K. Bald Cypress Knees, Taxodium Distichum (Cupressaceae): An Anatomical Study, with Functional Implications. Flora 2021, 278, 151788. [Google Scholar] [CrossRef]
- Martin, C.E.; Francke, S.K. Root Aeration Function of Baldcypress Knees (Taxodium distichum). Int. J. Plant Sci. 2015, 176, 170–173. [Google Scholar] [CrossRef]
- Wang, G.-B.; Cao, F.-L. Formation and Function of Aerenchyma in Baldcypress (Taxodium distichum (L.) Rich.) and Chinese Tallow Tree (Sapium Sebiferum (L.) Roxb.) under Flooding. S. Afr. J. Bot. 2012, 81, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Middleton, B. Carbon Stock Trends of Baldcypress Knees along Climate Gradients of the Mississippi River Alluvial Valley Using Allometric Methods. For. Ecol. Manag. 2020, 461, 117969. [Google Scholar] [CrossRef]
- Sou, H.-D.; Masumori, M.; Kurokochi, H.; Tange, T. Histological Observation of Primary and Secondary Aerenchyma Formation in Adventitious Roots of Syzygium kunstleri (King) Bahadur and R.C.Gaur Grown in Hypoxic Medium. Forests 2019, 10, 137. [Google Scholar] [CrossRef] [Green Version]
- Grosse, W.; Schulte, A.; Fujita, H. Pressurized Gas Transport in Two Japanese Alder Species in Relation to Their Natural Habitats. Ecol. Res. 1993, 8, 151–158. [Google Scholar] [CrossRef]
- Takaishi, T.; Sensui, Y. Thermal Transpiration Effect of Hydrogen, Rare Gases and Methane. Trans. Faraday Soc. 1963, 59, 2503–2514. [Google Scholar] [CrossRef]
- Denbigh, K.G. Thermo-Osmosis of Gases through a Membrane. Nature 1949, 163, 60. [Google Scholar] [CrossRef]
- Denbigh, K.G.; Raumann, G. The Thermo-Osmosis of Gases through a Membrane. I. Theoretical. Proc. R. Soc. London Ser. A Math. Phys. Sci. 1952, 210, 377–387. [Google Scholar]
- Buchel, H.B.; Grosse, W. Localization of the Porous Partition Responsible for Pressurized Gas Transport in Alnus glutinosa (L.) Gaertn. Tree Physiol. 1990, 6, 247–256. [Google Scholar] [CrossRef]
- Dittert, K.; Wätzel, J.; Sattelmacher, B. Responses of Alnus Glutinosa to Anaerobic Conditions-Mechanisms and Rate of Oxygen Flux into the Roots. Plant Biol. 2006, 8, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, W.; Armstrong, J. Stem Photosynthesis Not Pressurized Ventilation Is Responsible for Light-Enhanced Oxygen Supply to Submerged Roots of Alder (Alnus glutinosa). Ann. Bot. 2005, 96, 591–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batzli, J.M.; Dawson, J.O. Development of Flood-Induced Lenticels in Red Alder Nodules Prior to the Restoration of Nitrogenase Activity. Can. J. Bot. 1999, 77, 1373–1377. [Google Scholar] [CrossRef]
- Huber, M.; Linder, H.P. The Evolutionary Loss of Aerenchyma Limits Both Realized and Fundamental Ecohydrological Niches in the Cape Reeds (Restionaceae). J. Ecol. 2012, 100, 1338–1348. [Google Scholar] [CrossRef] [Green Version]
- Bedoya, A.M.; Madriñán, S. Evolution of the Aquatic Habit in Ludwigia (Onagraceae): Morpho-Anatomical Adaptive Strategies in the Neotropics. Aquat. Bot. 2015, 120, 352–362. [Google Scholar] [CrossRef]
- Hoffmann, M.H. To the Roots of Carex: Unexpected Anatomical and Functional Diversity. Syst. Bot. 2019, 44, 26–31. [Google Scholar] [CrossRef]
- Visser, E.J.W.; Bogemann, G.M.; van de Steeg, H.M.; Pierik, R.; Blom, C.W.P.M. Flooding Tolerance of Carex Species in Relation to Field Distribution and Aerenchyma Formation. New Phytol. 2000, 148, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, W.; Armstrong, J. Plant Internal Oxygen Transport (Diffusion and Convection) and Measuring and Modelling Oxygen Gradients. In Low-Oxygen Stress in Plants; Springer: Berlin, Germany, 2014; Volume 21, pp. 267–297. [Google Scholar]
- Schröder, P.; Grosse, W.; Woermann, D. Localization of Thermo-Osmotically Active Partitions in Young Leaves of Nuphar Lutea. J. Exp. Bot. 1986, 37, 1450–1461. [Google Scholar] [CrossRef]
- Bazhin, N.M. Influence of Plants on the Methane Emission from Sediments. Chemosphere 2004, 54, 209–215. [Google Scholar] [CrossRef]
- Bridgham, S.D.; Cadillo-Quiroz, H.; Keller, J.K.; Zhuang, Q. Methane Emissions from Wetlands: Biogeochemical, Microbial, and Modeling Perspectives from Local to Global Scales. Glob. Chang. Biol. 2013, 19, 1325–1346. [Google Scholar] [CrossRef]
- Bansal, S.; Johnson, O.F.; Meier, J.; Zhu, X. Vegetation Affects Timing and Location of Wetland Methane Emissions. J. Geophys. Res. Biogeosci. 2020, 125, e2020JG005777. [Google Scholar] [CrossRef]
- Bhullar, G.S.; Iravani, M.; Edwards, P.J.; Olde Venterink, H. Methane Transport and Emissions from Soil as Affected by Water Table and Vascular Plants. BMC Ecol. 2013, 13, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turetsky, M.R.; Kotowska, A.; Bubier, J.; Dise, N.B.; Crill, P.; Hornibrook, E.R.C.; Minkkinen, K.; Moore, T.R.; Myers-Smith, I.H.; Nykänen, H.; et al. A Synthesis of Methane Emissions from 71 Northern, Temperate, and Subtropical Wetlands. Glob. Chang. Biol. 2014, 20, 2183–2197. [Google Scholar] [CrossRef]
- Kosten, S.; Piñeiro, M.; de Goede, E.; de Klein, J.; Lamers, L.P.M.; Ettwig, K. Fate of Methane in Aquatic Systems Dominated by Free-Floating Plants. Water Res. 2016, 104, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Henneberg, A.; Sorrell, B.K.; Brix, H. Internal Methane Transport through Juncus effusus: Experimental Manipulation of Morphological Barriers to Test Above- and Below-ground Diffusion Limitation. New Phytol. 2012, 196, 799–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lee, J.; Yun, J.; Yang, Y.; Ding, W.; Yuan, J.; Kang, H. Mechanisms of Enhanced Methane Emission Due to Introduction of Spartina anglica and Phragmites australis in a Temperate Tidal Salt Marsh. Ecol. Eng. 2020, 153, 105905. [Google Scholar] [CrossRef]
- van den Berg, M.; van den Elzen, E.; Ingwersen, J.; Kosten, S.; Lamers, L.P.M.; Streck, T. Contribution of Plant-Induced Pressurized Flow to CH4 Emission from a Phragmites Fen. Sci. Rep. 2020, 10, 12304. [Google Scholar] [CrossRef]
- Were, D.; Kansiime, F.; Fetahi, T.; Hein, T. Carbon Dioxide and Methane Fluxes from Various Vegetation Communities of a Natural Tropical Freshwater Wetland in Different Seasons. Environ. Processes 2021, 8, 553–571. [Google Scholar] [CrossRef]
- Jones, M.B. Methane Production and Emission from Papyrus-Dominated Wetlands. SIL Proc. 2000, 27, 1406–1409. [Google Scholar] [CrossRef]
- Van der Nat, F.J.W.A.; Middelburg, J.J.; van Meteren, D.; Wielemakers, A. Diel Methane Emission Patterns from Scirpus lacustris and Phragmites australis. Biogeochemistry 1998, 41, 1–22. [Google Scholar] [CrossRef]
- Sebacher, D.I.; Harriss, R.C.; Bartlett, K.B. Methane Emissions to the Atmosphere Through Aquatic Plants. J. Environ. Qual. 1985, 14, 40–46. [Google Scholar] [CrossRef]
- Inubushi, K.; Sugii, H.; Nishino, S.; Nishino, E. Effect of Aquatic Weeds on Methane Emission from Submerged Paddy Soil. Am. J. Bot. 2001, 88, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.F.; Liu, S.; Zhu, J.; Zhao, L.; Qi, T.; Liang, J.; Luo, J.; Xiao, X.; Fan, X. Limited Aerenchyma Reduces Oxygen Diffusion and Methane Emission in Paddy. J. Environ. Manag. 2021, 279, 111583. [Google Scholar] [CrossRef] [PubMed]
- Machacova, K.; Borak, L.; Agyei, T.; Schindler, T.; Soosaar, K.; Mander, Ü.; Ah-Peng, C. Trees as Net Sinks for Methane (CH4) and Nitrous Oxide (N2O) in the Lowland Tropical Rain Forest on Volcanic Réunion Island. New Phytol. 2021, 229, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Pangala, S.R.; Enrich-Prast, A.; Basso, L.S.; Peixoto, R.B.; Bastviken, D.; Hornibrook, E.R.C.; Gatti, L.v.; Marotta, H.; Calazans, L.S.B.; Sakuragui, C.M.; et al. Large Emissions from Floodplain Trees Close the Amazon Methane Budget. Nature 2017, 552, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Pangala, S.R.; Moore, S.; Hornibrook, E.R.C.; Gauci, V. Trees Are Major Conduits for Methane Egress from Tropical Forested Wetlands. New Phytol. 2013, 197, 524–531. [Google Scholar] [CrossRef]
- Laanbroek, H.J. Methane Emission from Natural Wetlands: Interplay between Emergent Macrophytes and Soil Microbial Processes. A Mini-Review. Ann. Bot. 2010, 105, 141–153. [Google Scholar] [CrossRef]










| Plant | ∆P(Pa) | Air Flow (mL/min) | Plant | ∆P(Pa) | Air Flow (mL/min) |
|---|---|---|---|---|---|
| Monocotyledons | Dicotyledons | ||||
| Cyperaceae | Nymphaeaceae | ||||
| Cyperus compactus Retz. | 20 | 0.60 | Nymphaea rubra Roxb. | 236 | 140 |
| Cyperus digitatus Roxb. | 14 | 1.29 | Nymphaea nouchali Burm. f. | 116 | 15.2 |
| Eleocharis dulcis (Burm. f.) Trin. ex Hensch | 628 | 11.9 | Nelumbonaceae | ||
| Eleocharis acutangula Schultes | 15 | 0.10 | Nelumbo nucifera Gaertn. | 295 | 288 |
| Scirpus grossus L. f. | 3 | 0.22 | Menyanthaceae | ||
| Scirpus littoralis Shrad. | 83 | 0.39 | Nymphoides indica (L.) Kuntze | 485 | 36 |
| Scleria poaeformis Retz. | 22 | 1.11 | Convolvulaceae | ||
| Poaceae | Ipomoea aquatica Forssk. | 3 | 0.18 | ||
| Phragmites vallatoria (L.) Veldkamp | 482 | 1.59 | |||
| Urochloa mutica (Forsk T.Q. Nguyen | 11 | 0.09 | |||
| Hymenachne acutigluma (Steud.) Gilliland | 141 | 0.55 | |||
| Oryza rufipogon Griff. | 23 | 0.32 | |||
| Leersia hexandra Swartz. | 62 | 0.15 | |||
| Pontederiaceae | |||||
| Eichhornia crassipes (Mart.) Solms | 8 | 0.12 | |||
| Araceae | |||||
| Colocasia esculenta (L.) Schott | 3 | 0.10 | i | ||
| Limnocharitaceae | |||||
| Limnocharis flava (L.) Buchenau | 6 | 0.81 | |||
| Plant Group | Taxonomic Group | Source of Gasses | Ventilation Principle | Special Features | Reference |
|---|---|---|---|---|---|
| Submerged | Isoetids | Water, metabolism, Sediment | Diffusion, aeration of rhizosphere via buried leaves | Aerenchyma, CAM | [38,44] |
| Angiosperms | Water, metabolism, Sediment | Diffusion | Metabolic gasses trapped in aerenchyma | [29,31] | |
| Floating | Nuphar spp., Nymphaea spp. | Air, metabolism, Sediment | Pressurized ventilation, thermo-osmotic gas transport, | ‘Heat pump’ drives gasses from the atmosphere via young natant leaves, petioles to roots and back, via older leaves to the atmosphere | e.g., [45,48,51] |
| Nelumbo nucifera | Air, metabolism, Sediment | Pressurized ventilation, influx via laminal stomata of natant leaves through aerenchyma to rhizomes; back from rhizomes through aerenchyma in petiole through stomata in leaf central part | Leaf lamina with fewer and smaller stomata, leaf central part with larger and denser stomata, which actively regulate the airflow by opening and closing | [53,54,55] | |
| Helophytes | Equisetum spp.—4 out of 9 have through-flow convection | Air, possibly also metabolism, sediment | Pressurized ventilation, humidity-induced diffusion, | Air moves through stomata through branches, via interconnecting aerenchyma channels in stem and rhizomes, with venting through the previous year’s stubble or damaged shoot. | [60,61] |
| Phragmites spp. | Air, possibly also metabolism, sediment | Pressurized ventilation, suction via old broken stems (Venturi-effect), air films on leaves when submerged | Via leaves, stems to root system, partly to sediment (ROL), and back to stems, leaves, and atmosphere | [58,63,64,65,126] | |
| Typha spp. | Air, sediment, possibly metabolism, oxygen in the rhizosphere may be obtained from the decomposition of hydrogen peroxide by catalase | Pressurized ventilation, leaf stomata create inner pressure, air films on leaves when submerged | Air enters through middle-aged leaves, and exits through the oldest ones | [58,73,74,76] | |
| Oryza sativa | Air films on leaves when submerged | Flow from above-ground parts via roots by diffusion, and possibly also by mass flow | Water-repellent leaf surface; air layer up to 25 µm, large air spaces inside leaves and roots, the porosity of adventitious roots, a barrier in roots to prevent radial O2 loss from roots | [81,83,89] | |
| Species of mangrove forest | Acrostichum spp. | All plant parts have large air spaces | [94,95] | ||
| Nypa fruticans | Bases of abscised leaves function as air inlets, by developing a network of lenticels covering the leaf base connected to aerenchyma | “snorkeling palm” leaf bases function up to 4 years after leaf abscission | [96] | ||
| Mangroves | High oxygen pressure in the roots is maintained via ventilation through the lenticels on different root structures connected with aerenchyma | Special structures, i.e., pneumatophores, knee roots, stilt roots, or plant roots, provide ventilation during low tides | [99] | ||
| Other wetland species | Alnus spp. | Thermo-osmotically driven gas flow | In Alnus glutinosa, the flow is from the external atmosphere through the stems to the roots | Thermo-osmotic flow in alder is related to the lenticels in the bark of the stem, stem photosynthesis | [4,51,120,127] |
| Taxodium distichum | “knees” emerging from the roots to the surface of the water, flooding increases the porosity of roots, stems, and leaves, and enhanced O2 diffusion to roots. | Snorkeling | [109] | ||
| Syzygium kunstleri | Oxygen transportation occurs through aerenchyma in the root tips, periderm near the root base, and secondary aerenchyma between layers of phellem | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Björn, L.O.; Middleton, B.A.; Germ, M.; Gaberščik, A. Ventilation Systems in Wetland Plant Species. Diversity 2022, 14, 517. https://doi.org/10.3390/d14070517
Björn LO, Middleton BA, Germ M, Gaberščik A. Ventilation Systems in Wetland Plant Species. Diversity. 2022; 14(7):517. https://doi.org/10.3390/d14070517
Chicago/Turabian StyleBjörn, Lars Olof, Beth A. Middleton, Mateja Germ, and Alenka Gaberščik. 2022. "Ventilation Systems in Wetland Plant Species" Diversity 14, no. 7: 517. https://doi.org/10.3390/d14070517
APA StyleBjörn, L. O., Middleton, B. A., Germ, M., & Gaberščik, A. (2022). Ventilation Systems in Wetland Plant Species. Diversity, 14(7), 517. https://doi.org/10.3390/d14070517







