Morphological and Genetic Variability in Radix auricularia (Mollusca: Gastropoda: Lymnaeidae) of Lake Baikal, Siberia: The Story of an Unfinished Invasion into the Ancient Deepest Lake
Abstract
:1. Introduction
2. Material and Methods
2.1. Material Sampling
2.2. Morphological Treatment and Taxonomic Identification of Radix Specimens
2.3. Molecular Techniques and Sequence Analyses
3. Results
3.1. Molecular Phylogeny and Phylogeography
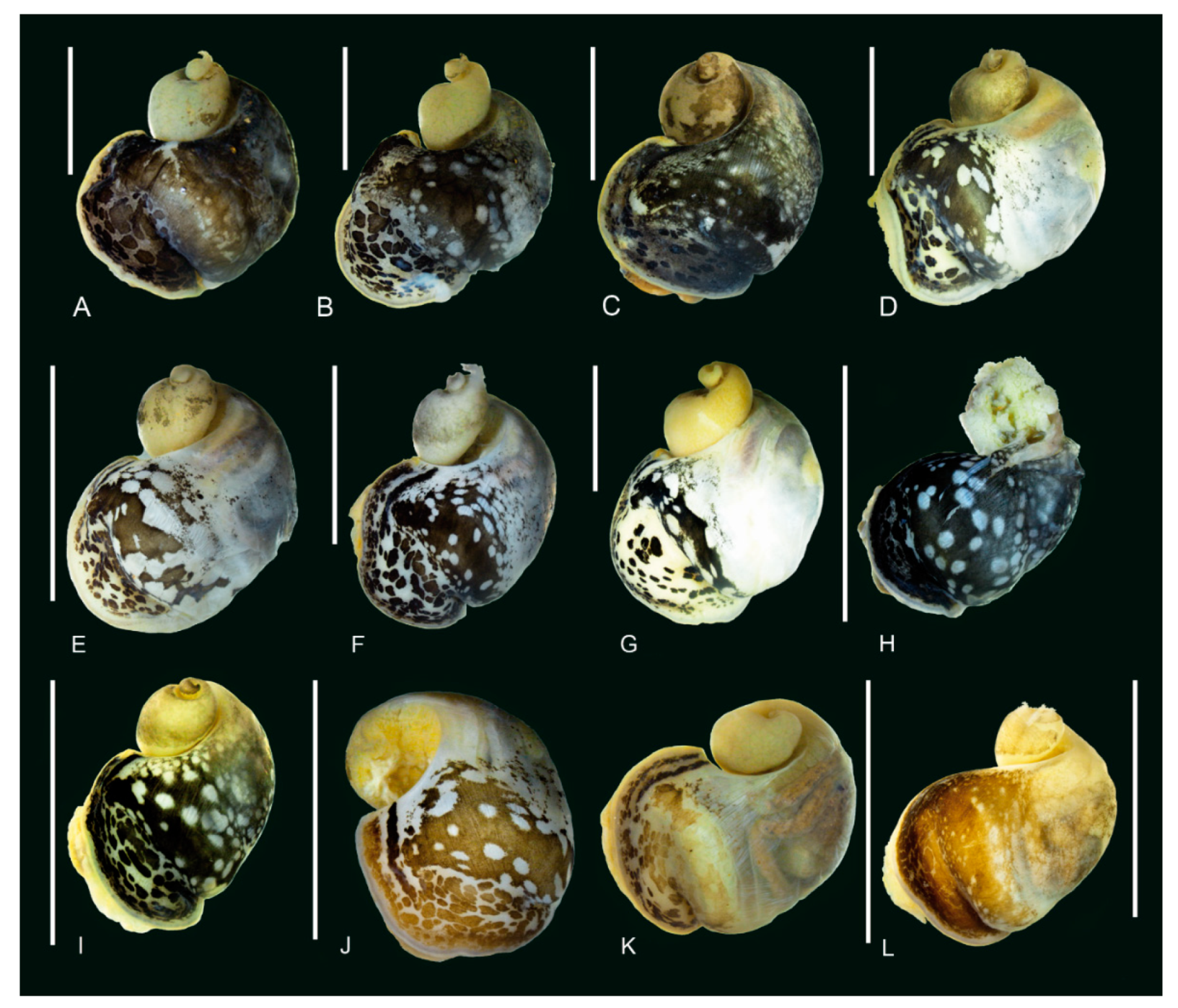
3.2. Morphology of the Baikalian Representatives of Radix
3.3. Morphological Comparison between Baikalian and Extra-Baikalian Populations of Radix auricularia
4. Discussion
4.1. Molecular Phylogeny and Phylogeography
4.2. Morphology
4.3. The Process of Radix auricularia Invasion to Lake Baikal Ecosystem
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozhov, M. Lake Baikal and Its Life; Junk, W.: The Hague, The Netherlands, 1963; p. vii+344. [Google Scholar]
- Martin, P. Lake Baikal. Arch. Für Hydrobiol. 1995, 44, 3–11. [Google Scholar]
- Kozhova, O.M.; Izmest’eva, L.R. (Eds.) Lake Baikal: Evolution and Biodiversity; Backhuys: Leiden, The Netherlands, 1998; 447p. [Google Scholar]
- Sherbakov, D.Y. Molecular phylogenetic studies on the origin of biodiversity in Lake Baikal. Trends Ecol. Evol. 1995, 14, 92–95. [Google Scholar] [CrossRef]
- Roth, R.A. Freshwater Aquatic Biomes; Greenwood Press: Westport, CT, USA; London, UK, 2009; 237p. [Google Scholar]
- Martens, K.; Goddeeris, B.; Coulter, G. (Eds.) Speciation in Ancient Lakes. In Archiv für Hydrobiologie; E. Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1994; Volume 44, pp. 1–508. [Google Scholar]
- Martens, K. Speciation in Ancient Lakes. Trends Ecol. Evol. 1997, 12, 177–182. [Google Scholar] [CrossRef]
- Sideleva, V.G. The Endemic Fishes of Lake Baikal; Backhuys: Leiden, The Netherlands, 2003; 270p. [Google Scholar]
- Sitnikova, T.Y. Recent views on the history and diversity of the Baicalian malacofauna. Arch. Für Hydrobiol. 1994, 44, 319–326. [Google Scholar]
- Sitnikova, T.Y. Baikal gastropods described by W.A. Lindholm. Proc. Zool. Inst. RAS 2019, 323, 214–252. [Google Scholar] [CrossRef]
- Sitnikova, T.Y.; Starobogatov, Y.I.; Shirokaya, A.A.; Shibanova, I.V.; Korobkova, N.V.; Adov, F.V. The phylum mollusca (gastropoda). In Index of Animal Species Inhabiting Lake Baikal and Its Catchment Area; Timoshkin, O.A., Ed.; Nauka: Novosibirsk, Russia, 2014; Volume 2, pp. 937–1002. (In Russian) [Google Scholar]
- Martinson, G.G. Baikalian mollusks in the Tertiary deposits of Xinjiang. In Memory of Academician, L.S. Berg: Collected Essays on Geography and Biology; The USSR Academy of Sciences Press: Moscow, Russia; Leningrad, Russia, 1955; pp. 524–527. (In Russian) [Google Scholar]
- Martinson, G.G. The problem of the Baikal fauna origin. Zool. Zhurnal 1967, 46, 1594–1598. (In Russian) [Google Scholar]
- Starobogatov, Y.I. Fauna of Mollusks and the Zoogeograpghic Regionalization of Fresh Waterbodies of the World; Nauka: Leningrad, Russia, 1970; 372p. (In Russian) [Google Scholar]
- Kozhov, M.M. Mollusks of Baikal Lake. Taxonomy, distribution, ecology, some data on genesis and history. Tr. Baikal’skoi Limnol. Stantsii (Trav. De La Stn. Limnol. Du Lac Bajkal) 1936, 8, 1–320. (In Russian) [Google Scholar]
- Boss, K.J. On the evolution of gastropods in ancient lakes. In Pulmonates: Volume 2A. Systematics, Evolution and Ecology; Fretter, V., Peake, J., Eds.; Academic Press: London, UK, 1978; pp. 385–428. [Google Scholar]
- Starobogatov, Y.I.; Sitnikova, T.Y. The pathway of species formation of the Lake Baikal mollusks. Zhurnal Obs. Biol. 1990, 51, 499–512. (In Russian) [Google Scholar]
- Sitnikova, T.Y. Review of Valvatidae (Gastropoda: Heterobranchia) endemic to Lake Baikal, with taxonomic and morphological notes. Arch. Für Molluskenkd. 2018, 147, 181–201. [Google Scholar] [CrossRef]
- Stelbrink, B.; Shirokaya, A.A.; Clewing, C.; Sitnikova, T.Y.; Prozorova, L.A.; Albrecht, C. Conquest of the deep, old and cold: An exceptional limpet radiation in Lake Baikal. Biol. Lett. 2015, 11, 1–4. [Google Scholar] [CrossRef]
- Hampton, S.E.; Izmest’eva, L.R.; Moore, M.V.; Katz, S.L.; Dennis, B.; Silow, E.A. Sixty years of environmental change in the world’s largest freshwater lake—Lake Baikal, Siberia. Glob. Change Biol. 2008, 14, 1947–1958. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.V.; Hampton, S.E.; Izmest’eva, L.R.; Silow, E.A.; Peshkova, E.A.; Pavlov, B.K. Climate Change and the World’s “Sacred Sea”—Lake Baikal, Siberia. BioScience 2009, 59, 405–417. [Google Scholar] [CrossRef] [Green Version]
- Timoshkin, O.; Samsonov, D.; Yamamuro, M.; Moore, M.; Belykh, O.; Malnik, V.; Sakirko, M.; Shirokaya, A.; Bondarenko, N.; Domysheva, V.; et al. Rapid ecological change in the coastal zone of Lake Baikal (East Siberia): Is the site of the world’s greatest freshwater biodiversity in danger? J. Great Lakes Res. 2016, 42, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Timoshkin, O.; Moore, M.; Kulikova, N.; Tomberg, I.; Malnik, V.; Shimaraev, M.; Troitskaya, E.; Shirokaya, A.; Sinyukovich, V.; Zaitseva, E.; et al. Groundwater contamination by sewage causes benthic algal outbreaks in the littoral zone of Lake Baikal (East Siberia). J. Great Lakes Res. 2018, 44, 230–244. [Google Scholar] [CrossRef]
- Bondarenko, N.A.; Tomberg, I.V.; Shirokaya, A.A.; Belykh, O.I.; Tikhonova, I.V.; Fedorova, G.A.; Netsvetaeva, O.G.; Eletskaya, E.V.; Timoshkin, O.A. Dolichospermum lemmermannii (Nostocales) bloom in world’s deepest Lake Baikal (East Siberia): Abundance, toxicity and factors influencing growth. Limnol. Freshw. Biol. 2021, 1, 1101–1110. [Google Scholar] [CrossRef]
- Hubendick, B. Recent Lymnaeidae. Their Variation, Morphology, Taxonomy, Nomenclature and Distribution. K. Sven. Vetensk. Handl. Ser. 1951, 3, 1–223. [Google Scholar]
- Jackiewicz, M. Błotniarki Europy (Gastropoda: Pulmonata: Lymnaeidae); Kontekst: Poznań, Poland, 2000; 115p. [Google Scholar]
- Kruglov, N.D. Lymnaeid Snails of Europe and Northern Asia; Smolensk State Pedagogical University Press: Smolensk, Russia, 2005; 508p. (In Russian) [Google Scholar]
- Glöer, P. Die Süßwassergastropoden Nord- und Mitteleuropas: Bestimmungsschlüssel, Lebensweise, Verbreitung; Conchbooks: Hackenheim, Germany, 2002; 327p. [Google Scholar]
- Glöer, P. The Freshwater Gastropods of the West-Palaearctis. Vol. I Fresh- and Brackish Waters Except Spring and Subterranean Snails; Glöer, P.: Hetlingen, Germany, 2019; 399p. [Google Scholar]
- Vinarski, M.V.; Aksenova, O.V.; Bolotov, I.N. Taxonomic assessment of genetically-delineated species of radicine snails (Mollusca, Gastropoda, Lymnaeidae). Zoosystematics Evol. 2020, 96, 577–608. [Google Scholar] [CrossRef]
- Burch, J.B. North American Freshwater Snails; Malacological Publications: Hamburg, MI, USA, 1989; p. viii + 365. [Google Scholar]
- Maack, R. Noticen über einige Land- und Süsswassermollusken, gesammelt auf einer Reise zu den Privatgoldwäschen des Jenisseischen Kreises. Mélanges Biol. Tirés Du Bull. Phys.-Math. De L’académie Impériale Des Sci. De St. Pétersbourg 1854, 2, 8–18. [Google Scholar]
- Lindholm, W.A. Die Mollusken des Baikalsees (Gastropoda et Pelecypoda) systematisch und zoogeographisch bearbeitet. In Wissenschaftliche Ergebnisse Einer Zoologische Expedition Nach Dem Baikal-See unter Leitung des. Prof. Alexis Korotneff i. d. J. 1900–1902; Friedländer und Sohn: Kiew, Ukraine; Berlin, Germany, 1909; Volume 4, pp. 1–104. [Google Scholar]
- Dybowski, B. Bemerkungen und Zusatze zu der Arbeit von Dr. W. Dybowski “Mollusken aus der Uferregion des Baicalsees”. Ezhegodnik Zool. Muzeya Imp. Akad. Nauk. 1912, 17, 165–218. [Google Scholar]
- Starobogatov, Y.I.; Streletzkaja, E.A. Composition and zoogeographical characteristics of freshwater malacofauna of the East Siberia and northern part of the Far East. Tr. Zool. Inst. AN SSSR 1967, 42, 221–268. (In Russian) [Google Scholar]
- Izzatullaev, Z.I.; Kruglov, N.D.; Starobogatov, Y.I. New and ill-known species of mollusks of the subgenus Radix of the genus Lymnaea of the USSR fauna from the Central Asia (Gastropoda, Pulmonata). Izv. Akad. Nauk. Tajikskoy SSR Otd. Biol. Nauk. 1983, 4, 53–57. (In Russian) [Google Scholar]
- Kruglov, N.D.; Starobogatov, Y.I. Annotated and illustrated catalogue of species of the family Lymnaeidae (Gastropoda Pulmonata Lymnaeiformes) of Palaearctic and adjacent river drainage areas. Part, I. Ruthеnica. Russ. Malacol. J. 1993, 3, 65–92. [Google Scholar]
- Andreyeva, S.I.; Andreyev, N.I.; Vinarski, M.V. Key to Freshwater Gastropods of Western Siberia (Mollusca: Gastropoda). V. 1. Gastropoda: Pulmonata. Fasc. 1. Families Acroloxidae and Lymnaeidae; The Authors: Omsk, Russia, 2010; 200p. (In Russian) [Google Scholar]
- Stift, M.; Michel, E.; Sitnikova, T.Y.; Mamonova, E.Y.; Sherbakov, D.Y. Palaearctic gastropod gains a foothold in the dominion of endemics: Range expansion and morphological change of Lymnaea (Radix) auricularia in Lake Baikal. Hydrobiologia 2004, 513, 101–108. [Google Scholar] [CrossRef]
- De Boer, M.G.; Stift, M.; Michel, E. Two new highly polymorphic microsatellite loci and inadvertent minisatellite loci for Lymnaea auricularia. J. Molluscan Stud. 2004, 70, 115–116. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, C.T.; de Souza, C.P.; de Moura Soares, D. Possible competitive displacement of planorbids by Melanoides tuberculata in Minas Gerais, Brazil. Mem. Do Inst. Oswaldo Cruz 2001, 96 (Suppl. S1), 173–176. [Google Scholar] [CrossRef] [Green Version]
- Cianfanelli, S.; Lori, E.; Bodon, M. Alien freshwater molluscs in Italy and their distribution. In Biological Invaders in Inland Waters: Profiles, Distribution, and Threats; Gherardi, F., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 103–121. [Google Scholar]
- Zukowski, S.; Walter, K.F. Freshwater snails in competition: Alien Physa acuta (Physidae) and native Glyptophysa gibbosa (Planorbidae) in the River Murray, South Australia. Mar. Freshw. Res. 2009, 60, 999–1005. [Google Scholar] [CrossRef]
- Rohlf, F.J. tpsDig, version 2.36; Stony Brook: State University of New York: New York, NY, USA, 2017. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST. Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. Available online: https://palaeo-electronica.org/2001_1/past/past.pdf (accessed on 15 April 2022).
- Rohlf, F.J. tpsRelw, version 1.67; Stony Brook: State University of New York: New York, NY, USA, 2017. [Google Scholar]
- Schniebs, K.; Glöer, P.; Vinarski, M.V.; Hundsdoerfer, A.K. Intraspecific morphological and genetic variability in Radix balthica (Linnaeus, 1758) (Gastropoda: Basommatophora: Lymnaeidae) with morphological comparison to other European Radix species. J. Conchol. 2011, 40, 657–678. [Google Scholar]
- Schniebs, K.; Glöer, P.; Vinarski, M.V.; Hundsdoerfer, A.K. Intraspecific morphological and genetic variability in the European freshwater snail Radix labiata (Rossmaessler, 1835) (Gastropoda: Basommatophora: Lymnaeidae). Contrib. Zool. 2013, 82, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Schniebs, K.; Glöer, P.; Georgiev, D.; Hundsdoerfer, A.K. A molecular genetic evidence of the occurrence of the freshwater snail Radix lagotis (Schrank, 1803) (Gastropoda: Lymnaeidae) in Bulgaria. Ecologica 2015, 3, 29–39. [Google Scholar] [CrossRef]
- Schniebs, K.; Glöer, P.; Vinarski, M.V.; Quinonero-Salgado, S.; Lopez-Soriano, J.; Hundsdoerfer, A.K. A new alien species in Europe: First record of Austropeplea viridis (Quoy & Gaimard, 1833) (Mollusca, Gastropoda, Lymnaeidae) in Spain. J. Conchol. 2017, 42, 357–370. [Google Scholar]
- Schniebs, K.; Glöer, P.; Quinonero-Salgado, S.; Lopez-Soriano, J.; Hundsdoerfer, A.K. The first record of Galba cubensis (L. Pfeiffer, 1839) (Mollusca, Gastropoda, Lymnaeidae) from open fields in Europe. Folia Malacol. 2018, 26, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Schniebs, K.; Glöer, P.; Vinarski, M.V.; Beran, L.; Hundsdoerfer, A. Intraspecific morphological and genetic variability in the Palaearctic freshwater snail Radix ampla (Hartmann, 1821) (Gastropoda: Basommatophora: Lymnaeidae). J. Conchol. 2019, 43, 245–267. [Google Scholar]
- Schniebs, K.; Bössneck, U.; Hundsdoerfer, A.K. On the identity of Radix peregra (O. F. Müller, 1774) (Gastropoda: Basommatophora: Lymnaeidae) in the Azores. J. Conchol. 2020, 43, 531–541. [Google Scholar]
- Vinarski, M.; Schniebs, K.; Glöer, P.; Hundsdoerfer, A. The taxonomic status and phylogenetic relationships of the genus Aenigmomphiscola Kruglov and Starobogatov, 1981 (Gastropoda: Pulmonata: Lymnaeidae). J. Nat. Hist. 2011, 45, 2049–2068. [Google Scholar] [CrossRef]
- Vinarski, M.V.; Aksenova, O.V.; Bespalaya, Y.V.; Bolotov, I.N.; Schniebs, K.; Gofarov, M.Y.; Kondakov, A.V. Radix dolgini: The integrative taxonomic approach supports the species status of a Siberian endemic snail (Mollusca, Gastropoda, Lymnaeidae). Comptes Rendus Biol. 2016, 339, 24–36. [Google Scholar] [CrossRef]
- Merritt, T.J.S.; Shi, L.; Chase, M.C.; Rex, M.A.; Etter, R.J.; Quattro, J.M. Universal cytochrome b primers facilitate intraspecific studies in molluscan taxa. Mol. Mar. Biol. Biotechnol. 1998, 7, 7–11. [Google Scholar]
- Bargues, M.V.; Vigo, M.; Horak, P.; Dvorak, J.; Patzner, R.A.; Pointier, J.-P.; Jackiewicz, M.; Meier-Brook, C.; Mas-Coma, S. European Lymnaeidae (Mollusca: Gastropoda), intermediate hosts of trematodiases, based on nuclear ribosomal DNA ITS-2 sequences. Infect. Genet. Evol. 2001, 1, 85–107. [Google Scholar] [CrossRef]
- Almeyda-Artigas, R.J.; Bargues, M.D.; Mas-Coma, S. ITS-2 rDNA sequencing of Gnathostoma species (Nematoda) and elucidation of the species causing human gnathostomiasis in the Americas. J. Parasitol. 2000, 6, 537–544. [Google Scholar] [CrossRef]
- Aksenova, O.V.; Bolotov, I.N.; Gofarov, M.Y.; Kondakov, A.V.; Vinarski, M.V.; Bespalaya, Y.V.; Kolosova, Y.S.; Palatov, D.M.; Sokolova, S.E.; Spitsyn, V.M.; et al. Species richness, molecular taxonomy and biogeography of the radicine pond snails (Gastropoda: Lymnaeidae) in the Old World. Sci. Rep. 2018, 8, 11199. [Google Scholar] [CrossRef] [Green Version]
- Hall, T.A. BioEdit: A user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Simmons, M.P.; Ochoterena, H. Gaps as characters in sequence-based phylogenetic analyses. Syst. Biol. 2000, 49, 369–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swofford, D.L. PAUP*—Phylogenetic Analysis Using Parsimony (*and Other Ethods); Version 4; Sinauer Associates Inc.: Sunderland, MA, USA, 2002. [Google Scholar]
- Silvestro, D.; Michalak, I. raxmlGUI: A Graphical Front-End for RAxML. 2012. Available online: http://sourceforge.net/projects/raxmlgui/ (accessed on 20 February 2018).
- Stamatakis, A.; Ludwig, T.; Meier, H. Raxml-iii: A fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics 2005, 21, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Vinarski, M.V.; Kantor, Y.I. Analytical Catalogue of Fresh and Brackish Water Molluscs of Russia and Adjacent Countries; A.N. Severtsov Institute of Ecology and Evolution of RAS: Moscow, Russia, 2016; 543p. [Google Scholar]
- Shirokaya, A.A.; Sitnikova TYa Vinarski, M.V.; Palatov, D.M.; Kijashko, P.V.; Izzatullaev, Z.I. Fresh- and brackish-water gastropod taxa described by Zuvaidullo I. Izzatullaev. Arch. Für Molluskenkd. 2019, 148, 197–261. [Google Scholar] [CrossRef]
- Sitnikova, T.Y.; Takhteev, V.V. Gastropods of the hot springs of Transbaikalia. In Hydrobiology of the Eastern Siberian Waterbodies. Trudy Biologo-Pochvennogo Fakulteta IGU; Irkutsk State University Press: Irkutsk, Russia, 2006; Volume 6, pp. 137–150. (In Russian) [Google Scholar]
- Piechocki, A.; Wawrzyniak-Wydrowska, B. Guide to Freshwater and Marine Mollusca of Poland; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2016; 279p. [Google Scholar]
- Stadnichenko, A.P. Lymnaeids and Acroloxids (Lymnaeidae, Acroloxidae) of the Ukraine; Tsentr Uchebnoy Literatury: Kiev, Ukraine, 2004; 327p. (In Russian) [Google Scholar]
- Khokhutkin, I.M.; Vinarski, M.V.; Grebennikov, M.E. Molluscs of the Urals and the Adjacent Areas. The Family Lymnaeidae (Gastropoda, Pulmonata, Lymnaeiformes); Goshchitsky Publishers: Ekaterinburg, Russia, 2009; 162p. (In Russian) [Google Scholar]
- Vinarski, M.V. On the reality of local and ecological races in lymnaeid snails (Mollusca, Gastropoda, Lymnaeidae). Biol. Bull. 2016, 43, 1003–1017. [Google Scholar] [CrossRef]
- Aksenova, O.; Vinarski, M.; Bolotov, I.; Kondakov, A.; Bespalaya Yu Tomilova, A.; Paltser, I.; Gofarov, M. Two Radix spp. (Gastropoda: Lymnaeidae) endemic to thermal springs around Lake Baikal represent ecotypes of the widespread Radix auricularia. J. Zool. Syst. Evol. Res. 2017, 55, 298–309. [Google Scholar] [CrossRef]
- Glöer, P. Süßwassermollusken. Ein Bestimmungsschlüssel für die Bundesrepublik Deutschlands; 14. überarbeitete Auflage; Deutscher Jugendbund für Naturbeobachtung: Göttingen, Germany, 2015; p. 135S. [Google Scholar]
- Glöer, P.; Pešić, V. Radix skutaris n. sp., a new species from Montenegro (Gastropoda: Lymnaeidae). Mollusca 2008, 26, 83–88. [Google Scholar]
- Albrecht, C.; Wolff, C.; Glöer, P.; Wilke, T. Concurrent evolution of ancient sister lakes and sister species: The freshwater gastropod genus Radix in lakes Ohrid and Prespa. Hydrobiologia 2008, 615, 157–167. [Google Scholar] [CrossRef]
- Shimaraev, M.N.; Domysheva, V.M. Trends in hydrological and hydrochemical processes in Lake Baikal under conditions of modern climate change. In Climatic Change and Global Warming of Inland Waters. Impacts and Mitigation for Ecosystems and Societies; Goldman, C.R., Kumagai, M., Robarts, R.D., Eds.; John Wiley & Sons: Chichester, UK, 2013; pp. 43–66. [Google Scholar]
- Voznesenskiy, A.V. Outline of the climate peculiarities of Lake Baikal. In Lotsia i Fiziko-Geographicheskiy Ocherk Ozera Baikal (Maps and Physical-Geographical Outline of Lake Baikal); Drizhenko, O.K., Ed.; Glavnoe Gidrographicheskoe upravlenie: St. Petersburg, Russia, 1908; pp. 178–329. (In Russian) [Google Scholar]
- Timoshkin, O.A.; Zaytseva, E.P. Water temperature dynamics in the shallow zone of western coast of Southern Baikal (area of Bolshie Koty Bay, rivers Bolshaya Kotinka and Zhilische) as revealed from measurements by Onset Stowaway Tidbit loggers. In Index of Animal Species Inhabiting Lake Baikal and Its Catchment Area; Timoshkin, O.A., Ed.; Nauka: Novosibirsk, Russia, 2009; Volume 2, pp. 732–759. (In Russian) [Google Scholar]
- Koryakov, E.A.; Glazunov, I.V.; Vilisova, I.K. Coastal lakes of Baikal before its regulation. In Limnologiya Pribrezhno-Sorovoy Zony Baikala; Lut, B.F., Ed.; Nauka: Novosibirsk, Russia, 1977; pp. 4–44. (In Russian) [Google Scholar]
- Shimaraev, N.M.; Kuimova, L.N. The temperature regime and thermal balance. In Limnologiya Pribrezhno-Sorovoy Zony Baikala; Lut, B.F., Ed.; Nauka: Novosibirsk, Russia, 1977; pp. 82–106. (In Russian) [Google Scholar]
- Vereshchagina, K.; Kondrateva, E.; Mutin, A.; Jakob, L.; Bedulina, D.; Shchapova, E.; Madyarova, E.; Axenov-Gribanov, D.; Luckenbach, T.; Pörtner, H.-O.; et al. Low annual temperature likely prevents the Holarctic amphipod Gammarus lacustris from invading Lake Baikal. Sci. Rep. 2021, 11, 10532. [Google Scholar] [CrossRef] [PubMed]
- Jakob, L.; Axenov-Gribanov, D.V.; Gurkov, A.N.; Ginzburg, M.; Bedulina, D.S.; Timofeyev, M.A.; Luckenbach, T.; Lucassen, M.; Sartoris, F.J.; Pörtner, H.O. Lake Baikal amphipods under climate change: Thermal constraints and ecological consequences. Ecosphere 2016, 7, e01308. [Google Scholar] [CrossRef] [Green Version]
- Lipaeva, P.; Vereshchagina, K.; Drozdova, P.; Jakob, L.; Kondrateva, E.; Lucassen, M.; Bedulina, D.; Timofeev, M.; Stadler, P.; Luckenbach, T. Different ways to play it cool: Transcriptomic analysis sheds light on different activity patterns of three amphipod species under long-term cold exposure. Mol. Ecol. 2021, 30, 5735–5751. [Google Scholar] [CrossRef] [PubMed]
- Aksenova, O.V.; Vinarski, M.V.; Bolotov INBeslapaya, Y.u.V.; Kondakov, A.V.; Paltser, I.S. An overview of Radix species of the Kamchatka Peninsula (Gastropoda: Lymnaeidae). Bull. Russ. Far East. Malacol. Soc. 2016, 20, 5–27. [Google Scholar]
- Von Oheimb, P.V.; Landler, L.; Von Oheimb, K.C.M. Cold snails in hot springs: Observations from Patagonia and the Tibetan Plateau. Malacologia 2016, 59, 313–320. [Google Scholar] [CrossRef]
- Vinarski, M.V.; Bolotov, I.N.; Aksenova, O.V.; Babushkin, E.S.; Bespalaya, Y.V.; Makhrov, A.A.; Nekhaev, I.O.; Vikhrev, I.V. Freshwater Mollusca of the Circumpolar Arctic: A review on their taxonomy, diversity and biogeography. Hydrobiologia 2021, 848, 2891–2918. [Google Scholar] [CrossRef]
- Johnson, L.E.; Ricciardi, A.; Cartlon, J.T. Overland dispersal of aquatic invasive species: A risk assessment of transient recreational boating. Ecol. Appl. 2001, 11, 1789–1799. [Google Scholar] [CrossRef]
- De Ventura, L.; Weissert, N.; Tobias, R.; Kopp, K.; Jokela, J. Overland transport of recreational boats as a spreading vector of zebra mussel Dreissena polymorpha. Biol. Invasions 2016, 18, 1451–1466. Available online: https://link.springer.com/article/10.1007/s10530-016-1094-5. (accessed on 12 April 2022). [CrossRef]
- Bacela-Spychalska, K.; Grabowski, M.; Rewicz, T.; Konopacka, A.; Wattier, R. The ‘killer shrimp’ Dikerogammarus villosus (Crustacea, Amphipoda) invading Alpine lakes: Overland transport by recreational boats and scuba-diving gear as potential entry vectors? Aquat. Conserv. Mar. Freshw. Ecosyst. 2013, 23, 606–618. [Google Scholar] [CrossRef]
- Verbolov, V.I. Currents and water exchange in Lake Baikal. Vodn. Resur. 1996, 23, 413–423. (In Russian) [Google Scholar]
- Forel, F.-A. Introduction à l’étude de la faune profonde du lac Léman. Bull. De La Société Vaud. Des Sci. Nat. 1869, 10, 217–223. [Google Scholar]
- Forel, F.-A. Matériaux pour servir à l’étude de la faune profonde du lac Léman. 1” série. Bull. De La Société Vaud. Des Sci. Nat. 1874, 13, 1–164. [Google Scholar]
- Roszkowski, W. Contribution a l’étude des limnées du Lac Léman. Rev. Suisse De Zool. 1914, 22, 457–539. [Google Scholar]
- Dybowski, W. Die Gastropoden-Fauna des Baicalsees Anatomisch und Systematisch Bearbeitet; Memories de L’Academie Imperiale des Sciences de St. Petersbourg: St. Petersbourg, Russia, 1875; Volume 22, pp. 1–73. [Google Scholar]
- Piaget, J. Les récents dragages malacologiques de M. le Prof. Émile Yung dans le lac Léman. J. De Conchyliol. 1912, 60, 205–233. [Google Scholar]
- Germain, L. Mollusques terrestres et fluviatiles. Fauna De Fr. 1931, 22, 479–897. [Google Scholar]
- Clessin, S. Die Mollusken der Tiefenfauna unserer Alpenseen. Malakozool. Blätter 1877, 24, 159–185. [Google Scholar]
- Brot, A. Matériaux Pour Servir à l’étude de la Faune Profonde du lac Léman. Bull. De La Société Vaud. Des Sci. Nat. 1874, 13, 109–113. [Google Scholar]
- Piaget, J. Les mollusques sublittoraux du Léman recueillis par M. le Prof. Yung. Zool. Anz. 1913, 42, 615–624. [Google Scholar]
- Roszkowski, W. Notes sur les Limnées de la faune profonde du lac Leman. Zool. Anz. 1912, 40, 375–381. [Google Scholar]
- Albrecht, C.; Wilke, T.; Kuhn, K.; Streit, B. Convergent evolution of shell shape in freshwater limpets: The African genus Burnupia. Zool. J. Linn. Soc. 2004, 140, 577–588. [Google Scholar] [CrossRef]
- Plam, M.; Jørgensen, A.; Kristensen, T.h.K.; Madsen, H. Sympatric Biomphalaria species (Gastropoda: Planorbidae) in lake Albert, Uganda, show homoplasies in shell morphology. Afr. Zool. 2008, 43, 34–44. [Google Scholar] [CrossRef]
- Moore, A.C.; Burch, J.B.; Duda, T.F., Jr. Recognition of a highly restricted freshwater snail lineage (Physidae: Physella) in southeastern Oregon: Convergent evolution, historical context, and conservation considerations. Conserv. Genet. 2015, 16, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Colautti, R.I.; Lau, J.A. Contemporary evolution during invasion: Evidence for differentiation, natural selection, and local adaptation. Mol. Evol. 2015, 24, 1999–2017. [Google Scholar] [CrossRef]
- Lande, R. Evolution of phenotypic plasticity in colonizing species. Mol. Ecol. 2015, 24, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Chevin, L.-M.; Hoffmann, A.A. Evolution of phenotypic plasticity in extreme environments. Philos. Transacions R. Society. Ser. B 2017, 372, 20160138. [Google Scholar] [CrossRef]
- Stanley, S.M. Adaptive morphology of the shell in bivalves and gastropods. In The Mollusca. Form and Function; Trueman, E.R., Clarke, M.R., Eds.; Academic Press: Londion, UK, 1988; Volume 11, pp. 105–141. [Google Scholar]
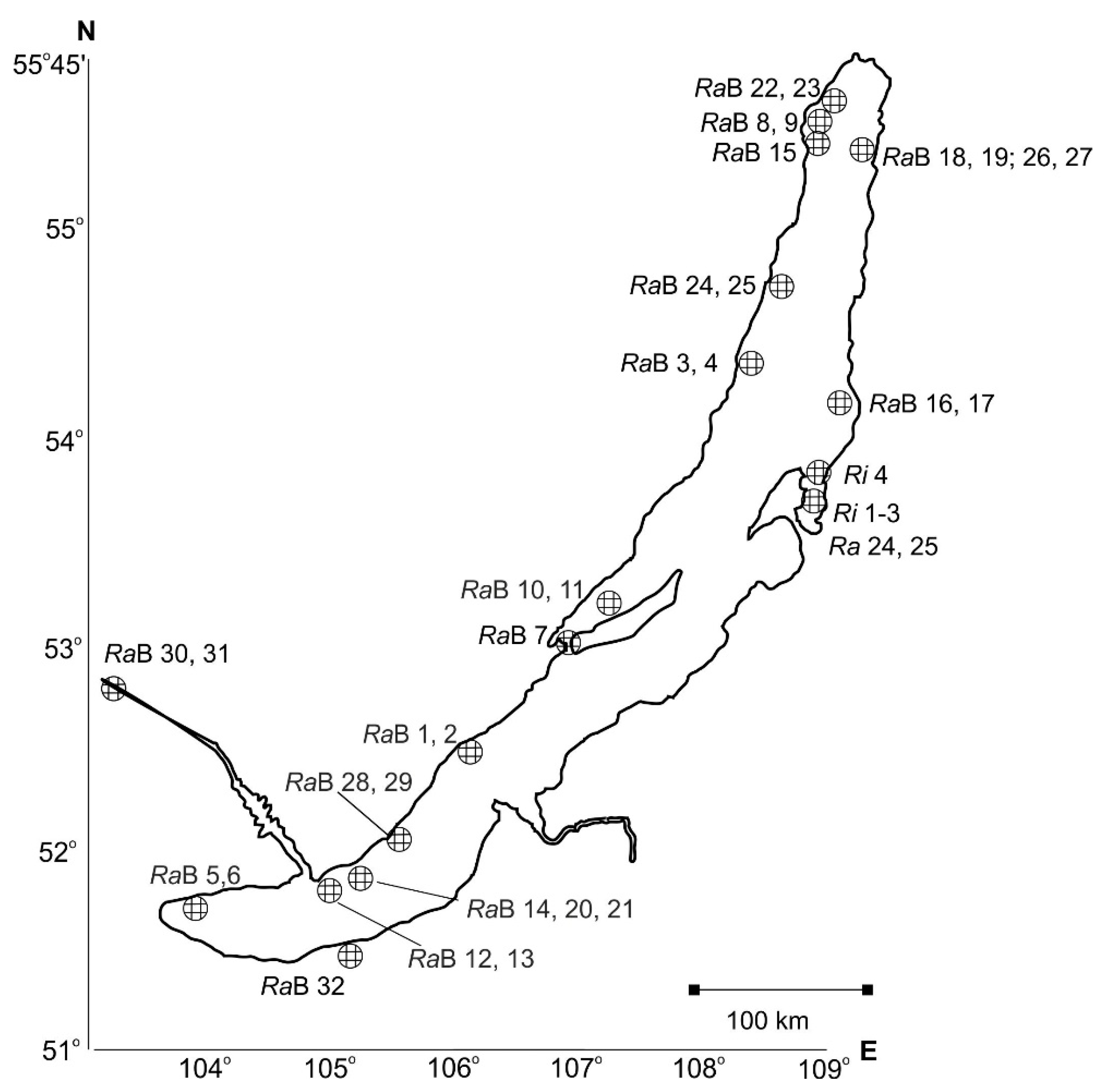










| Region | Locality, Coordinates (Snail Numbers Correspond to Figure 1) | N |
|---|---|---|
| Baikal Lake | ||
| Northwestern Baikal | Bolshaya Kosa Bay, N 54°45′; E 108°50′ (RaB 24, 25) | 5 |
| Ayaya Bay, N 55°27′, E 109°52′ (RaB 18, 19, 26, 27) | 15 | |
| Near Zarechnoye settlement, N 55°27′, E 109°52′ (RaB 18, 19, 26, 27) | 7 | |
| Near Cape Slyudyanskiy N 55°30′; E 109°12′ (RaB 15) | 9 | |
| Muzhinay Cape, N 54°51′, E 108°54″ | 6 | |
| Near Valukan Cape, N 54°18′; E 109°26′ (RaB 16, 17) | 5 | |
| Near Elokhin Cape, N 54°33′; E 108°39′ (RaB 3, 4) | 10 | |
| Near Solontsovyi Cape, N 54°10′, E 108°22′ | 2 | |
| Onokochanskaya Bay, N 55°32′, E 109°11′ | 2 | |
| Senogda Bay, N 55°34′; E 109°13′ (RaB 8, 9) | 5 | |
| Maloye More Strait (=Small Sea) | Zagli-nuur Bay, N 53°02′; E 106°75′ * | 2 |
| Kharin-Irgi Bay, N 53°03′, E 106°55′ (RaB 7) * | 2 | |
| Shibeteyskaya Bay, 53°08.08′; E 107°05.53′ (RaB 10, 11) | 5 | |
| Southwestern Baikal | Babushka Bay, N 52°15′; E 105°42′ (RaB 28, 29) | 2 |
| Peschanaya Bay, N 52°15′41″, E 105°42′9″ | 3 | |
| Near Bolshiye Koty settlement (Zhilishche Ravine), N 51°53.96′; E 105°03.84′ (RaB 14, 20, 21) | 12 | |
| Near Buguldeyka settlement, N 52°32′; E 106°05′ (RaB 1, 2) | 8 | |
| Near Listvennichnoye settlement, N 51°51′; E 104°50′ (RaB 12, 13) | 13 | |
| Near Cape Zub (Khabartuy), N 51°43′; E 103°52′ (RaB 5, 6) | 2 | |
| Chivyrkuy Bay and eastern Baikal | Near Cape Fertik, N 53°48′, E 109°03′ (Ri 4) * | 23 |
| Near Katun’ village, N 53°41’ E 109°17’ (Ri 1, 2, 3) * | 24 | |
| Kotovo Bay, N 53° 39’ E 108° 58′ (Ra 24, 25) * | 6 | |
| Zmeinaya Bight, N 53°46′ E 109°0′ * | 20 | |
| Total: | 188 | |
| Other territories | ||
| Western Mongolia | Khar-Nuur Lake, N 48°22′ E 95°37′ (near Ra 23, 26) | 32 |
| Eastern Siberia | Irkutsk Region, an oxbow of the Kirenga River, N 54°42′ E 105°47′ | 22 |
| Western Siberia | Omsk Region, Krivoye Lake, N 56°46′ E 74°36′ (near Ra 3) | 36 |
| Total: | 90 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schniebs, K.; Sitnikova, T.Y.; Vinarski, M.V.; Müller, A.; Khanaev, I.V.; Hundsdoerfer, A.K. Morphological and Genetic Variability in Radix auricularia (Mollusca: Gastropoda: Lymnaeidae) of Lake Baikal, Siberia: The Story of an Unfinished Invasion into the Ancient Deepest Lake. Diversity 2022, 14, 527. https://doi.org/10.3390/d14070527
Schniebs K, Sitnikova TY, Vinarski MV, Müller A, Khanaev IV, Hundsdoerfer AK. Morphological and Genetic Variability in Radix auricularia (Mollusca: Gastropoda: Lymnaeidae) of Lake Baikal, Siberia: The Story of an Unfinished Invasion into the Ancient Deepest Lake. Diversity. 2022; 14(7):527. https://doi.org/10.3390/d14070527
Chicago/Turabian StyleSchniebs, Katrin, Tatiana Ya. Sitnikova, Maxim V. Vinarski, Anke Müller, Igor V. Khanaev, and Anna K. Hundsdoerfer. 2022. "Morphological and Genetic Variability in Radix auricularia (Mollusca: Gastropoda: Lymnaeidae) of Lake Baikal, Siberia: The Story of an Unfinished Invasion into the Ancient Deepest Lake" Diversity 14, no. 7: 527. https://doi.org/10.3390/d14070527
APA StyleSchniebs, K., Sitnikova, T. Y., Vinarski, M. V., Müller, A., Khanaev, I. V., & Hundsdoerfer, A. K. (2022). Morphological and Genetic Variability in Radix auricularia (Mollusca: Gastropoda: Lymnaeidae) of Lake Baikal, Siberia: The Story of an Unfinished Invasion into the Ancient Deepest Lake. Diversity, 14(7), 527. https://doi.org/10.3390/d14070527







