Abstract
We conducted an extensive literature review in search of records of dendrolimnetic Psychodinae, with additional field sampling of the European species of Psychodinae associated with water-filled tree holes. After checking more than 100 publications, only 11 specific published records involving dendrolimnetic Psychodinae were found. Our results show that six genera, represented by 13 species of Psychodinae, are associated with 13 species of plant trees. As a result of our field sampling, we report Lepiseodina latipennis (Sarà, 1953) and Telmatoscopus bartai (Ježek, 2004) comb. nov. for the first time in Germany. Furthermore, we redescribe L. latipennis based on freshly collected material with a closer examination of the holotype. Derived from our findings, we review the genera Lepiseodina Enderlein, 1937 and Telmatoscopus Eaton, 1904, providing an identification key for the males of both genera. In addition, we synonymyze Krivosheinoscopus Ježek, 2001 syn. nov. under Telmatoscopus, changing combination of Telmatoscopus ussuricus (Ježek, 2001) comb. nov. and Telmatoscopus bartai (Ježek, 2004), additionally, we change combination and a sononymy of Tematoscopus wagneri (Salmanna, 1982) comb. et syn. nov. under Telmatoscopus advena (Eaton, 1893). Furthermore, we describe for the first time the female and eggs of Telmatoscopus advena. Moreover, we provide the first published DNA barcodes (COI) for Telmatoscopus bartai, Lepiseodina latipennis (Sarà, 1953), Lepiseodina rothschildi (Eaton, 1912), and Lepiseodina tristis (Meigen, 1830). Finally, we also discuss the taxonomy and ecology of the European dendrolimnetic species of the subfamily Psychodinae.
1. Introduction
Phytotelmata (singular phytotelma) derives from the Greek words “phyto”, which means plant, and “telma”, meaning pond, and was first proposed by Varga [1], based on his observations, to define the microhabitats product of water-filled bodies in plant surfaces [2]. Within the phytotelmata, we can find the dendrotelmata (singular dendrotelma), deriving from the Greek words “dendron”, meaning tree, and “telma”, which circumscribe the body waters only to water-filled tree holes. These dendrotelmata are considered an integral microhabitat inside forest ecosystems, as they provide a suitable space for development, prey, and water sources for many organisms ranging from invertebrates to vertebrates [3,4]. Species that relate to or inhabit these water-filled tree holes are known as dendrolimnetic, from the Greek words “dendron”, and “limnḗtēs”, which means relating to or inhabiting the open water of a body of fresh water.
Dendrotelmata are commonly found in old trees, dead or alive, and are a considered crucial component of ecosystems, especially with the modern forestry management where the number of mature and over-mature trees (commonly referred as old trees) has decreased [5]. The main sources of nutrients flowing in the tree holes consist of leaf litter and arthropod cadavers, while the quality and composition of these nutrients vary across species and habitats [6,7]. The fact that these microhabitats have a small size, discrete boundaries, and are naturally replicated in nature, makes them attractive for studies of community structure and functionalities [7]. Researchers have reported the usage of dendrotelmata by amphibians during development, as a water source for reptiles and small mammals, and, as bathing sites for birds and bats [4]. Nonetheless, mainly invertebrates with aquatic development have been reported developing inside these microhabitats, with some reports of other invertebrates that use them as a water source [4,8]. Among them, the most common organisms found inside are immature stages of Diptera and Coleoptera [7,8,9,10].
Moth flies (Insecta: Diptera: Psychodidae) are commonly found in water bodies, as the majority of psychodid species develop in water during larval stages, with a few exceptions that develop in soil, dung, or fungi [11,12,13]. There are six subfamilies recognized worldwide, namely Bruchomyiinae, Horaiellinae, Phlebotominae, Psychodinae, Sycoracinae, and Trichomyiinae; all of them except Horaiellinae are present in Europe [14,15]. Species in the subfamily Trichomyiinae develop inside tree holes or rotting wood and their larvae are considered xylophagous (from Greek “xylon”, meaning wood, and “faguein”, meaning eating) [5]. Species of Bruchomyiinae and Phlebotominae have been reported developing on the ground and leaf litter feeding on decaying organic matter [15]. The larval stages of Sycoracinae species have been found developing in aquatic mosses and leaf litter [15]. On the contrary, species of the subfamily Psychodinae are commonly reported developing in water bodies such as ponds and streams, but there are a few genera present in Europe whose larvae develop inside of tree holes, namely Clogmia, Clytocerus, Lepiseodina, Pneumia, Psychoda, and Telmatoscopus [13,16,17,18,19,20,21,22,23,24,25]. Although the larval development and habitat for some European species are known, the moth flies associated with water-filled tree holes have been poorly studied in Europe [25].
In the present study, we revise the available literature about the European species of the subfamily Psychodinae known to develop in dendrotelmata, with a special emphasis on the genera Lepiseodina and Telmatoscopus. Additionally, we study the dendrolimnetic moth flies collected in Germany resulting from the German Barcode of Life (GBOL) project [26] (www.bolgermany.de, accessed on 02 April 2022). As an outcome, we redescribe Lepiseodina latipennis (Sarà, 1953) from Germany (Nordrhein-Westfalen) and Italy (Sicily) based on new morphological and molecular data, and we synonymize Krivosheinoscopus Ježek, 2001 syn. nov. under Telmatoscopus Eaton, 1904. Furthermore, we change combination and synonymize Telmatoscopus wagneri (Salamanna, 1982) comb. nov. et syn. nov. under Telmatoscopus advena (Eaton, 1893), and we describe for the first time the female and eggs of Telmatoscopus advena (Eaton, 1893) based on morphological and molecular data. Moreover, we provide the first record of Lepiseodina latipennis (Sarà, 1953) and Telmatoscopus bartai Ježek 2004 comb. nov. from Germany. Furthermore, we provide the first COI (5′-end of the cytochrome c oxidase subunit I) sequences, also known as DNA barcode, for Telmatoscopus bartai (Ježek, 2001) comb. nov., Lepiseodina latipennis (Sarà, 1953), Lepiseodina rothschildi (Eaton, 1912), and Lepiseodina tristis (Meigen, 1830).
2. Material and methods
2.1. Geographic Scope
We follow the proposed geographic boundaries of Europe by de Jong et al. [27] with the following limits: East: Ural (E 60°), West: Atlantic Ocean (W 30°), South: Mediterranean (N 35°), and North: Atlantic Islands (N 82°).
2.2. Literature Records
Literature search was conducted by tracking references from known literature with the help of search engines (e.g., www.scholar.google.com, accessed on 5 Jaunary 2022) and scientific databases (e.g., www.jstor.org, www.scopus.com, www.webofscience.com, 5 January 2022) using the search keywords “Psychodidae, Psychodinae, dendrolimnetic, Diptera, water-filled tree holes”. Literature used for the study encompasses records since the description of the species to the most recent published works until the beginning of 2022, focusing on Psychodinae and dendrolimnetic studies. More than 100 items were analyzed; however, only 11 included records of dendrolimnetic Psychodinae (as listed in Table 1).
2.3. Sampling
Specimens were collected using Malaise traps during the years 2013–2021 as part of the German Barcode of Life (GBOL) project [26] (www.bolgermany.de, accessed on 2 April 2022). Specimens were preserved in 96% ethanol and stored at −20 °C until they were dissected for DNA extraction and preparing permanent slides. All sampled specimens are stored at the Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany (ZFMK). Further material was bred from organic matter sampled from dendrotelmata, and, occasionally collected with a Malaise trap and directly collected in Italy, during 2010–2022 (AM).
Additional examined material is deposited in the following natural history collections mentioned in the text using their acronyms:
AM: Alessio Morelli private collection, Pianella (PE), Italy; later to be deposited at the Natural History Museum of Genova.
BNHM: British National History Museum, London, United Kingdom.
MNGD: Museo di Storia Naturale Giacomo Doria, Comune di Genova, Genova, Italy.
NMP: Národní Muzeum, Prague, Czech Republic.
ZFMK: Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany.
2.4. Study of the Collected Material and Terminology
After lysis (see Genetics below), the specimens were cleared using NaOH 10%, dissected, and permanently mounted using Euparal (Waldeck GmbH & Co. KG, Division Chroma, Havixbecker Straße, Münster, Germany) as mounting medium, following the procedure detailed in Ibáñez-Bernal [28], with the modification that prior to the diaphanization process, we performed the dissection of the head, wings, and terminal abdominal segments to macerate them in NaOH 10% and continue with the procedure, while preserving the remaining tissue (thorax, legs, first abdominal segments) in ethanol for posterior DNA extraction. Additional material was macerated in KOH 10%, transferred in acetic acid 10%, and dehydrated in acetone 99%. Specimens were dissected in clove oil and parts mounted on microscope slides with Canada balm. For the additional material, some specimens were photographed to keep a quality image of the habitus.
We follow the general terminology proposed by Cumming et al. and Kvifte and Wagner [15,29], except for the male terminalia that we use the term “hypopods” for the posterior genitalic appendages, which have been treated in the literature as cercopodia or surstyli originating in the 9th abdominal segment, or 10th segment, or a combination of both, as proposed by Kvifte and Wagner [30]. For the female Terminalia we follow Kotrba [31]. Egg terminology follows De Almeida et al. [32].
In the examined material of each species, a code is provided for each examined specimen (e.g., ZFMK-DIP-000852020), and all the label information is found as Supplementary Material (Table S1).
2.5. Genetics
Specimens were processed at the ZFMK, where lysis and PCR were performed following the protocol and primers by [33,34]. After the PCR, samples were sent to Beijing Genomics Institute (BGI) for bidirectional sequencing. Raw data were curated manually using Geneious (v. 7.1.9). Final COI sequences were 658 bp long. All sequences will be publicly available at www.bolgermany.de. BOLD and genebank accession IDs can be found in the Supplementary Material.
3. Results
Based on our literature review, we report 13 species of Psychodinae associated with dendrotelmata belonging to six genera (Table 1), namely Clogmia [C. albipunctata (Williston, 1893)], Clytocerus [C. xylophilus Vaillant, 1983], Lepiseodina [L. latipennis (Sarà, 1953), L. rothschildi (Eaton, 1912) and L. tristis (Meigen, 1830)], Pneumia [P. canescens (Meigen, 1804) and P. trivialis (Eaton, 1893)], Psychoda [P. alternata Say, 1824, P. cinerea Banks, 1894 and P. minuta Banks, 1894] and Telmatoscopus [T. advena (Eaton, 1893), T. bartai (Ježek, 2004) comb. nov., and T. thuringicus Beran, Doczkal, Pfister & Wagner, 2010]. Additionally, we list Telmatoscopus bartai (Jezek, 2004) comb. nov. as a potential dendrolimnetic species, giving arguments for this decision.

Table 1.
Dendrolimnetic Psychodinae taxa reported for Europe, tree species where it was reported and references. Double asterisk (**) denotates a new record of tree species for the Psychodinae species.
Table 1.
Dendrolimnetic Psychodinae taxa reported for Europe, tree species where it was reported and references. Double asterisk (**) denotates a new record of tree species for the Psychodinae species.
| Taxon | Tree Species | Reference |
|---|---|---|
| Clogmia albipunctata (Williston, 1893) | Oak (Quercus sp.), not specified | [35,36] |
| Clytocerus xylophilus Vaillant, 1983 | Lime tree (Tilia sp.) | [37] |
| Lepiseodina tristis (Meigen, 1830) | Ash (Fraxinus sp.), beech (Fagus sp.), birch (Betula sp.), cherry (Prunus sp.), elm (Ulmus sp.), maple (Acer sp.), oak (Quercus sp.), mulberry (Morus sp.), lime (Tilia sp.), not specified, ** Populus nigra L. | [13,22,25,36,38] |
| Lepiseodina rothschildi (Eaton, 1912) | Maple (Acer sp.), oak (Quercus sp.), not specified | [9,13,38] |
| Lepiseodina latipennis (Sarà, 1953) | ** Maple tree (Acer sp.), ** Oak (Quercus sp.) | |
| Pneumia canescens (Meigen, 1804) | Not specified | [18,25] |
| Pneumia trivialis (Eaton, 1893) | Not specified | [22,25] |
| Psychoda alternata Say, 1824 | Not specified | [23] |
| Psychoda cinerea Banks, 1894 | Apple (Malus sp.), oak (Quercus sp.), ** Hornbeam (Carpinus or Ostrya sp.) | [25] |
| Psychoda minuta Banks, 1894 | Maple (Acer sp.), oak (Quercus sp.) | [25] |
| Telmatoscopus advena (Eaton, 1893) | Ash (Fraxinus sp.), birch (Betula sp.), elm (Ulmus sp.), oak (Quercus sp.), sycamore (Platanus sp.) | [13,22,25,38] |
| Telmatoscopus thuringicus Beran, Doczkal, Pfister & Wagner, 2010 | Not specified (assumption of development) | [5] |
| Telmatoscopus laurencei Freeman, 1953 | Lime tree (Tilia sp.) | [39] |
3.1. Key to the Males of European Psychodinae Genera Found in Dendrotelmata
Differential diagnosis. Adults of the subfamily Psychodinae can be easily differentiated from the adults of the exclusively xylophagous subfamily Trichomyiinae, which can also be found in tree holes, by the presence of an eye bridge in Psychodinae (absent in Trichomyiinae) and wing vein R with five branches in Psychodinae, with two longitudinal veins between radial and medial forks (vein R with four branches in Trichomyiinae, with one longitudinal vein between radial and medial forks).
- 1.
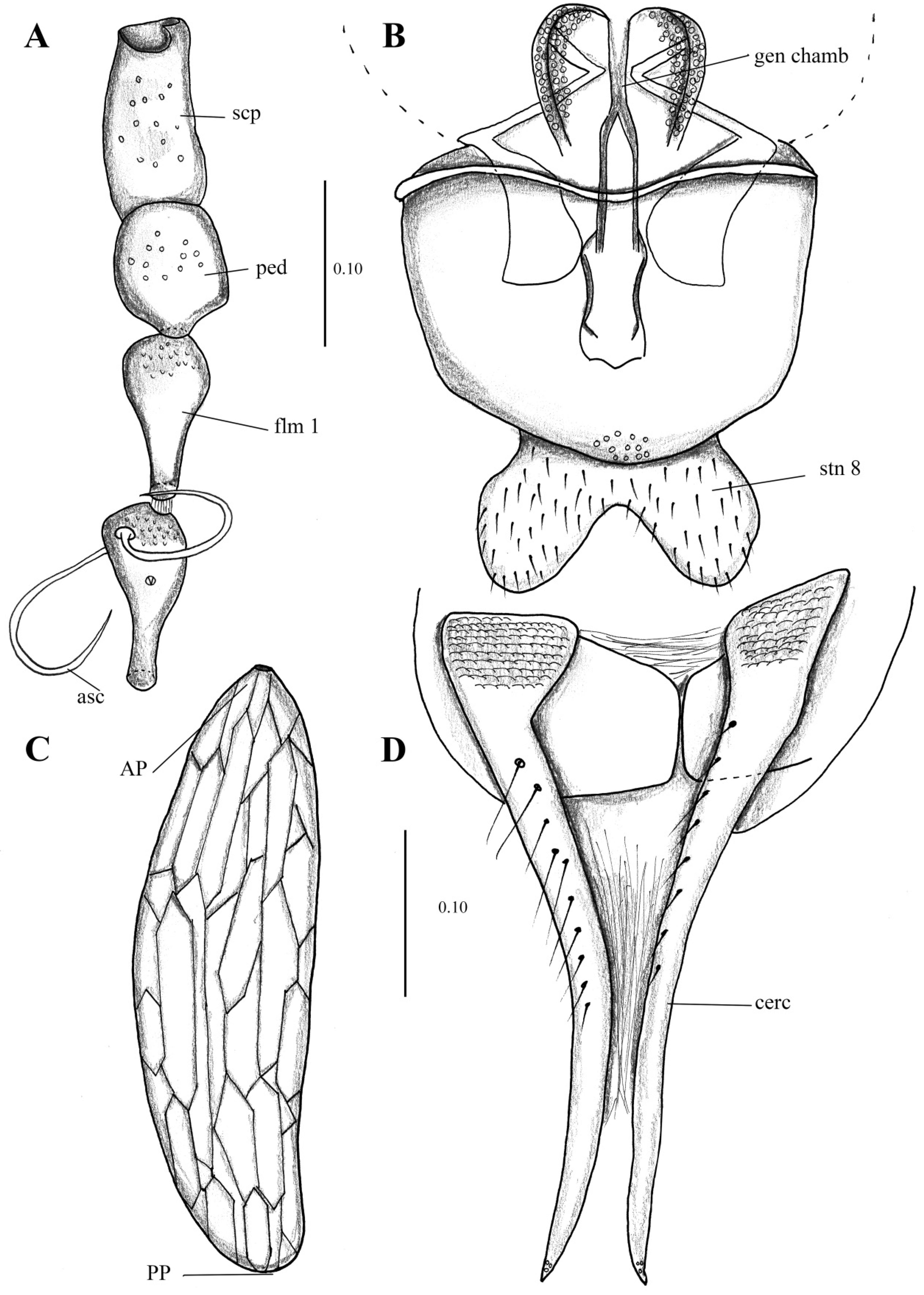
- Antenna with at least flagellomeres 2–10 nodiform, divided into a basal nod and a distal neck (Figure 1B,D)…3
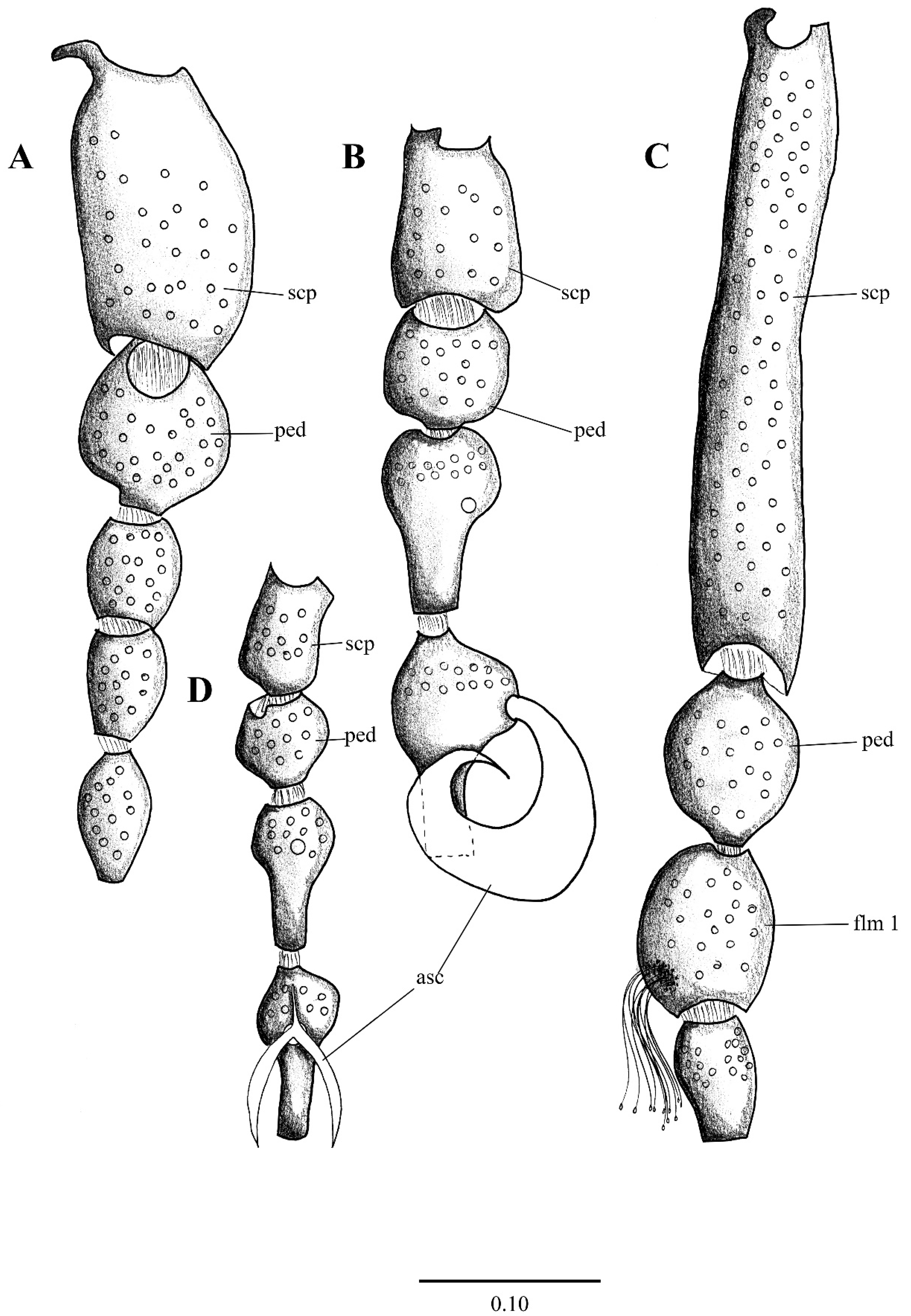
 Figure 1. First antennal segments (left antenna) of: (A). Pneumia trivialis (Eaton, 1893), (B) Telmatoscopus advena (Eaton, 1893), (C). Clytocerus ocellaris (Meigen, 1818), (D). Psychoda sp. Abbreviations: asc = ascoids, flm = flagellomere, ped = pedicel, scp = scape. Scale (A–C) in millimeters (mm).
Figure 1. First antennal segments (left antenna) of: (A). Pneumia trivialis (Eaton, 1893), (B) Telmatoscopus advena (Eaton, 1893), (C). Clytocerus ocellaris (Meigen, 1818), (D). Psychoda sp. Abbreviations: asc = ascoids, flm = flagellomere, ped = pedicel, scp = scape. Scale (A–C) in millimeters (mm).
- -
- Antenna with flagellomeres cylindrical or fusiform (Figure 1A,C)…2
- 2.
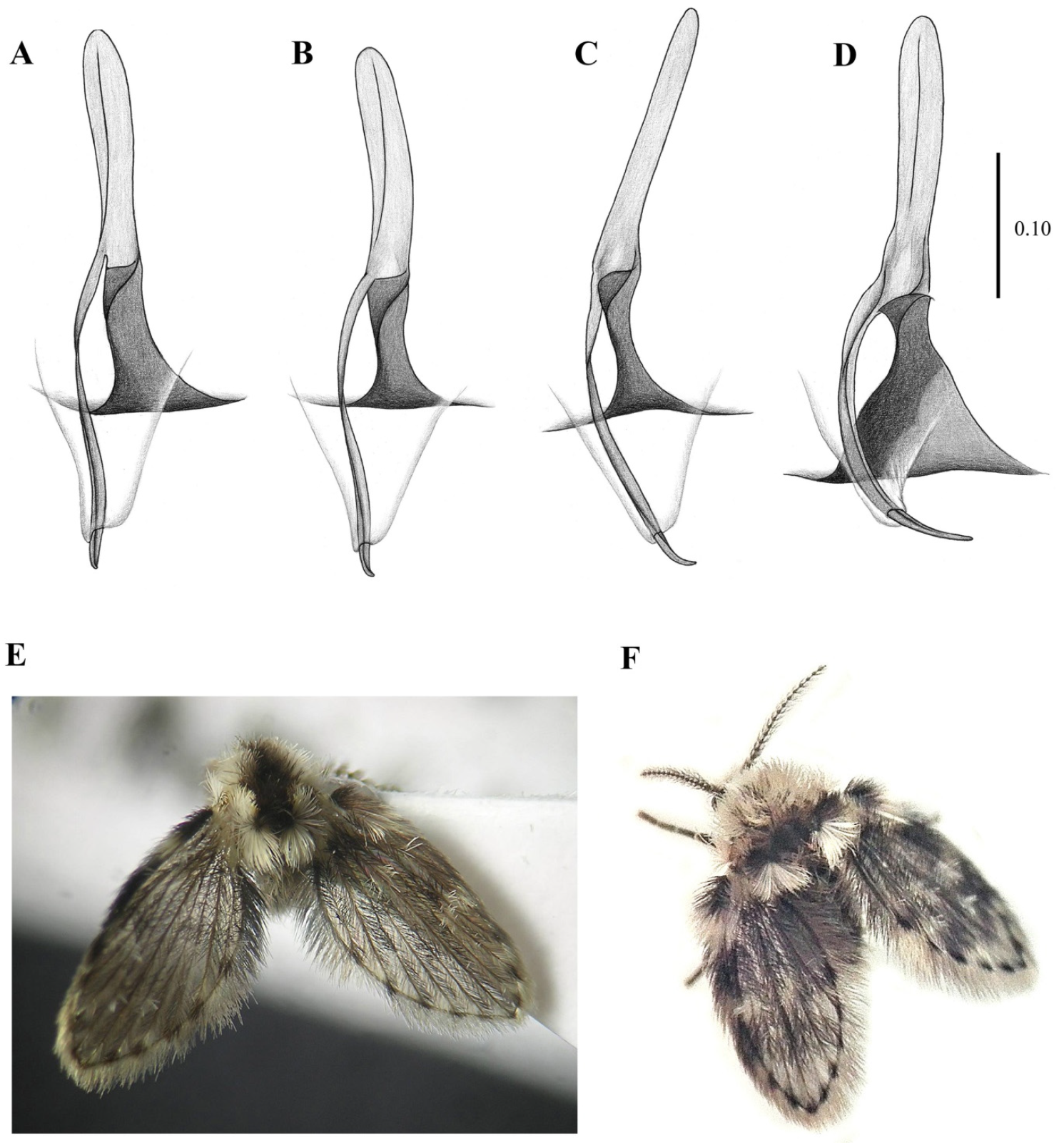
- Cornicula (everted sac-shaped structures on the back surface of the head (presumed scent organs); also known as patagia) usually present; antennal scape more than three times longer than pedicel; flagellomere 1 with a distal brush of wavy setae (Figure 1C)…Clytocerus Eaton, 1904
- -
- Cornicula always absent; antennal scape less than three times the length of pedicel; flagellomere 1 without a distal brush of wavy setae (Figure 1A)…Pneumia Enderlein, 1935
- 3.
- Ascoids with anterior and posterior branches (ascoids Y-shaped) (Figure 1D); hypopods of males with only one apical tenaculum…Psychoda Latreille, 1797
- -
- Ascoids with a single curved branch or carrying only anterior branches (ascoids digitiform and not Y-shaped) (Figure 1B); hypopods of males with four or more apical tenacula…4
- 4.
- Ascoids bifurcate (as in Kvifte & Wagner [15], Figure 16; also in Ibáñez-Bernal [40], Figures 55 and 56); tenacula distally knife-shaped and shorter than basal width of hypopods (as in Ibáñez-Bernal [40], Figure 58)…Clogmia Enderlein, 1937
- -
- Ascoids with a single digitiform or leaf-shaped branch, not bifurcate (Figure 1B); tenacula distally feathered and longer than the basal width of hypopods (Figure 2B, Figure 3B, Figure 4D, Figure 5B, Figure 6B, Figure 7B, Figure 8A,C, Figure 9A–C, Figure 10B and Figure 11B)…5
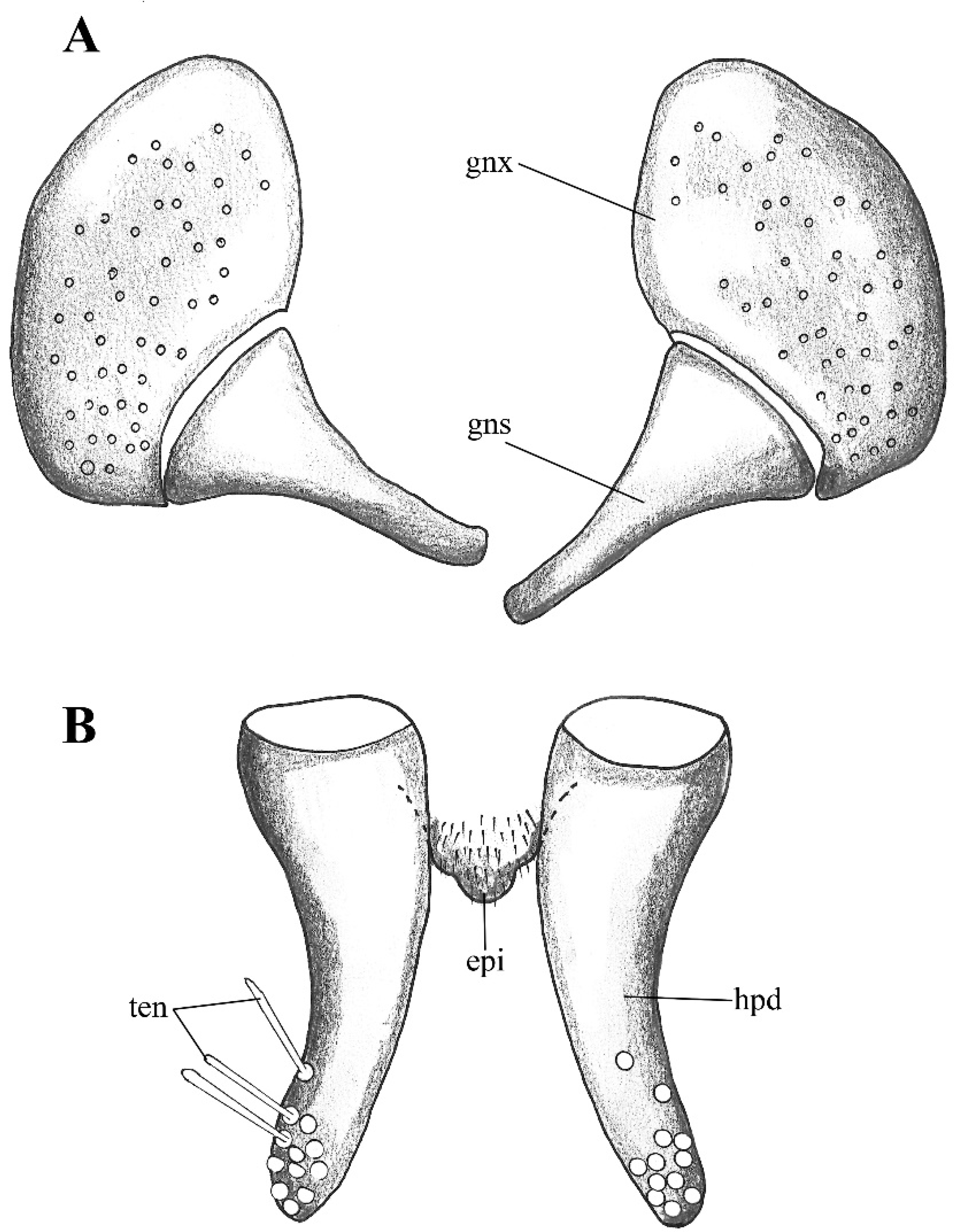
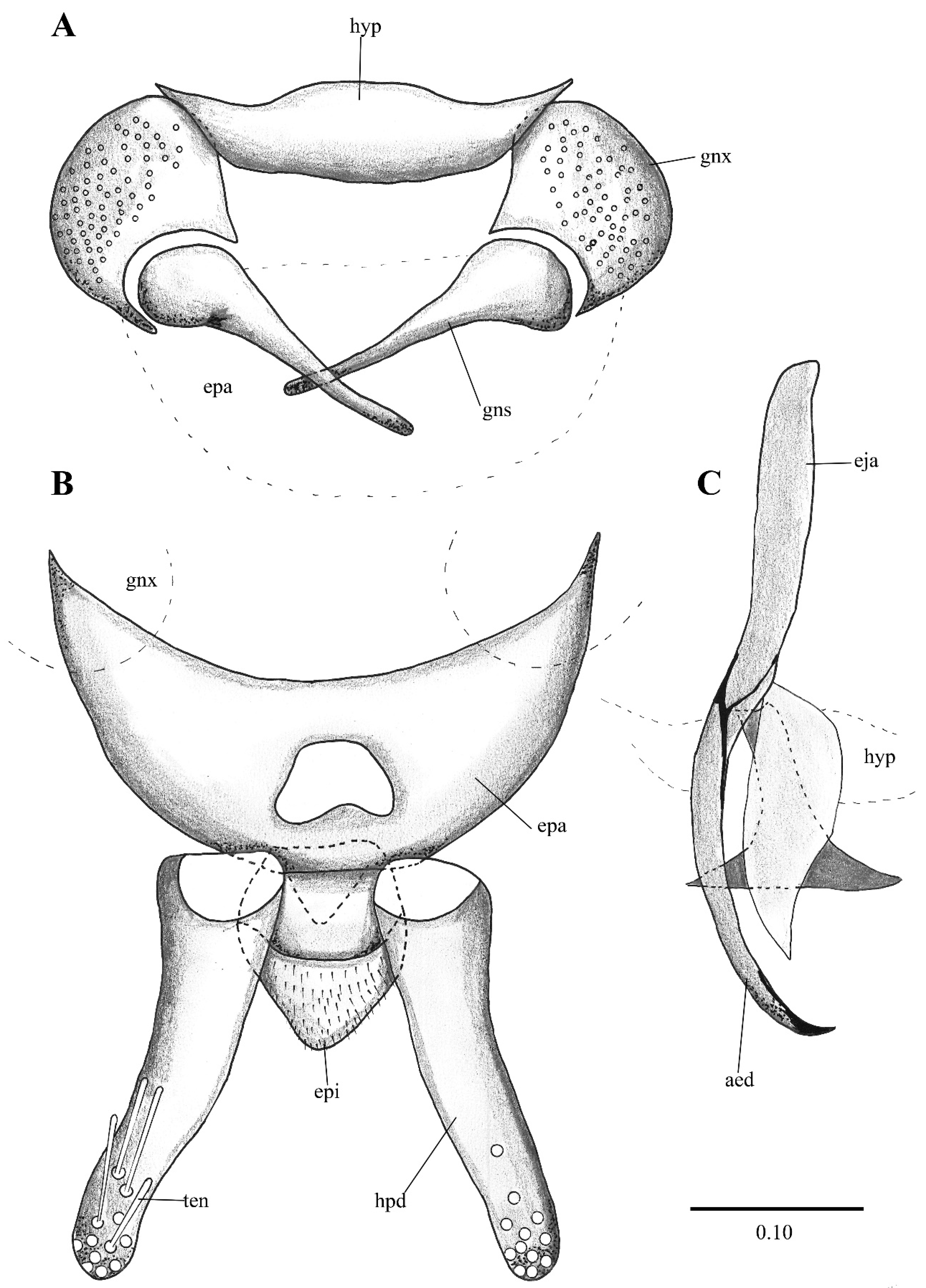
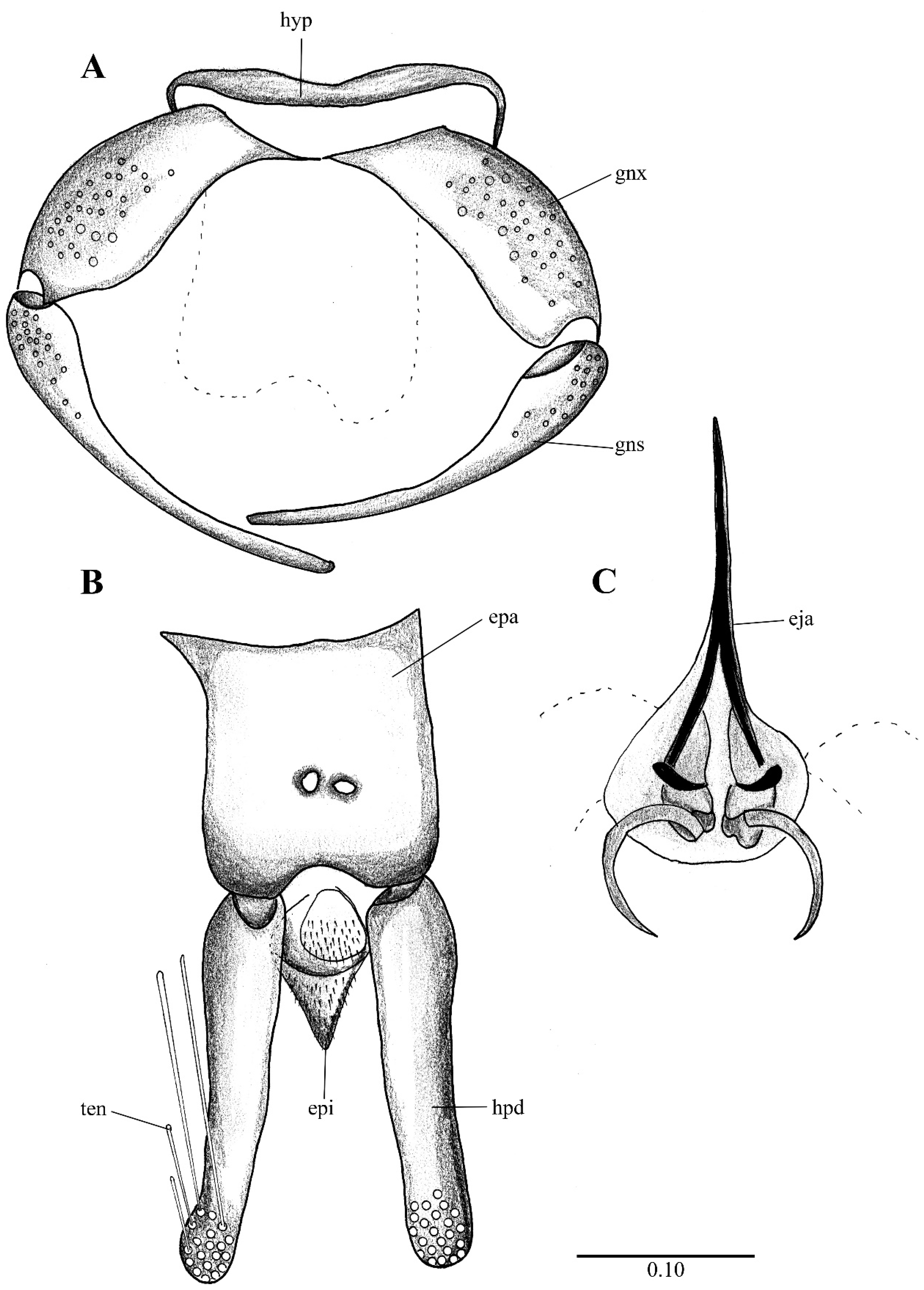
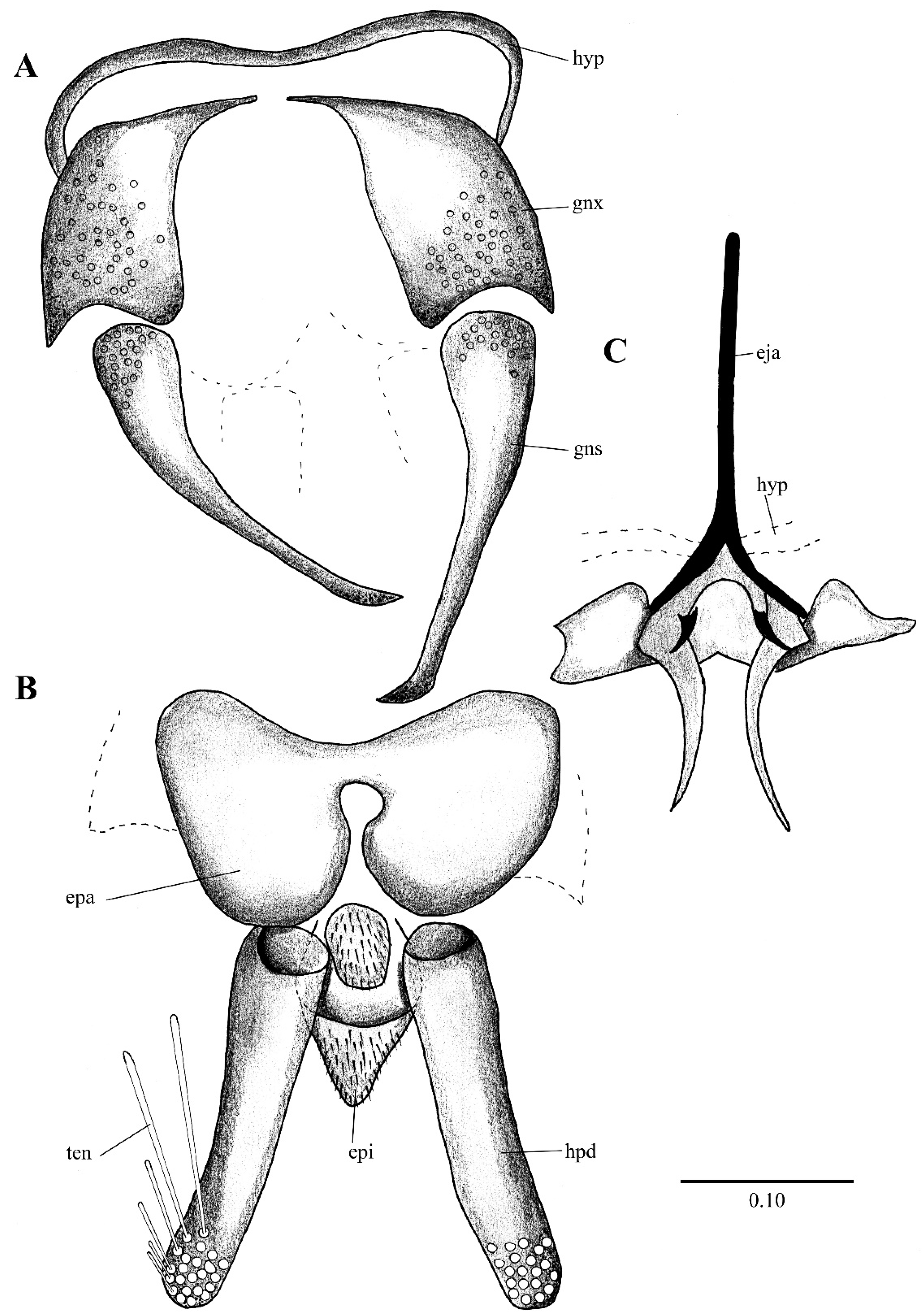
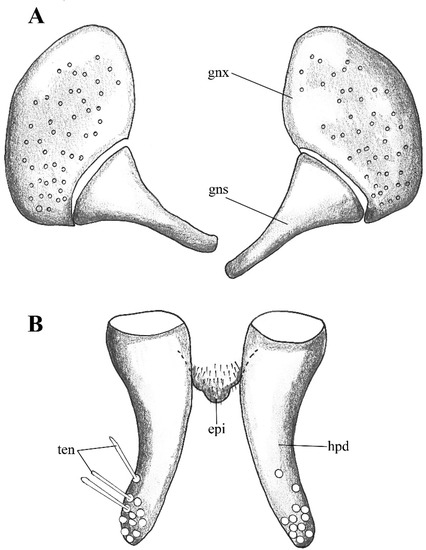 Figure 2. Lepiseodina latipennis (Sarà 1953), male genitalia adapted from the original description by Sarà (1953). (A). Gonocoxites and gonostyli. (B). Hypopods and hypoproct. Abbreviations: gnx = gonocoxite, gns = gonostyli, hpd = hypopod, epi = epiproct, ten = tenacula. No scale available based on original drawing.
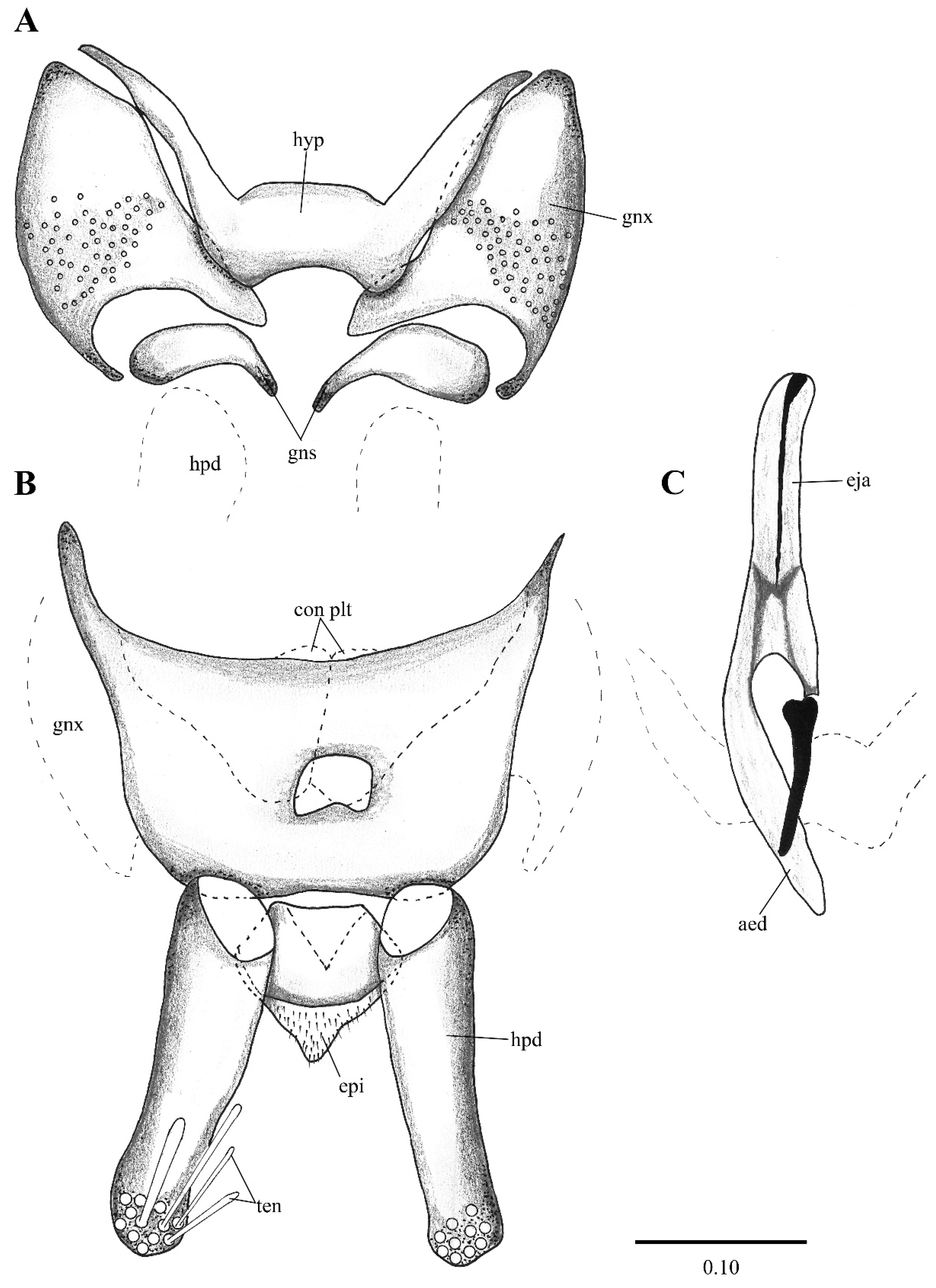
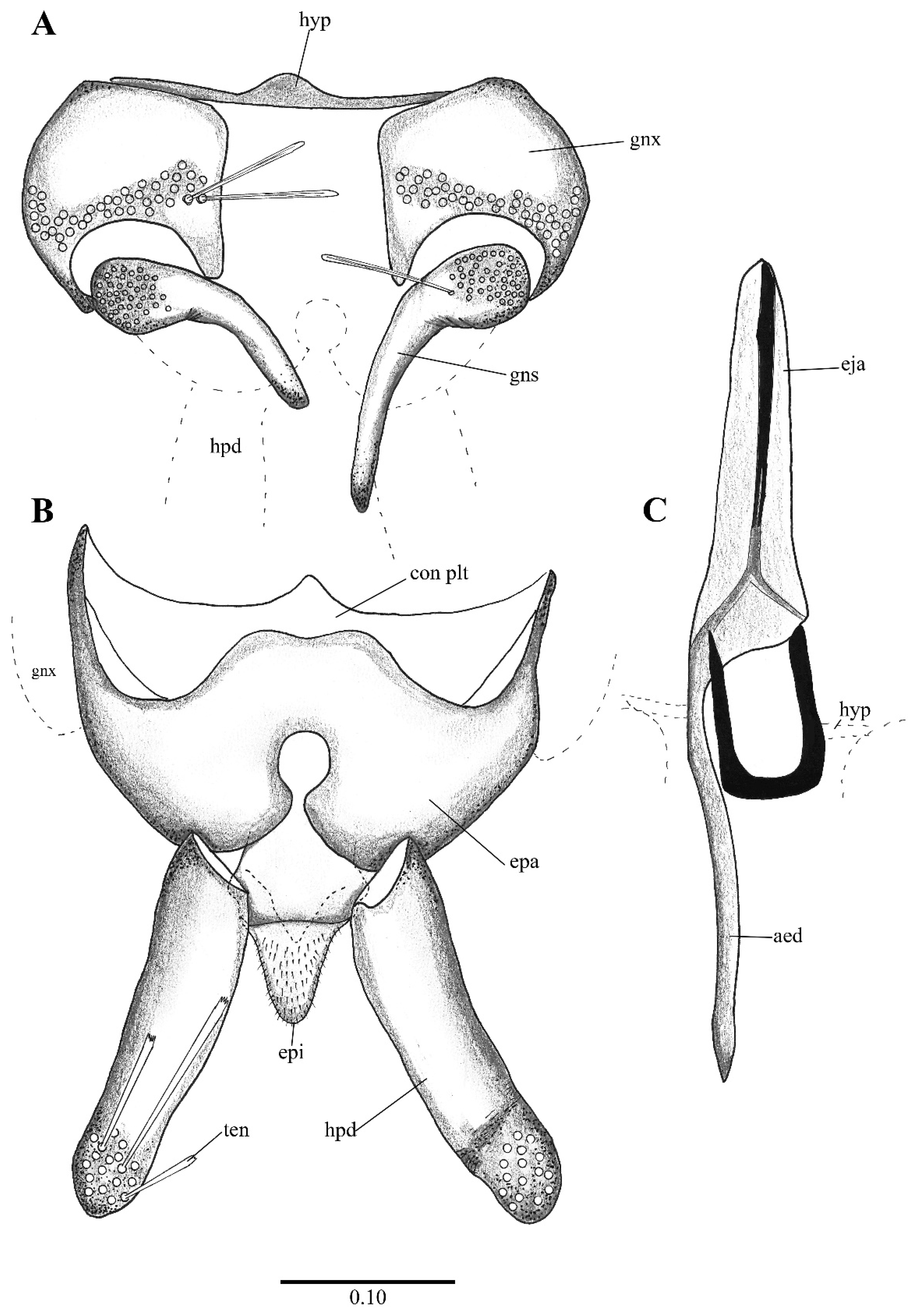
Figure 2. Lepiseodina latipennis (Sarà 1953), male genitalia adapted from the original description by Sarà (1953). (A). Gonocoxites and gonostyli. (B). Hypopods and hypoproct. Abbreviations: gnx = gonocoxite, gns = gonostyli, hpd = hypopod, epi = epiproct, ten = tenacula. No scale available based on original drawing.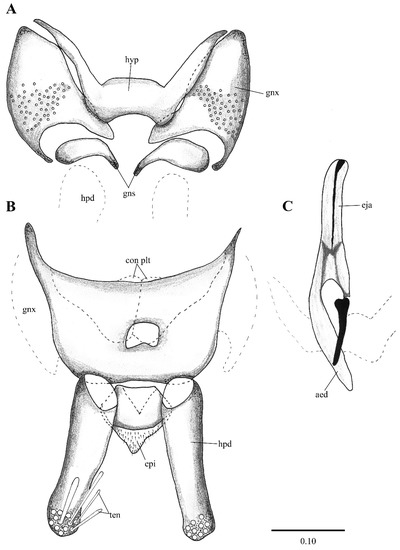 Figure 3. Lepiseodina rothschildi (Eaton, 1912), male genitalia. (A). Hypandrium, gonocoxites and gonostylus. (B) Epandrium, hypopods, tenacula, hypoproct. (C) Aedeagus. Abbreviations: aed = aedeagus, con plt = condyle plate-like, eja = ejaculatory apodeme, epi = epiproct, gnx = gonocoxite gns = gonostylus, ten = tenacula, hyp = hypandrium. Scale (A–C) in millimeters (mm).
Figure 3. Lepiseodina rothschildi (Eaton, 1912), male genitalia. (A). Hypandrium, gonocoxites and gonostylus. (B) Epandrium, hypopods, tenacula, hypoproct. (C) Aedeagus. Abbreviations: aed = aedeagus, con plt = condyle plate-like, eja = ejaculatory apodeme, epi = epiproct, gnx = gonocoxite gns = gonostylus, ten = tenacula, hyp = hypandrium. Scale (A–C) in millimeters (mm).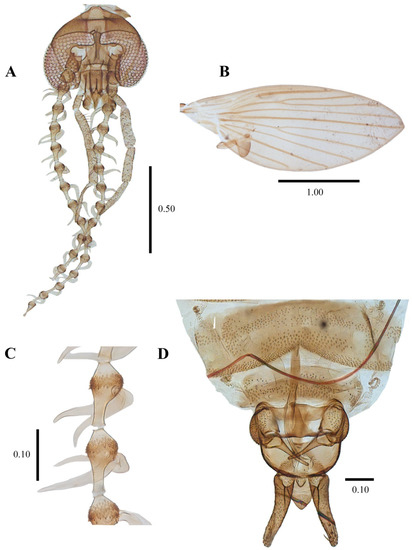 Figure 4. Lepiseodina latipennis (Sarà, 1953), male. (A) Head. (B) Wing. (C) Flagellomeres and ascoids. (D) Genitalia. Scale (A–D) in millimeters (mm).
Figure 4. Lepiseodina latipennis (Sarà, 1953), male. (A) Head. (B) Wing. (C) Flagellomeres and ascoids. (D) Genitalia. Scale (A–D) in millimeters (mm).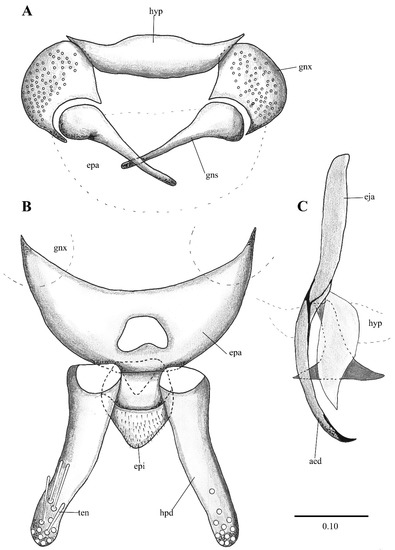 Figure 5. Lepiseodina latipennis (Sarà, 1953), male genitalia. (A) Hypandrium, gonocoxites and gonostylus. (B) Epandrium, hypopods, tenacula, hypoproct. (C) Aedeagus. Abbreviations: aed = aedeagus, epa = epandrium, eja = ejaculatory apodeme, epi = epiproct, gnx = gonocoxite, gns = gonostylus, ten = tenacula, hpd = hypopods, hyp = hypandrium. Scale (A–C) in millimeters (mm).
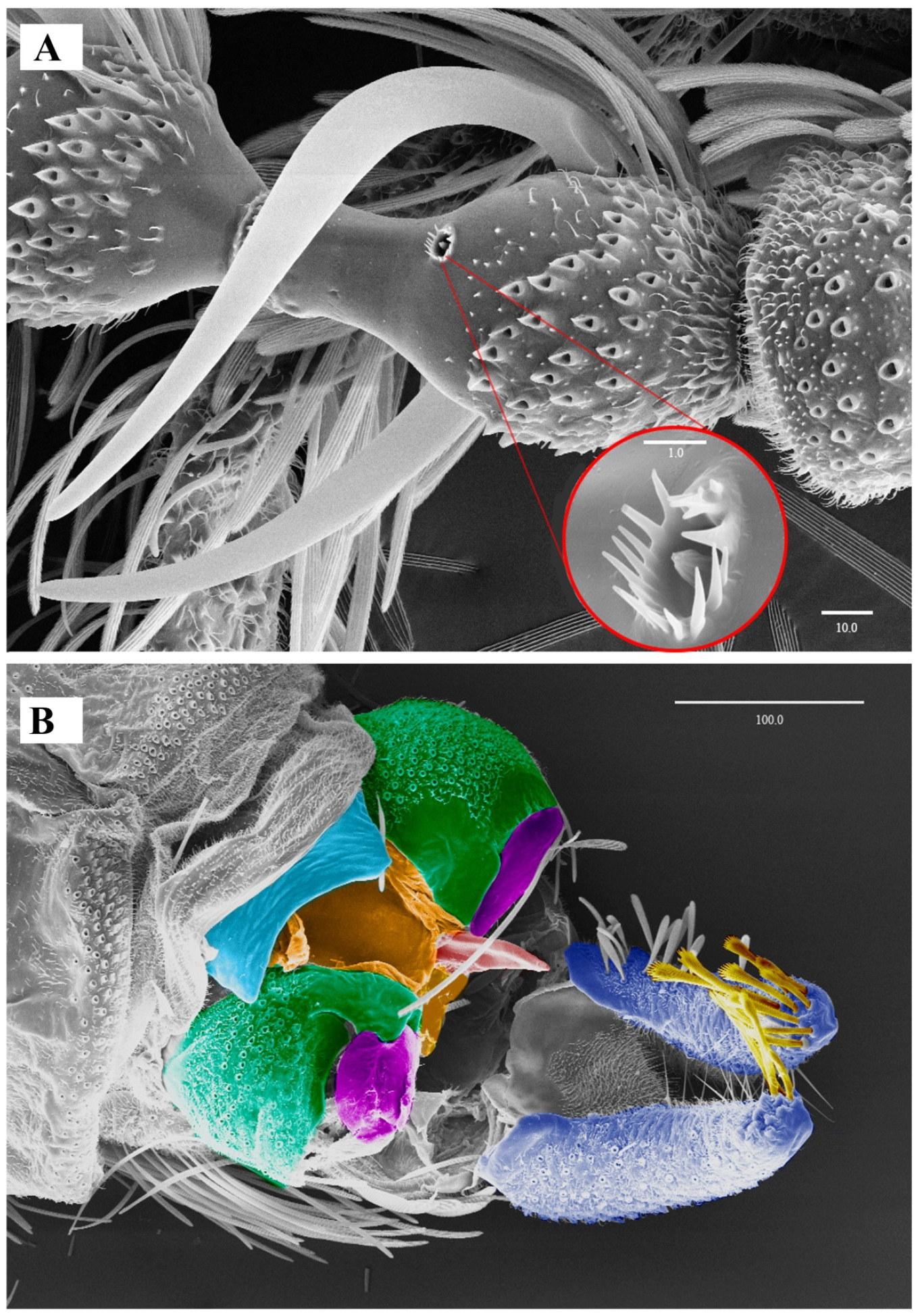
Figure 5. Lepiseodina latipennis (Sarà, 1953), male genitalia. (A) Hypandrium, gonocoxites and gonostylus. (B) Epandrium, hypopods, tenacula, hypoproct. (C) Aedeagus. Abbreviations: aed = aedeagus, epa = epandrium, eja = ejaculatory apodeme, epi = epiproct, gnx = gonocoxite, gns = gonostylus, ten = tenacula, hpd = hypopods, hyp = hypandrium. Scale (A–C) in millimeters (mm).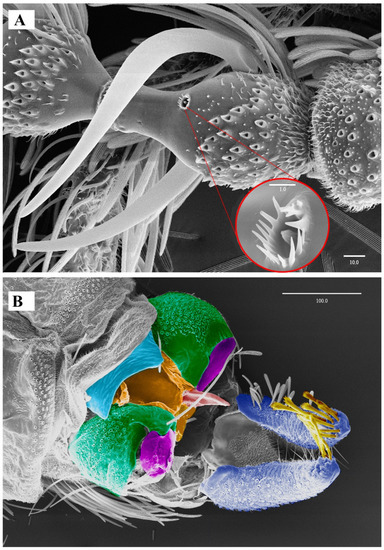 Figure 6. Lepiseodina latipennis (Sarà, 1953), male. SEM pictures. (A) First flagellomere (3rd antennal segment), red circle higher magnification of sensilla. (B) Genitalia, light blue = hypandrium, orange = aedeagal sheath, green = gonocoxites, purple = gonostyli, red = aedeagus, dark blue = hypopods, yellow = tenacula. Scale (A,B) in micrometers (μm).
Figure 6. Lepiseodina latipennis (Sarà, 1953), male. SEM pictures. (A) First flagellomere (3rd antennal segment), red circle higher magnification of sensilla. (B) Genitalia, light blue = hypandrium, orange = aedeagal sheath, green = gonocoxites, purple = gonostyli, red = aedeagus, dark blue = hypopods, yellow = tenacula. Scale (A,B) in micrometers (μm).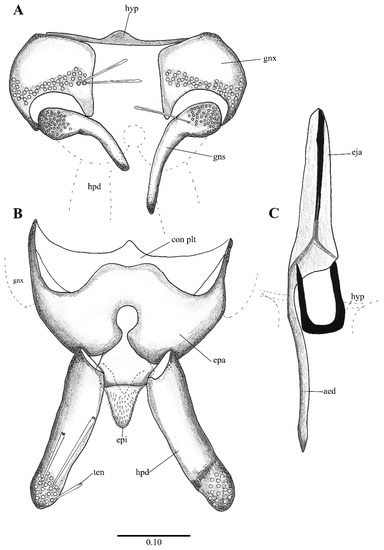 Figure 7. Lepiseodina tristis (Meigen, 1830), male genitalia. (A) Hypandrium, gonocoxites and gonostylus. (B) Epandrium, hypopods, tenacula, hypoproct. (C) Aedeagus. Abbreviations: aed = aedeagus, con plat = condyles plate-like, epa = epandrium, eja = ejaculatory apodeme, epi = epiproct, gnx = gonocoxite, gns = gonostylus, ten = tenacula, hpd = hypopods, hyp = hypandrium. Scale (A–C) in millimeters (mm).
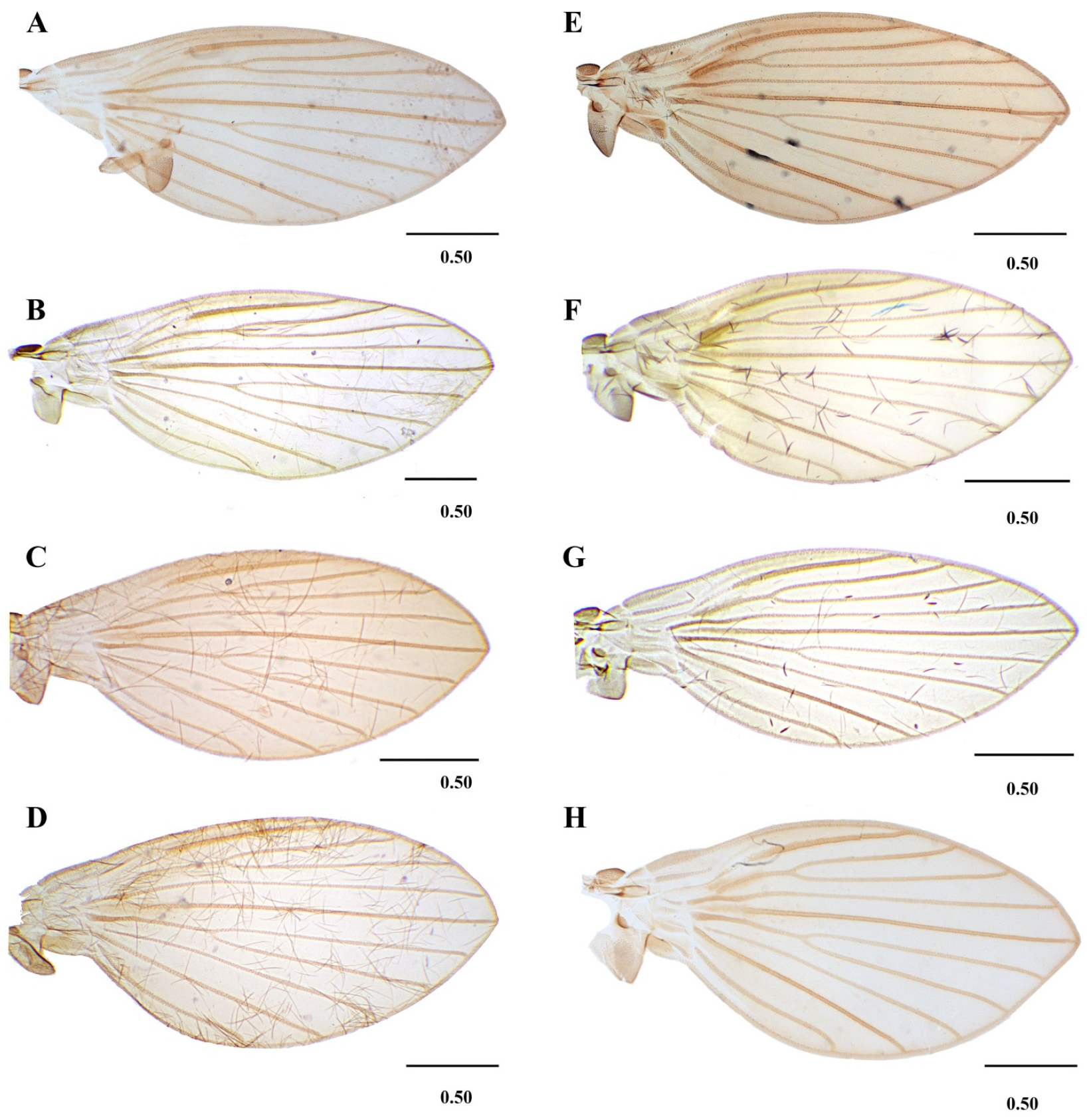
Figure 7. Lepiseodina tristis (Meigen, 1830), male genitalia. (A) Hypandrium, gonocoxites and gonostylus. (B) Epandrium, hypopods, tenacula, hypoproct. (C) Aedeagus. Abbreviations: aed = aedeagus, con plat = condyles plate-like, epa = epandrium, eja = ejaculatory apodeme, epi = epiproct, gnx = gonocoxite, gns = gonostylus, ten = tenacula, hpd = hypopods, hyp = hypandrium. Scale (A–C) in millimeters (mm).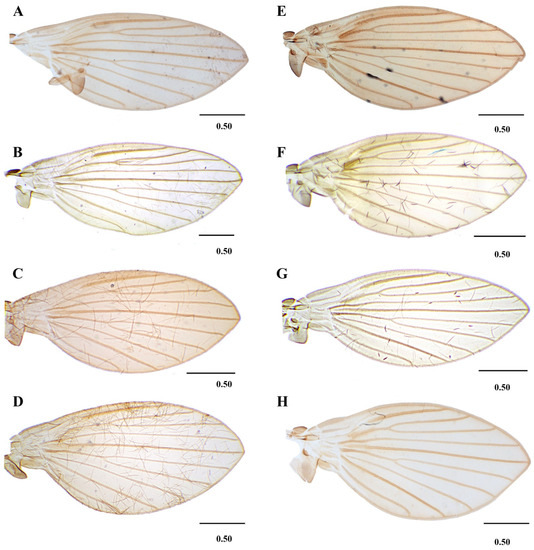 Figure 8. Wings variability of: (A–D) Lepiseodina latipennis (Sarà 1953). (E,F) L. tristis (Meigen, 1830), (G,H) L. rothschildi (Eaton, 1912). Scale (A–H) in millimeters (mm).
Figure 8. Wings variability of: (A–D) Lepiseodina latipennis (Sarà 1953). (E,F) L. tristis (Meigen, 1830), (G,H) L. rothschildi (Eaton, 1912). Scale (A–H) in millimeters (mm).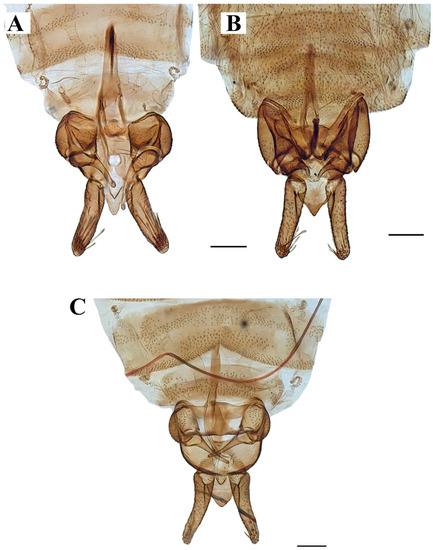 Figure 9. Genitalia of: (A) Lepiseodina tristis (Meigen, 1830), (B) Lepiseodina rothschildi (Eaton, 1912), (C) Lepiseodina latipennis (Sarà, 1953). Scale (A–C) in millimeters (mm), scale lines = 0.10 mm.
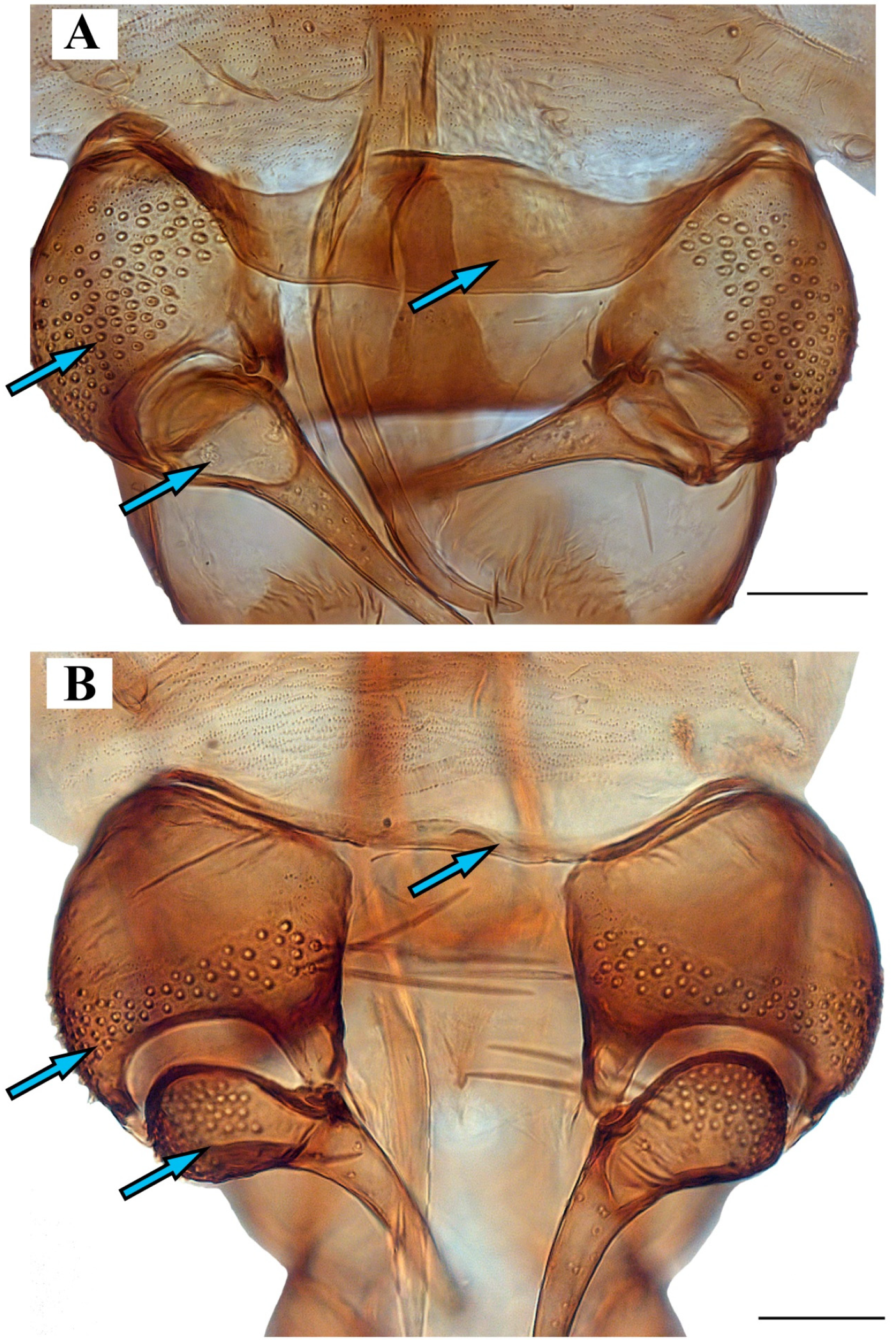
Figure 9. Genitalia of: (A) Lepiseodina tristis (Meigen, 1830), (B) Lepiseodina rothschildi (Eaton, 1912), (C) Lepiseodina latipennis (Sarà, 1953). Scale (A–C) in millimeters (mm), scale lines = 0.10 mm.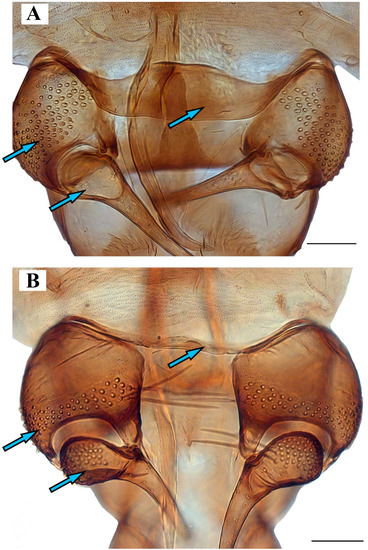 Figure 10. Genitalia of: (A) Lepiseodina latipennis (Sarà, 1953), (B) Lepiseodina tristis (Meigen, 1830). Blue arrows show differences in hypandrium, gonocoxite, and gonostyli. Scale (A,B) in millimeters (mm), scale lines = 0.10 mm.
Figure 10. Genitalia of: (A) Lepiseodina latipennis (Sarà, 1953), (B) Lepiseodina tristis (Meigen, 1830). Blue arrows show differences in hypandrium, gonocoxite, and gonostyli. Scale (A,B) in millimeters (mm), scale lines = 0.10 mm.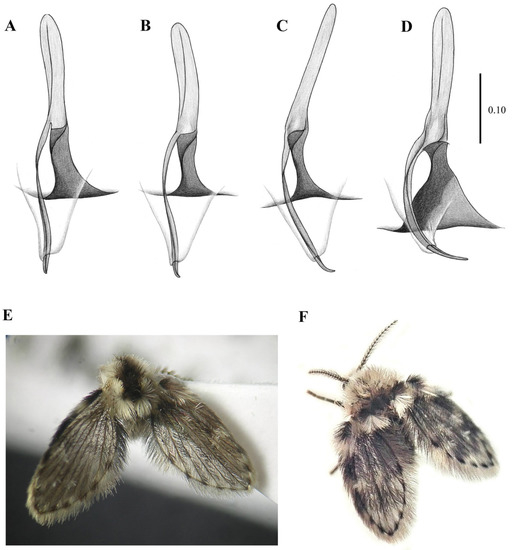 Figure 11. Genitalia variability of Lepiseodina latipennis (Sarà, 1953): (A–C) Aedeagal complex in dorsal view. (D) same in posterodorsal view. (E) Habitus of L. latipennis (Sarà, 1953), (F) Habitus of L. rothschildi (Eaton, 1912). Scale (A–D) in millimeters (mm); without scale (E,F).
Figure 11. Genitalia variability of Lepiseodina latipennis (Sarà, 1953): (A–C) Aedeagal complex in dorsal view. (D) same in posterodorsal view. (E) Habitus of L. latipennis (Sarà, 1953), (F) Habitus of L. rothschildi (Eaton, 1912). Scale (A–D) in millimeters (mm); without scale (E,F).
- 5.
- -
- Aedeagus symmetrical, with ejaculatory apodeme narrower than distal elements (Figure 12C and Figure 13C); ascoids leaf-shaped or digitiform…Telmatoscopus Eaton, 1904
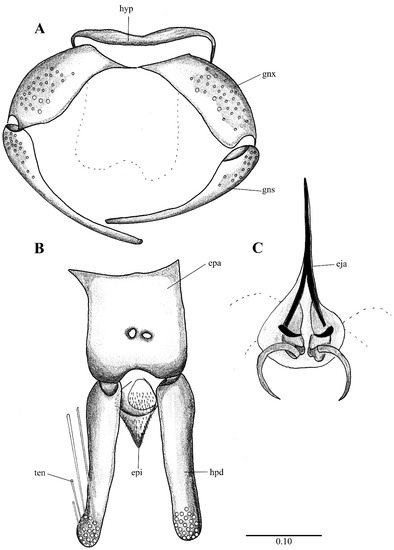 Figure 12. Telmatoscopus advena (Eaton, 1893), male genitalia. (A) Hypandrium, gonocoxites and gonostylus. (B) Epandrium, hypopods, tenacula, hypoproct. (C) Aedeagus. Abbreviations: epa = epandrium, epi = epiproct, eja = ejaculatory apodeme, gnx = gonocoxite gns = gonostylus, hpd = hypopod, ten = tenacula, hyp = hypandrium. Scale (A–C) in millimeters (mm).
Figure 12. Telmatoscopus advena (Eaton, 1893), male genitalia. (A) Hypandrium, gonocoxites and gonostylus. (B) Epandrium, hypopods, tenacula, hypoproct. (C) Aedeagus. Abbreviations: epa = epandrium, epi = epiproct, eja = ejaculatory apodeme, gnx = gonocoxite gns = gonostylus, hpd = hypopod, ten = tenacula, hyp = hypandrium. Scale (A–C) in millimeters (mm).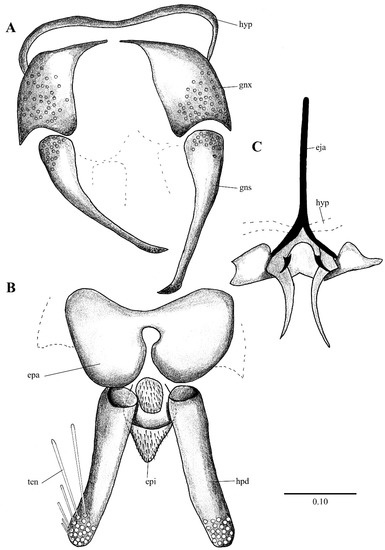 Figure 13. Telmatoscopus bartai (Ježek, 2004), male genitalia. (A) Hypandrium, gonocoxites and gonostylus. (B) Epandrium, hypopods, tenacula, hypoproct. (C) Aedeagus. Abbreviations: aed = aedeagus, epa = epandrium, epi = epiproct, eja = ejaculatory apodeme, gnx = gonocoxites, hpd = hypopod, hyp = hypandrium, ten = tenacula. Scale (A–C) in millimeters (mm).
Figure 13. Telmatoscopus bartai (Ježek, 2004), male genitalia. (A) Hypandrium, gonocoxites and gonostylus. (B) Epandrium, hypopods, tenacula, hypoproct. (C) Aedeagus. Abbreviations: aed = aedeagus, epa = epandrium, epi = epiproct, eja = ejaculatory apodeme, gnx = gonocoxites, hpd = hypopod, hyp = hypandrium, ten = tenacula. Scale (A–C) in millimeters (mm).
3.2. Systematic Assessment
Genus Clogmia Enderlein, 1937
Clogmia Enderlein, 1937: 87. Type species: Psychoda albipennis Williston (=albipunctata Williston).
Diagnosis. Scape less than 2.5 times its width; flagellomeres symmetrically nodiform, with ascoids variable in shape; flagellomere 14 with elongated apiculus tapering towards apex; wing with radial fork basal to median fork; ejaculatory apodeme Y-shaped, narrow in dorsal view; aedeagus symmetrical in dorsal view.
Species present in Europe associated with dendrotelmata: C. albipunctata (Williston, 1893) [35,36] (Table 1).
Clogmia albipunctata (Williston, 1893)
Psychoda albipunctata Williston, 1893: 113. Type locality: Cuba, La Havana.
Pericoma meridionalis Eaton, 1894: 194. Type locality: East Africa.
Psychoda snowii Haseman, 1907: 311. Type locality: USA, Texas, Galveston.
Psychoda legnothisa Spieser, 1909: 44. Type locality: Tanzania.
Psychoda erecta Curran, 1926: 102. Type locality: West Indies.
Pschoda nocturna Abreu, 1930: 115. Type locality: Not given, probably Canary Islands (see Ibáñez-Bernal [40]).
Psychoda nocturna var. nigrithorax Abreu, 1930: 115. Type locality: not given, probably Canary Islands.
Telmatoscopus haranti Mirouse, 1958: 93. Type locality: Midi de la France.
Telmatoscopus albipunctatus (Williston): [41] (p. 185).
Clogmia albipunctata (Williston): [42] (p. 42); 351 [11] (p. 351) (see Ibáñez-Bernal [40]).
Diagnosis. Antenna with symmetrically nodiform flagellomeres, each flagellomere with a bifurcate ascoids; eyes separated by 1 facet diameter, eye bridge with 4 facet rows; aedeagus symmetrical, ejaculatory apodeme straight and narrow in dorsal view; hypopods with five-six tenacula, tenacula decreasing in size towards the apex of hypopod.
Examined material: ZFMK-DIP-00081299, ZFMK-DIP-00081508, ZFMK-DIP-00081514, ZFMK-DIP-00081543, ZFMK-DIP-00081624, ZFMK-DIP-00081591, ZFMK-DIP-00082118 [ZFMK].
Distribution. This is one of the most widespread species of Psychodidae in the world, highly invasive and synanthropic. In Europe, it is recorded in Azores Archipelago, Belgium, Canary Islands, Corsica, Croatia, Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Italy, Luxemburg, Madeira Islands, Sardinia, Slovakia, Slovenia, Spain, Sweden and United Kingdom [9,43,44,45,46,47].
Genus Clytocerus Eaton, 1904
Clytocerus Eaton, 1904: 59. Type species: Clytocerus ocellaris (Meigen, 1804: 44) by subsequent monotypy of Malloch (1907) (see [48]).
Diagnosis: Cornicula (everted sac-shaped structures on the back surface of the head) are usually present; eye bridge with 3–6 facet rows, separated by 1–3 facet diameters; antennal scape elongate, three-six times longer than wide; flagellomere I and II fused to form a compound flagellomere, with a distal brush of wavy setae [14].
Species present in Europe associated with dendrotelmata: C. xylophilus Vaillant, 1983 [37] (Table 1).
Clytocerus xylophilus Vaillant, 1983
Clytocerus xylophilus Vaillant, 1983: 355. Type locality: France.
Diagnosis. Eyes separated by 2 facet diameters, interocular suture absent. Antennal scape 4.75 times longer than wide. Hypopods with 6–7 tenacula.
Examined material. None.
Distribution. France [43].
Remarks. Vaillant [37] mentions under the description of C. xylophilus that the larvae of Clytocerus pulvereus Vaillant, 1983 can be found in the same substrate as C. xylophilus; however, it is referring to the “black humus” found near a river (same substrate mentioned by Krek [49]), and later Vaillant [37] clarifies that only C. xylophilus is known as a dendrolimnetic species.
Genus Lepiseodina Enderlein, 1937
Lepiseodina Enderlein, 1937: 91. Type species: Psychoda tristis Meigen, 1830: 272.
Diagnosis. Frons and clypeus separated and not protruding over eye margin; scape short, less than 2 times length of pedicel; flagellomeres symmetrically nodiform in dorsal view, with a pair of digitiform sinuous ascoids; flagellomere 14 with elongated apiculus; wing vein R2+3 not connected to R4; apex of R5 ending on, or below wing apex; gonocoxites short, with dorsal projection undeveloped; ejaculatory apodeme narrow in dorsal view; aedeagal complex asymmetric, with a parameral structure more or less sclerotized, connecting aedeagus to the gonocoxal apodemes; hypopods with indistinctly fringed tenacula; epandrium with a single foramen.
Species present in Europe associated with dendrotelmata: L. rothschildi (Eaton, 1912), L. latipennis (Sarà, 1953), and L. tristis (Meigen, 1830) [9,13,23,25,36,38] (Table 1).
Lepiseodina latipennis (Sarà, 1953)
Telmatoscopus latipennis Sarà, 1953: 13. Type locality: Italy, Messina.
Telmatoscopus latipennis Sarà: [50] (p. 53).
Clogmia latipennis (Sarà): [51] (p. 60).
Lepiseodina latipennis (Sarà): [52] (p. 146).
Diagnosis. Male hypandrium broad along its entire length; gonocoxal alveoli distributed in the entire gonocoxal surface; gonostyli without alveoli on the base, twice the length of gonocoxites; epandrium not divided, with a single kidney-shaped foramen; aedeagus incurved; paramere not strongly sclerotized; hypoproct tongue-shaped.
Differential diagnosis. This species is closely related to Lepiseodina tristis, but it can be distinguished by the combination of the following characters: hypandrium broad along its entire length with convexity in the basal margin in L. latipennis (hypandrium narrow with a medial projection in the basal margin in L. tristis); gonocoxal alveoli distributed in the entire gonocoxal surface in L. latipennis (gonocoxal alveoli restricted to the apical margin in two or three irregular rows in L. tristis); gonostyli without alveoli on the base in L. latipennis (gonostyli with alveoli in the base in L. tristis); epandrium not divided in the apical margin, with a kidney-shaped foramen in L. latipennis (epandrium divided in the apical margin with a rounded foramen in L. tristis); aedeagus curved in L. latipennis (aedeagus straight in L. tristis); parameres not strongly sclerotized and almost not visible in L. latipennis (parameres strongly sclerotized in L. tristis).
Lepiseodina latipennis can be differentiated from Lepiseodina rothschildi by the gonostyli twice the length of gonocoxites in L. latipennis (gonostyli less than half of the length of gonocoxite in L. rothschildi), apical margin of hypandrium convex in L. latipennis (apical margin of hypandrium concave in L. rothschildi), and parameres not strongly sclerotized and almost not visible in L. latipennis (paramere strongly sclerotized in L. rothschildi).
Redescription. Measurements in mm (mean, SD = 0.005, n = 2). Wing length 2.53 (2.20–3.30), wing width 0.98 (1.02–1.32). Head length 0.50 (0.48–0.59), head width 0.63 (0.53–0.65). Antennal segments, scape length: 0.11 (0.09–0.11), pedicel: 0.08 (0.70–0.90), postpedicel: 0.10 (0.10–0.13), flagellomeres average length 0.11; Palpomeres 1: 0.10 (0.08–0.10), 2: 0.25 (0.20–0.27), 3: 0.23 (0.20–0.24), 4: 0.29 (0.24–0.29).
Male. Head: eye bridge with rows of four facets (rarely 3 facets); frontal patch undivided, with an irregular row of alveoli extending towards the interocular suture, the whole frontal patch together with the irregular row resemble the shape of a handbell; interocular suture as an inverted “v”. No alveoli of supra ocular setae are present. Labella bulbous, longer than wide, with 10–12 small setae. Antenna with scape cylindrical, 1.34 times longer than its width, 1.43 (1.2–1.4) times the length of pedicel; pedicel spherical; 14 nodiform flagellomeres with basal bulb and distal neck, apical flagellomere with long apiculus, apiculus almost half the length of the flagellomere (Figure 4A); antennal ascoids slightly flattened, digitiform, sinous (S-shaped), total length about 1.88 the length of flagellomeres; flagellomeres with a sensilla (as shown in Figure 6A). Palpal segment proportions 1.0:2.63:2.37:3.03.
Thorax with no particular characters.
Wing: Length about 2.5 (2–2.5) times its width, variable in shape, with anterior margin more or less curved; membrane bare except on veins; hyaline with a slight infuscation on costal cell; Sc straight, ending at level of the origin of R2+3; Origin of R2+3 not joining R4, a little distal to the origin of M1+2; origin of M1+2 broad and rounded; forks of R2+3 and M1+2 almost at the same level, the fork of R2+3 being slightly distal to M1+2; R5 ending at wing apex; CuA ending in wing margin at the level of R2+3 fork.
Abdomen. Without any particular characters.
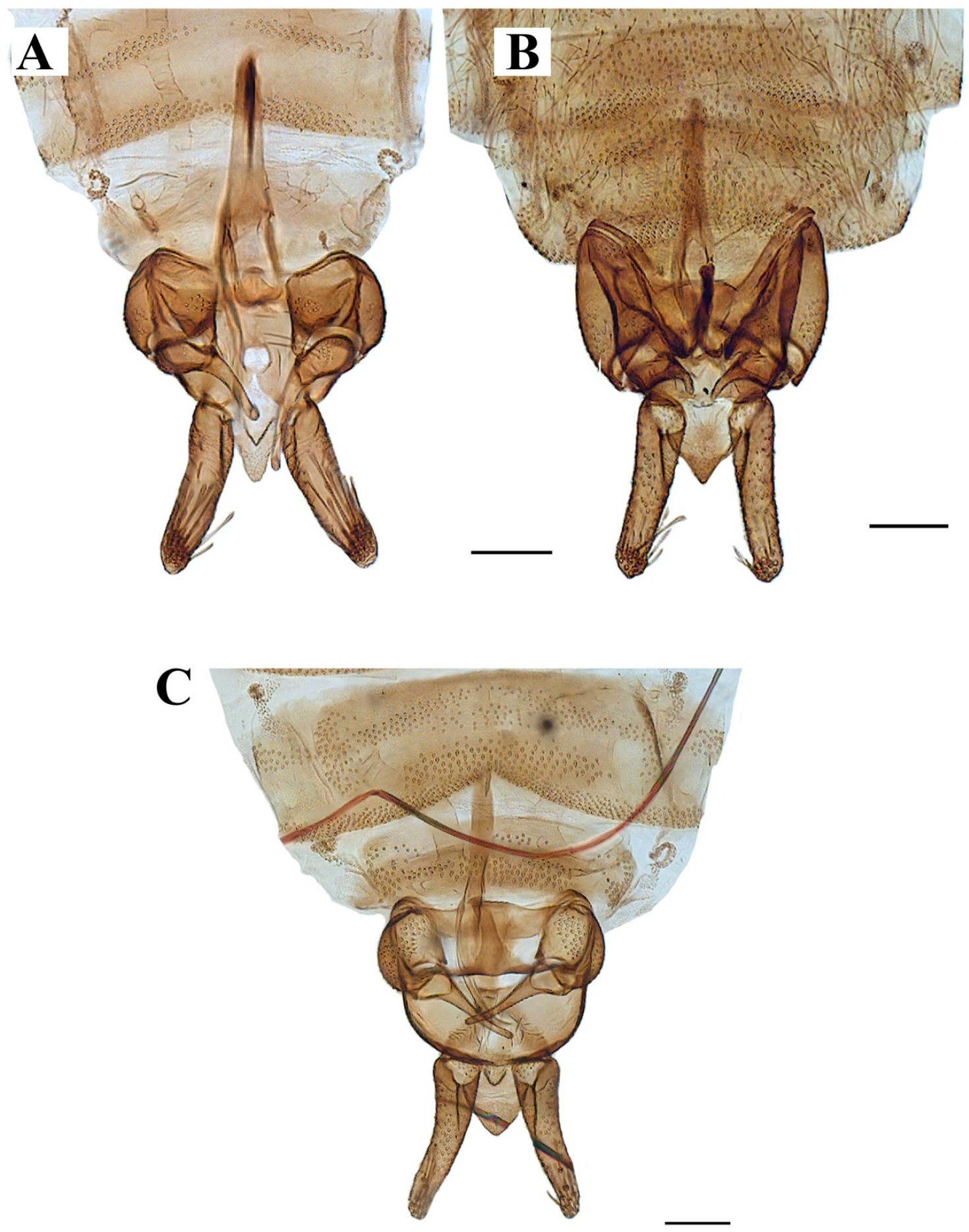
Genitalia. Hypandrium plate-like, broad, basal margin with medial projection widely rounded, distal margin almost straight; gonocoxites longer than wide, covered in alveoli on almost all the surface; gonostyli about 1.5 times the length of gonocoxites, tapering towards apex, almost straight; gonocoxal apodemes poorly distinguishable in dorsal view, in ventral view plate-like, strongly sinuous, fused and narrow at livel of the midline; parameres forming a single slight sclerotized structure, pyriform, resembling the upper half of a bowling pin connecting like a bridge aedeagus to gonocoxal apodemes (as in Figure 4D, Figure 5C, Figure 6B, Figure 9C and Figure 10A); epandrium subrectangular, about twice wider than long with a single kidney-shaped foramen; hypopods slightly out curved, apex rounded, with 9–13 tenacula (as in Figure 4B, Figure 5C and Figure 9C); epiproct short, triangular and covered in pilosity; hypoproct broad and tongue-shaped, covered in pilosity, extending towards mid of hypopods. Aedeagus with ejaculatory apodeme digitiform, narrow 7.5 times longer than its width, about 1.5 times the length of gonocoxites; distiphallus about the same length of ejaculatory apodeme, incurved, extending towards the apex of gonostyli, both ejaculatory apodeme and distiphallus form a single complex, jointed with parameral structure and encircles by a membranous parameral sheath.
Female. Unknown.
Remarks. The holotype (slide mounted) is quite dark, making the observation of structures difficult. The head is dissected, and quite difficult to see clearly, one antenna is dissected and the other antenna is missing, one complete palpus is dissected, the other palpus is missing. The hypopods are dissected and placed apart from each other, one gonocoxites and gonostylus are dissected and placed apart from the genitalia, the remaining parts of the genitalia (aedeagal complex, one gonocoxites and gonostylus) are found together. One wing is dissected and separated from the thorax. On the original description Sarà (1953) mentions the shape of the hypoproct being trilobed (Figure 2), after examination of the holotype in the preparation the hypoproct looks indeed trilobed; however, we consider this a malformation on the slide itself, and not the natural shape of the hypoproct, all other examined material present a broad tongue-shaped hypoproct. On the original description Sarà abstained to illustrate or describe the aedeagal complex; however, after examination we are sure all our specimens belong to the same species.
Biology. Some specimens (No. 0190-0193) emerged from organic matter sampled from an Acer sp. dendrotelma (Figure 14), in a mixed submontan forest, additionally, the specimen used for the SEM pictures was collected in a Malaise trap next to dendrotelmata of an oak (Quercus sp.) tree, suggesting that also L. latipennis is a dendrolimnetic species. In Germany altitude ranges from 250–280 m a.s.l. (meters above sea level), while in Italy it has a range from 40–800 m a.s.l.

Figure 14.
(A,B) Habitat of Psychoda cinerea Banks, 1894, dendrotelma of hornbeam (Carpinus or Ostrya sp.). (C,D) Habitat of Lepiseodina tristis (Meigen, 1830), rotting tree hole with basal dendrotelma of Populus nigra. All in a mixed submontan forest (Foro Valley, central Italy).
Examined material. Holotype examined [MNGD], ZFMK-DIP-00081595, ZFMK-DIP-00081552 [ZFMK], Specimen No. 0039, 0488, 0487, 0439, 0190, 0191, 0192, 0193 [AM].
Distribution: Previously known only from the type locality in Messina (Sicily, Italy). The records here reported are the first from Germany (Rheinland-Pfalz) and for northern and central Italy.
Lepiseodina rothschildi (Eaton, 1912)
Telmatoscopus rothschildii Eaton, 1912: 7. Type locality: England, London, Hyde Park.
Telmatoscopus rotschildi (Eaton): Lapsus calami of [50], followed by Salamanna in Dahl et al. [53] (see below).
Clogmia rothschildi (Eaton) [ICZN (1999): art. 33.3.1: incorrect subsequent spelling in prevailing usage is deemed to be a correct original spelling and maintained; see Ježek (2004)]: Vaillant (1982a): 298; (1982b): 206; Wagner (1990): 60; Bernotienè (2002): 7.
Clogmia rotschildi (Eaton): Dahl et al. [53] (p. 33).
Lepiseodina rothschildi (Eaton): Ježek [52] (p. 146).
Diagnosis. Male gonostyli are half the length of gonocoxites; paramere strongly sclerotized, overlapping to the lateral branch of aedeagus; hypandrium W-shaped, broad on the entire surface with the apical margin concave.
Examined material: ZFMK-DIP-00081311, ZFMK-DIP-00081322, ZFMK-DIP-00081323, ZFMK-DIP-00081324, ZFMK-DIP-00081327, ZFMK-DIP-00081328, ZFMK-DIP-00081329, ZFMK-DIP-00081330, ZFMK-DIP-00081510, ZFMK-DIP-00081537, ZFMK-DIP-00081551, ZFMK-DIP-00081558, ZFMK-DIP-00081559, ZFMK-DIP-00081567, ZFMK-DIP-00081574, ZFMK-DIP-00081623, ZFMK-DIP-00082122, ZFMK-DIP-00082126, ZFMK-DIP-00082147, ZFMK-DIP-00082149 [ZFMK]. Specimens number: 13666, 13689, 13694, 13625, 13628, 13822, 18655, 18490, 18225, 19261, 19110, 16352, 16489, 18408, 19469, 17902, 21769, 12319, 12320, 21533, 21114 [NMP]. Specimens number: 0489 [AM].
Distribution. Austria, Belgium, Bulgaria, Czech Republic, Finland, France, Germany, Ireland, Italy, Lithuania, Netherlands, Slovakia, Spain, United Kingdom [9,13,54,55].
Lepiseodina tristis (Meigen, 1830)
Psychoda tristis Meigen, 1830: 272. Type locality: Not specified, probably Belgium or Germany.
Psychoda (Pericoma) tristis (Meigen): [56] (p. 16).
Pericoma tristis (Meigen): [57] (p. 17).
Telmatoscopus tristis (Meigen): [58] (p. 170).
Lepiseodina tristis (Meigen): [42]) (p. 91); [52] (p. 146).
Clogmia tristis (Meigen): [53] (p. 33); [59] (p. 7).
Diagnosis. Male hypandrium narrow, with a small abrupt median projection; gonocoxal alveoli restricted to two irregular lines on the apical surface; gonocoxites with a patch of alveoli at base; two parameres strongly sclerotized.
Examined material: ZFMK-DIP-00081512, ZFMK-DIP-00081513, ZFMK-DIP-00081563, ZFMK-DIP-00081583, DIP-ZFMK-00081622, ZFMK-DIP-00082119, ZFMK-DIP-00082124, ZFMK-DIP-00082125, ZFMK-DIP-00082128, ZFMK-DIP-00082130, ZFMK-DIP-00082133, ZFMK-TIS-2628581, ZFMK-TIS-2628542, ZFMK-TIS-2628588, ZFMK-TIS-2628587, ZFMK-TIS-2628608 [ZFMK]. Specimen number: 24339, 13701, 13668, 13633, 13823, 17331, 17270, 20777, 20243, 20077, 20076, 20080, 20079, 20088, 20067, 3226, 33282, 34041, 34042, 34043, 34044, 20987, 21365, 23772, 24339 [NMP]. Specimen number: 0490, 0491 [AM].
Distribution. Algeria, Austria, Belgium, Croatia, Czech Republic, France (incl. Corsica), Germany, Ireland, Italy, Lithuania, Slovakia, and United Kingdom [25,36,43,50,53,59,60]. In Italy, the species is known only from two old and uncertain records for the northern and peninsular region [53,61]. The specimens here reported confirm the occurrence of this species in the Italian peninsula.
Key to the European adult males of Lepiseodina
- 1.
- Gonostyli longer than gonocoxites…2
- -
- Gonostyli about half the length of gonocoxites (Figure 3A)… L. rothschildi
- 2.
- Hypandrium narrow, with a small medial projection on basal margin (Figure 7A); alveoli in gonocoxites restricted in two or three irregular rows in the apical margin (Figure 9A and Figure 10B); gonostyli with alveoli on the base (Figure 9A and Figure 10B); two parameres strongly sclerotized (Figure 7C)… L. tristis
- -
Genus Pneumia Enderlin, 1935
Pneumia Enderlein, 1935: 247. Type species: Pericoma palustris Meigen, 1804 (see [62]).
Diagnosis. Eye bridge with 6 facet rows. Antennal flagellomeres barrel-shaped, basal flagellomeres lacking spines or clusters of stiff setae; apical flagellomere with a digitiform apical protuberance about as long as or longer than the basal part of the flagellomere carrying it. Aedeagal complex symmetrical; gonostyli bulbose and elongate at the base, tapering towards the apex.
Species present in Europe associated with dendrotelmata: Pneumia canescens (Meigen, 1818) and Pneumia trivialis (Eaton, 1893) [18,23,25] (Table 1).
Pneumia canescens (Meigen, 1818)
Trichoptera canescens Meigen, 1818: 45. Type locality: not given, probably Germany.
Pericoma canescens (Meigen): 20 (p. 156).
Pneumia canescens (Meigen): [63] (p. 124).
Diagnosis. Eye bridge with 5–6 facet rows: wing vein fork R2+3 with a backward projection (as in [11] (Figure 12)); hypopods with 10 tenacula; ejaculatory apodeme wider at the base than apex; aedeagal complex longer than gonocoxites, tapering towards apex.
Examined material. NMP-2687, NMP-2913, NMP-10787, NMP-17243 [NMP].
Distribution: Afghanistan, Armenia, Austria, Azerbaijan, Belgium, Bulgaria, China, Czech Republic, Denmark, France, Georgia, Germany, Greece, Hungary, Kyrgyzstan, Lithuania, Netherlands, Poland, Romania, Russia (Novosibirsk Region), Slovakia, Sweden, Turkey and United Kingdom [40,56,57].
Pneumia trivialis (Eaton, 1893)
Pericoma trivialis Eaton, 1893: 121. Type locality: Great Britain.
Pneumia trivialis (Eaton): [23] (p. 202); [63] (p. 116).
Diagnosis. Eye bridge with 7 facet rows; frons not extending beyond first palpal segment; all pal segments of similar width; fore tibia straight and not engrossed; wing vein fork R2+3 without a backward projection; hypopods with 7 or fewer tenacula; ejaculatory apodeme straight, not wider at base; aedeagal complex shorter than gonocoxites, not tapering towards apex, distal part of aedeagus smooth without wrinkles.
Examined material. ZFMK-DIP-00081529, ZFMK-DIP-00081536, ZFMK-DIP-00081541, ZFMK-DIP-00081576, ZFMK-DIP-00081601, ZFMK-DIP-00081602, ZFMK-DIP-00081603, ZFMK-DIP-00081604, ZFMK-DIP-00081605, ZFMK-DIP-00081606, ZFMK-DIP-00081607, ZFMK-DIP-00081610, ZFMK-DIP-00081999, ZFMK-DIP-00082001, ZFMK-DIP-00082002, ZFMK-DIP-00082003, ZFMK-DIP-00082006, ZFMK-DIP-00082007, ZFMK-DIP-00082008, ZFMK-DIP-00082009, ZFMK-DIP-00082010, ZFMK-DIP-00082011, ZFMK-DIP-00082012, ZFMK-DIP-00082013, ZFMK-DIP-00082014, ZFMK-DIP-00082015, ZFMK-DIP-00082016, ZFMK-DIP-00082017, ZFMK-DIP-00082029, ZFMK-DIP-00082031, ZFMK-DIP-00082032, ZFMK-DIP-00082033, ZFMK-DIP-00082034, ZFMK-DIP-00082035, ZFMK-DIP-00082036, ZFMK-DIP-00082037, ZFMK-DIP-00082039, ZFMK-DIP-00082040, ZFMK-DIP-00082041, ZFMK-DIP-00082042, ZFMK-DIP-00082043, ZFMK-DIP-00082044, ZFMK-DIP-00082045, ZFMK-DIP-00082046, ZFMK-DIP-00082047, ZFMK-DIP-00082048, ZFMK-DIP-00082060, ZFMK-DIP-00082061, ZFMK-DIP-00082062, ZFMK-DIP-00082063, ZFMK-DIP-00082064, ZFMK-DIP-00082065, ZFMK-DIP-00082066, ZFMK-DIP-00082068, ZFMK-DIP-00082069, ZFMK-DIP-00082070, ZFMK-DIP-00082071, ZFMK-DIP-00082072, ZFMK-DIP-00082073, ZFMK-DIP-00082074, ZFMK-DIP-00082076, ZFMK-DIP-00082077, ZFMK-DIP-00082078, ZFMK-DIP-00082079, ZFMK-DIP-00082080, ZFMK-DIP-00082081 [ZFMK].
Distribution: Austria, Azerbaijan, Belgium, Bosnia–Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Finland, France, Georgia, Germany, Hungary, Ireland, Netherlands, Norway, Poland, Portugal, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey, Ukraine and United Kingdom [43,55,64,65].
Genus Psychoda Latreille, 1797
Psychoda Latreille, 1796: 152. Type species: Tipula phalaenoides Linnaeus, 1758 by subsequent designation of Quate [41] (p. 191).
Diagnosis. Species with short vertex; labellum flattened and carrying digitiform setae; eyebridge without interocular suture; antenna with 12–14 flagellomeres, those beyond 11th always reduced in size and showing different types of fusion; flagellomeres nodiform with a neck (except apical flagellomeres); ascoids usually with three branches, two anterior and one posterior branch, Y-shaped or with four branches, shaped like a plus sign (+); aedeagus often asymmetrical; hypopods with a single apical tenaculum.
Species present in Europe associated with dendrotelmata: P. alternata Say, 1824, P. cinerea Banks, 1894 and P. minuta Banks, 1894. [23,25] (Table 1).
Psychoda alternata Say, 1824
Psychoda alternata Say, 1824: 358. Type locality: USA, Pennsylvania, Philadelphia.
Psychoda tripunctata Macquart, 1838: 85. Type locality: not given, probably France.
Psychoda marginepunctata von Roser, 1840: 50. Type locality: not given.
Psychoda sexpunctata Phillipi, 1865: 631. Type locality: not given.
Psychoda schizura Kincaid, 1899: 21. Type locality: USA, Seattle, Washington.
Psychoda nocturnala Haseman, 1907: 319. Type locality: USA, Missouri, Columbia.
Psychoda floridica Haseman, 1907: 324. Type locality: USA, Florida.
Psychoda bengalensis Brunetti, 1908: 371. Type locality: India, Calcutta and Simla.
Psychoda albimaculata Welch, 1912: 411. Type locality: USA, Illinois.
Psychoda dakotensis Dyar, 1926: 109. Type locality: USA, South Dakota.
Psychoda alternata var. marmosa Abreu, 1930: 123. Type locality: not given.
Psychoda alternata var. floridica Haseman: Johannsen, 1934: 25.
Tinearia alternata (Say): [66] (p. 142); [63] (p. 114) [67] (online catalogue); [51] (p. 46); [68](p. 96); [69] (p. 89); [70] (p. 107);
Psychoda (Tinearia) alternata Say: Bravo et al. [71] (p 5, 11).
Psychoda alternata Say: [40] (p. 97). [41] (p. 218) [72] (p. 216); [73] (p. 195); [74] (p. 16); [75] (p. 12); [76] (p 238); [77] (p. 67); [78] (p. 21); [79] (p. 48).
Diagnosis. Eyes separated by 1–3 facet diameters, without interocular suture; antenna with 13 flagellomeres, last three flagellomeres small, flagellomere 11–12 fused, flagellomere 13 smaller and partially fused to 12; labellum with one short and four long teeth and four setae; aedeagus asymmetrical, with on paramere thicker than aedeagus, ejaculatory apodeme broad posteriorly, longer than aedeagal complex; hypopods 2.3 times longer than gonostyli. [40].
Examined material. None.
Distribution. Cosmopolitan [40,55]. In Europe, it is present in Austria, Balearctic Islands, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Dennmark, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Netherlands, Norway, Poland, Portugal, Romania, Sardinia, Slovakia, Slovenia, Spain, Sweden, Switzerland, and United Kingom [55].
Psychoda cinerea Banks, 1894
Psychoda cinerea Banks, 1894: 331. Type locality: USA, New York, Sea Cliff, L. I.
Threticus compar Eaton, 1904: 57. Type locality: Algeria, England, Ireland, and Maderia.
Psychoda prudens Curran, 1924: 219. Type locality: Canada, Alberta.
Psychodocha cinerea (Banks): [66] (p. 135); [80] (p. 100).
Diagnosis. Eyes separated by 1–2 facet diameters, without interocular suture; antenna with 14 flagellomeres, last 3 separated not fused; labellum with four terminal digitiform setae and up to 5 setiform setae; medial fork with basal swelling; aedeagal complex slightly S-shaped, asymmetrical, with unpaired bent paramere; aedeagus subtriangular, covering a squarish structure; ejaculatory apodeme triangular at base in dorsal view; hypandrium trapezoidal; hypopods as long as gonostyli, gradually narrowing toward the apex, but without basal swelling.
Examined material. Specimens number: No. 0480-4081 [AM] Reared from dendrotelmata of a hornbeam (Carpinus or Ostrya sp.).
Distribution. Cosmopolitan [71]. In Europe, it is present in Austria, Azores archipelago, Belgium, Bosnia–Herzegovina, Bulgaria, Canary Islands, Corsica, Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Luxemburg, Madeira Islands, Netherlands, Norway, Poland, Romania, Sardina, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, and United Kingdom [43,49].
Psychoda minuta Banks, 1894
Psychoda minuta Banks, 1894: 331. Type locality: USA, New York, near Sea Cliff.
Psychoda marylandana Del Rosario, 1936: 111.
Psychoda spreta Tonnoir, 1940: 57.
Psychodula minuta (Banks): [52] (p. 56).
Diagnosis. Eyes separated by 1 facet diameters, without interocular suture; antenna with 16 flagellomeres, last 4 flagellomeres reduced in size and fused; labellum with four terminal digitiform setae and two trichiform setae; aedeagus asymmetrical, flanked by two large triangular plates of morphologically unknown origin; gonostyli narrowing to tapered point in apical fifth; hypandrium narrow, not trapezoidal; hypopods much longer than gonostyli, with basal swelling.
Examined material. None.
Distribution. Holarctic. In Europe, it is present in Austria, Balearic Islands, Belgium, Bulgaria, Corsica, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Lithuania, Madeira Islands, Netherlands, Norway, Romania, Sardinia, Slovakia, Slovenia, Spain, Sweden, Switzerland, and United Kingdom [25,43,59,81].
Genus Telmatoscopus Eaton, 1904
Telmatoscopus Eaton, 1904: 58. Type species: Pericoma advena Eaton, 1893, by designation of Quate [82].
Sciria Enderlein, 1935: 247. Type species: Pericoma advena Eaton, 1893, by original designation (see [83]).
Krivosheinoscopus Ježek, 2001: 57 Type species: Telmatoscopus ussuricus (Ježek, 2001) syn. nov.
Diagnosis (modified from Kvifte, [83]). Frons and clypeus separated and not protruding over eye margin; flagellomeres asymmetrically nodiform, with paired leaf-shaped to digitiform ascoids; flagellomere 14 with elongated apiculus; wing veins R2+3 not connected to R4; apex of R5 ending at wing apex; ejaculatory apodeme narrow in dorsal view and distally ending in two short branches with membranous connection to aedeagal complex; aedeagal complex symmetrical; aedeagal complex encapsulated in a parameral sheath; hypopods with indistinctly fringed tenacula; epandrium with a single foramen.
Remarks: Ježek [84] described Krivosheinoscopus based on five male specimens of the type species K. ussuricus Ježek, 2001 (now Telmatoscopus ussuricus comb. nov.) from the Russian Far East. Later, he described K. bartai Ježek, 2004 (now Telmatoscopus bartai comb. nov.) from the Czech Republic (Ježek 2004). Ježek [84] provided a diagnostic table of closely related telmatoscopoid genera, namely, Lepiseodina, Sciria (=Telmatoscopus), Iranotelmatoscopus, Krivosheinoscopus, Telmatoscopus auctt. (=Seoda) [84] (p. 60).
In the table, seven morphological characters were provided to differentiate Krivosheinoscopus from Sciria (=Telmatoscopus), they are numbered and discussed below.
- 1.
- The scape/pedicel proportion is 2:1 in Kvirosheinoscopus (1:1 in Sciria); however, even while the proportion of scape/pedicel length proposed by Ježek [84] is 2:1 in T. ussuricus, this is not the case for T. bartai where it is 1:1.
- 2.
- The ascoids are very long, thin and coiled in Krivosheinoscopus (large, flat, leaf or hood-shaped in Sciria). This character is contradicted by Telmatoscopus laurencei [38], which has digitiform ascoids and coiled, and these character states are polymorphic in Vaillantodes Wagner, 2001 and Panimerus Eaton, 1913. Intermediary forms also occur in some genera of Telmatoscopoids. Due to widespread polymorphism of this character we do not consider this a reliable genus-level character unless supported by other, independent lines of evidence.
- 3.
- The first palpal segment very short and keg-shaped in Krivosheinoscopus (long and cylindrical in Sciria). This character is variable inside the genus Telmatoscopus (e.g., long in Telmatosocpus advena and shorter in Telmatoscopus thuringicus Beran, Doczkal, Pfister & Wagner, 2010), therefore it is here considered as intrageneric variability, and thus not a diagnostic character.
- 4.
- The position of the radial and medial forks of wing venation, at the same distance in Krivosheinoscopus (medial fork distad to radial fork in Sciria). This is another character presenting as variable within the genus Telmatoscopus (e.g., medial fork distal in T. advena, medial fork basal in T. thuringicus, forks at the same level in Telmatoscopus bartai).
- 5.
- Two pairs of protuberances in the aedeagal complex in Krivosheinoscopus (one pair in Sciria). According to the homologization of [83], the absence of protruding parameres in Telmatoscopus are not due to absence of parameres, they are present as transverse sclerites within the aedeagal–parameral complex. The difference between T. advena, T. bartai and T. ussuricus in the parameres is a question of degree of development rather than a clear-cut presence/absence question, and intermediate cases occur in Nearctic species (e.g., T. patibulus Quate).
- 6.
- The hypoproct has a terminal projection, long and narrow in Krivosheinoscopus (without a terminal projection, triangular in Sciria). This character is contradicted by Telmatoscopus bartai comb. nov. where the hypoproct is triangular, and not thin and elongated.
With three shared characters between Krivosheinoscopus and Telmatoscopus (=Sciria sensu Ježek) including the wing vein R5 ending at wing apex, vein Cu ending distal to M1+2 and, the ejaculatory apodeme laterally compressed (=basal apodeme of the aedeagal complex in Ježek) [84].
Furthermore, Ježek listed seven (five are commented here) morphological characters separating Krivosheinoscopus from Seoda Enderlein (considered as Telmatoscopus auctt. In Ježek [84]), including:
1. The scape/pedicel proportion, being 2:1 in Krivosheinoscopus (3-4:1 in Seoda); this remains a diagnostic difference.
2. First palpal segment short and keg-shaped in Krivosheinoscopus (long and cylindrical in Seoda). This character is variable inside Telmatoscopus, and is thus not a diagnostic character.
3. Vein Cu ending distal to M1+2 in Krivosheinoscopus (samel level or distad to M1+2 in Seoda) yet again, a variable character;
4. Wing vein R5 ending at wing apex in Krivosheinoscopus (ending below wing apex in Seoda), this character is diagnostic between Seoda and Telmatoscopus as pointed out in [83].
5. The ejaculatory apodeme laterally compressed in Krivosheinoscopus (dorso-ventrally compressed in Seoda) also a diagnostic character pointed out in [83].
Diagnostic morphological characters to separate Telmatoscopus from Seoda presented in [83] include single pair of digitiform to filiform ascoids (an additional ring of small setiform ascoids + the main pair of digitiform ascoids in Seoda); frons clearly separated from clypeus not protruding over the mesal margin of eyes in Telmatoscopus (frons fused with clypeaus and protruding over the mesal margin of eyes in Seoda); parameral sclerites not fused in Telmatoscopus (parameral sclerites fused in Seoda).
Our currently reformulated conscription of Telmatoscopus includes 10 species globally, namely T. advena (Eaton, 1893), Palaearctic; T. bartai (Ježek, 2004) comb. nov., Europe; T. dendrophilus Vaillant, 1983, USA; T. frondeus Tokunaga & Etsuko, 1955, Japan; T. laurencei Freeman, 1953, Europe; T. pappi (Wagner, 1979), Afghanistan; T. patibulus Quate, 1955, USA; T. ussuricus (Ježek, 2001) comb. nov., Russia; T. tanegashimensis (Ježek & Mogi, 1995), Japan; T. thuringicus Beran, Doczkal, Pfister & Wagner, 2010, Europe.
Notes. Previously, Kvifte, [83] included Panimerus wagneri Salamanna, 1982 as Seoda, however the inclusion of T. wagneri (=T. advena) comb. nov. et syn. nov. inside of Telmatoscopus is based on the male genital morphology presented in the original description, therefore the new combination from Seoda to Telmatoscopus. Additionally, the characters separating T. wagneri (=T. advena) syn. nov. and, T. advena are quite inconspicuous, however, we did not examine the holotype of T. wagneri (=T. advena) syn. nov., nonetheless, based on the original description and figures, we did not find any morphological characters that support them as separates species, and we treat T. wagneri as a new synonym of T. advena.
Additional characters and closer examination of the species T. pappi, T. frondeus, and, T. tanegashimensis is desirable as they share some characters with the genus Lepiseodina, however, these species are outside the geographical scope of this work, and therefore are included as Telmatoscopus following [83] until a broader revision of the genus is available.
Species present in Europe associated with dendrotelmata: T. advena (Eaton, 1904), T. bartai (Ježek, 2004) comb. nov., T. laurencei Freeman, 1953, T. thuringicus Beran, Doczkal, Pfister & Wagner, 2010 [5,13,25,38,39] (Table 1).
Telmatoscopus advena (Eaton, 1893)
Pericoma advena Eaton, 1893: 127. Type locality: Great Britain.
Telmatoscopus advena (Eaton): [81] (pp. 205–209)
Sciria advena (Eaton): [85] (p. 87).
Telmatoscopus advenus (Eaton): [86] (p. 86).
Panimerus advenus (Eaton): [50] (p. 80).
Panimerus havelkai Wagner, 1975: 1; (see [83]).
Panimerus wagneri Salamanna, 1982 syn. nov.
Telmatoscopus seguyi Vaillant, 1990: 378; (see [83]).
Diagnosis. Male. Ascoids broad, leaf-shaped and coiled; phallomeres incurved, symmetrical, less than half the length of ejaculatory apodeme; hypopods with tenacula restricted to apex.
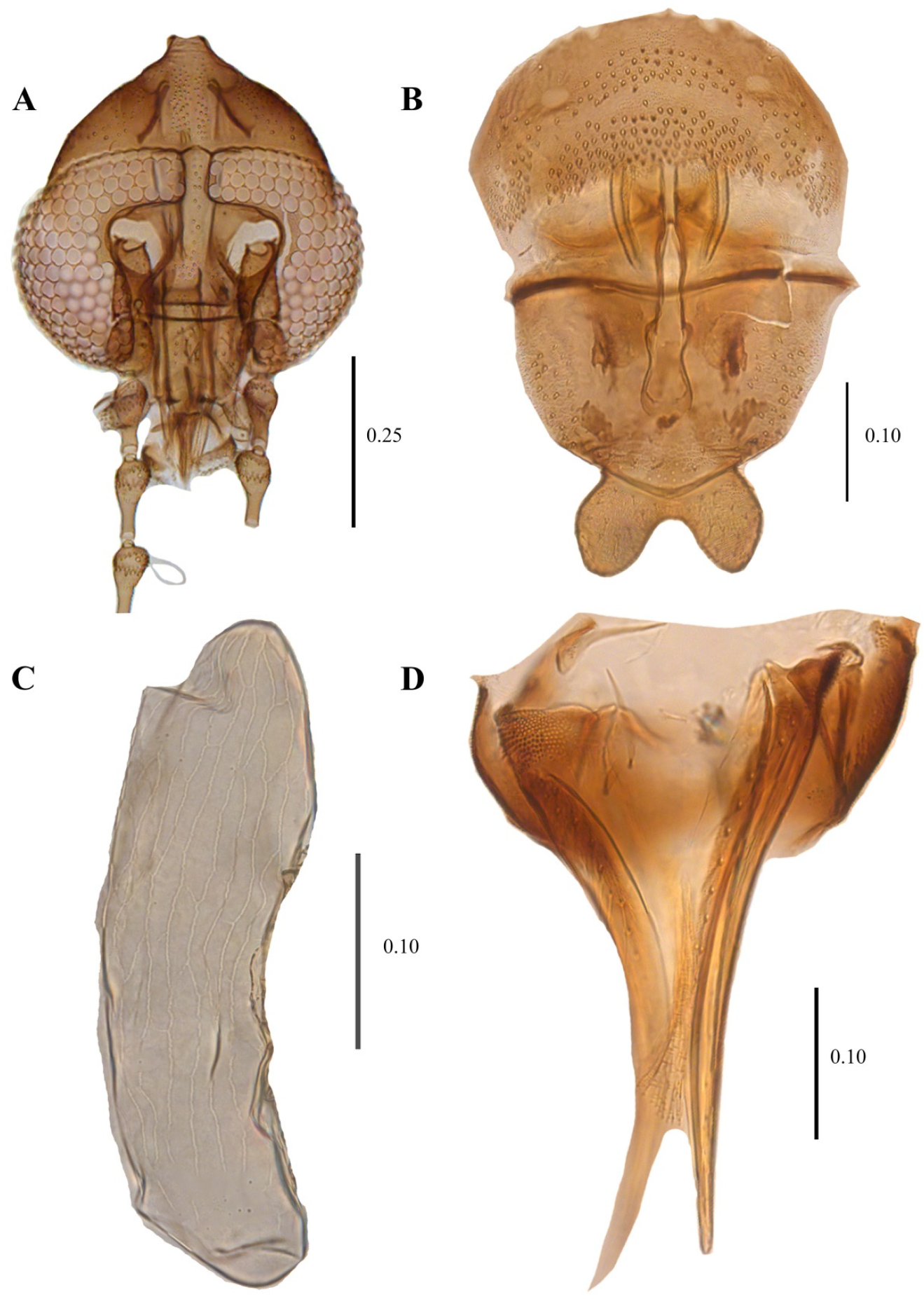
Female description (Figure 15 and Figure 16) based on the description of the male in [83] the female is similar to the male except: Head slightly longer than wide (0.5 mm wide, 0.6 mm length). Eye bridge with four facet rows, separated by 1.5 facet diameter; only first palpal segment present in examined material; apical antennal flagellomeres absent in examined material, ascoids s-shaped, coiled, thin (not broad leaf-shaped as in male). Wing length, wing width, length x times its width.
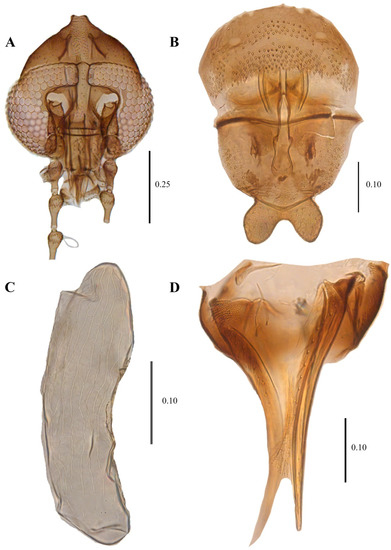
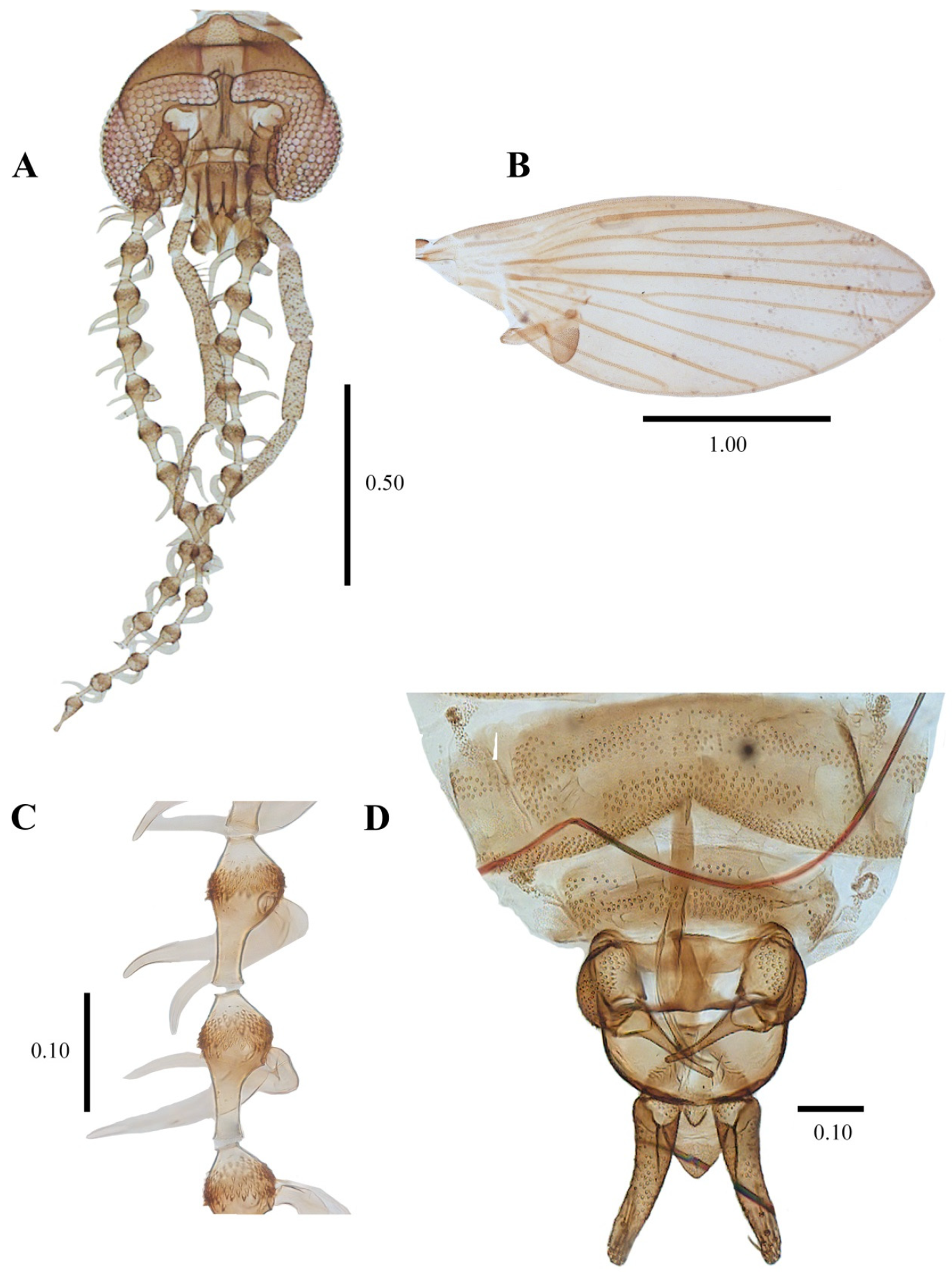
Figure 15.
Telmatoscopus advena (Eaton, 1983), female. (A) Head. (B) Genital chamber. (C) Egg. (D) Cerci. Scale (A–D) in millimeters (mm).
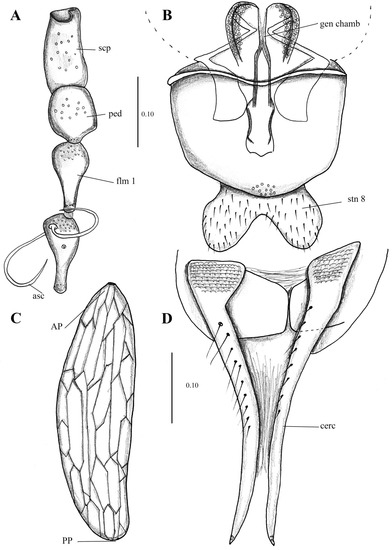
Figure 16.
Telmatoscopus advena (Eaton, 1893), female. (A) First antennal segments. (B) Sternite 8 and genital chamber. (C) Egg. (D) Cerci. Abbreviations: AP = anterior pole, asc = ascoids, cerc = cerci, flm = flagellomere, gen chamb = genital chamber, ped = pedicel, PP = posterior pole, scp = scape, stn = sternite. Scale (A–D) in millimeters (mm).
Wing 2.7 times longer than wide (1 mm wide, 2.7 mm long), Sc long, ending at junction of R4 + R2+3. Infuscated in area between C and R1.
Sternite 8 (Subgenital plate) about the same length and width (0.25 mm wide, 0.24 mm long), waisted in the middle; basal margin straight, apical margin covered in small setae and strongly concave in the middle. Cerci long (0.4 mm length), 1.6 times the length of the sternite 8, with 10 setae on the dorsal surface, the surface in the base of the cerci presents multiple plate-shaped protuberances, resembling the texture of crocodile skin. Subgenital lobes rectangular.
Egg description. (Mean length 0.32 mm, width 0.1 mm, n = 15) Ovoid, longer than wide (0.1 mm wide, 0.32 long). No Micropyle structure is visible in the anterior pole; however, it does seem to be a circular aperture where the micropyle could be located. Exochorion with both longitudinal and transversal single, flattened ridges that when joined, form cells (mainly rectangular or hexagonal) across the exochorion.
Material examined. Holotype examined: no. 235444 [BMNH], ZFMK-DIP-00081296, ZFMK-DIP-00081297, ZFMK-DIP-00081298, ZFMK-DIP-00081306, ZFMK-DIP-00081307, ZFMK-DIP-00081308, ZFMK-DIP-00081309, ZFMK-DIP-00081310, ZFMK-DIP00081313, ZFMK-DIP-00081314, ZFMK-DIP-00081315, ZFMK-DIP-00081316, ZFMK-DIP-00081317, ZFMK-DIP-00081318, ZFMK-DIP-00081319, ZFMK-DIP-00081325, ZFMK-DIP-00081320, ZFMK-DIP-00081331, ZFMK-DIP-00081571, ZFMK-DIP-00081572, ZFMK-DIP-00081573, ZFMK-DIP-00081581, ZFMK-DIP-00081582, ZFMK-DIP-00081620, ZFMK-DIP-00081621, ZFMK-DIP-00081312, ZFMK-DIP-00081500, ZFMK-DIP-00081501, ZFMK-DIP-00081502, ZFMK-DIP-00081509, ZFMK-DIP-00081511, ZFMK-DIP-00081517, ZFMK-DIP-00081520, ZFMK-DIP-00081522, ZFMK-DIP-00081538, ZFMK-DIP-00081545, ZFMK-DIP-00081546, ZFMK-DIP-00081547, ZFMK-DIP-00081549, ZFMK-DIP-00081550, ZFMK-DIP-00081553, ZFMK-DIP-00082120, ZFMK-DIP-00082121, ZFMK-DIP-00082123, ZFMK-DIP-00082127, ZFMK-DIP-00082129, ZFMK-DIP-00082131, ZFMK-DIP-00082132, ZFMK-DIP-00082134, ZFMK-DIP-00082135, ZFMK-DIP-00081570, ZFMK-DIP-00081579, ZFMK-DIP-00081596 [ZFMK]. Specimen number: 10047, 13667, 13602, 13527, 12687, 18493, 13193, 18215, 18237, 20784, 20750, 20823, 0, 11356, 11264, 11357/34235, 21212, 21315, 22648, 22649, 22650, 22651, 22652, 22653 [NMP].
Distribution. Aegan Islands, Belgium, Finland, France, Germany, Ireland, Norway, Slovakia, United Kingdom [13,43,87].
Telmatoscopus bartai (Ježek, 2004) comb. nov.
Krivosheinoscopus bartai Ježek, 2004: 117, Type locality: Czech Republic: Bohemia or., Železné hory Mts Protected landscape area.
Diagnosis. Male. Ascoids digitiform, thin, long, and coiled; phallomeres symmetrical, out curved, more than half the length of ejaculatory apodeme; hypopods with tenacula restricted to apex.
Examined material. Holotype examined: Cat. No. 34243 [NMP]. ZFMK-DIP-00081598, ZFMK-DIP-00081597, ZFMK-DIP-00081599 [ZFMK].
Distribution. Czech Republic [88], Germany. Our three reported specimens are the first record of this species in Germany.
Telmatoscopus laurencei Freeman, 1953
Telmatoscopus laurencei Freeman, 1953: 71. Type locality: Great Britain. Herts, Harpenden, Rothamsted Experimental Station.
Diagnosis. Male. Ascoids digitiform, thin, coiled; phallomeres coiled, less than half the length of ejaculatory apodeme; hypopods with tenacula restricted to apex.
Examined material. None.
Distribution. Only known from Great Britain [13,39,43].
Telmatoscopus thuringicus Beran, Doczkal, Pfister & Wagner, 2010
Telmatoscopus thuringicus Beran, Doczkal, Pfister & Wagner, 2010: 63. Type locality: Germany, Thuringia, National Park Hainich, Weberstedt, Birkensee.
Diagnosis. Male. Ascoids are broad, leaf-shaped, tapering towards the apex and coiled; phallomeres out curved, less than half the length of ejaculatory apodeme; hypopods with a cluster of approximately 30 tenacula at apex, and more tenacula scattered along the entire surface of hypopods.
Examined material. None.
Distribution. Only known from Germany [5].
Key to the European adult males of Telmatoscopus
- 1.
- More than 30 tenacula; tenacula not restricted to the apical portion of hypopods, scattered in all the hypopods length (as in [5] (Figure 10))…T. thuringicus
- -
- Less than 30 tenacula; tenacula restricted to the apical portion of hypopods and not scattered in the hypopods length…2
- 2.
- -
- Ascoids narrow, never broader than 1/3 of the width of the basal node of flagellomere carrying them; parameres coiled or out curved (Figure 13C)…3
- 3.
- Parameres coiled with strong incurvation after the coil and apical tips hook-out curved (as in [38] (Figure 2D)); hypopods with less than 20 tenacula…T. laurencei
- -
- Parameres not coiled and out curved (Figure 13C); hypopods with more than 20 tenacula…T. bartai
4. Discussion
All of the above-mentioned species (also in Table 1) are recorded to be associated with dendrotelmata to a certain degree, whether they are specialized to complete their life cycle in this ecosystem or they are only opportunistic is a different matter. As mentioned by Oboňa and Ježek [25] the extreme variation of environmental conditions such as pH, temperature, frequent water loss with rapid flooding and, oxygen deficit can cause the death of non-specialized species, especially in larval stages that are using dendrotelmata as an irregular breeding/developing site, while specialized species can endure these harsh 822 environmental variations thriving through all their life cycle.
The occurrence of Clogmia albipunctata, C. xylophilus, Pneumia canescens, P. trivialis, Psychoda alternata, P. cinerea, and P. minuta in dendrotelmata is rather incidental or highly understudied [23,25]. In other words, the presence of these species could be an extension of their regular development sites (e.g., small water bodies, streams, etc.) and adults happen to find water-filled tree holes that are a potential development site for their offspring, thus, they could develop in Dendrotelmata and survive, but their long-term usage of this site is not well documented. Further studies in different habitat could provide key information to better understand the ecological relationship between water-filled tree holes and the species of Psychodinae that develop in them. To the date, only a handful of studies specifically targeted moth flies in dendrotelmata.
Clogmia albipunctata is broadly distributed both in Europe and worldwide and is the most synanthropic species inside the Psychodidae fauna, it can be commonly found inside buildings, and sewage treatment plants in almost every city. The high synanthropy is shared with some species of the genus Psychoda, which are also often found in cities, including P. alternata and P. cinerea, this species can certainly adapt and develop in a wide range of habitats, including dendrotelmata, therefore, these species could be classified as generalists when it comes to sites for larval development and not bound to develop in water-filled tree holes. Some further Psychoda species may be found in dendrotelmata, but the development of most species is still unknown.
Lepiseodina tristis, L. rothschildi, Telmatoscopus advena and T. laurencei are well-documented species that develop inside dendrotelmata (Table 1) with multiple records of both adults and larvae. On the contrary, Telmatoscopus thuringicus and T. bartai comb. nov., are only assumed to be dendrolimnetic based on observations of the closely related species T. advena [5]. In the case of the herein described species Lepiseodina latipennis—some specimens collected in Italy were reared from decaying organic matter collected in a dendrotelmata from a maple tree (Acer sp.), further specimens collected in Germany came from a Malaise Trap placed next to a water-filled tree whole in an Oak tree, thus proving that this species develop inside dendrotelmata as congeneric species.
5. Conclusions
In Europe, only 13 species of Psychodinae are known to develop inside water-filled tree holes, after our extensive record search we report that the Psychodinae species develop in 13 different tree species, including two new tree species in which no previous record of larval development was reported. We redescribed Lepiseodina latipennis through holotype and new material examination, and we report it for the first time in Germany. We also report Telmatoscopus bartai comb. nov. for the first time in Germany, and we provide a generic discussion of Telmatoscopus. Water-filled tree holes (dendrotelmata) are usually associated with old trees which are commonly endangered through the forest management strategies applied in European countries, and they are becoming rare to find inside forests. To summarize, there is a gap in the knowledge of the ecological interactions inside the Psychodinae and their environment, further studies can potentially provide new information, new records and new interactions that would be beneficial to better understand the ecosystems. Furthermore, old tree individuals remain a key component in the forests, as they harbor high biodiversity that is still understudied and they should remain untouched until the natural decomposition takes place.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14070532/s1, Table S1: Examined material collection Data.
Author Contributions
Conceptualization, S.J.-S., G.M.K. and X.M.; investigation, S.J.-S., A.M., G.M.K. and X.M.; Methodology, S.J.-S., A.M., G.M.K. and X.M.; Resources, S.J.-S., A.M., G.M.K. and X.M.; Writing—original draft, S.J.-S.; Writing—review & editing, A.M., G.M.K. and X.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Bundesministerium für Bildung und Forschung, Berlin, Germany, project “German Barcode of Life III: Dark Taxa” (FKZ 16LI1901B).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Björn Müller for his incredible help during the DNA extraction process of specimens. We extend our gratitude to Björn Rulik for collecting the some of the specimens examined for this work. We are thankful to Michal Tkoč for allowing the first author to visit the Psychodidae collection in Prague. We are thankful to all the GBOL III: Dark Taxa team for their constant support. Last but not least we are thankful to Greg Curler and Weia Reinboud for valuable discussions on taxonomy of dendrolimnetic Psychodidae.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Varga, L. Ein interessanter Biotop der Bioconose von Wasserorganismen. Biol. Zentralbl. 1928, 48, 143–162. [Google Scholar]
- Kitching, R.K. An Ecological Study of Water-Filled Tree-Holes and their Position in the Woodland Ecosystem. J. Anim. Ecol. 1971, 40, 281–302. Available online: https://www.jstor.org/stable/pdf/3247.pdf (accessed on 5 April 2022). [CrossRef]
- Kitching, R.L. Food Webs and Container Habitats: The Natural History and Ecology of Phytotelmata; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Kirsch, J.J.; Sermon, J.; Jonker, M.; Asbeck, T.; Gossner, M.M.; Petermann, J.S.; Basile, M. The use of water-filled tree holes by vertebrates in temperate forests. Wildl. Biol. 2021, 1, 1–4. [Google Scholar] [CrossRef]
- Beran, B.; Doczkal, D.; Pfister, K.; Wagner, R. Two new species of Psychodidae (subfamilies: Trichomyiinae and Psychodinae) from Germany associated with decaying wood. Zootaxa 2010, 2386, 59–64. [Google Scholar] [CrossRef]
- Fish, D.; Carpenter, S.R. Leaf litter and larval mosquito dynamics in tree-hole ecosystems. Ecology 1982, 63, 283–288. [Google Scholar] [CrossRef]
- Schmidl, J.; Sulzer, P.; Kitching, R.L. The insect assemblage in water-filled tree-holes in a European temperate deciduous forest: Community composition reflects structural, trophic, and physicochemical factors. Hydrobiologia 2008, 598, 285–303. [Google Scholar] [CrossRef]
- Gossner, M.M. A three-year study of the phenology of insect larvae (Coleoptera, Diptera) in water-filled tree holes in the canopy of a beech tree. Eur. J. Entomol. 2018, 115, 524–534. [Google Scholar] [CrossRef]
- Oboňa, J.; Ježek, J.; Kanasová, K.; Mango, P. Hiding in plain sight: New records and endangered flies (Diptera) from a tree-hole in an urban park (Prešov, Slovakia). Acta Musei Sil. Sci. Nat. 2021, 70, 75–81. [Google Scholar] [CrossRef]
- Oboňa, J.; Ježek, J.; Fogašová, K.; Manko, P.; Korneyev, V.A. The moth fly Clogmia albipunctata (Diptera: Psychodidae) in Ukraine. Ukr. Entomofaunistyka 2021, 12, 13–16. [Google Scholar]
- Duckhouse, D.A. Non-phlebotomine Psychodidae (Diptera, Nematocera) of southern Africa. II. Neoarisemus and the brunettoid and telmatoscopoid genera. Ann. Natal Mus. 1978, 23, 305–359. [Google Scholar]
- Withers, P. Moth Flies (Diptera: Psychodidae). Dipter. Dig. 1989, 4, 1–83. [Google Scholar]
- Withers, P. Some further records of Irish rot-hole moth-flies (Diptera: Psychodidae), with a first record for Telmatoscopus rothschildii, and a figure of the male terminalia of that species. Ir. Nat. J. 1989, 23, 16–17. [Google Scholar]
- Curler, G.R.; Moulton, K. A review of Nearctic Clytocerus (Diptera: Psychodidae: Psychodinae). Can. Entomol. 2012, 144, 186–195. [Google Scholar] [CrossRef]
- Kvifte, G.M.; Wagner, R. Psychodidae (Sand Flies, Moth Flies or Owl Flies). In Manual of Afrotropical Diptera. Nematocerous Diptera and Lower Brachycera; Kirk-Spriggs, A.H., Sinclair, B.J., Eds.; South African National Biodiversity Institute: Pretoria, South Africa, 2017; Volume 2, pp. 607–632. [Google Scholar]
- Feuerborn, H.J. Der sexuelle Reizapparat (Schmuck-, Duft- und berührungsorgane) der Psychodiden nach biologischen und physiologischen Gesichtspunkten untersucht. Arch. Nat. A 1922, 4, 1–137. [Google Scholar]
- Feuerborn, H.J. Die Larven der Psychodiden oder Schmetterlingsmücken. ein beitrag zur “Ökologie des Feuchten”. Int. Ver. Theor. Angew. Limnol. Verh. 1923, 1, 181–213. [Google Scholar] [CrossRef]
- Mayer, K. Zür kenntnis der buchenhöhlenfauna. Arch. Hydrobiol. 1938, 33, 388–400. [Google Scholar]
- Röhnert, U. Wassererfüllte baumhöhlen und ihre besiedlung. ein beitrag zur Fauna dendrolimnetica. Arch. Hydrobiol. 1950, 44, 472–516. [Google Scholar]
- Jung, H.F. Beiträge zur biologie, Morphologie und Systematik der europäischen Psychodiden (Diptera). Deut. Entomol. Z. 1956, 3, 97–257. [Google Scholar]
- Mirouse, R.; Vaillant, F. Les Telmatoscopus des arbres creux (Dipteres Psychodidae). L’Entomologiste 1960, 16, 7–16. [Google Scholar]
- Withers, P. Some moth-flies (Diptera: Psychodidae) reared from tree rot-holes in Ireland including a first breeding record of Telmatoscopus advenus. Ir. Nat. J. 1987, 22, 201–202. [Google Scholar]
- Vaillant, F. Les Psychodinae dendrolimnophiles et dendrolimnobiontes palearctiques et nearctiques (insecta, Diptera, nematocera, Psychodidae). Spixiana 1989, 12, 193–208. [Google Scholar]
- Vaillant, F. Les Diptères Psychodidae dendrolimnobiontes du sud-est de la France et leur microendémisme. Ann. Soc. Entomol. Fr. 1990, 26, 371–379. [Google Scholar]
- Oboňa, J.; Ježek, J. First records of dendrolimnetic moth flies (Diptera: Psychodidae) from Slovakia. Klapalekiana 2012, 48, 279–289. [Google Scholar]
- Geiger, M.F.; Astrin, J.J.; Borsch, T.; Burkhardt, U.; Grobe, P.; Hand, R.; Hausmann, A.; Hohberg, K.; Krogmann, L.; Lutz, M.; et al. How to tackle the molecular species inventory for an industrialized nation—Lessons from the first phase of the German Barcode of Life initiative GBOL (2012–2015). Genome 2016, 59, 661–670. [Google Scholar] [CrossRef] [PubMed]
- De Jong, Y.; Verbeek, M.; Michelsen, V.; de Place Bjørn, P.; Los, W.; Steeman, F.; Bailly, N.; Basire, C.; Chylarecki, P.; Stloukal, E.; et al. Fauna Europaea—All European animal species on the web. Biodivers. Data J. 2014, 2, e4034. [Google Scholar] [CrossRef]
- Ibáñez-Bernal, S. Phlebotominae (Diptera: Psychodidae) de Mexico. V. Clave ilustrada para la identificacion de los machos de Lutzomyia França. Folia Entomol. Mex. 2005, 44, 49–66. [Google Scholar]
- Cumming, J.M.; Wood, D.M. 3. Adult morphology and terminology. In Manual of Afrotropical Diptera. Introductory Chapters and Keys to Diptera Families; Kirk-Spriggs, A.H., Sinclair, B.J., Eds.; SANBI Graphics & Editing: Pretoria, South Africa, 2017; Volume 1, pp. 89–133. [Google Scholar]
- Kvifte, G.M.; Wagner, R. Review of Neurosystasis Satchell, with two new species from Cuba and a discussion of cerci and surstyli in Psychodinae (Diptera: Psychodidae). Zootaxa 2017, 4306, 81–90. [Google Scholar] [CrossRef]
- Kotrba, M. Morphology and terminology of the female postabdomen. In Contributions to a Manual of Palaearctic Diptera; Papp, L., Darvas, B., Eds.; Science Herald: Budapest, Hungary, 2000; Volume 1, pp. 75–84. [Google Scholar]
- De Almeida, N.; Oliveira, R.S.; Brazil, B.G.; Soares, M.J. Pattern of exochorion ornaments on eggs of seven South American species of Lutzomyia sand flies (Diptera: Psychodidae). J. Med. Entomol. 2004, 41, 819–825. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Astrin, J.J.; Stüben, P.E. Phylogeny in cryptic weevils: Molecules, morphology and new genera of western Palaearctic Cryptorhynchinae (Coleoptera: Curculionidae). Invertebr. Syst. 2008, 22, 503–522. [Google Scholar] [CrossRef]
- Ježek, J.; Lukáš, J.; Kvifte, G.M.; Oboňa, J. New faunistic records of non-biting moth flies (Diptera: Psychodidae) from the Czech Republic and Slovakia. Klapalekiana 2012, 48, 121–126. [Google Scholar]
- Kvifte, G.M.; Ivković, M.; Klarić, A. New records of moth flies (Diptera: Psychodidae) from Croatia, with the description of Berdeniella keroveci sp. nov. Zootaxa 2013, 3737, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, F. 9d. Psychodidae-Psychodinae. In Die Fliegen der Palaearktischen Region 2; Lindner, E., Ed.; Leif.. 328, E. Schweizerbart’scheVerlagsbuchhandlung: Stuttgart, Germany, 1983; pp. 311–358. [Google Scholar]
- Kroča, J.; Ježek, J. Moth Flies (Diptera: Psychodidae) of the Moravskoslezské Beskydy Mts and Podbeskydská pahorkatina Upland, Czech Republic, II. Acta Musei Sil. Sci. Nat. 2019, 68, 201–232. [Google Scholar] [CrossRef]
- Freeman, P. Two new species of Psychodidae (Diptera, Nematocera) from Britain. Proc. R. Entomol. 1953, 22, 69–71. [Google Scholar] [CrossRef]
- Ibáñez-Bernal, S. New Records and Descriptions of Mexican Moth Flies (Diptera: Psychodidae, Psychodinae). Trans. Am. Entomol. Soc. 2008, 134, 87–131. [Google Scholar] [CrossRef]
- Quate, L.W. A revision of the Psychodidae (Diptera) in America north of Mexico; University of California Press: Berkeley, CA, USA, 1955; Volume 10, pp. 103–273. [Google Scholar]
- Enderlein, G. Klassifikation der Psychodiden (Dipt.). Deut. Entomol. Z. 1937, 1936, 81–112. [Google Scholar]
- Wagner, R. Fauna Europaea: Psychodidae. In Fauna Europaea: Diptera: Nematocera; Fauna Europea Version 1.1; de Jong, Y., Ed.; 2004; Available online: http://www.faunaeur.org (accessed on 5 February 2022).
- Boumans, L.; Zimmer, J.-Y.; Verheggen, F. First record of the ‘bathroom mothmidge’ Clogmia albipunctata, a conspicuous element of the Belgian fauna that went unnoticed (Diptera: Psychodidae). Phegea 2009, 37, 153–160. Available online: http://dare.uva.nl/document/2/71517 (accessed on 5 April 2022).
- Sivell, D.; Irwin, T. Clogmia albipunctata (Williston) (Diptera, Psychodidae) in London. Dipter. Dig. 2016, 23, 111–115. [Google Scholar]
- Salmela, J.; Keskitalo, M.; Metsälä, P. Perhossääski Clogmia albipunctata (Williston) havaittu Suomesta (Diptera, Psychodidae). Sahlbergia 2019, 25, 15–17. [Google Scholar]
- Kvifte, G.M. Citizen science reveals the establishment of the invasive container breeder Clogmia albipunctata in Sweden and Denmark (Diptera: Psychodidae). Manag. Biol. Invasions, 2022; accepted. [Google Scholar]
- Evenhuis, N.L. Reassessment of Clytocerus Eaton, 1904 (Diptera: Psychodidae) based on a recently discovered type species designation by Malloch in 1907, with a checklist of world species. Systema Dipterorum Nomenclatural Notes. II. Occas. Pap. Bishop Mus. 2022, 143, 11–15. Available online: http://hbs.bishopmuseum.org/sd/pdf/op143p11-15.pdf (accessed on 22 May 2022).
- Krek, S. Psychodidae (Diptera: Insecta) Balkanskog Poluotoka; Studentska Stamparija Univerziteta: Sarajevo, Bosnia and Herzegovina, 1999; 417p. [Google Scholar]
- Vaillant, F. Psychodidae-Psychodinae. In Die Fliegen Der Palearktischen Region; Lindner, E., Ed.; Lief.. 291, E. Schweizerbart’scheVerlagsbuchhandlung: Stuttgart, Germany, 1972; pp. 49–78. [Google Scholar]
- Wagner, R. Family Psychodidae, 11–65. In Psychodidae–Chironomidae; Catalogue of Palaearctic Diptera; Soós, A., Ed.; Akadémiai Kiadó: Budapest, Hungary, 1990; Volume 2, 499p. [Google Scholar]
- Ježek, J. Redescriptions of nine common Palearctic and Holarctic species of Psychodini End. (Diptera: Psychodidae). Acta Entomol. Musei Natl. Pragae 1990, 43, 33–83. [Google Scholar]
- Dahl, C.; Krivosheina, N.P.; Krzeminska, E.; Lucchi, A.; Nicolai, P.; Salamanna, G.; Santini, L.; Skuhrava, M.; Zwick, P. Diptera Blephariceromorpha, Bibionomorpha, Psychodomorpha, Ptychopteromorpha. In Checklist Delle Specie Della Fauna Italiana; Minelli, A., Ruffo, S., La Posta, S., Eds.; Calderini: Bologna, Italy, 1995; pp. 1–39. [Google Scholar]
- Kvifte, G.M.; Stokkan, M.; Wagner, R. Review of the Psychodinae from Mallorca, Spain, with description of Pericoma unipennata, sp. n. (Diptera, Psychodidae). Zookeys 2016, 577, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Ježek, J.; Manko, P.; Oboňa, J. Synopsis of the Psychodidae (Diptera) fauna of Bulgaria. Zootaxa 2020, 4877, 201–240. [Google Scholar] [CrossRef]
- Neuhaus, G.H. Diptera Marchica. Systematisches Verzeichniss der Zweiflügler (Mücken und Fliegen) der Mark Brandenburg mit Kurzer Beschreibung und analytischen Bestimmungs-Tabellen; Nicolaische verlags-buchhandlung: Berlin, Germany, 1886; 371p. [Google Scholar] [CrossRef]
- Schiner, I.R. Catalogus Systematicus Dipterorum Europae; impensis Societatis zoologico-botanicae editus, Vindobonae: Viena, Austria, 1864; 144p. [Google Scholar] [CrossRef]
- Tonnoir, A.L. Nouvelle contribution A l’etude des Psychodidae (Diptera) et description de dix espsces nouvelles d’Europe. Ann. Soc. Entomol. Belg. 1922, 62, 153–181. [Google Scholar]
- Bernotienè, R. Moth flies (Diptera, Psychodidae) new for Lithuanian fauna. Ekologija 2002, 2, 4–8. [Google Scholar]
- Ježek, J. Psychodidae Newman, 1834. In Checklist of Diptera of the Czech Republic and Slovakia; Jedlička, L., Kúdela, M., Stloukalová, V., Eds.; 2009; Available online: http://www.edvis.sk/diptera2009/ (accessed on 12 April 2022).
- Bezzi, M. Contributo alla fauna ditterologica della provincia di Pavia. II. Boll. Soc. Entomol. Ital. 1892, 24, 74–75. [Google Scholar]
- Omelková, M.; Ježek, J. Two new species of Pneumia Enderlein (Diptera, Psychodidae, Psychodinae) from the Palearctic region. Zootaxa 2012, 3180, 1–18. [Google Scholar] [CrossRef]
- Ježek, J.; Goutner, V. Psychodidae (Diptera) of Greece. Acta Musei Natl. Pragae Ser. B 1995, 50, 107–124. [Google Scholar]
- Ježek, J.; Grootaert, P.; Lock, K.; Manko, O.; Oboňa, J. Moth flies (Diptera: Psychodidae) from the Belgian transition of the Atlantic to the central European Faunal zones. Biodivers. Environ. 2018, 10, 5–16. [Google Scholar]
- Ježek, J.; Manko, P.; Oboňa, J. Psychodidae (Diptera) of Azerbaijan and Georgia-faunistics with biodiversity notes. ZooKeys 2021, 1049, 15–42. [Google Scholar] [CrossRef] [PubMed]
- Ježek, J. Nomenclatural changes of some higher taxa of Palearctic Psychodinae (Diptera, Psychodidae). Acta Faun. Entomol. Musei Natl. Pragae 1984, 17, 155–170. [Google Scholar]
- Duckhouse, D.A.; Lewis, D.J. Superfamily Psychodoidea 15. Family Psychodidae. Catalog of the Diptera of Australasian and Oceanian Regions. Bish. Mus. Spec. Publ. 1989, 86, 166–179. [Google Scholar]
- Jezek, J. New and interesting moth flies (Diptera, Psychodidae) from the Australian museums of Perth and Sydney. Čas. Nár. Muz. Rada Přír. 2000, 169, 23–33. [Google Scholar]
- Wagner, R. Contribution to the knowledge of Spanish Psychodidae (Diptera) with description of two new species. Zool. Baetica 2001, 12, 83–90. [Google Scholar]
- Jezek, J. The first account of Slovenian moth flies (Psychodidae, Diptera). Čas. Nár. Muz. Rada Přír. 2002, 171, 89–116. [Google Scholar]
- Bravo, F.; Cordeiro, D.; Chagas, C. Two new species and new records of Psychoda Latreille (Diptera: Psychodidae: Psychodinae) from Brazil, with comments on supraespecific classification of the genus. Zootaxa 2006, 1298, 1–15. [Google Scholar] [CrossRef]
- Quate, L.W. Taxonomy of Neotropical Psychodidae (Diptera) 1. Psychoda species of West Indies and Central America, with a key to Trinidad species. Pan-Pac. Entomol. 1959, 35, 213–220. [Google Scholar]
- Duckhouse, D.A. Psychodidae (Diptera, Nematocera) of Southern Australia: Subfamily Psychodinae. Trans. R. Entomol. Soc. Lond. 1966, 118, 153–220. [Google Scholar] [CrossRef]
- Pellerano, G. Notas sobre Psychodidae (Diptera) argentinos. I. Redescripción de Psychoda alternata Say, P. cinerea Banks y Telmatoscopus albipunctatus (Williston). Physis 1967, 27, 9–26. [Google Scholar]
- Duckhouse, D.A. Family Psychodidae. In A Catalogue of the Diptera of the Americas South of the United States; Papavero, N., Ed.; Museu de Zoologia, Universidade de Sao Paulo: São Paulo, Brazil, 1973; pp. 1–29. [Google Scholar]
- Duckhouse, D.A. Family Psychodidae. In A catalog of the Diptera of the Oriental Region. Vol. 1. Suborder Nematocera; Delfinado, M.D., Hardy, D.E., Eds.; University Press of Hawaii: Honolulu, HI, USA, 1973; pp. 226–244. [Google Scholar]
- Quate, L.W. Preliminary taxonomy of Costa Rican Psychodidae (Diptera), exclusive of Phlebotominae. Rev. Biol. Trop. 1996, 44, 1–81. [Google Scholar] [PubMed]
- Collantes, F.; Martínez-Ortega, E. Nuevas citas de especies conocidas de Psychodinae (Diptera: Psychodidae) en Nicaragua. Rev. Nicaragüense Entomol. 1999, 48, 17–27. [Google Scholar]
- Bejarano, E.E. Lista actualizada de los psicódidos (Diptera: Psychodidae) de Colombia. Folia Entomol. Mex. 2006, 45, 47–56. [Google Scholar]
- Wagner, R.; Andrade, R.; Gonçalves, A. Moth flies (Diptera, Psychodidae) from Portugal with descriptions of a new genus, new species and additions to the fauna of the Iberian Peninsula. Zootaxa 2022, 5129, 37–59. [Google Scholar] [CrossRef]
- Tjeder, B. Östskånska insekter 2. Hymenoptera (forts.) och Diptera Nematocera (partim.). Opusc. Entomol. 1954, 19, 205–209. [Google Scholar]
- Quate, L.W. Family Psychodidae. In A Catalog of the Diptera of America North of Mexico; Stone, A., Sabrosky, C.W., Wirth, W.W., Foote, R.H., Coulson, J.R., Eds.; U.S. Government Printing Office: Washington, DC, USA, 1965; pp. 91–97. [Google Scholar]
- Kvifte, G.M. Nomenclature and taxonomy of Telmatoscopus Eaton and Seoda Enderlein; with a discussion of parameral evolution in Paramormiini and Pericomaini (Diptera: Psychodidae, Psychodinae). Zootaxa 2014, 3878, 390–400. [Google Scholar] [CrossRef]
- Ježek, J. New Palearctic taxa of moth flies (Diptera: Psychodidae) from very small accidental spirituous samples of insects. Acta Carol. Biol. 2001, 45, 53–66. [Google Scholar]
- Enderlein, G. Zur Klassifikation der Psychodinen. Sber. Ges. Naturf. Freunde Berl. 1935, 1935, 246–249. [Google Scholar]
- Freeman, P. British Psychodidae. Handb. Identif. Br. Insects 1950, 9, 77–96. [Google Scholar]
- Kvifte, G.M.; Boumans, L. Further records and DNA barcodes of Norwegian moth flies (Diptera, Psychodidae). Nor. J. Entomol. 2014, 61, 11–14. [Google Scholar]
- Ježek, J. New and interesting moth flies (Diptera: Psychodidae) from protected and underestimated natural areas of Czech Republic. J. Nat. Muz. Prague Nat. Hist. Ser. 2004, 173, 113–128. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).