Prioritizing Plants around the Cross-Border Area of Greece and the Republic of North Macedonia: Integrated Conservation Actions and Sustainable Exploitation Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Botanical Collections of Target Plants
2.2. Hierarchical Prioritization of Target Plants
- Taxa assessed as threatened in the Global IUCN Red List website (https://www.iucnredlist.org/, accessed on 13 June 2022) and/or the 1997 Global IUCN Red List [58] present in sites of the INTERREG eligible region,
- Other Important Species of the NATURA 2000 Network (EU Directive 92/43/EEC) of the Greek INTERREG eligible regions [59],
- Species of special conservation interest of the Emerald Network Areas of (http://emerald.eea.europa.eu/#, accessed on 13 June 2022) of sites of the Republic of North Macedonian included in the INTERREG eligible region,
- Trigger species of Important Plant Areas (IPA) and Key Biodiversity Areas (KBA) of the Republic of North Macedonian [8] included in the INTERREG eligible region,
- Other local Balkan endemic taxa (some extending to Italy, Anatolia and/or Romania) present in the INTERREG eligible regions according to widely scattered literature sources (e.g., [60], etc.).

- (i)
- The Euro + Med Plantbase (http://www.emplantbase.org/home, accessed 13 June 2022) and/or the official Flora of Greece (http://portal.cybertaxonomy.org/flora-greece/, accessed 13 on June 2022),
- (ii)
- The Global IUCN Red List website (http://www.iucnredlist.org/, accessed on 13 June 2022),
- (iii)
- The Kew Botanic Garden’s database (http://plantsoftheworldonline.org/, accessed on 13 June 2022).
2.3. In-Situ Documentation of Threats-Risks of Target Plants
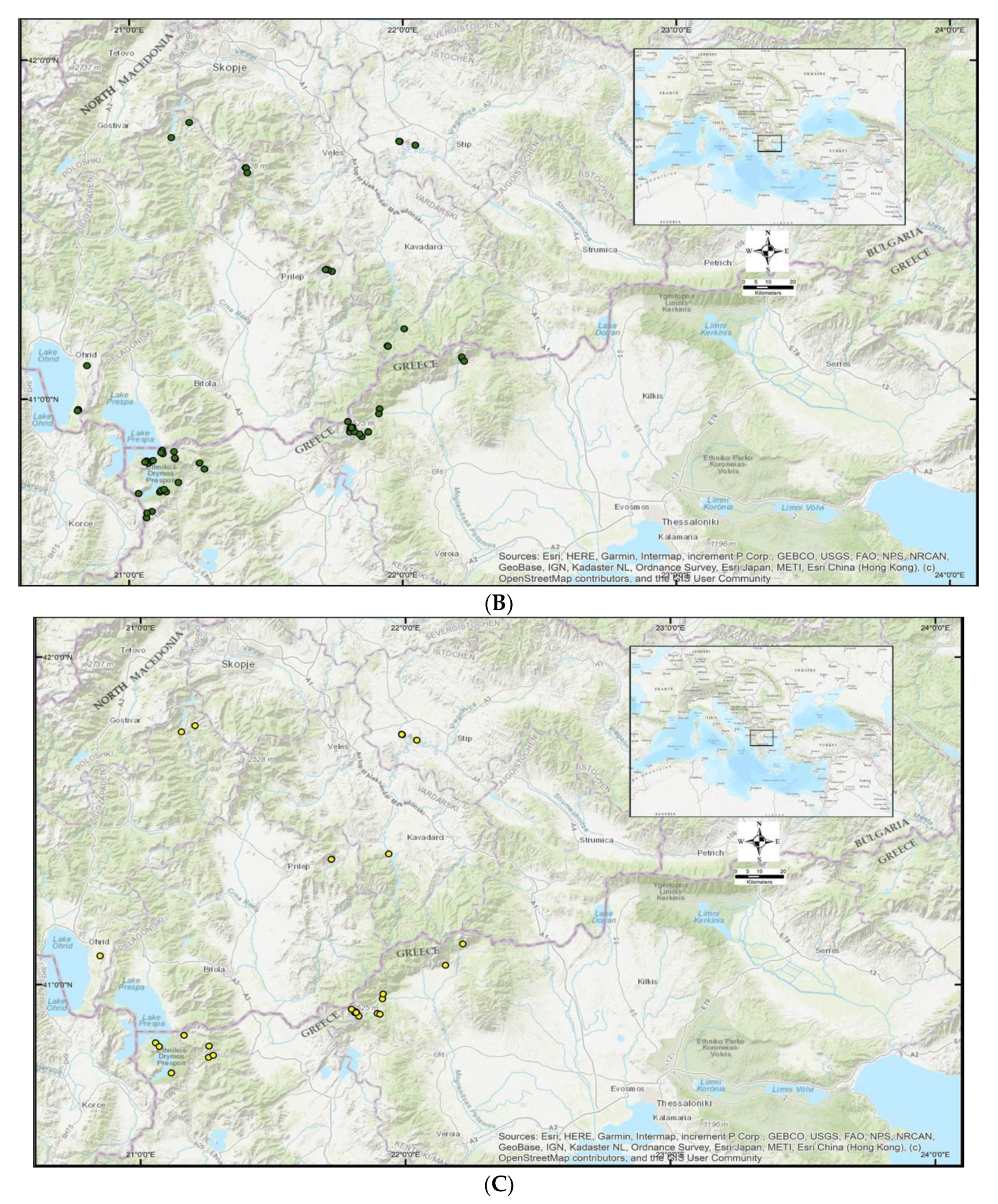
2.4. Spatial Analysis of the Habitats of Target Plants
2.5. Worldwide Ex Situ Conservation of Target Plants
2.6. Seed Germination Trials of Target Plants
2.7. Cutting Propagation Trials of Target Plants
2.8. Plant Division Trials of Target Plants
2.9. Statistical Analysis of Datasets
2.10. Ornamental Characteristics of Target Plants for Gardening-Landscaping Applications
3. Results
3.1. Overview of Botanical Collections
3.2. Extinction Risk Assessments
3.3. Threats-Risks Analysis
3.4. Worldwide Ex-Situ Conservation
3.5. Ex-Situ Propagation Trials
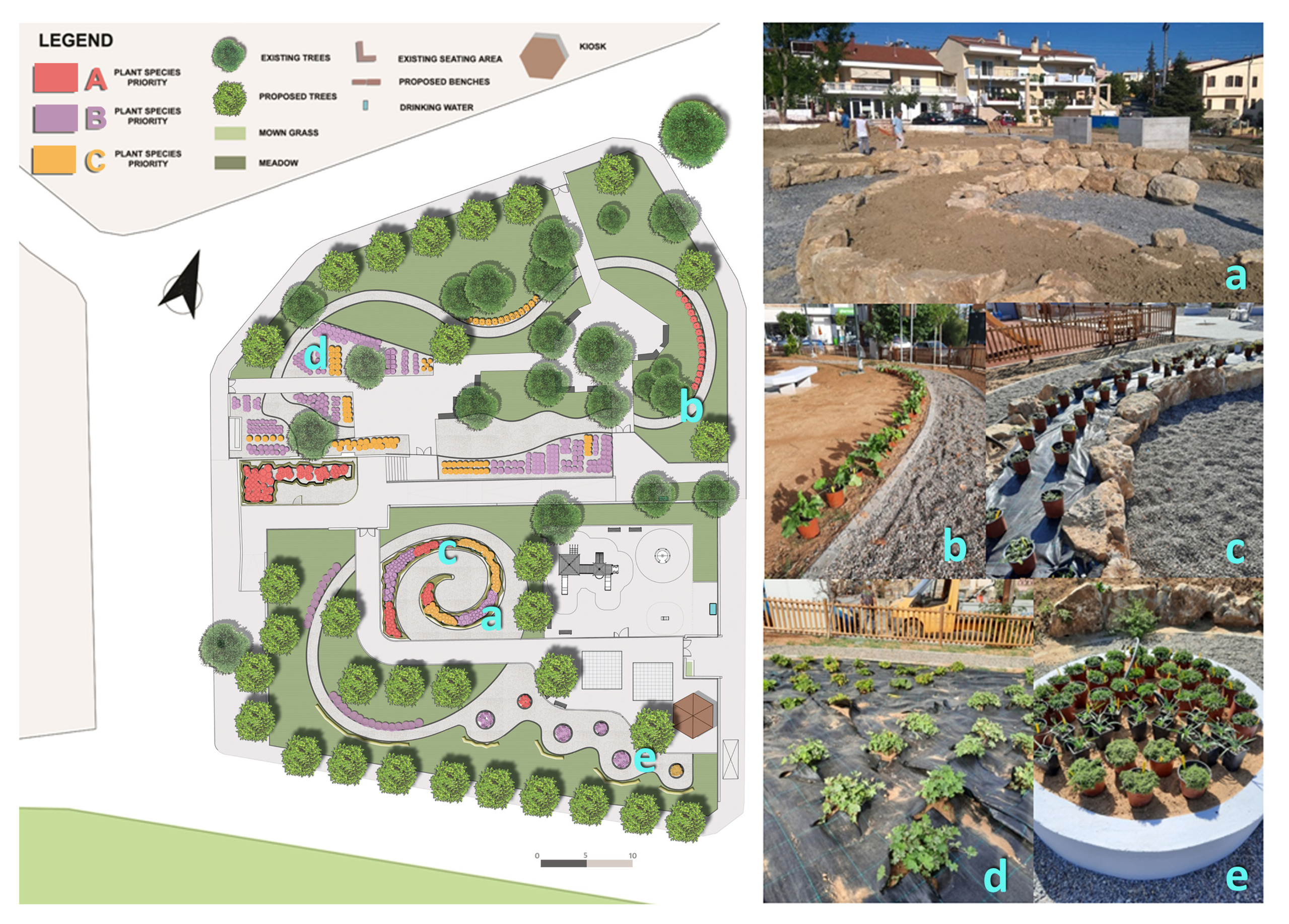
3.6. Establishment in Awareness-Raising Sites
4. Discussion
4.1. Conservation Concern and Plant Prioritization Process
4.2. Facilitating Informed In-Situ Conservation Efforts in the Cross-Border Area
4.3. Ex-Situ Conservation Efforts for Future In-Situ Actions
4.4. Priority Plants in Awareness-Raising Sites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fenu, G.; Bacchetta, G.; Christodoulou, C.; Cogoni, D.; Fournaraki, C.; del Galdo, G.; Pietro, G.; Gotsiou, P.; Kyratzis, A.; Piazza, C.; et al. A common approach to the conservation of threatened island vascular plants: First results in the Mediterranean basin. Diversity 2020, 12, 157. [Google Scholar] [CrossRef]
- Butchart, S.H.M.; Walpole, M.; Collen, B.; van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global biodiversity: Indicators of recent declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef]
- Convention on Biological Diversity. CBD/WG2020/3/7 Report of the Open-Ended Working Group on the Post-2020 Global Biodiversity Framework on Its Third Meeting (Part II). In Proceedings of the Third Meeting of the Open-Ended Working Group on the Post-2020 Global Biodiversity Framework, Geneva, Switzerland, 14–19 March 2022; Available online: https://www.cbd.int/doc/c/50c9/a685/3844e4030802e9325bc5e0b4/wg2020-03-07-en.pdf (accessed on 13 June 2022).
- Le Roux, J.J.; Hui, C.; Castillo, M.L.; Iriondo, J.M.; Keet, J.H.; Khapugin, A.A.; Médail, F.; Rejmánek, M.; Theron, G.; Yannelli, F.A.; et al. Recent anthropogenic plant extinctions differ in biodiversity hotspots and coldspots. Curr. Biol. 2019, 29, 2912–2918.e2. [Google Scholar] [CrossRef]
- Pimm, S.L.; Russell, G.J.; Gittleman, J.L.; Brooks, T.M. The Future of Biodiversity. Science 1995, 269, 347–350. [Google Scholar] [CrossRef]
- Heywood, V.H. In situ conservation of plant species–An unattainable goal? Isr. J. Plant Sci. 2016, 63, 211–231. [Google Scholar] [CrossRef]
- Melovski, L.; Velevski, M.; Matevski, V.; Avucatov, V.; Sarov, A. Using Important Plant Areas and Important Bird Areas to Identify Key Biodiversity Areas in the Republic of Macedonia. J. Threat. Taxa 2012, 4, 2766–2778. Available online: https://threatenedtaxa.org/JoTT/article/view/782/1399 (accessed on 13 June 2022). [CrossRef]
- Bilz, M.; Kell, S.P.; Maxted, N.; Lansdown, R.V. European Red List of Vascular Plants; Publications Office of the European Union: Luxembourg, 2011; Available online: https://www.iucn.org/sites/dev/files/import/downloads/rl_4_016.pdf (accessed on 13 June 2022).
- IUCN. IUCN Red List Categories and Criteria: Version 3.1, 2nd ed.; IUCN: Gland, Switzerland, 2012; Available online: https://www.iucnredlist.org/resources/categories-and-criteria (accessed on 13 June 2022).
- Rodrigues, A.; Pilgrim, J.; Lamoreux, J.; Hoffmann, M.; Brooks, T. The value of the IUCN Red List for conservation. Trends Ecol. Evol. 2006, 21, 71–76. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Strid, A.; Raus, T. Flora of Greece Web. Vascular Plants of Greece: An Annotated Checklist. 2020. Available online: https://portal.cybertaxonomy.org/flora-greece/content (accessed on 13 June 2022).
- Matevski, V.; Čarni, A.; Avramoski, O.; Juvan, N.; Kostadinovski, M.; Košir, P.; Paušič, P.; Šilc, U. Forest Vegetation of Galičica Mountain Range in Macedonia; Založba ZRC, ZRC SAZU: Ljubljana, Slovenia, 2011; Available online: https://omp.zrc-sazu.si/zalozba/catalog/book/634 (accessed on 13 June 2022).
- Convention on Biological Diversity. CBD: Country Profiles—North Macedonia, Sixth National Report to the United Nations Convention on Biological Diversity; CBD: Skopje, Macedonia, 2022; Available online: https://www.cbd.int/doc/nr/nr-06/mk-nr-06-en.pdf (accessed on 13 June 2022).
- Phitos, D.; Strid, A.; Snogerup, S.; Greuter, W. The Red Data Book of Rare and Threatened Plants of Greece; WWF: Athens, Greece, 1995. [Google Scholar]
- Phitos, D.; Konstantinidis, T.; Kamari, G. The Red Data Book of Rare and Threatened Plants of Greece; Hellenic Botanical Society: Patras, Greece, 2009. [Google Scholar]
- Krigas, N.; Vokou, D.; Bandi, A. Identified Threats and Proposed Protection Measures for the Rare and Threatened Plants of Greece. In European Botanic Gardens in a Changing World—Insights into Eurogard VI; Kigas, N., Tsoktouridis, G., Cook, C.M., Mylona, P., Maloupa, E., Eds.; Balkan Botanic Garden of Kroussia and Botanic Gardens Conservation International: Thessaloniki, Greece, 2014; pp. 43–52. Available online: http://www.botanicgardens.eu/eurogard/eurogard6/eurogardVI.pdf (accessed on 13 June 2022).
- Maxted, N.; Ford-Lloyd, B.V.; Hawkes, J.G. Complementary conservation strategies. In Plant Genetic Conservation; Maxted, N., Ford-Lloyd, B.V., Hawkes, J.G., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 15–39. [Google Scholar] [CrossRef]
- Krigas, N.; Menteli, V.; Vokou, D. Analysis of the ex situ conservation of the Greek endemic flora at national European and global scales and of its effectiveness in meeting GSPC Target 8. Plant Biosyst. 2016, 150, 573–582. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Sarropoulou, V.; Krigas, N.; Maloupa, E.; Tsoktouridis, G. GIS-Facilitated effective propagation protocols of the Endangered local endemic of Crete Carlina diae (Rech. f.) Meusel and A. Kástner (Asteraceae): Serving ex situ conservation needs and its future sustainable utilization as an ornamental. Plants 2020, 9, 1465. [Google Scholar] [CrossRef]
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Lamchouri, F.; Tsiridipis, I.; Tsiafouli, M.A.; et al. Exploring the potential of neglected local endemic plants of three Mediterranean regions in the ornamental sector: Value chain feasibility and readiness timescale for their sustainable exploitation. Sustainability 2021, 13, 2539. [Google Scholar] [CrossRef]
- Libiad, M.; Khabbach, A.; El Haissoufi, M.; Anestis, I.; Lamchouri, F.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Greveniotis, V.; Tsiripidis, I.; et al. Agro-alimentary potential of the neglected and underutilized local endemic plants of Crete (Greece), Rif-Mediterranean coast of Morocco and Tunisia: Perspectives and challenges. Plants 2021, 10, 1770. [Google Scholar] [CrossRef]
- Bourgou, S.; Ben Haj Jilani, I.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V.; et al. Medicinal-cosmetic potential of the local endemic plants of Crete (Greece), Northern Morocco and Tunisia: Priorities for conservation and sustainable exploitation of neglected and underutilized phytogenetic resources. Biology 2021, 10, 1344. [Google Scholar] [CrossRef] [PubMed]
- Hatzilazarou, S.; El Haissoufi, M.; Pipinis, E.; Kostas, S.; Libiad, M.; Khabbach, A.; Lamchouri, F.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; et al. GIS-Facilitated seed germination and multifaceted evaluation of the Endangered Abies marocana Trab. (Pinaceae) enabling conservation and sustainable exploitation. Plants 2021, 10, 2606. [Google Scholar] [CrossRef]
- Pipinis, E.; Hatzilazarou, S.; Kostas, S.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; et al. Facilitating conservation and bridging gaps for the sustainable exploitation of the Tunisian local endemic Plant Marrubium aschersonii (Lamiaceae). Sustainability 2022, 14, 1637. [Google Scholar] [CrossRef]
- Kostas, S.; Hatzilazarou, S.; Pipinis, E.; Bourgou, S.; Ben Haj Jilani, I.; Ben Othman, W.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; et al. DNA Barcoding, GIS-facilitated seed germination and pilot cultivation of Teucrium luteum subsp.gabesianum (Lamiaceae), a Tunisian local endemic with potential medicinal and ornamental value. Biology 2022, 11, 462. [Google Scholar] [CrossRef]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Kalogiannakis, K.; Debouba, F.J.; Ipsilantis, I.; Tsoktouridis, G.; et al. Pilot cultivation of the Vulnerable Cretan endemic Verbascum arcturus L. (Scrophulariaceae): Effect of fertilization on growth and quality features. Sustainability 2021, 13, 14030. [Google Scholar] [CrossRef]
- Fanourakis, D.; Paschalidis, K.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Liapaki, E.; Jouini, M.; Ipsilantis, I.; Maloupa, E.; et al. Pilot cultivation of the local endemic Cretan marjoram Origanum microphyllum (Benth.) Vogel (Lamiaceae): Effect of fertilizers on growth and herbal quality features. Agronomy 2022, 12, 94. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Maloupa, E.; Tsoktouridis, G. Vegetative propagation and ex-situ conservation of Acantholimon androsaceum and Limonium chersonesum, two promising local endemics of Crete (Greece) available for floricultural and pharmaceutical sustainable exploitation. Not. Bot. Hort. Agrobot. Cluj-Napoca 2021, 49, 12261. [Google Scholar] [CrossRef]
- Mohammad Esmaeili, M.; Sattarian, A.; Bonis, A.; Bouzillé, J. Ecology of seed dormancy and germination of Carex divisa Huds.: Effects of stratification, temperature and salinity. Intern. J. Plant Product. 2012, 3, 27–40. [Google Scholar] [CrossRef]
- Zarghani, H.; Mijani, S.; Nasrabadi, S.E.; Ghias-Abadi, M.; Khorramdel, S.; Azimi, R. Temperature effects on the seed germination of some perennial and annual species of Asteraceae family. Plant Breed. Seed Sci. 2014, 69, 3–14. [Google Scholar] [CrossRef]
- Asha Rani, N.S.; Prasad, M.P. In-Vitro Studies on the Germination of Atropa belladonna Seeds under Different Conditions. Intern. J. Sci. Res. 2014, 3, 552–555. Available online: https://www.ijsr.net/get_abstract.php?paper_id=OCT14184 (accessed on 20 May 2022).
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Paparella, S.; Araujo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Statwick, J.M. Germination pretreatments to break hard-seed dormancy in Astragalus cicer L. (Fabaceae). PeerJ 2016, 4, e2621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yao, L.; Zhang, Y.; Baskin, J.M.; Baskin, C.C.; Xiong, Z.; Tao, J. A review of the seed biology of Paeonia species (Paeoniaceae), with particular reference to dormancy and germination. Planta 2019, 249, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.J.; Kester, D.E.; Davies, F.I.; Geneve, R.L. Plant Propagation: Principles and Practices, 7th ed.; Prentice Hall: Hoboken, NJ, USA, 2000. [Google Scholar]
- Raju, N.L.; Prasad, M.N.V. Influence of growth hormones on adventitious root formation in semi-hardwood cuttings of Celasturs paniculatus Willd: A contribution for rapid multiplication and conservation management. Agrofor. Syst. 2010, 79, 249–252. [Google Scholar] [CrossRef]
- Waman, A.A.; Smitha, G.R.; Bohra, P.A. Review on clonal propagation of medicinal and aromatic plants through stem cuttings for promoting their cultivation and conservation. Curr. Agric. Res. J. 2019, 7, 2. [Google Scholar] [CrossRef]
- Bryant, P.H.; Trueman, S.J. Stem anatomy and adventitious root formation in cuttings of Angophora, Corymbia and Eucalyptus. Forests 2015, 6, 1227–1238. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Krigas, N.; Grigoriadou, K.; Tsoktouridis, G.; Maloupa, E. Vegetative Propagation of the Range-Restricted Greek Endemic Silene fabaria (L.) Sm. in Sibth. & Sm. subsp.domokina Greuter (Caryophyllaceae). Intern. J. Bot. Stud. 2018, 3, 37–40. Available online: http://www.botanyjournals.com/archives/2018/vol3/issue5/3-5-14 (accessed on 19 May 2022).
- Sarropoulou, V.; Maloupa, E. Asexual propagation of four medicinal Greek endemic plants of Lamiaceae family with conservation priority from the collection of the Balkan Botanic Garden of Kroussia, N. Greece. J. Adv. Agric. 2019, 10, 1611–1622. [Google Scholar] [CrossRef]
- Flynn, C. The value of ecological plantings in public areas. Sibbaldia 2009, 7, 43–60. [Google Scholar] [CrossRef]
- Krigas, N.; Panagiotidou, M.; Maloupa, E. Incorporating biogeographical principles in horticulture: Design of the Ionian Islands Unique Rock Garden in Thessaloniki, Greece. Sibbaldia 2017, 15, 129–146. [Google Scholar] [CrossRef]
- Villagra-Islas, P. Newer plant displays in botanical gardens: The role of design in environmental interpretation. Landsc. Res. 2011, 36, 573–597. [Google Scholar] [CrossRef]
- Sanders, D. Making public the private life of plants: The contribution of informal learning environments. Intern. J. Sci. Educ. 2007, 29, 1209–1228. [Google Scholar] [CrossRef]
- Hood, A.; Reaney, C. Native plant project at the University of Dundee botanic garden. Sibbaldia 2013, 11, 175–184. [Google Scholar] [CrossRef]
- Phondani, P.C.; Bhatt, A.; Elsarrag, E.; Alhorr, Y.M.; El-Keblawy, A. Criteria and indicator approach of global sustainability assessment system for sustainable landscaping using native plants in Qatar. Ecol. Ind. 2016, 69, 381–389. [Google Scholar] [CrossRef]
- Alam, H.; Khattak, J.Z.; Ppoyil, S.B.; Kurup, S.S.; Ksiksi, T. Landscaping with native plants in the United Arab Emirates: A review. Emir. J. Food Agric. 2017, 29, 729–741. [Google Scholar] [CrossRef]
- Krigas, N.; Maloupa, E. The Balkan Botanic Garden of Kroussia, Northern Greece: A garden dedicated to the conservation of native plants of Greece and the Balkans. Sibbaldia 2008, 6, 9–27. [Google Scholar] [CrossRef]
- Maloupa, E.; Krigas, N.; Grigoriadou, K.; Lazari, D.; Tsoktouridis, G. Conservation strategies for native plant species and their sustainable exploitation: Case of the Balkan Botanic Garden of Kroussia, N. Greece. In Floriculture Ornamental Plant Biotechnology: Advances and Topical Issues, 1st ed.; Texeira Da Silva, J.A., Ed.; Global Science Books: Isleworth, UK, 2008; Volume 4, pp. 37–56. [Google Scholar]
- Horvat, I.; Glavač, V.; Ellenberg, H. Vegetation Sudosteuropas; Gustav Fischer Verlag: Stuttgart, Germany, 1974. [Google Scholar]
- Stevanovic, V. Analysis of the Central European and Mediterranean orophylic element on the mountains of W. and Central Balkan Peninsula, with special reference to endemics. Bocconea 1996, 5, 77–97. [Google Scholar]
- Strid, A.; Tan, K. Flora Hellenica; Koeltz Scientific Books: Königstein, Germany, 1997; Volume 1. [Google Scholar]
- Orsenigo, S.; Montagnani, C.; Fenu, G.; Gargano, D.; Peruzzi, L.; Thomas, A.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Bovio, M.; et al. Red listing plants under full national responsibility: Extinction risk and threats in the vascular flora endemic to Italy. Biol. Conserv. 2018, 224, 213–222. [Google Scholar] [CrossRef]
- Orsenigo, S.; Fenu, G.; Gargano, D.; Montagnani, C.; Abeli, T.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Carta, A.; Castello, M.; et al. Red list of threatened vascular plants in Italy. Plant Biosyst. 2020, 155, 310–355. [Google Scholar] [CrossRef]
- Gillett, H.; Walter, J.; Kerry, S. 1997 IUCN Red List of Threatened Plants; International Union for the Conservation of Nature: Gland, Switzerland, 1998; Available online: https://portals.iucn.org/library/sites/library/files/documents/RL-1997-001.pdf (accessed on 13 June 2022).
- Kokkini, S.; Iatrou, G.; Georghiou, K.; Artelari, P.; Bazos, I.; Georghiadis, T.; Georgiou, U.; Drossos, E.; Hanlidou, E.; Karousou, R.; et al. Other Important Plant Species. In Directive 92/43/EEC, The Greek “Habitat” Project Natura 2000: An Overview; Dafis, S., Papastergiadou, K., Georghiou, K., Babalonas, D., Georghiadis, T., Papageorgiou, M., Lazaridou, T., Tsiaoussi, V., Eds.; Commission of the European Communities DG XI, The Goulandris Natural History Museum—Greek Biotope/Wetland Centre: Athens, Greece, 1997; pp. 468–486, 768–777, 801–839. [Google Scholar]
- Strid, A.; Bergmayer, E.; Fotiadis, G. Flora and Vegetation of the Prespa National Park, Greece; Society for the Protection of Prespa: Agios Germanos, Greece, 2020. [Google Scholar]
- ISTA. International Rules for Seed Testing. Seed Sci. Technol. 1999, 27, 333. Available online: https://www.seedtest.org/en/publications/international-rules-seed-testing-1168.html (accessed on 20 June 2022).
- Güleryüz, G.; Kırmızı, S.; Arslan, H.; Sakar, F.S. Dormancy and germination in Stachys germanica L. subsp.bithynica (Boiss.) Bhattacharjee seeds: Effects of short-time moist chilling and plant growth regulators. Flora 2011, 206, 943–948. [Google Scholar] [CrossRef]
- Koutsovoulou, K.; Daws, M.I.; Thanos, C.A. Campanulaceae: A family with small seeds that require light for germination. Ann. Bot. 2014, 113, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Preece, J.E. A century of progress with vegetative plant propagation. HortScience 2003, 38, 1015–1025. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction risk assessment of the Greek endemic flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef]
- Khela, S. Sideritis scardica, Near Threatened. The IUCN Red List of Threatened Species. e.T203271A2762714. 2013. Available online: https://dx.doi.org/10.2305/IUCN.UK.2013-2.RLTS.T203271A2762714.en (accessed on 18 June 2022).
- Domínguez Lozano, F.; Atkins, K.; Moreno Saiz, J.; Sims, A.; Dixon, K. The nature of threat category changes in three Mediterranean biodiversity hotspots. Biol. Conserv. 2013, 157, 21–30. [Google Scholar] [CrossRef]
- Burgman, M.; Keith, D.; Hopper, S.; Widyatmoko, D.; Drill, C. Threat syndromes and conservation of Australian flora. Biol. Conserv. 2007, 134, 73–82. [Google Scholar] [CrossRef]
- Hayward, M.W. The need to rationalize and prioritize threatening processes used to determine threat status in the IUCN Red List. Conserv. Biol. 2009, 23, 1568–1576. [Google Scholar] [CrossRef]
- Cassini, M. Ranking threats using species distribution models in the IUCN Red List assessment process. Biodivers. Conserv. 2011, 20, 3689–3692. [Google Scholar] [CrossRef]
- Krigas, Ν.; Mouflis, G.; Grigoriadou, K.; Maloupa, E. Conservation of important plants from the Ionian Islands at the Balkan Botanic Garden of Kroussia, N Greece: Using GIS to link the in situ collection data with plant propagation and ex situ cultivation. Biodivers. Conserv. 2010, 19, 3583–3603. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Tsoktouridis, G.; Maloupa, E. In vitro propagation of medicinal and aromatic plants: The case of selected Greek species with conservation priority. In Vitro Cell. Dev. Biol.-Plant 2019, 55, 635–646. [Google Scholar] [CrossRef]
- Csontos, P.; Rucińska, A.; Puchalski, J. Germination of Erysimum pieninicum and Erysimum odoratum seeds after various storage conditions. J. Landsc. Ecol. 2010, 8, 389–394. [Google Scholar]
- Karimi, M.; Berrichi, A.; Boukroute, A. Study of vegetative propagation by cuttings of Thymus satureioides. J. Mater. Environ. Sci. 2014, 5, 1320–1325. [Google Scholar]
- Akoumianaki–Ioannidou, A.; Gerasimidou, A.; Salta, A. Sexual and Asexual Propagation of Teucrium brevifolium. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Hortic. 2018, 75, 111–114. [Google Scholar] [CrossRef]
- Reed, B.M.; Sarasan, V.; Kane, M.; Bunn, E.; Pence, V.C. Biodiversity conservation and conservation biotechnology tools. In Vitro Cell. Dev. Biol.-Plant 2011, 47, 1–4. [Google Scholar] [CrossRef]
- Volis, S. Conservation-oriented restoration—A two for one method to restore both threatened species and their habitats. Plant Divers. 2019, 41, 50–58. [Google Scholar] [CrossRef]
- Bunn, E.; Turner, S.R.; Dixon, K.W. Biotechnology for saving rare and threatened flora in a biodiversity hotspot. In Vitro Cell. Dev. Biol.-Plant 2011, 47, 188–200. [Google Scholar] [CrossRef]
- Oseni, O.M.; Pande, V.; Kumar, N.T. A review on plant tissue culture, a technique for propagation and conservation of endangered plant species. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3778–3786. [Google Scholar] [CrossRef]
- Chin, H.F. Strategies for Conservation of Recalcitrant Species; Plant Biotechnology Laboratory, Faculty of Life Sciences, University of Kebangsaan Malaysia: Bangi, Malaysia, 1996. [Google Scholar]
- Kueffer, C.; Kaiser-Bunbury, C.N. Reconciling conflicting perspectives for biodiversity conservation in the Anthropocene. Front. Ecol. Environ. 2014, 12, 131–137. [Google Scholar] [CrossRef]
- Cristea, V.; Jarda, L.; Holobiuc, I. Ex situ conservation of three endemic and/or endangered Dianthus species. Not. Bot. Hort. Agrobot. Cluj-Napoca 2013, 41, 73–78. [Google Scholar] [CrossRef]
- Maloupa, E.; Karapatzak, E.; Ganopoulos, I.; Karydas, A.; Papanastasi, K.; Kyrkas, D.; Yfanti, P.; Nikisianis, N.; Zahariadis, A.; Kosma, I.S.; et al. Molecular authentication, phytochemical evaluation and asexual propagation of wild-growing Rosa canina L. (Rosaceae) genotypes of Northern Greece for sustainable exploitation. Plants 2021, 10, 2634. [Google Scholar] [CrossRef] [PubMed]
- Karapatzak, E.; Krigas, N.; Ganopoulos, I.; Papanastasi, K.; Kyrkas, D.; Yfanti, P.; Nikisianis, N.; Karydas, A.; Manthos, I.; Kosma, I.S.; et al. Documenting Greek indigenous germplasm of Cornelian cherry (Cornus mas L.) for sustainable utilization: Molecular authentication, asexual propagation, and phytochemical evaluation. Plants 2022, 11, 1345. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Lai, L.; Zhou, J.; Yi, S.; Liu, X.; Guo, J.; Zheng, Y. Differences in ecological traits between plants grown in situ and ex situ and implications for conservation. Sustainability 2022, 14, 5199. [Google Scholar] [CrossRef]
- Krigas, N.; Panagiotidou, M.; Maloupa, E. Horticulture and Landscaping with Greek Native Plants in Urbanised Areas: The Experience of the Balkan Botanic Garden of Kroussia, Northern Greece. Autochthonous Plants in the Urban Environment. In Proceedings of the 2nd Slovenian Meeting of European Botanic Gardens Consortium with Symposium, Ljubljana, Slovenia, 25–29 May 2016. [Google Scholar]
- Grigoriadou, K.; Krigas, N.; Lazari, D.; Maloupa, E. Chapter 4—Sustainable Use of Mediterranean Medicinal-Aromatic Plants. In Feed Additives: Aromatic Plants and Herds in Animal Nutrition and Health; Florou-Paneri, P.E., Christaki, E., Giannenas, I., Eds.; Elsevier Academic Press: London, UK, 2020; pp. 57–74. Available online: https://doi.org/10.1016/B978-0-12-814700-9.00004-2 (accessed on 13 June 2022).











| PPs in Ex-Situ Conservation | Taxa | PCA Taxa | PCB Taxa | PCC Taxa |
|---|---|---|---|---|
| Successfully propagated and planted in awareness sites | 92 | 16 | 48 | 28 |
| Ex-situ maintained with restricted use in awareness sites | 38 | 8 | 26 | 4 |
| Total | 130 | 24 | 74 | 32 |
| Propagation Methods | Type of Propagation Method | Total PPs | PCA Taxa | PCB Taxa | PCC Taxa |
|---|---|---|---|---|---|
| One | Seed germination trials | 34 | 6 | 19 | 9 |
| Cutting trials | 45 | 7 | 21 | 17 | |
| Plant division | 31 | 6 | 19 | 6 | |
| Two | Seed germination trials and cutting trials | 3 | - | 2 | 1 |
| Seed germination trials and plant division | 6 | 1 | 3 | 2 | |
| Cutting trials and plant division | 11 | 2 | 7 | 2 | |
| Three | Seed germination trials, cutting trials, plant division | 2 | - | 1 | 1 |
| Propagation Method | Total Individuals | PCA Individuals | PCB Individuals | PCC Individuals |
|---|---|---|---|---|
| Seed germination trials | 1044 | 40 | 822 | 182 |
| Cutting trials | 1617 | 231 | 842 | 544 |
| Plant division | 586 | 156 | 276 | 154 |
| All methods | 3247 | 219 | 1940 | 880 |
| Propagation Method | Total Taxa Propagated | HSS | HSS and HPS | HSS and HPS—PCA Taxa | HSS and HPS—PCB Taxa | HSS and HPS—PCC Taxa |
|---|---|---|---|---|---|---|
| Seed germination trials | 34 | 29 | 12 | 1 | 10 | 1 |
| Cutting trials | 45 | 38 | 35 | 6 | 16 | 13 |
| Plant division | 31 | 10 | 10 | 3 | 6 | 1 |
| PPs in Ex-Situ Conservation | Number of Taxa | Total Seeds Counted | Number of Seedlots (Counted Seeds) of PCA Taxa | Seedlots (Counted Seeds) of PCB Taxa | Seedlots (Counted Seeds) of PCC Taxa |
|---|---|---|---|---|---|
| Successfully propagated and planted in awareness sites | 64 | 480,246 | 12 (9090) | 33 (215,122) | 19 (256,034) |
| Ex-situ maintained with restricted use in awareness sites | 21 | 112,166 | 4 (57,275) | 15 (54,826) | 2 (65) |
| Total | 85 | 592,412 | 16 (66,365) | 48 (269,948) | 21 (256,099) |
| Awareness Site | Total Taxa (Total Individuals) | Families | PCA Taxa | PCB Taxa | PCC Taxa |
|---|---|---|---|---|---|
| BBGK | 104 (850) | 26 | 17 | 60 | 27 |
| BAPP | 70 (1000) | 23 | 12 | 42 | 16 |
| GEA | 130 (130) | 37 | 26 | 74 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krigas, N.; Karapatzak, E.; Panagiotidou, M.; Sarropoulou, V.; Samartza, I.; Karydas, A.; Damianidis, C.K.; Najdovski, B.; Teofilovski, A.; Mandzukovski, D.; et al. Prioritizing Plants around the Cross-Border Area of Greece and the Republic of North Macedonia: Integrated Conservation Actions and Sustainable Exploitation Potential. Diversity 2022, 14, 570. https://doi.org/10.3390/d14070570
Krigas N, Karapatzak E, Panagiotidou M, Sarropoulou V, Samartza I, Karydas A, Damianidis CK, Najdovski B, Teofilovski A, Mandzukovski D, et al. Prioritizing Plants around the Cross-Border Area of Greece and the Republic of North Macedonia: Integrated Conservation Actions and Sustainable Exploitation Potential. Diversity. 2022; 14(7):570. https://doi.org/10.3390/d14070570
Chicago/Turabian StyleKrigas, Nikos, Eleftherios Karapatzak, Marina Panagiotidou, Virginia Sarropoulou, Ioulietta Samartza, Antonis Karydas, Christos K. Damianidis, Boris Najdovski, Aco Teofilovski, Dejan Mandzukovski, and et al. 2022. "Prioritizing Plants around the Cross-Border Area of Greece and the Republic of North Macedonia: Integrated Conservation Actions and Sustainable Exploitation Potential" Diversity 14, no. 7: 570. https://doi.org/10.3390/d14070570
APA StyleKrigas, N., Karapatzak, E., Panagiotidou, M., Sarropoulou, V., Samartza, I., Karydas, A., Damianidis, C. K., Najdovski, B., Teofilovski, A., Mandzukovski, D., Stipanović, V. B., Papanastasi, K., Kapagianni, P. D., Fotakis, D., Grigoriadou, K., Tsoktouridis, G., Andonovski, V., & Maloupa, E. (2022). Prioritizing Plants around the Cross-Border Area of Greece and the Republic of North Macedonia: Integrated Conservation Actions and Sustainable Exploitation Potential. Diversity, 14(7), 570. https://doi.org/10.3390/d14070570










