Altitudinal Patterns of Species Richness of Seed Plants in Qingling Mountains: A Test of Rapoport’s Rule
Abstract
:1. Introduction
2. Data and Methods
2.1. Natural Conditions of the Study Area
2.2. Datasources
2.3. Dataanalysis
2.3.1. Analysis of Species Richness along an Altitudinal Gradient
2.3.2. Predictions of Species Richness along an Altitudinal Gradient
2.3.3. Verification of Rapoport’s Rule
3. Results
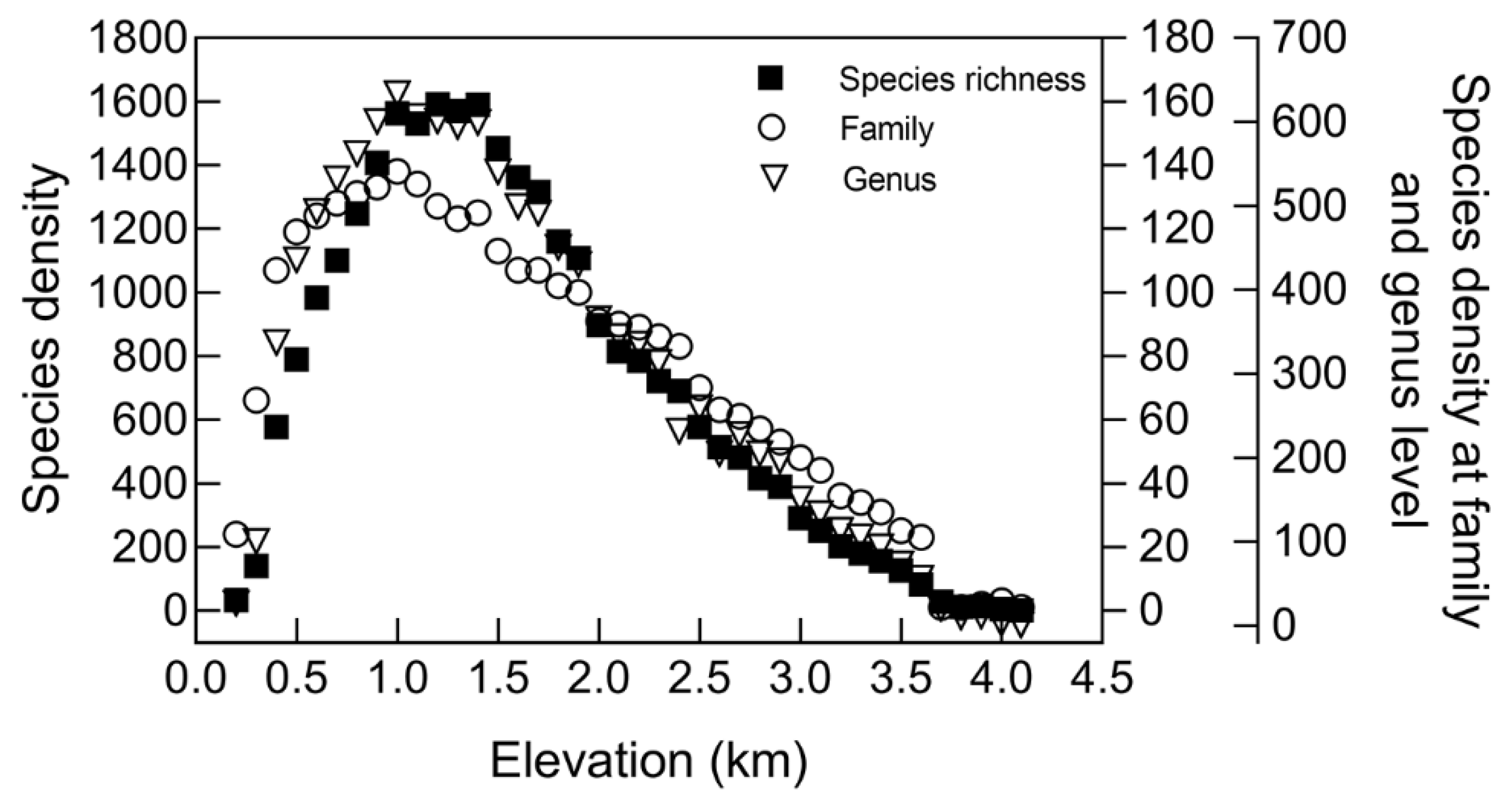
3.1. Altitudinal Species Richness Patterns of Seed Plants in Qinling Mountains
3.2. Explanation of Altitudinal Patterns of Species Richness in Qinling Mountains by Different Hypotheses
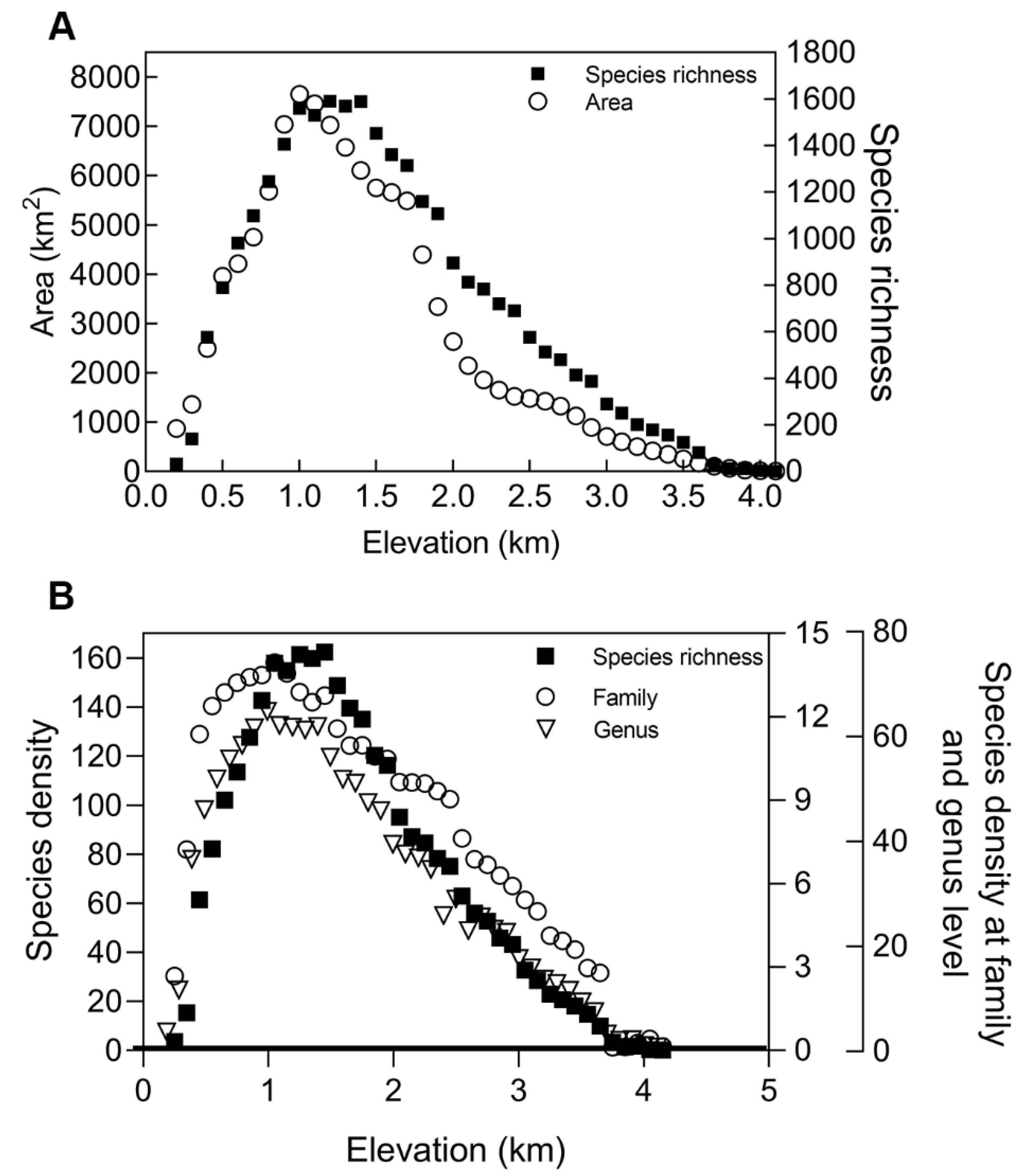
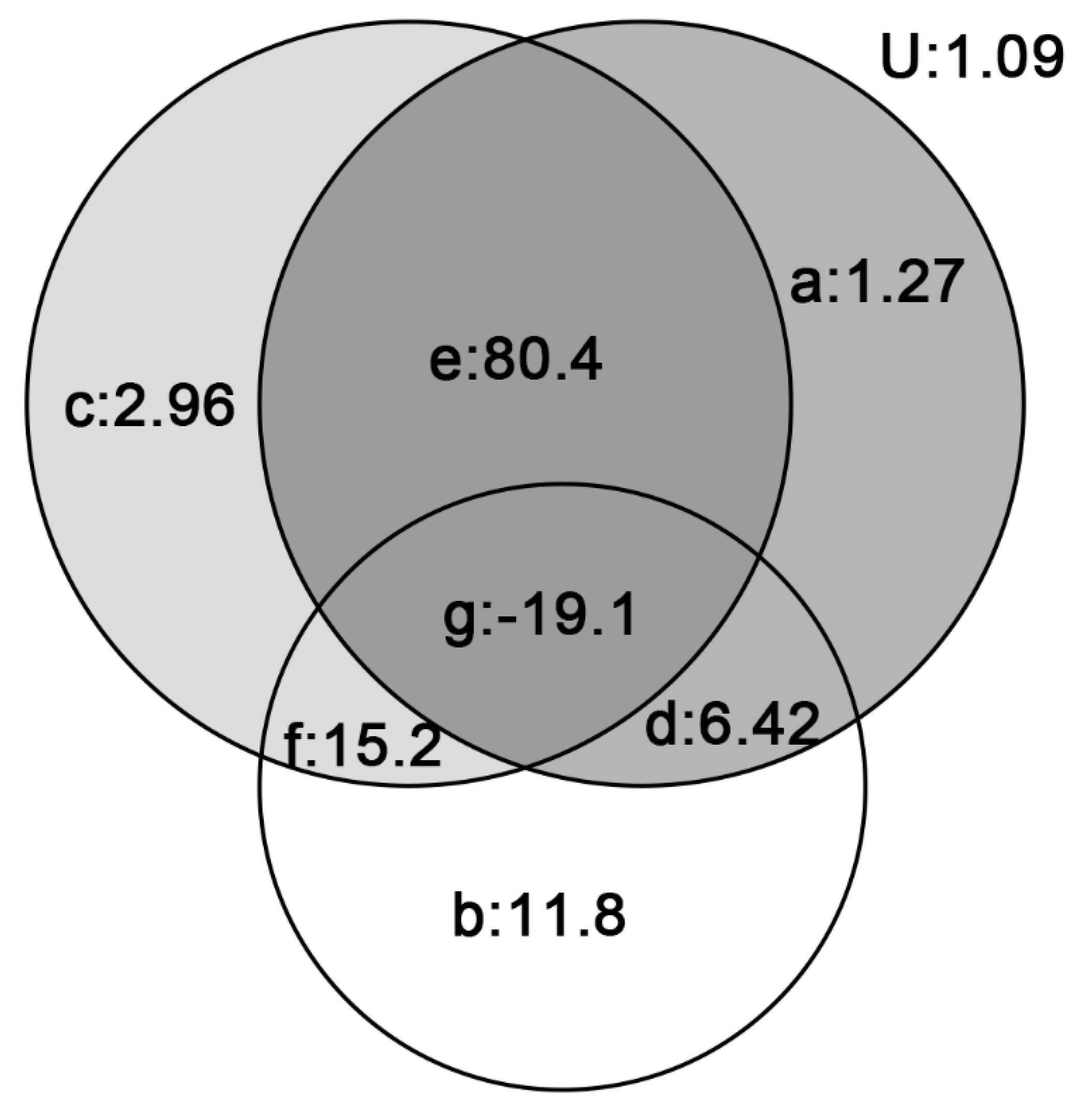
3.2.1. Species–Area Relationship Hypothesis
3.2.2. The Mid-Domain Effect Hypothesis
3.2.3. Explanatory Quantity of Different Hypotheses
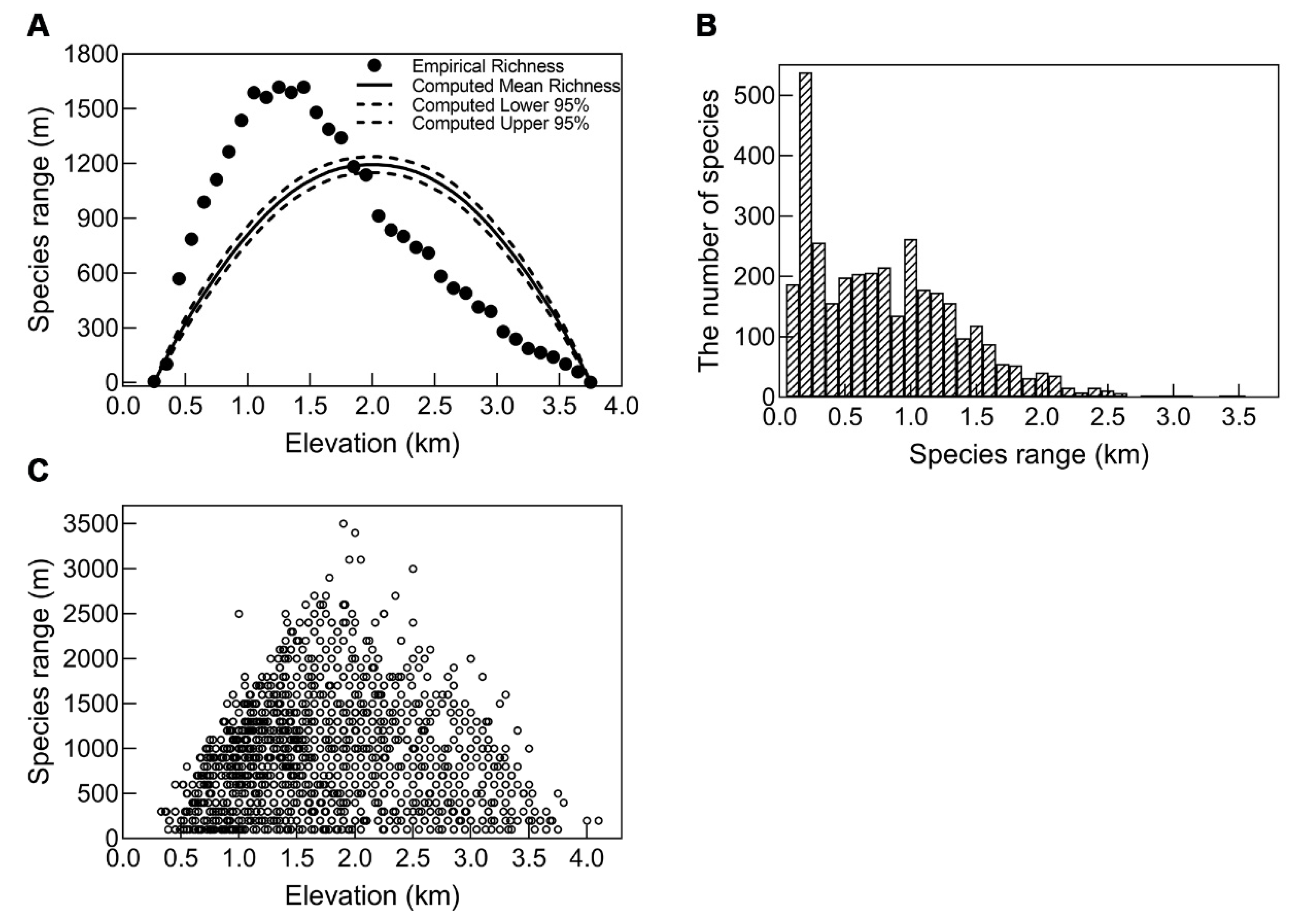
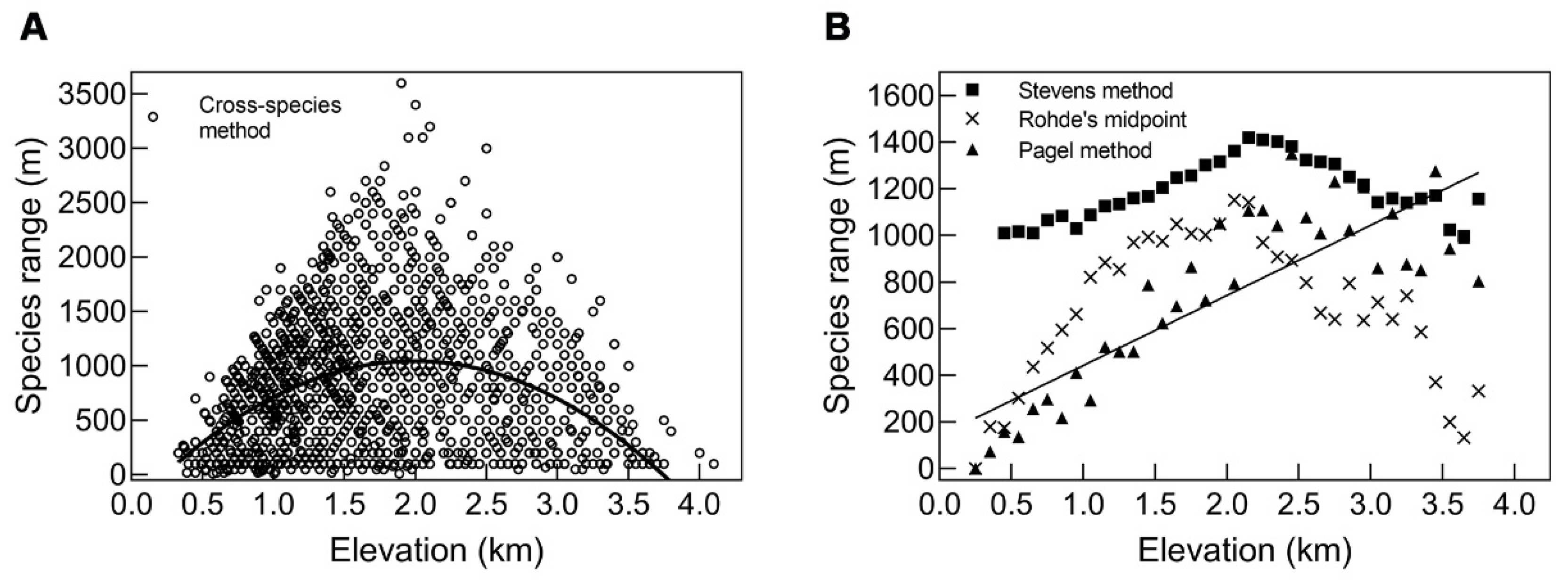
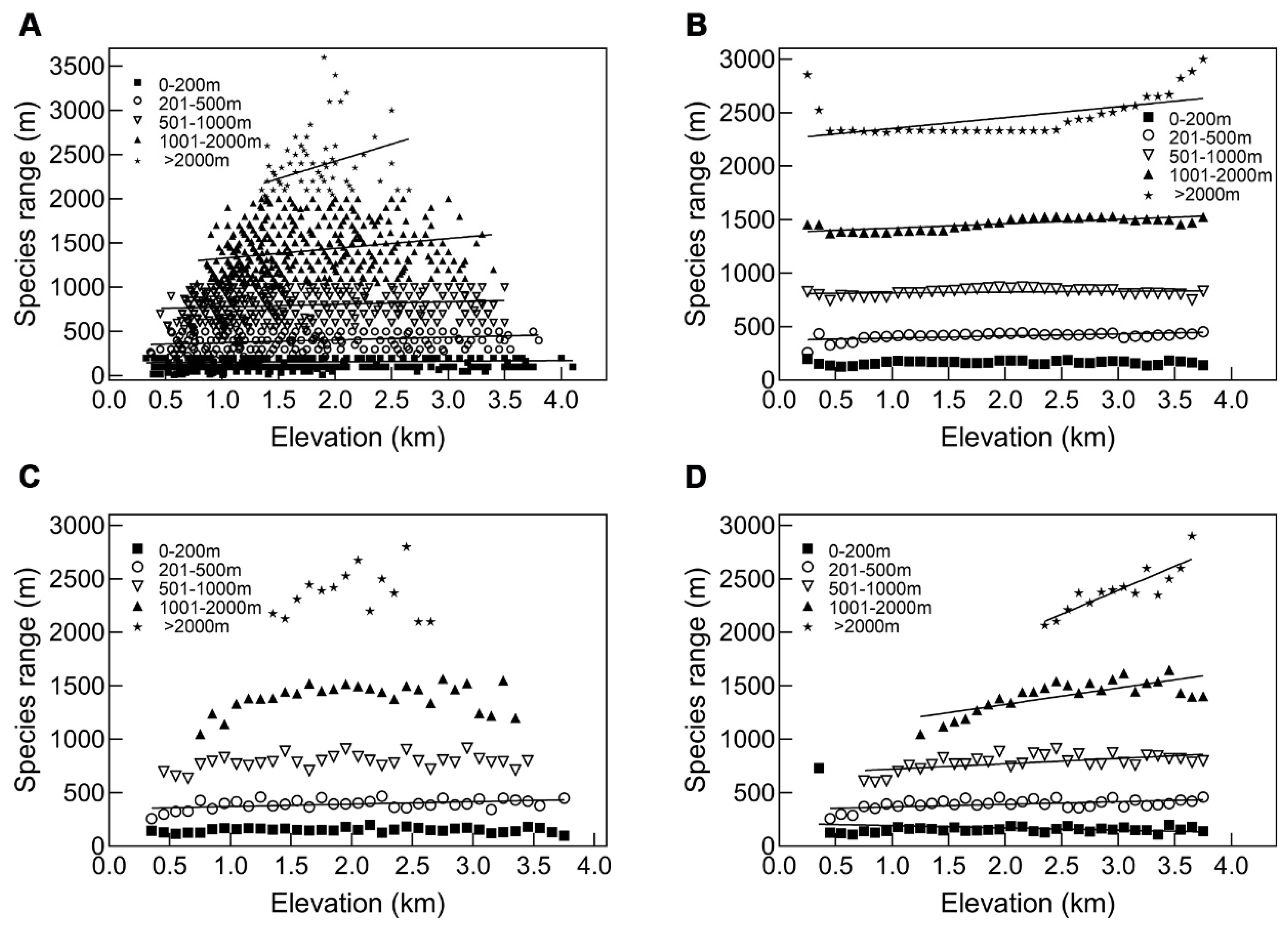
3.3. A Test of Rapoport’s Rule with Altitude
3.3.1. A Test of Rapoport’s Rule by Four Methods
3.3.2. A Test of Rapoport’s Rule by Grouping Method
4. Discussion and Conclusions
4.1. Species Richness along an Altitudinal Gradient in Qinling Mountains and the Explanatory Power of Different Hypotheses
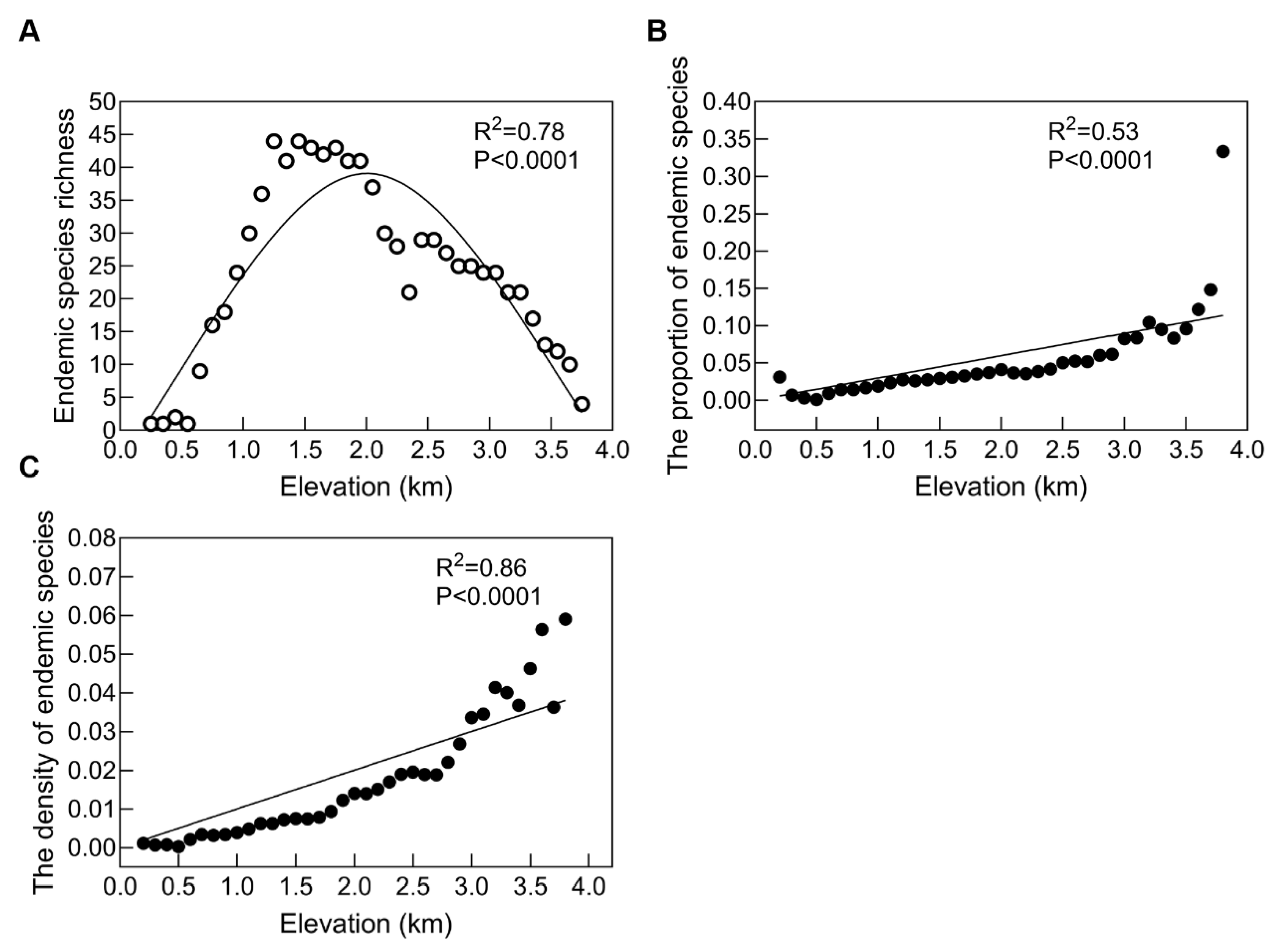
4.2. Endemic Species Richness Patterns along an Altitudinal Gradient in Qinling Mountains
4.3. A Test of Rapoport’s Rule
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thiel-Egenter, C.; Gugerli, F.; Alvarez, N.; Brodbeck, S.; Colli, L. Effects of species traits on the genetic diversity of high-mountain plants: A multi-species study across the Alps and the Carpathians. Glob. Ecol. Biogeogr. 2010, 18, 78–87. [Google Scholar] [CrossRef]
- Jiang, S.; Kong, F.H.; Hu, R.; Zhang, J.X.; Zhang, M.H. Effects of the climatic region on richness correlations between vascular plants and vertebrates in nature reserves of China. Diversity 2022, 14, 499. [Google Scholar] [CrossRef]
- Bradshaw, A.D. Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 1965, 13, 115–155. [Google Scholar]
- Leopold, C.R.; Hess, S.C. Facilitating adaptation to climate change while restoring a montane plant community. PLoS ONE 2019, 14, e218516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef]
- Acharya, B.K.; Chettri, B.; Vijayan, L. Distribution pattern of trees along an elevation gradient of Eastern Himalaya, India. Acta Oecol. 2011, 37, 329–336. [Google Scholar] [CrossRef]
- Gurvich, D.E.; Zeballos, S.R.; Demaio, P.H. Diversity and composition of cactus species along an altitudinal gradient in the Sierras del Norte Mountains (Cordoba, Argentina). S. Afr. J. Bot. 2014, 93, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Shimwell, D.W. Areography. Geographical Strategies of Species. by E. H. Rapoport. J. Ecol. 1983, 71, 1037–1038. [Google Scholar] [CrossRef]
- Rapoport, E.H. Ecological Areography. In Areography; Elsevier: Amsterdam, The Netherlands, 1982; pp. 210–241. [Google Scholar]
- Stevens, G.C. The latitudinal gradient in geographical range: How so many species coexist in the tropics. Am. Nat. 1989, 133, 240–256. [Google Scholar] [CrossRef]
- Stevens, G.C. The elevational gradient in altitudinal range: An extension of Rapoport’s latitudinal rule to altitude. Am. Nat. 1992, 140, 893–911. [Google Scholar] [CrossRef]
- Egoh, B.; Reyers, B.; Rouget, M.; Bode, M.; Richardson, D.M. Spatial congruence between biodiversity and ecosystem services in South Africa. Biol. Conserv. 2009, 142, 553–562. [Google Scholar] [CrossRef]
- Lee, P.Y.; Rotenberry, J.T. Relationships between bird species and tree species assemblages in forested habitats of eastern North America. J. Biogeogr. 2010, 32, 1139–1150. [Google Scholar] [CrossRef]
- Fady, B.; Conord, C. Macroecological patterns of species and genetic diversity in vascular plants of the Mediterranean Basin. Divers. Distrib. 2009, 16, 53–64. [Google Scholar] [CrossRef]
- Li, X.; Tian, H.; Wang, Y.; Li, R.; Song, Z.; Zhang, F.; Xu, M.; Li, D. Vulnerability of 208 endemic or endangered species in China to the effects of climate change. Reg. Environ. Chang. 2013, 13, 843–852. [Google Scholar] [CrossRef]
- Araújo, M.B.; Rahbek, C. How does climate change affect biodiversity? Science 2006, 313, 1396–1397. [Google Scholar] [CrossRef]
- Heikkinen, R.K.; Kartano, L.; Leikola, N.; Aalto, J.; Virkkala, R. High-latitude EU habitats directive species at risk due to climate change and land use. Glob. Ecol. Conserv. 2021, 28, e1664. [Google Scholar] [CrossRef]
- Panitsa, M.; Trigas, P.; Iatrou, G.; Sfenthourakis, S. Factors affecting plant species richness and endemism on land-bridge islands—An example from the East Aegean archipelago. Acta Oecol. 2010, 36, 431–437. [Google Scholar] [CrossRef]
- Trigas, P.; Iatrou, G.; Panitsa, M. Vascular plant species diversity, biogeography and vulnerability in the aegean islands as exemplified by Evvia Island (W Aegean, Greece). Fresen. Environ. Bull. 2015, 17, 48–57. [Google Scholar]
- Gehrke, B.; Linder, H.P. Species richness, endemism and species composition in the tropical Afroalpine flora. Alp. Bot. 2014, 124, 165–177. [Google Scholar] [CrossRef]
- Lamoreux, J.F.; Morrison, J.C.; Ricketts, T.H.; Olson, D.M.; Dinerstein, E.; McKnight, M.W.; Shugart, H.H. Global tests of biodiversity concordance and the importance of endemism. Nature 2006, 440, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Legendre, P.; Boisclair, D.; Gauthier, S.; Smith, S.J. Effects of spatial scale and choice of statistical model (linear versus tree-based) on determining species-habitat relationships. Can. J. Fish. Aquat. Sci. 2012, 69, 2095–2111. [Google Scholar] [CrossRef]
- Chi, X.; Tang, Z. Effects of area, temperature and geometric constraints on elevational patterns of species richness:a case study in the Mountain Taibai, Qinling Mountains, China. Chin. J. Plant Ecol. 2011, 35, 362–370. [Google Scholar] [CrossRef]
- Yue, M.; Zhang, L.; Dang, G.; Tian, G.U. The relationships of higher plants diversity and elevation gradient in Foping National Reserve. Sci. Geogr. Sin. 2002, 22, 349–354. [Google Scholar]
- Fu, Z.; Zhang, X.; Shunyi, L.; Ming, T. The summarize of research on the flora and vegetation of the Qinling Mountain range. Acta Bot. Boreall.-Occident. Sin. 1996, 16, 93–106. [Google Scholar]
- Liang, J.; Ding, Z.; Lie, G.; Zhou, Z.; Hu, Z. Species richness patterns of vascular plants and their drivers along an elevational gradient in the central Himalayas. Glob. Ecol. Conserv. 2020, 24, e01279. [Google Scholar] [CrossRef]
- Namgail, T.; Rawat, G.S.; Mishra, C.; Van Wieren, S.E.; Prins, H.H.T. Biomass and diversity of dry alpine plant communities along altitudinal gradients in the Himalayas. J. Plant Res. 2012, 125, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Mittelbach, G.G.; Schemske, D.W.; Cornell, H.V.; Allen, A.P.; Brown, J.M.; Bush, M.B.; Harrison, S.P.; Hurlbert, A.H.; Knowlton, N.; Lessios, H.A. Evolution and the latitudinal diversity gradient: Speciation, extinction and biogeography. Ecol. Lett. 2007, 10, 315–331. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Diniz-Filho, J.A.; Jaramillo, C.A.; Soeller, S.A. Climate, niche conservatism, and the global bird diversity gradient. Am. Nat. 2007, 170 (Suppl. S2), S16–S27. [Google Scholar] [CrossRef] [PubMed]
- Terborgh, J. On the notion of favorableness in plant ecology. Am. Nat. 1973, 107, 481–501. [Google Scholar] [CrossRef]
- Rosenzweig, M.L. Species Diversity Gradients: We know more and less than we thought. J. Mammal. 1992, 73, 715–730. [Google Scholar] [CrossRef]
- Colwell, R.K.; Rahbek, C.; Gotelli, N.J. The middomain effect and species richness patterns: What have we learned so far? Am. Nat. 2004, 163, E1–E23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardelus, C.L.; Colwell, R.K.; Watkins, J.E. Vascular epiphyte distribution patterns: Explaining the mid-elevation richness peak. J. Ecol. 2006, 94, 144–156. [Google Scholar] [CrossRef]
- Trigas, P.; Panitsa, M.; Tsiftsis, S. Elevational gradient of vascular plant species richness and endemism in Crete—The effect of Post-Isolation mountain uplift on a continental island system. PLoS ONE 2013, 8, e59425. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M. The elevational gradient of Andean plant endemism: Varying influences of taxon-specific traits and topography at different taxonomic levels. J. Biogeogr. 2002, 29, 1159–1165. [Google Scholar] [CrossRef]
- Colwell, R.K. RangeModel: Tools for exploring and assessing geometric constraints on species richness (the mid-domain effect) along transects. Ecography 2008, 31, 4–7. [Google Scholar] [CrossRef]
- Selvi, E. Rare plants on Mount Amiata, Italy: Vulnerability to extinction on an ecological ‘island’. Biol. Conserv. 1997, 81, 257–266. [Google Scholar] [CrossRef]
- González-Suárez, M.; Gómez, A.; Revilla, E. Which intrinsic traits predict vulnerability to extinction depends on the actual threatening processes. Ecosphere 2013, 4, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Myers, E.M.; Harwell, T.I.; Yale, E.L.; Lamb, A.M.; Anthony, F.W.; Norman, J. Multifaceted, cross-generational costs of hybridization in sibling Drosophila species. PLoS ONE 2013, 8, e80331. [Google Scholar] [CrossRef] [Green Version]
- Inouye, D.W.; Barr, B.; Armitage, K.B.; Inouye, B.D. Climate change is affecting altitudinal migrants and hibernating species. Proc. Natl. Acad. Sci. USA 2000, 97, 1630–1633. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.D. Translocation of species, climate change, and the end of trying to recreate past ecological communities. Trends Ecol. Evol. 2011, 26, 216–221. [Google Scholar] [CrossRef]
- Feng, J.; Hu, X.; Wang, J.; Wang, Y. Support for the elevational Rapoport’s rule among seed plants in Nepal depends on biogeographical affinities and boundary effects. Ecol. Evol. 2016, 6, 7246–7252. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Lee, M.H.; Song, J.H.; Byeon, S.Y.; Lee, G.E.; Kim, H.J.; Chae, S.B.; Yun, C.W.; Kim, J.D. Empirical evaluation of the elevational Rapoport’s rule: The species richness patterns for vascular plants on Seorak Mountain, Korea: Relationship between group of life forms and phytogeography affinity along the elevational gradient. Ecol. Evol. 2021, 11, 12872–12881. [Google Scholar] [CrossRef]
- Saito, S.; Kato, N.; Kudara, H.; Kagawa, S. Structure and floristic composition of a beech forest at the upper limit of the altitudinal distribution. Bull. Gumma Prefect. Womens Coll. 1983, 3, 115–122. [Google Scholar]
- Takahashi, K.; Azuma, H.; Yasue, K. Effects of climate on the radial growth of tree species in the upper and lower distribution limits of an altitudinal ecotone on Mount Norikura, central Japan. Ecol. Res. 2010, 18, 549–558. [Google Scholar] [CrossRef] [Green Version]
| Hypothesis | R2 |
|---|---|
| SAR | 0.689 |
| MTE | 0.143 |
| MDE | 0.794 |
| SAR + MTE | 0.959 |
| SAR + MDE | 0.871 |
| MTE + MDE | 0.976 |
| SAR + MTE + MDE | 0.989 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Li, T.; Yue, M. Altitudinal Patterns of Species Richness of Seed Plants in Qingling Mountains: A Test of Rapoport’s Rule. Diversity 2022, 14, 603. https://doi.org/10.3390/d14080603
Li Q, Li T, Yue M. Altitudinal Patterns of Species Richness of Seed Plants in Qingling Mountains: A Test of Rapoport’s Rule. Diversity. 2022; 14(8):603. https://doi.org/10.3390/d14080603
Chicago/Turabian StyleLi, Qian, Ting Li, and Ming Yue. 2022. "Altitudinal Patterns of Species Richness of Seed Plants in Qingling Mountains: A Test of Rapoport’s Rule" Diversity 14, no. 8: 603. https://doi.org/10.3390/d14080603
APA StyleLi, Q., Li, T., & Yue, M. (2022). Altitudinal Patterns of Species Richness of Seed Plants in Qingling Mountains: A Test of Rapoport’s Rule. Diversity, 14(8), 603. https://doi.org/10.3390/d14080603





