Reproductive Mode of Corbicula tobae (Martens, 1900): Brooding and Larval Morphology in Lake Toba (Indonesia)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
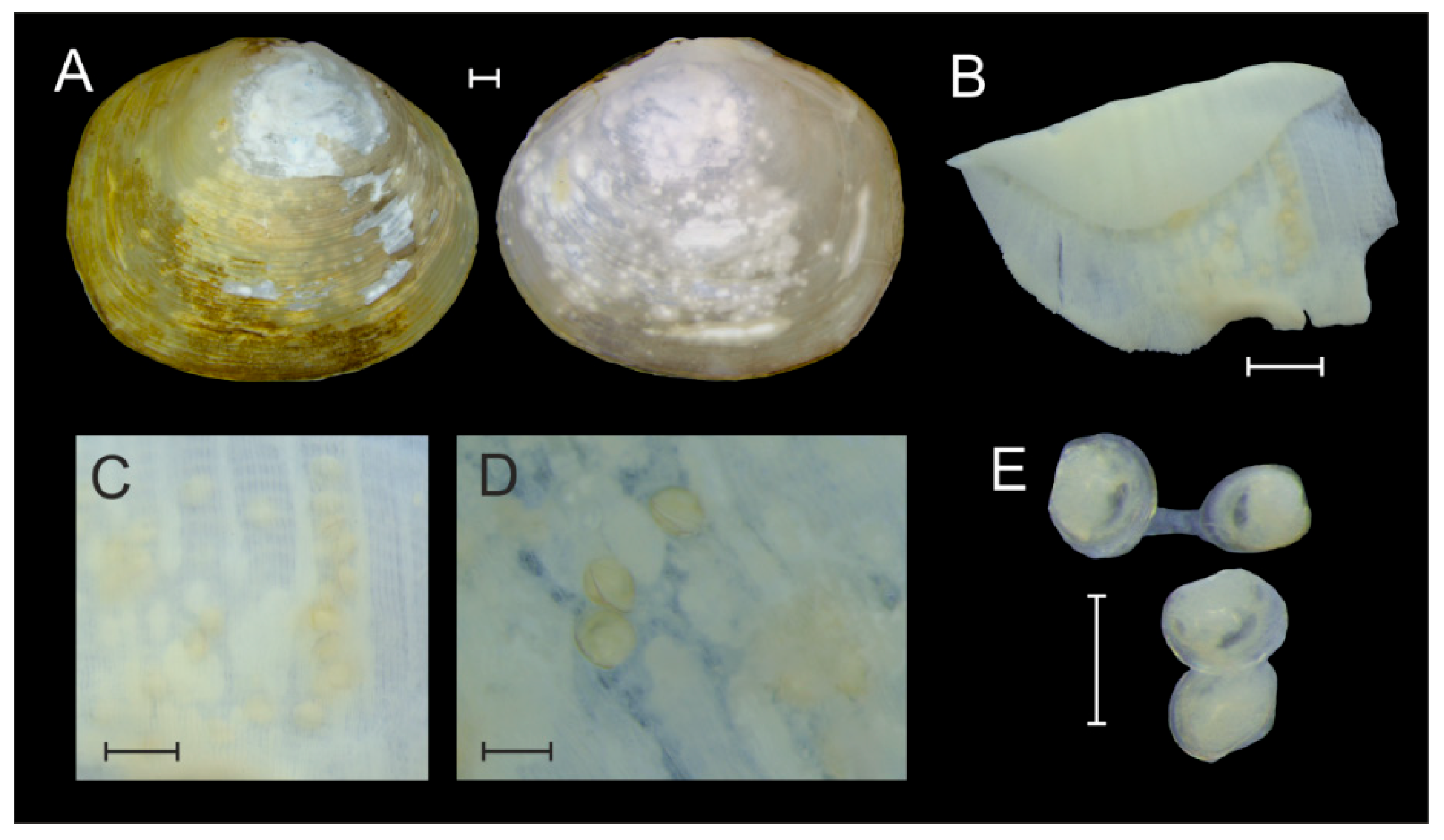
3.1. Larval Morphology
3.2. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajagopal, S.; van der Velde, G.; de Vaate, A.B. Reproductive biology of the Asiatic clams Corbicula fluminalis and Corbicula fluminea in the River Rhine. Arch. Hydrobiol. 2000, 149, 403–420. [Google Scholar] [CrossRef]
- Korniushin, A.V.; Glaubrecht, M. Novel reproductive modes in freshwater clams: Brooding and larval morphology in Southeast Asian taxa of Corbicula (Mollusca, Bivalvia, Corbiculidae). Acta Zool. 2003, 84, 293–315. [Google Scholar] [CrossRef]
- Glaubrecht, M.; von Rintelen, T.; Korniushin, A.V. Towards a systematic revision of brooding freshwater Corbiculidae in Southeast Asia (Bivalvia, Veneroida): On shell morphology, anatomy and molecular phylogenetics of endemic taxa from islands in Indonesia. Malacologica 2003, 45, 1–40. [Google Scholar]
- Glaubrecht, M.; Feher, Z.; Von Rintelen, T. Brooding in Corbicula madagascariensis (Bivalvia, Corbiculidae) and the repeated evolution of viviparity in corbiculids. Zool. Scr. 2006, 35, 641–654. [Google Scholar] [CrossRef]
- Mito, T.; Tanaka, T.; Aranishi, F. Genetic Variability and reproduction structure of Corbicula japonica in major fishing brackish lakes in Japan. Open J. Mar. Sci. 2014, 4, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Bespalaya, Y.V.; Aksenova, O.V.; Kropotin, A.V.; Shevchenko, A.R.; Travina, O.V. Reproduction of the androgenetic population of the Asian Corbicula clam (Bivalvia: Cyrenidae) in the Northern Dvina River basin, Russia. Diversity 2021, 13, 316. [Google Scholar] [CrossRef]
- Sousa, R.; Novais, A.; Costa, R.; Strayer, D.L. Invasive bivalves in fresh waters: Impacts from individuals to ecosystems and possible control strategies. Hydrobiologia 2014, 735, 233–251. [Google Scholar] [CrossRef] [Green Version]
- Bespalaya, Y.V.; Aksenova, O.V.; Gofarov, M.Y.; Kondakov, A.V.; Kropotin, A.V.; Kononov, O.D.; Bolotov, I.N. Who inhabits the world’s deepest crater lake? A taxonomic review of Corbicula (Bivalvia: Cyrenidae) clams from Lake Toba, North Sumatra, Indonesia. J. Zool. Syst. Evol. Res. 2021, 59, 400–410. [Google Scholar] [CrossRef]
- Byrne, M.; Phelps, H.; Church, T.; Adair, V.; Selvakumaraswamy, P.; Potts, J. Reproduction and development of the freshwater clam Corbicula australis in southeast Australia. Hydrobiologia 2000, 418, 185–197. [Google Scholar] [CrossRef]
- Hedtke, S.M.; Stanger-Hall, K.; Baker, R.J.; Hillis, D.M. All male asexuality: Origin and maintenance of androgenesis in the Asian clam Corbicula. Evolution 2008, 62, 1119–1136. [Google Scholar] [CrossRef]
- Lee, T.; Siripattrawan, S.; Ituarte, C.F.; Foighil, D.O. Invasion of the clonal clams: Corbicula lineages in the New World. Am. Malacol. Bull. 2005, 20, 113–122. [Google Scholar]
- Mackie, G.L. Biology of Freshwater Corbiculid and Sphaeriid Clams of North America; Ohio Biological Survey Bulletin New Series: Columbus, OH, USA, 2007; Volume 15, 436p. [Google Scholar]
- Pigneur, L.M.; Hedtke, S.M.; Etoundi, E.; Van Doninck, K. Androgenesis: A review through the study of the selfish shellfish Corbicula spp. Heredity 2012, 108, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurukawa, M.; Mizumoto, S. An ecological study on the bivalve ‘Seta-shijimi’, Corbicula sandai Reinhardt on the Lake Biwa. II. On the development. Bull. Jpn. Soc. Sci. Fish. 1953, 19, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.; Soutome, Y.; Sekiguchi, H. Larval development of the brackish water clam Corbicula japonica (Bivalvia: Corbiculidae), with special reference to Morphological Comparison with Concurrent Tidal Flat Bivalves. Venus 2004, 63, 33–48. [Google Scholar]
- Nanbu, R.; Mizuno, T.; Sekiguchi, H. Post-settlement growth and mortality of brackish water clam Corbicula japonica in the Kiso Estuaries, Central Japan. Fish. Sci. 2008, 74, 1254–1268. [Google Scholar] [CrossRef]
- Bespalaya, Y.V.; Kropotin, A.V.; Kondakov, A.V.; Aksenova, O.V.; Gofarov, M.Y.; Kim, S.K.; Lee, J.H.; Travina, O.V.; Vikhrev, I.V.; Vinarski, M.V.; et al. A taxonomic reassessment of native and invasive species of Corbicula clams (Bivalvia: Cyrenidae) from the Russian Far East and Korea. Zool. J. Linn. Soc. 2022. [Google Scholar] [CrossRef]
- von Rintelen, T.; Glaubrecht, M. Rapid evolution of sessility in an endemic species flock of the freshwater bivalve Corbicula from ancient lakes on Sulawesi, Indonesia. Biol. Lett. 2006, 2, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Sahidin, A.; Muhammad, G.; Hasan, Z.; Arief, C.M.W.; Marwoto, R.M.; Komaru, A. Indonesian freshwater bivalves: A meta-analysis of endemicity, ecoregion distributions, and conservation status AACL. Bioflux 2021, 14, 3750–3775. Available online: http://www.bioflux.com.ro/aac (accessed on 31 January 2022).
- Konishi, K.; Kawamura, K.; Furuita, H.; Komaru, A. Spermatogenesis of the freshwater clam Corbicula aff. fluminea Müller (Bivalvia: Corbiculidae). J. Shellfish Res. 1998, 17, 185–189. [Google Scholar]
- Qiu, A.; Shi, A.; Komaru, A. Yellow and brown shell color morphs of Corbicula fluminea (Bivalvia. Corbiculidae) from Sichuan Province, China, are triploids and tetraploids. J. Shellfish Res. 2001, 20, 323–328. [Google Scholar]
- Komaru, A.; Konishi, K. Ultrastructure of biflagellate spermatozoa in the freshwater clam, Corbicula leana. Invertebr. Reprod. Dev. 1996, 29, 193–197. [Google Scholar] [CrossRef]
- Rybalkina, S.M.; Maiorova, M.A.; Anisimov, A.P.; Kravchenko, D.N. The Gametogenesis and sexual cycle of the bivalve Corbicula japonica Prime (1864) in the Mouth of the Kievka River (Sea of Japan). Russ. J. Mar. Biol. 2013, 39, 253–264. [Google Scholar] [CrossRef]
- Ostrovsky, A.N.; Lidgard, S.; Gordon, D.P.; Schwaha, T.; Genikhovich, G.; Ereskovsky, A.V. Matrotrophy and placentation in invertebrates: A new paradigm. Biol. Rev. 2016, 91, 673–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bespalaya, Y.; Bolotov, I.; Aksenova, O.; Kondakov, A.; Paltser, I.; Gofarov, M. Reproduction of Pisidium casertanum (Poli, 1791) in Arctic lake. R. Soc. Open Sci. 2015, 2, 140212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bespalaya, Y.V.; Joyner-Matos, J.; Bolotov, I.N.; Aksenova, O.V.; Gofarov, M.Y.; Sokolova, S.E.; Shevchenko, A.R.; Travina, O.V.; Zubry, N.A.; Aksenov, A.; et al. Reproductive ecology of Pisidium casertanum (Poli, 1791) (Bivalvia: Sphaeriidae) in contrasting habitats of Arctic lakes. J. Molluscan Stud. 2019, 85, 11–23. [Google Scholar] [CrossRef]


| Species | Maximum Shell Length, mm | Spermatozoon Type | Head Length of Spermatozoon, µm | Location of Brood | Larval Size, mm | Type of Released Larvae (Juveniles) | Type of Brooding | References |
|---|---|---|---|---|---|---|---|---|
| C. moltkiana Prime, 1878 | 30 | Monoflagellate | 11–12 | Inner demibranches | 0.25–0.40 | D-shaped | Synchronous | [2,3,4] |
| C. linduensis Bollinger, 1914 | 17 | Monoflagellate | 8–9 | Inner demibranches | Up to 1.5 | Umbonal | Sequential | [2,3,4] |
| C. matannensis Sarasin & Sarasin, 1898 | 32.5 | Monoflagellate | 11–12 | Inner demibranches | 0.30–0.42 | D-shaped | Synchronous | [2,3,4] |
| C. loehensis Kruimel, 1913 | 18 | Monoflagellate | 9–10 | Inner demibranches | ~0.35 | D-shaped | Synchronous | [2,3,4] |
| C. possoensis Sarasin & Sarasin, 1898 | 29.4 | Monoflagellate | 10–11 | Both demibranches | 0.25–0.30 | D-shaped | Synchronous | [2,3,4] |
| C. tobae (Martens, 1900) | 15.5 | Monoflagellate | 3.4–4.4 | Inner demibranches | 0.23–0.29 | D-shaped | Synchronous | [8]; this study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kropotin, A.V.; Bespalaya, Y.V.; Aksenova, O.V.; Bolotov, I.N. Reproductive Mode of Corbicula tobae (Martens, 1900): Brooding and Larval Morphology in Lake Toba (Indonesia). Diversity 2022, 14, 700. https://doi.org/10.3390/d14090700
Kropotin AV, Bespalaya YV, Aksenova OV, Bolotov IN. Reproductive Mode of Corbicula tobae (Martens, 1900): Brooding and Larval Morphology in Lake Toba (Indonesia). Diversity. 2022; 14(9):700. https://doi.org/10.3390/d14090700
Chicago/Turabian StyleKropotin, Alexander V., Yulia V. Bespalaya, Olga V. Aksenova, and Ivan N. Bolotov. 2022. "Reproductive Mode of Corbicula tobae (Martens, 1900): Brooding and Larval Morphology in Lake Toba (Indonesia)" Diversity 14, no. 9: 700. https://doi.org/10.3390/d14090700
APA StyleKropotin, A. V., Bespalaya, Y. V., Aksenova, O. V., & Bolotov, I. N. (2022). Reproductive Mode of Corbicula tobae (Martens, 1900): Brooding and Larval Morphology in Lake Toba (Indonesia). Diversity, 14(9), 700. https://doi.org/10.3390/d14090700






