Topographical Based Significance of Sap-Sucking Heteropteran in European Wheat Cultivations: A Systematic Review
Abstract
:1. Introduction
2. Methodology
3. Evaluation of Heteropteran Pests Harmful to Cereal Cultivation in Europe
3.1. Main Qualitative and Quantitative Properties of Wheat Cultivation in the European Continent
3.2. Taxonomic Order and General Distribution of Heteropteran Pests Registered in European Cereal Cultivation
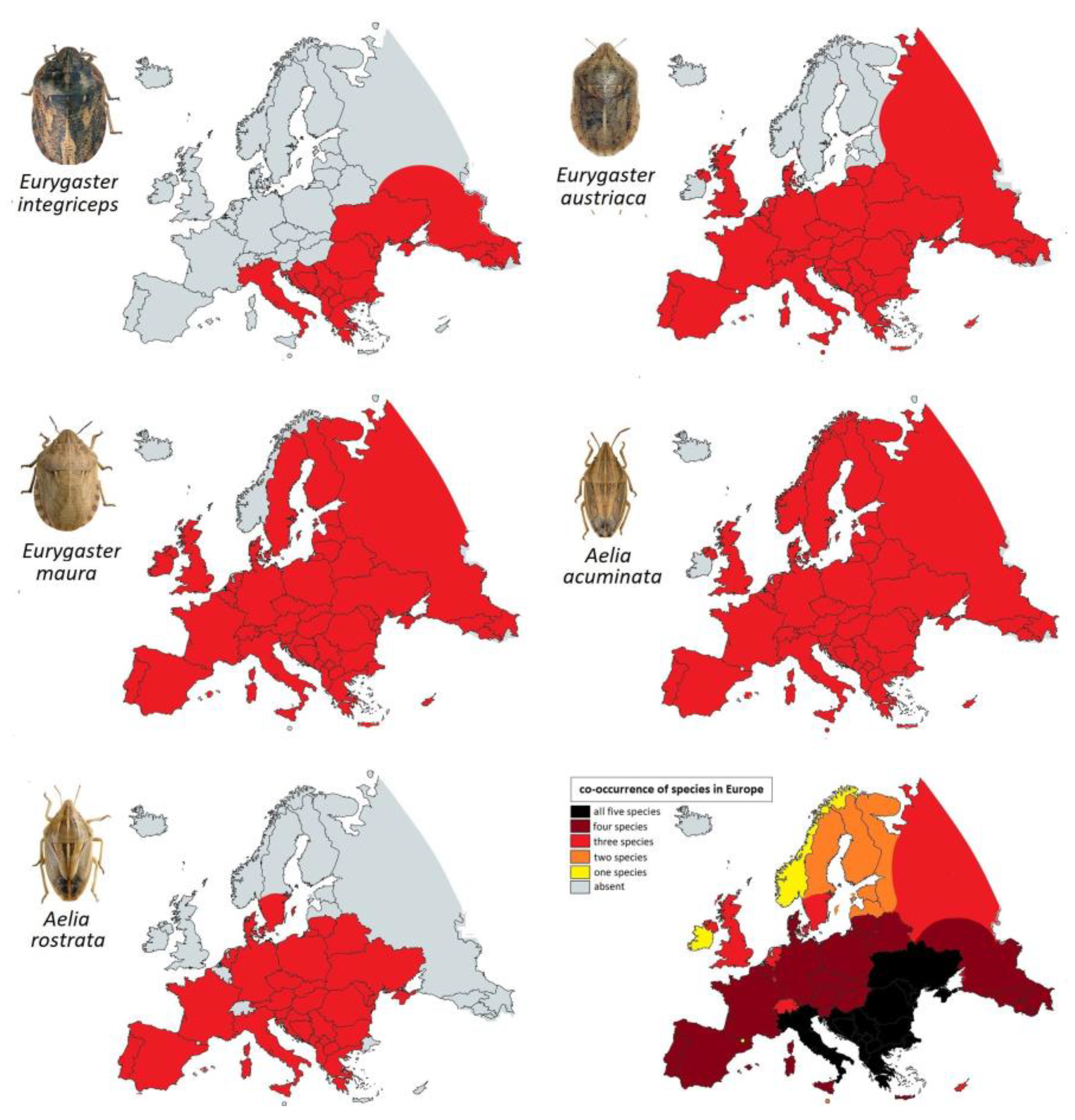
3.3. The Distribution of Main European Species of Heteropteran Registered as Cereals Pests
3.4. Species Ranking Based on Temperature Requirements
3.5. The Main Biological Characteristics of Wheat Bugs
3.6. Voltinism and Diapause
3.7. Migration
3.8. Seasonal Activity as a Function of the Host Preferences
3.9. Morphological Feeding Characteristics of the Sap-Sucking (Hemiptera: Heteropetra) Insects
3.10. Registered Quantitative Damage in Wheat Caused by Different Cereal Bugs
3.11. Physiological Consequences of Saliva Injected by Heteropteran Pests in Cereals
3.12. Comparison and Evaluation of Cereal Bugs According to Their Damage Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Other Possible Wheat Pests from the Heteroptera Suborder
Appendix B
Main Identifying Features of Heteropteran Adult Cereal Pests
References
- Poutanen, K.S.; Kårlund, A.O.; Gómez-Gallego, C.; Johansson, D.P.; Scheers, N.M.; Marklinder, I.M.; Eriksen, A.K.; Silventoinen, P.C.; Nordlund, E.; Sozer, N.; et al. Grains–a Major Source of Sustainable Protein for Health. Nutr. Rev. 2022, 80, 1648–1663. [Google Scholar] [CrossRef]
- Schuh, R.T.S.; Weirauch, C. True Bugs of the World (Hemiptera: Heteroptera): Classification and Natural History, 2 nd ed.; Siri Scientific Press: Rochdale, UK, 2020; ISBN 9780995749696. [Google Scholar]
- Javahery, M.; Schaefer, C.W.; Lattin, J.D. Shield Bugs (Scutelleridae). In Heteroptera of Economic Importance; Schaefer, C.W., Panizzi, A.C., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 475–486. [Google Scholar]
- Critchley, B.R. Literature Review of Sunn Pest Eurygaster Integriceps Put. (Hemiptera, Scutelleridae). Crop Prot. 1998, 17, 271–287. [Google Scholar] [CrossRef]
- de Jong, Y.; Verbeek, M.; Michelsen, V.; Bjørn, P.D.P.; Los, W.; Steeman, F.; Bailly, N.; Basire, C.; Chylarecki, P.; Stloukal, E.; et al. Fauna Europaea—All European Animal Species on the Web. Biodivers. Data J. 2014, 2, e4034. [Google Scholar] [CrossRef] [Green Version]
- Memisoglu, H.; Melan, K.; Ozkan, M.; Kilic, A.U.; Dortbudak, Y. Investigations on the Hibernation and Migration of Aelia Rostrata Boh. In the Central Anatolia. Bitki Koruma Bül. 1996, 36, 115–142. [Google Scholar]
- Bandani, A.R.; Kazzazi, M.; Mehrabadi, M. Purification and Characterization of Midgut A-Amylases of Eurygaster Integriceps. Entomol. Sci. 2009, 12, 25–32. [Google Scholar] [CrossRef]
- Sivri, D.; Köksel, H.; Bushuk, W. Effects of Wheat Bug (Eurygaster Maura) Proteolytic Enzymes on Electrophoretic Properties of Gluten Proteins. New Zeal. J. Crop Hortic. Sci. 1998, 26, 117–125. [Google Scholar] [CrossRef]
- Zoccatelli, G.; Vincenzi, S.; Corbelini, M.; Vaccino, P.; Tavella, L.; Curioni, A. Breakdown of Gluten Polymers by Eurygaster Maura Protease. In Proceedings of the Program and abstracts of the 8th Gluten Workshop; Viterbo, Italy, 8–10 September 2003; p. 82. [Google Scholar]
- Aja, S.; Pérez, G.; Rosell, C.M. Wheat Damage by Aelia Spp. and Erygaster Spp.: Effects on Gluten and Water-Soluble Compounds Released by Gluten Hydrolysis. J. Cereal Sci. 2004, 39, 187–193. [Google Scholar] [CrossRef]
- Karababa, E.; Ozan, A.N. Effect of Wheat Bug (Eurygaster Integriceps) Damage on Quality of a Wheat Variety Grown in Turkey. J. Sci. Food Agric. 1998, 77, 399–403. [Google Scholar] [CrossRef]
- Hariri, G.; Williams, P.C.; El-Haramein, F.J. Influence of Pentatomid Insects on the Physical Dough Properties and Two-Layered Flat Bread Baking Quality of Syrian Wheat. J. Cereal Sci. 2000, 31, 111–118. [Google Scholar] [CrossRef]
- Benedek, P. Differences in the Seasonal Activity of Central European Cereal Bugs Concerning Their Population Dynamics and Origin. Z. Für Angew. Entomol. 1971, 67, 238–246. [Google Scholar] [CrossRef]
- Benedek, P. On Differences in the Seasonal Activity of Cereal Bugs and Notes on the Specific Composition of Their Populations in Hungary. Acta Phytopathol. Entomol. Hung. 1971, 6, 119–200. [Google Scholar]
- Kapustkina, A.V.; Khilevskiy, V.A. Population and Harmfulness Dynamics of the Sunn Pest Eurygaster Integriceps Put. (Heteroptera, Scutelleridae) in Wheat Crops of the Ciscaucasia Steppe Zone. Entomol. Rev. 2020, 100, 173–178. [Google Scholar] [CrossRef]
- Huang, J.; Sedano, F.; Huang, Y.; Ma, H.; Li, X.; Liang, S.; Tian, L.; Zhang, X.; Fan, J.; Wu, W. Assimilating a Synthetic Kalman Filter Leaf Area Index Series into the WOFOST Model to Improve Regional Winter Wheat Yield Estimation. Agric. For. Meteorol. 2016, 216, 188–202. [Google Scholar] [CrossRef]
- FAOSTAT Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 5 May 2022).
- Beres, B.L.; Rahmani, E.; Clarke, J.M.; Grassini, P.; Pozniak, C.J.; Geddes, C.M.; Porker, K.D.; May, W.E.; Ransom, J.K. A Systematic Review of Durum Wheat: Enhancing Production Systems by Exploring Genotype, Environment, and Management (G × E × M) Synergies. Front. Plant Sci. 2020, 11, 568657. [Google Scholar] [CrossRef] [PubMed]
- Day, L.; Augustin, M.A.; Batey, I.L.; Wrigley, C.W. Wheat-Gluten Uses and Industry Needs. Trends Food Sci. Technol. 2006, 17, 82–90. [Google Scholar] [CrossRef]
- Bloksma, A.H. Rheology of the Breadmaking Process. Cereal Foods World 1990, 35, 228–236. [Google Scholar]
- Edwards, N.M.; Preston, K.R.; Paulley, F.G.; Gianibelli, M.C.; McCaig, T.N.; Clarke, J.M.; Ames, N.P.; Dexter, J.E. Hearth Bread Baking Quality of Durum Wheat Varying in Protein Composition and Physical Dough Properties. J. Sci. Food Agric. 2007, 87, 2000–2011. [Google Scholar] [CrossRef]
- Andersson, R.; Hämäläinen, M.; Åman, P. Predictive Modelling of the Bread-Making Performance and Dough Properties of Wheat. J. Cereal Sci. 1994, 20, 129–138. [Google Scholar] [CrossRef]
- Dowell, F.E.; Maghirang, E.B.; Pierce, R.O.; Lookhart, G.L.; Bean, S.R.; Xie, F.; Caley, M.S.; Wilson, J.D.; Seabourn, B.W.; Ram, M.S.; et al. Relationship of Bread Quality to Kernel, Flour, and Dough Properties. Cereal Chem. 2008, 85, 82–91. [Google Scholar] [CrossRef]
- Borla, O.P.; Motta, E.L.; Saiz, A.I.; Fritz, R. Quality Parameters and Baking Performance of Commercial Gluten Flours. LWT–Food Sci. Technol. 2004, 37, 723–729. [Google Scholar] [CrossRef]
- Edde, P.A. Arthropod Pests of Small Grains: Wheat (Triticum Aestivum L.) and Barley (Hordeum Vulgare L.). In Field Crop Arthropod Pests of Economic Importance; Elsevier: London, UK, 2022; pp. 536–611. ISBN 978-0-12-818621-3. [Google Scholar]
- Neimorovets, V. Review of the Genus Eurygaster (Hemiptera: Heteroptera: Scutelleridae) of Russia. Zootaxa 2020, 4722, 501–539. [Google Scholar] [CrossRef]
- Neimorovets, V. Distribution of the Sunn Pests from the Genus Eurygaster (Heteroptera: Scutelleridae). Plant Prot. News 2019, 4, 36–48. [Google Scholar] [CrossRef]
- Afonin, A.N.S.L.G.; Dzyubenko, N.I.; Frolov, A.N. Interactive Agricultural Ecological Atlas of Russia and Neighboring Countries Economic Plants and Their Diseases, Pests and Weeds. Available online: http://www.agroatlas.ru (accessed on 9 May 2022).
- Kivan, M. Development Rate and Lower Temperature Threshold in the Eggs of Eurygaster Integriceps (Heteroptera: Scutelleridae). J. Insect Sci. 2008, 15, 163–166. [Google Scholar] [CrossRef]
- Konjević, A.; Štrbac, P.; Petrić, D.; Popović, A.; Ignjatović-Ćupina, A. Temperature-Dependent Development Model of Pest Wheat Bugs Eurygaster and Aelia Spp. (Heteroptera: Scutelleridae and Pentatomidae). Entomol. Gen. 2014, 35, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.S. Notes on the Systematics and Distribution of Some Species of Aelia Fabr. (Hemiptera, Pentatomidae) in the Middle East, with Special Reference to the Rostrata Group. Ann. Mag. Nat. Hist. 1962, 5, 129–145. [Google Scholar] [CrossRef]
- Lodos, N. Aelia Species and Their Economic Importance in Turkey. EPPO Bull. 1981, 11, 29–32. [Google Scholar] [CrossRef]
- Saulich, A.K.; Musolin, D.L. Seasonal Cycles in Stink Bugs (Heteroptera, Pentatomidae) from the Temperate Zone: Diversity and Control. Entomol. Rev. 2014, 94, 785–814. [Google Scholar] [CrossRef]
- Panov, A.A. Control of Activity of Corpora Allata in the Bug Eurygaster Integriceps with Obligate Imaginal Diapause. Dokolady Biol. Sci. 1977, 223, 71–74. [Google Scholar]
- Guz, N.; Toprak, U.; Dageri, A.; Oktay Gurkan, M.; Denlinger, D.L. Identification of a Putative Antifreeze Protein Gene That Is Highly Expressed during Preparation for Winter in the Sunn Pest. Eurygaster Maura. J. Insect Physiol. 2014, 68, 30–35. [Google Scholar] [CrossRef]
- Saulich, A.K.; Musolin, D.L. Diapause in the Seasonal Cycle of Stink Bugs (Heteroptera, Pentatomidae) from the Temperate Zone. Entomol. Rev. 2012, 92, 1–26. [Google Scholar] [CrossRef]
- Brown, E.S. Notes on the Migration and Direction of Flight of Eurygaster and Aelia Species (Hemiptera, Pentatomoidea) and Their Possible Bearing on Invasions of Cereal Crops. J. Anim. Ecol. 1965, 34, 93. [Google Scholar] [CrossRef]
- Salis, L.; Goula, M.; Izquierdo, J.; Gordún, E. Population Density and Distribution of Wheat Bugs Infesting Durum Wheat in Sardinia, Italy. J. Insect Sci. 2013, 13, 50. [Google Scholar] [CrossRef] [Green Version]
- Dikyar, R. Biology and Control of Aelia Rostrata in Central Anatolia. EPPO Bull. 1981, 11, 39–41. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in Global Climate Change Research: Direct Effects of Rising Temperature on Insect Herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- McPherson, J.E.; McPherson, R.M. Stink Bugs of Economic Importance in America North of Mexico; CRC Press: Boca Raton, FL, USA, 2000; ISBN 9781420042429. [Google Scholar]
- Peiffer, M.; Felton, G.W. Insights into the Saliva of the Brown Marmorated Stink Bug Halyomorpha Halys (Hemiptera: Pentatomidae). PLoS ONE 2014, 9, e88483. [Google Scholar] [CrossRef] [Green Version]
- Baptist, B.A. The Morphology and Physiology of the Salivary Glands of Hemiptera-Heteroptera. J. Cell Sci. 1941, s2–83, 91–139. [Google Scholar] [CrossRef]
- Mehrabadi, M.; Bandani, A.R.; Dastranj, M. Salivary Digestive Enzymes of the Wheat Bug, Eurygaster Integriceps (Insecta: Hemiptera: Scutelleridae). C. R. Biol. 2014, 337, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Miles, P.W. The Saliva of Hemiptera. Adv. Insect Phys. 1972, 9, 183–255. [Google Scholar] [CrossRef]
- Memisoglu, H.; Melan, K.; Ozkan, M.; Kilic, A.U. Investigations on the Crop Losses the Wheat Caused by Cereal Bug (Aelia Rostrata Boh.) in the Region of Central Anatolia. Bitki Koruma Bülteni 1994, 34, 111–121. [Google Scholar]
- Canhilal, R.; Kutuk, H.; Kanat, A.D.; Islamoglu, M.; El-Haramein, F.; El-Bouhssini, M. Economic Threshold for the Sunn Pest, Eurygaster Integriceps Put. (Hemiptera: Scutelleridae), on Wheat in Southeastern Turkey. J. Agric. Urban Entomol. 2005, 22, 191–201. [Google Scholar]
- Gul, A.; Akbay, C.; Direk, M. Sunn Pest Control Policies and Effect of Sunn Pest Damage on Wheat Quality and Price in Turkey. Qual. Quant. 2006, 40, 469–480. [Google Scholar] [CrossRef]
- Kazemi Alamuti, M.; Majdi, M.; Hossini Salekdeh, G. A Historical Perspective to Sunn Pest (Eurygaster Integriceps) Management of Wheat from Traditional to Modern Methods. J. Biosaf. 2021, 14, 101–116. [Google Scholar]
- Bandani, A.R.; Alizadeh, M.; Talebi, K. Toxicity of Fenitrothion, an Organophosphorus Pesticide, against Summer Population of Sunn Pest, Eurygaster Integriceps Put. (Hemiptera: Scutelleridae). Commun. Agric. Appl. Biol. Sci. 2005, 70, 775–777. [Google Scholar] [PubMed]
- Kivan, M.; Kilic, N. Effects of Storage at Low-Temperature of Various Heteropteran Host Eggs on the Egg Parasitoid, Trissolcus Semistriatus. BioControl 2005, 50, 589–600. [Google Scholar] [CrossRef]
- Dizlek, H.; Islamoglu, M. Effects of Sunn Pest (Eurygaster Maura L. Heteroptera; Scutelleridae) Sucking Number on Physical and Physicochemical Characteristics of Wheat Varieties. J. Appl. Bot. Food Qual. 2015, 88, 10–15. [Google Scholar] [CrossRef]
- Mehrabadi, M.; Bandani, A.R.; Mehrabadi, R.; Alizadeh, H. Inhibitory Activity of Proteinaceous α-Amylase Inhibitors from Triticale Seeds against Eurygaster Integriceps Salivary α-Amylases: Interaction of the Inhibitors and the Insect Digestive Enzymes. Pestic. Biochem. Physiol. 2012, 102, 220–228. [Google Scholar] [CrossRef]
- Vaccino, P.; Corbellini, M.; Reffo, G.; Zoccatelli, G.; Migliardi, M.; Tavella, L. Impact of Eurygaster Maura (Heteroptera: Scutelleridae) Feeding on Quality of Bread Wheat in Relation to Attack Period. J. Econ. Entomol. 2006, 99, 757–763. [Google Scholar] [CrossRef]
- Vaccino, P.; Corbellini, M.; Curioni, A.; Zoccatelli, G.; Migliardi, M.; Tavella, L. Relationships between Timing of Eurygaster Maura Attacks and Gluten Degradation in Two Bread Wheat Cultivars. In The Gluten Proteins; Lafiandra, D., Masci, S., D’Ovidio, R., Eds.; RSC Publishing: Cambridge, UK, 2004; pp. 425–428. [Google Scholar]
- Aljaryian, R.; Kumar, L.; Taylor, S. Modelling the Current and Potential Future Distributions of the Sunn Pest Eurygaster Integriceps (Hemiptera: Scutelleridae) Using CLIMEX. Pest Manag. Sci. 2016, 72, 1989–2000. [Google Scholar] [CrossRef]
- Syromyatnikov, M.Y.; Golub, V.B.; Kokina, A.V.; Soboleva, V.A.; Popov, V.N. DNA Barcoding and Morphological Analysis for Rapid Identification of Most Economically Important Crop-Infesting Sunn Pests Belonging to Eurygaster Laporte, 1833 (Hemiptera, Scutelleridae). Zookeys 2017, 706, 51–71. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.J.; Ventura, M.U.; Silva, F.A.C.; Panizzi, A.R. Population Dynamics of Dichelops Melacanthus (Dallas) (Heteroptera: Pentatomidae) on Host Plants. Neotrop. Entomol. 2013, 42, 141–145. [Google Scholar] [CrossRef]
- Viator, H.P.; Pantoja, A.; Smith, C.M. Damage to Wheat Seed Quality and Yield by the Rice Stink Bug and Southern Green Stink Bug (Hemiptera: Pentatomidae). J. Econ. Entomol. 1983, 76, 1410–1413. [Google Scholar] [CrossRef]
- Paulian, F.; Popov, C. Sunn Pest or Cereal Bug. In Wheat documenta; Häfliger, E., Ed.; Ciba-Geigy Ltd.: Basle, Switzerland, 1980; pp. 69–74. [Google Scholar]
- Leskey, T.C.; Hamilton, G.C.; Nielsen, A.L.; Polk, D.F.; Rodriguez-Saona, C.; Christopher Bergh, J.; Ames Herbert, D.; Kuhar, T.P.; Pfeiffer, D.; Dively, G.P.; et al. Pest Status of the Brown Marmorated Stink Bug, Halyomorpha Halys in the USA. Outlooks Pest Manag. 2012, 23, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, M.A.; Popov, C. Considerations on Variability in Eurygaster Integriceps (Heteroptera). Stud. Si Cercet. Biol. 1976, 28, 89–94. [Google Scholar]
- Stavraki, H.G. Data on the Spread in Greece of Pentatomids Injurious to Cereals. Biol. Gall. 1979, 9, 301–306. [Google Scholar]
- Khalis Ali, W.; Salih Khidhir, A.Q. Illustration of the Morphologic Characters of the Sunn Pest Eurygaster Integriceps Puton, 1881 (Hemiptera: Scutelleridae) Collected from Erbil Governorate-Kurdistan Region-Iraq. Entomol. Ornithol. Herpetol. Curr. Res. 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Durak, D.; Kalender, Y. Fine Structure and Chemical Analysis of the Metathoracic Scent Gland of Eurygaster Maura (Linnaeus, 1758) (Heteroptera: Scutelleridae). Folia Biol. 2007, 55, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Batzakis, B.D. Morphological Characters of the Greek Species of Eurygaster Laporte, 1832 (Heteroptera: Pentatomidae). Ann. l’Inst. Phytopathol. Benaki 1972, 10, 267–279. [Google Scholar]
- Scudder, G.G.E. Comparative Morphology of Insect Genitalia. Annu. Rev. Entomol. 1971, 16, 379–406. [Google Scholar] [CrossRef]
- China, W.E.; Lodos, N. A Study of the Taxonomic Characters of Some Species of Aelia F. (Heteroptera-Pentatomidae). Ann. Mag. Nat. Hist. 1959, 2, 577–602. [Google Scholar] [CrossRef]
- Panizzi, A.R.; McPherson, J.E.; James, D.G.; Javahery, M.; McPherson, R.M. Stink Bugs (Pentatomidae). In Heteroptera of Economic Importance; Schaefer, C.W., Panizzi, A.R., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 412–474. [Google Scholar]


| Eurygaster integriceps | Eurygaster austriaca | Eurygaster maura | Aelia acuminata | Aelia rostrata | ||
|---|---|---|---|---|---|---|
| Harvest area of wheat within the distribution area in 2020 | km2 | 429,234.24 | 600,925.24 | 614,537.24 | 614,864.34 | 588,633.17 |
| ranking | 5 | 3 | 2 | 1 | 4 | |
| Migratory inclination | 1: yes, 0: no | 1 | 1 | 1 | 0 | 1 |
| ranking | 1 | 1 | 1 | 0 | 1 | |
| Colonization temperature | °C | 13.0 | 19.5 | 18.5 | 17.5 | 13.0 |
| ranking | 1 | 5 | 4 | 3 | 1 | |
| Number of related sources on damage since 2000 | No. | 35 | 7 | 16 | 2 | 4 |
| ranking | 1 | 3 | 2 | 5 | 4 | |
| Degree of synchronization with host plants | No. | 5 | 4 | 1 | 2 | 3 |
| ranking | 1 | 2 | 5 | 4 | 3 | |
| Sum of rankings | total score | 9 | 14 | 14 | 18 | 13 |
| dam.pot. | 1 | 3 | 3 | 5 | 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibicsár, S.; Keszthelyi, S. Topographical Based Significance of Sap-Sucking Heteropteran in European Wheat Cultivations: A Systematic Review. Diversity 2023, 15, 109. https://doi.org/10.3390/d15010109
Gibicsár S, Keszthelyi S. Topographical Based Significance of Sap-Sucking Heteropteran in European Wheat Cultivations: A Systematic Review. Diversity. 2023; 15(1):109. https://doi.org/10.3390/d15010109
Chicago/Turabian StyleGibicsár, Szilvia, and Sándor Keszthelyi. 2023. "Topographical Based Significance of Sap-Sucking Heteropteran in European Wheat Cultivations: A Systematic Review" Diversity 15, no. 1: 109. https://doi.org/10.3390/d15010109






