Possible Population Growth of Astrospartus mediterraneus (Risso, 1826) (Ophiuroidea, Gorgonocephalidae) in the Mediterranean Sea
Abstract
:1. Introduction
2. Materials and Methods
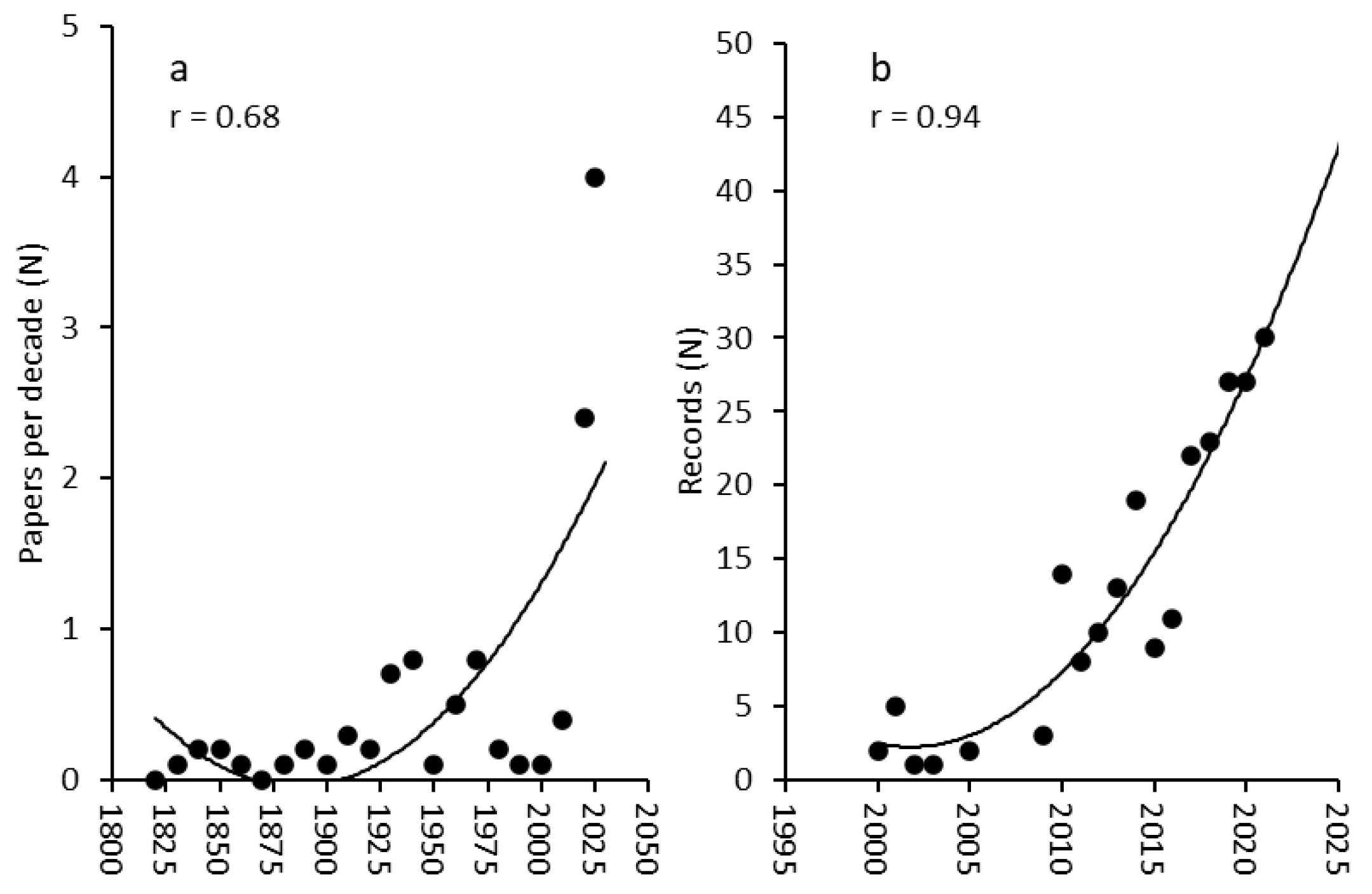
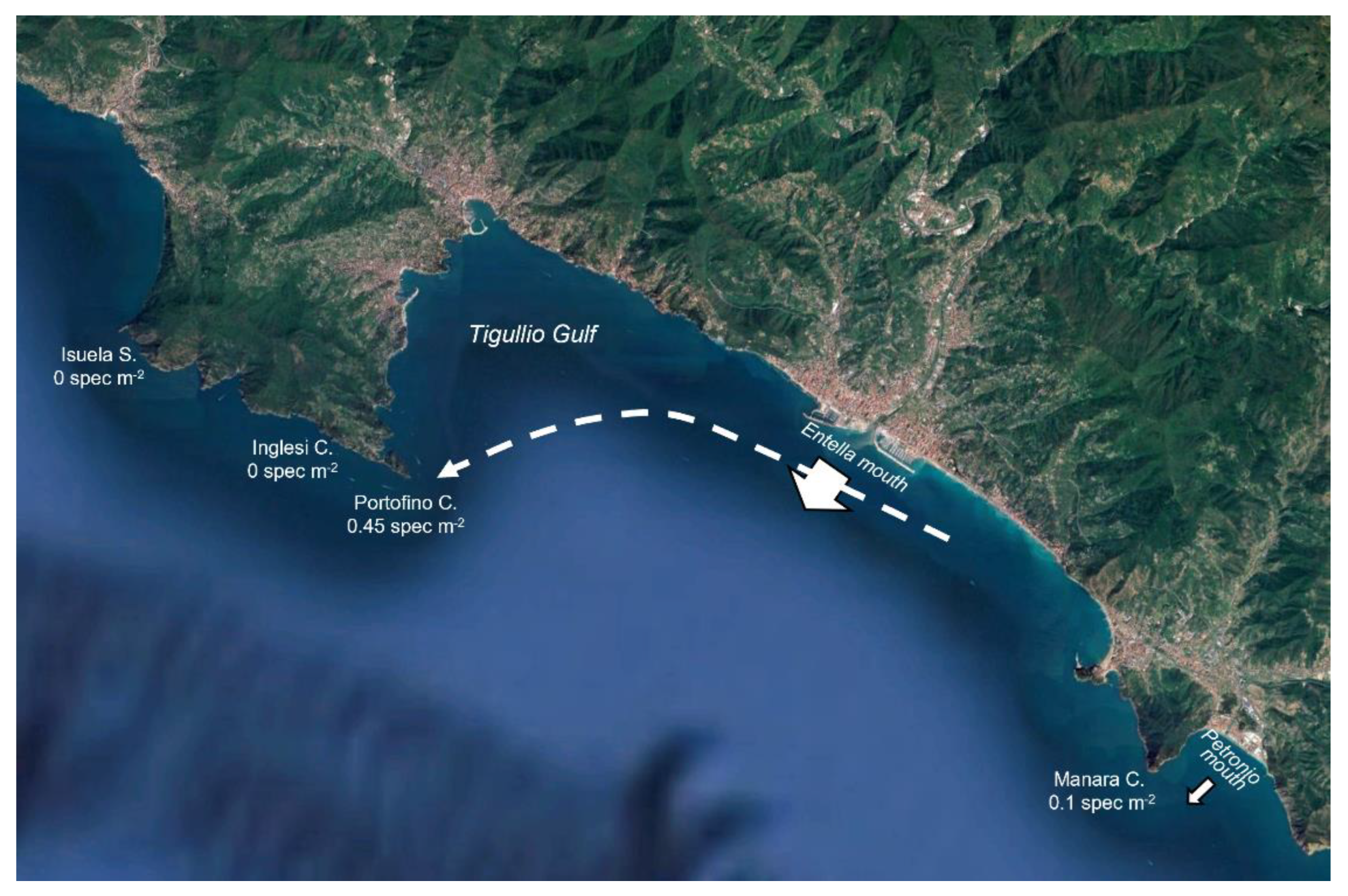
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ocaña, A.; Pérez-Ruzafa, A. Los equinodermos de las costas andaluzas. Acta Granatense 2004, 3, 83–136. [Google Scholar]
- Rondelet’s “Libri de Piscibus Marinis”: A Book About Science, Sea Monks and Sea Bishops; Macé Bonhomme: Lyon, France, 1554.
- Caruso, A.A. Lettere inedite di Domenico Cirillo a Giovanni Bianchi (Jano Planco). Nuova Riv. Stor. Med. 2021, 2, 15–34. [Google Scholar]
- Zibrowius, H. Nouvelles observations de l’ophiure gorgonocephale Astrospartus mediterraneus sur la côte méditerranéenne de France. Bibliographie annotee et repartition. Trav. Sci. Parc Natl. Port Cros 1978, 4, 157–169. Available online: http://pascal-francis.inist.fr/vibad/index.php? (accessed on 1 July 2022).
- Stöhr, S.; O’Hara, T.; Thuy, B. (Eds.) World Ophiuroidea Database. Astrospartus mediterraneus (Risso, 1826). 2022. Available online: http://www.marinespecies.org/aphia.php?p=taxdetails&id=124963#distributions (accessed on 1 July 2022).
- Tortonese, E. Distribution and Ecology of Endemic Elements in the Mediterranean Fauna (Fishes and Echinoderms). In Mediterranean Marine Ecosystems; Moraitou-Apostolopoulou, M., Kiortsis, V., Eds.; NATO Conference Series; Springer: Boston, MA, USA, 1985; Volume 8. [Google Scholar] [CrossRef]
- Hussein, K.B.; Bensahla Talet, L. A preliminary inventory of biodiversity and benthic habitats of “Plane” Island (Paloma) in Oran Bay, north western Algeria (western Mediterranean). J. Black Sea/Mediterr. Environ. 2019, 25, 49–72. Available online: http://hdl.handle.net/1834/40685 (accessed on 1 July 2022).
- Chammem, H.; Souissi, J.B.; Pérez-Ruzafa, A. Checklist with first records for the Echinoderms of northern Tunisia (central Mediterranean Sea). Sci. Mar. 2019, 83, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Fitori, A.; Fituri, A.E.; Aguilar, R.; Badreddine, A. First Record of Two Species of Echinodermata for Libyan Waters. J. Fish. Livest Prod. 2022, 10, 325. [Google Scholar]
- Montasell Bartrés, M. Astrospartus mediterraneus (Echinodermata: Ophiuroidea) in the Cap de Creus Marine Area: Ecological Characterization of an Emblematic Species. Final Grade. Bachelor’s Thesis, Universitat de Vic—Universitat Central de Catalunya, Facultat de Ciències i Tecnologia, Barcelona, Spain, 2022. Available online: http://hdl.handle.net/10854/6142 (accessed on 1 July 2022).
- Zibrowius, H. Observations biologiques au large du Lavandou (côte méditerranéenne de France) à l’aide du sous-marin Griffon de la marine nationale. Trav. Sci. Parc Natl. Port Cros 1978, 4, 171–176. [Google Scholar]
- Canessa, M.; Bavestrello, G.; Bo, M.; Enrichetti, F.; Trainito, E. Filling a Gap: A Population of Eunicella verrucosa (Pallas, 1766) (Anthozoa, Alcyonacea) in the Tavolara-Punta Coda Cavallo Marine Protected Area (NE Sardinia, Italy). Diversity 2022, 14, 405. [Google Scholar] [CrossRef]
- Canessa, M.; Bavestrello, G.; Bo, M.; Enrichetti, F.; Trainito, E. Leptogorgia sarmentosa (Anthozoa: Octocorallia) in NE Sardinia: Distribution and growth patterns. Mar. Biodivers. 2022; in press. [Google Scholar] [CrossRef]
- Santín, A.; Grinyó, J.; Ambroso, S.; Baena, P.; Biel Cabanelas, M.; Corbera, G.; Salazar, J.; Montseny, M.; Gili, J.M. Fishermen and scientists: Synergies for the exploration, conservation and sustainability of the marine environment. In The Ocean We Want: Inclusive and Transformative Ocean Science; Consejo Superior de Investigaciones Científicas (España): Madrid, Spain, 2022; pp. 77–79. [Google Scholar] [CrossRef]
- Bo, M.; Canese, S.; Spaggiari, C.; Pusceddu, A.; Bertolino, M.; Angiolillo, M.; Giusti, M.; Loreto, M.F.; Salvati, E.; Greco, S.; et al. Deep coral oases in the South Tyrrhenian Sea. PLoS ONE 2012, 7, e49870. [Google Scholar] [CrossRef] [Green Version]
- Bo, M.; Coppari, M.; Betti, F.; Enrichetti, F.; Bertolino, M.; Massa, F.; Bava, S.; Gay, G.; Cattaneo-Vietti, R.; Bavestrello, G. The high biodiversity and vulnerability of two Mediterranean bathyal seamounts support the need for creating offshore protected areas. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 543–566. [Google Scholar] [CrossRef]
- Cau, A.; Follesa, M.C.; Moccia, D.; Alvito, A.; Bo, M.; Angiolillo, M.; Canese, S.; Paliaga, E.M.; Orrù, P.E.; Sacco, F.; et al. Deepwater corals biodiversity along roche du large ecosystems with different habitat complexity along the south Sardinia continental margin (CW Mediterranean Sea). Mar. Biol. 2015, 162, 1865–1878. [Google Scholar] [CrossRef]
- Enrichetti, F.; Dominguez-Carrió, C.; Toma, M.; Bavestrello, G.; Betti, F.; Canese, S.; Bo, M. Megabenthic communities of the Ligurian deep continental shelf and shelf break (NW Mediterranean Sea). PLoS ONE 2019, 14, e0223949. [Google Scholar] [CrossRef] [Green Version]
- Angiolillo, M.; La Mesa, G.; Giusti, M.; Salvati, E.; Di Lorenzo, B.; Rossi, L.; Canese, S.; Tunesi, L. New records of scleractinian cold-water coral (CWC) assemblages in the southern Tyrrhenian Sea (western Mediterranean Sea): Human impacts and conservation prospects. Prog. Oceanogr. 2021, 197, 102656. [Google Scholar] [CrossRef]
- Consoli, P.; Altobelli, C.; Perzia, P.; Bo, M.; Rosso, A.; Alongi, G.; Serio, D.; Canese, S.; Romeo, T.; Andaloro, F. Species and habitats of conservation interest in the Ecologically and Biologically Significant Area of the Strait of Sicily: A contribution towards the creation of a Specially Protected Areas of Mediterranean Importance. Mediterr. Mar. Sci. 2021, 22, 297–316. [Google Scholar] [CrossRef]
- Moccia, D.; Cau, A.; Bramanti, L.; Carugati, L.; Canese, S.; Follesa, M.C.; Cannas, R. Spatial distribution and habitat characterization of marine animal forest assemblages along nine submarine canyons of Eastern Sardinia (central Mediterranean Sea). Deep. Sea Res. Part I Oceanogr. Res. Pap. 2020, 167, 103422. [Google Scholar] [CrossRef]
- Toma, M.; Bo, M.; Cattaneo-Vietti, R.; Canese, S.; Canessa, M.; Cannas, R.; Cardone, F.; Carugati, L.; Cau, A.; Corriero, G.; et al. Basin-scale occurrence and distribution of mesophotic and upper bathyal red coral forests along the Italian coasts. Med. Mar. Sci. 2022, 23, 484–498. [Google Scholar] [CrossRef]
- Toma, M.; Betti, F.; Bavestrello, G.; Cattaneo-Vietti, R.; Canese, S.; Cau, A.; Andaloro, F.; Greco, S.; Bo, M. Diversity and abundance of heterobranchs (Mollusca, Gastropoda) from the mesophotic and bathyal zone of the Mediterranean Sea. Eur. Zool. J. 2022, 89, 167–189. [Google Scholar] [CrossRef]
- Enrichetti, F.; Bo, M.; Morri, C.; Montefalcone, M.; Toma, M.; Bavestrello, G.; Tunesi, L.; Canese, S.; Giusti, M.; Salvati, E.; et al. Assessing the environmental status of temperate mesophotic reefs: A new, integrated methodological approach. Ecol. Ind. 2019, 102, 218–229. [Google Scholar] [CrossRef]
- Danovaro, R.; Corinaldesi, C.; Dell’Anno, A.; Snelgrove, P.V. The deep-sea under global change. Curr. Biol. 2017, 27, R461–R465. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo-Vietti, R.; Albertelli, G.; Aliani, S.; Bava, S.; Bavestrello, G.; Cecchi, L.B.; Bianchi, C.N.; Bozzo, E.; Capello, M.; Castellano, M.; et al. The Ligurian Sea: Present status, problems and perspectives. Chem. Ecol. 2010, 26, 319–340. [Google Scholar] [CrossRef]
- Ruggieri, N.; Castellano, M.; Misic, C.; Gasparini, G.; Cattaneo-Vietti, R.; Povero, P. Seasonal and interannual dynamics of a coastal ecosystem (Portofino, Ligurian Sea) in relation to meteorological constraints. Geophys. Res. Abstr. 2006, 8, 07774. [Google Scholar]
- Agrillo, G.; Bonati, V. Atlante Climatico della Liguria; ARPAL-Centro Funzionale Meteoidrologico di Protezione Civile: Genova, Italy, 2013; Volume 128. [Google Scholar]
- Hughes, R.N.; Hughes, D.J.; Smith, I.P. Citizen scientists and marine research: Volunteer participants, their contributions, and projection for the future. Oceanogr. Mar. Biol. Annu. Rev. 2014, 52, 257–314. [Google Scholar]
- Sandahl, A.; Tøttrup, A.P. Marine Citizen Science: Recent Developments and Future Recommendations. Citiz. Sci. Theory Pract. 2020, 5, 24. [Google Scholar] [CrossRef]
- Rossi, S.; Bramanti, L.; Gori, A.; Orejas, C. (Eds.) Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 1–1366. [Google Scholar] [CrossRef]
- Biel-Cabalenas, M.; Monastrell, M.; Santín, A.; Salazar, J.; Baena, P.; Vilandrich, N.; Gori, A.; Montseny, M.; Corbera, G.; Ambroso, S.; et al. “Can emblematic species become a pest? The case of Astrospartus mediterraneus (Risso, 1826) (Echinodermata: Ophiuroidea) in the artisanal fishing grounds of the Cap de Creus area (NW Mediterranean Sea)” UNEP/MAP—SPA/RAC, 2022. In Proceedings of the 4th Mediterranean Symposium on the Conservation of Coralligenous & Other Calcareous Bio-Concretions, Genova, Italy, 20–21 September 2022; Bouafif, C., Ouerghi, A., Eds.; SPA/RAC: Tunis, Tunisia, 2022; p. 184. [Google Scholar]
- Gatti, G.; Bianchi, C.N.; Parravicini, V.; Rovere, A.; Peirano, A.; Montefalcone, M.; Massa, F.; Morri, C. Ecological change, sliding baselines and the importance of historical data: Lessons from combing observational and quantitative data on a temperate reef over 70 years. PLoS ONE 2015, 10, e0118581. [Google Scholar] [CrossRef]
- Gatti, G.; Bianchi, C.N.; Montefalcone, M.; Venturini, S.; Diviacco, G.; Morri, C. Observational information on a temperate reef community helps understanding the marine climate and ecosystem shift of the 1980–90s. Mar. Pollut. Bull. 2017, 114, 528–538. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C.; Chiantore, M.; Montefalcone, M.; Parravicini, V.; Rovere, A. Mediterranean Sea biodiversity between the legacy from the past and a future of change. In Life in the Mediterranean Sea: A Look at Habitat Changes; Stambler, N., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 1–55. [Google Scholar]
- Puce, S.; Bavestrello, G.; Di Camillo, C.G.; Boero, F. Long-term changes in hydroid (Cnidaria, Hydrozoa) assemblages: Effect of Mediterranean warming? Mar. Ecol. 2009, 30, 313–326. [Google Scholar] [CrossRef]
- Calero, B.; Ramos, A.; Ramil, F. Distribution of suspension-feeder brittle stars in the Canary Current upwelling ecosystem (Northwest Africa). Deep Sea Res. Part I Oceanogr. Res. Pap. 2018, 142, 1–15. [Google Scholar] [CrossRef]
- Barry, J.P.; Dayton, P.K. Physical Heterogeneity and the Organization of Marine Communities. In Ecological Heterogeneity; Kolasa, J., Pickett, S.T.A., Eds.; Springer: New York, NY, USA, 1991; pp. 270–320. [Google Scholar]
- Drouin, G.; Himmelman, J.H.; Béland, P. Impact of tidal salinity fluctuations on echinoderm and mollusc populations. Can. J. Zool. 1985, 63, 1377–1387. [Google Scholar] [CrossRef]
- Tyler, P.A.; Young, C.M.; Clarke, A. Temperature and pressure tolerances of embryos and larvae of the Antarctic sea urchin Sterechinus neumayeri (Echinodermata: Echinoidea): Potential for deep-sea invasion from high latitudes. Mar. Ecol. Progr. Ser. 2020, 192, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Menge, B.A. Community Regulation: Under What Conditions Are Bottom-Up Factors Important on Rocky Shores? Ecology 1992, 73, 755–765. [Google Scholar] [CrossRef]
- Strathmann, R.R. Larval Feeding in Echinoderms. Am. Zool. 1975, 15, 717–730. [Google Scholar] [CrossRef] [Green Version]
- Lessios, H.A. Adaptation and phylogeny as determinants of egg size in echinoderms from the two sides of the Isthmus of Panama. Am. Nat. 1990, 135, 1–13. [Google Scholar] [CrossRef]
- Herrero-Pérezrul, M.D.; Reyes Bonilla, H.; García-Domínguez, F.; Cintra-Buenrostro, C.E. Reproduction and growth of Isostichopus fuscus (Echinodermata: Holothuroidea) in the southern Gulf of California, Mexico. Mar. Biol. 1999, 135, 521–532. [Google Scholar] [CrossRef]
- Brodie, J.; Fabricius, K.; De’ath, G.; Okaji, K. Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar. Pollut. Bull. 2005, 51, 266–278. [Google Scholar] [CrossRef]
- Coppari, M.; Zanella, C.; Rossi, S. The importance of coastal gorgonians in the blue carbon budget. Sci. Rep. 2019, 9, 13550. [Google Scholar] [CrossRef] [Green Version]
- Coppari, M.; Ferrier-Pages, C.; Castellano, M.; Massa, F.; Olivari, E.; Bavestrello, G.; Povero, P.; Bo, M. Seasonal variation of the stable C and N isotopic composition of the mesophotic black coral Antipathella subpinnata (Ellis & Solander, 1786). Estuar. Coast. Shelf Sci. 2020, 233, 106520. [Google Scholar] [CrossRef]
- Enrichetti, F.; Baldrighi, E.; Bavestrello, G.; Betti, F.; Canese, S.; Costa, A.; del Pasqua, M.; Giangrande, A.; Langeneck, J.; Misic, C.; et al. Ecological role and phylogenetic position of a new habitat-forming species (Canalipalpata, Sabellidae) from the Mediterranean mesophotic soft bottoms. Estuar. Coast. Shelf Sci. 2021, 265, 107737. [Google Scholar] [CrossRef]
- Marty, J.C.; Chiavérini, J. Hydrological changes in the Ligurian Sea (NW Mediterranean, DYFAMED site) during 1995–2007 and biogeochemical consequences. Biogeosciences 2010, 7, 2117–2128. [Google Scholar] [CrossRef] [Green Version]
- Bavestrello, G.; Cattaneo-Vietti, R.; Cerrano, C.; Danovaro, R.; Fabiano, M. Annual sedimentation rates and role of the resuspension processes along a vertical cliff (Ligurian Sea, Italy). J. Coast Res. 1995, 11, 690–696. Available online: https://www.jstor.org/stable/4298372 (accessed on 1 July 2022).
- Bavestrello, G.; Cattaneo-Vietti, R.; Danovaro, R.; Fabiano, M. Detritus rolling down a vertical cliff of the Ligurian Sea (Italy): The ecological role in hard bottom communities. Mar. Ecol. 1991, 12, 281–292. [Google Scholar] [CrossRef]
- Corradi, N.; Fanucci, F.; Firpo, M.; Piccazzo, M.; Traverso, M. L’Olocene della Piattaforma Continentale Ligure da Portofino alla Spezia; Istituto Idrografico della Marina, Università degli Studi di Genova: Genova, Italy, 1980. [Google Scholar]
- Cerrano, C.; Danovaro, R.; Gambi, C.; Pusceddu, A.; Riva, A.; Schiaparelli, S. Gold coral (Savalia savaglia) and gorgonian forests enhance benthic biodiversity and ecosystem functioning in the mesophotic zone. Biodivers. Conserv. 2010, 19, 153–167. [Google Scholar] [CrossRef] [Green Version]
- Gori, A.; Bavestrello, G.; Grinyó, J.; Dominguez-Carrió, C.; Ambroso, S.; Bo, M. Animal Forests in Deep Coastal Bottoms and Continental Shelf of the Mediterranean Sea. In Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–27. [Google Scholar] [CrossRef]
- Hendler, G. Echinodermata: Ophiuroidea. In Reproduction of Marine Invertebrates, Vol. VI, Echinoderms and Lophophorates; Giese, A., Pearse, J., Pearse, V.B., Eds.; Boxwood Press: Pacific Grove, CA, USA, 1991; pp. 355–511. [Google Scholar]

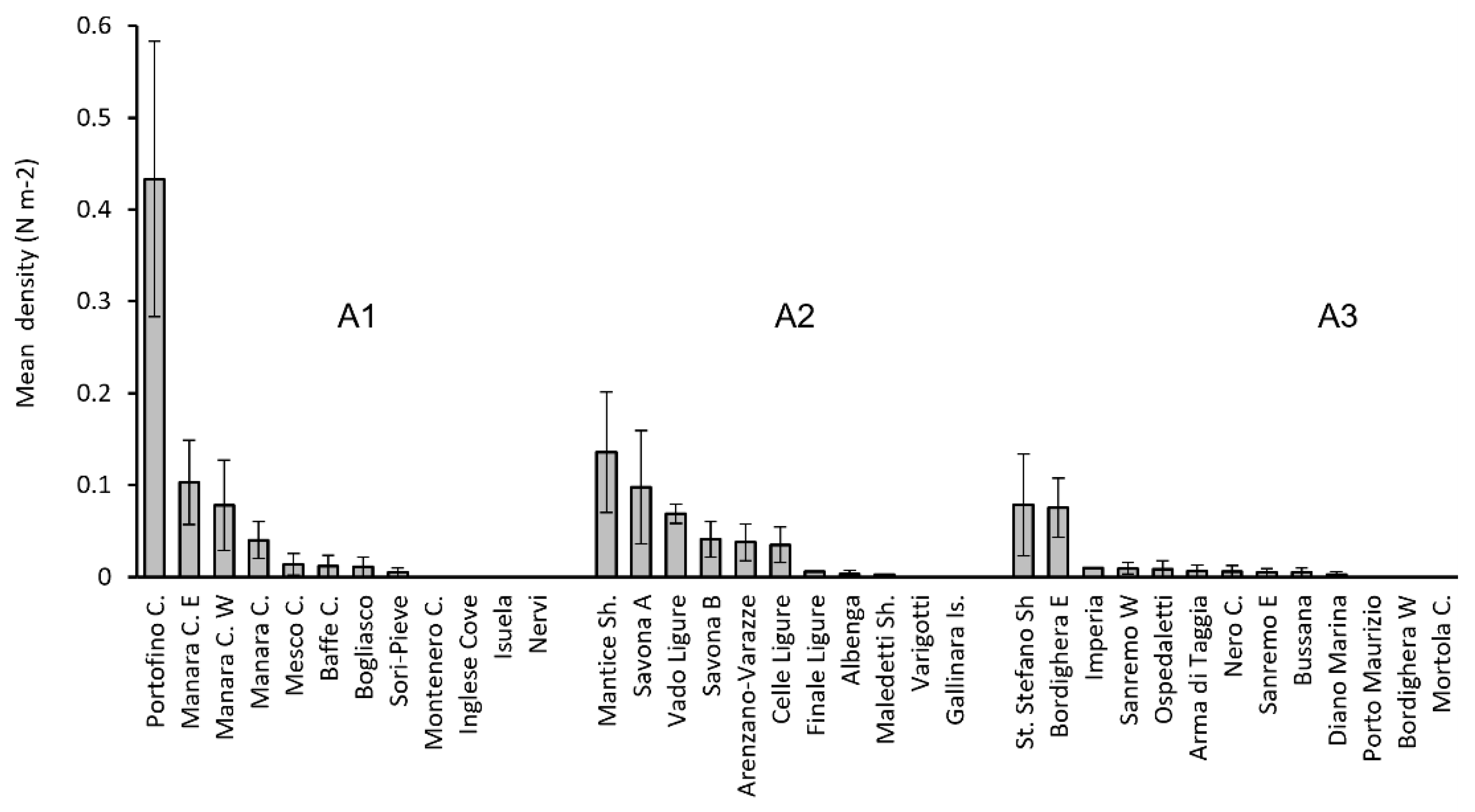





| Site | Date | Transect (N) | Lat. (N) | Long. (E) | Depth (m) | Hard Bottom in the Transect (%) | Specimens (N) | Specimens Mean Density (n m−2) ± SE | |
|---|---|---|---|---|---|---|---|---|---|
| Macro-area A1 | Montenero Cape | Aug. 2016 | 1 | 44.09043 | 9.73979 | 24–30 | 65.2 | 0 | 0 |
| Nov. 2020 | 1 | 24–30 | 63.2 | 0 | 0 | ||||
| Baffe Cape | Aug. 2015 | 3 | 44.23246 | 9.44129 | 30–55 | 42 | 0 | 0 | |
| Mesco Cape | Aug. 2016 | 2 | 44.13108 | 9.63407 | 41–56 | 57.3 ± 8.9 | 0 | 0 | |
| Sept. 2016 | 1 | 20–42 | 68.2 | 0 | 0 | ||||
| Nov. 2020 | 3 | 20–56 | 74.5 ± 25.5 | 6 | 0.03 ± 0.02 | ||||
| Manara Cape Est | Aug. 2015 | 2 | 44.24516 | 9.40409 | 53–69 | 54.3 ± 10.4 | 2 | 0.03 ± 0.01 | |
| Manara Cape | Aug. 2016 | 2 | 44.24234 | 9.40175 | 56–67 | 25 ± 8.4 | 2 | 0.04 ± 0.03 | |
| Manara Cape West | Aug. 2015 | 4 | 44.243216 | 9.40207 | 55–68 | 33.3 ± 2.5 | 2 | 0.03 ± 0.01 | |
| Jul. 2020 | 1 | 53–70 | 26 | 8 | 0.31 | ||||
| Oct. 2020 | 2 | 55–65 | 59 ± 2.8 | 13 | 0.06 ± 0.02 | ||||
| Portofino Cape | Jun. 2012 | 2 | 44.2923 | 9.22313 | 60–71 | 73 ± 19.8 | 38 | 0.31 ± 0.17 | |
| Aug. 2016 | 3 | 55–82 | 74.4 ± 15.4 | 64 | 0.28 ± 0.14 | ||||
| Jul. 2020 | 2 | 55–75 | 77 ± 23 | 101 | 0.79 ± 0.45 | ||||
| Inglesi Cove | Sept. 2016 | 1 | 44.30762 | 9.18748 | 29–45 | 65 | 0 | 0 | |
| Jul. 2020 | 1 | 29–45 | 73 | 0 | 0 | ||||
| Isuela Shoal | Sept. 2016 | 3 | 44.33713 | 9.14897 | 20–57 | 85.4 ± 6.7 | 0 | 0 | |
| Jun. 2020 | 2 | 20–57 | 83.5 ± 8.3 | 0 | 0 | ||||
| Sori-Pieve | Aug. 2015 | 3 | 44.36088 | 9.08745 | 32–54 | 97.5 ± 16.4 | 2 | 0.01 ± 0.01 | |
| Jun. 2020 | 1 | 44–54 | 100 | 0 | 0 | ||||
| Bogliasco | Aug. 2015 | 3 | 44.35950 | 9.06424 | 49–56 | 23.5 ± 3.2 | 0 | 0 | |
| Jun. 2020 | 1 | 51–56 | 38.4 | 1 | 0.04 | ||||
| Nervi | Aug. 2015 | 3 | 44.36460 | 9.03220 | 29–56 | 68.4 ± 15.8 | 0 | 0 | |
| Sites, 12 | Transects, 47 | Specimens, 239 | |||||||
| Macro-area A2 | Celle Ligure | Sept. 2016 | 3 | 44.33141 | 8.55200 | 36–52 | 50.3 | 5 | 0.04 ± 0.03 |
| Dec. 2020 | 3 | 36–52 | 42.2 ± 5.3 | 4 | 0.03 ± 0.03 | ||||
| Arenzano-Varazze | Aug. 2015 | 3 | 44.38512 | 8.69960 | 35–64 | 18.1 ± 9.7 | 0 | 0 | |
| Jul. 2019 | 3 | 35–64 | 15.1 ± 4.3 | 5 | 0.11 ± 0.11 | ||||
| Dec. 2021 | 3 | 39–64 | 22.6 ± 9.3 | 1 | 0.01 ± 0.01 | ||||
| Savona B | Sept. 2016 | 3 | 44.27878 | 8.52335 | 52–92 | 73.3 ± 6.6 | 7 | 0.03 ± 0.01 | |
| Dec. 2020 | 3 | 52–92 | 75.5 ± 3.5 | 12 | 0.05 ± 0.03 | ||||
| Savona A | Sept. 2016 | 3 | 44.28739 | 8.50042 | 42–55 | 41.6 ± 8.3 | 5 | 0.04 ± 0.01 | |
| Dec. 2020 | 3 | 42–55 | 42.6 ± 2.2 | 19 | 0.16 ± 0.1 | ||||
| Vado Ligure | Aug. 2015 | 3 | 44.26030 | 8.46638 | 43–70 | 66.6 ± 11.4 | 2 | 0.01 ± 0.01 | |
| Jul. 2019 | 3 | 43–70 | 52.4 ± 7.6 | 11 | 0.07 ± 0.07 | ||||
| Febr. 2022 | 3 | 43–70 | 48.2 ± 19.5 | 12 | 0.13 ± 0.07 | ||||
| Mantice Shoal | Jun. 2012 | 2 | 44.27057 | 8.52256 | 79–86 | 100 | 8 | 0.04 ± 0.01 | |
| Aug. 2015 | 3 | 76–94 | 66.6 ± 11.7 | 3 | 0.02 ± 0.01 | ||||
| Jul. 2019 | 3 | 76–94 | 56.3 ± 6.9 | 30 | 0.19 ± 0.11 | ||||
| Febr. 2022 | 3 | 76–94 | 85.7 ± 9.4 | 70 | 0.27 ± 0.15 | ||||
| Maledetti Shoal | Aug. 2015 | 3 | 44.22381 | 8.43657 | 52–123 | 43.3 ± 8.4 | 1 | 0.01 ± 0.01 | |
| Jul. 2019 | 9 | 52–106 | 55.8 ± 13.6 | 0 | 0 | ||||
| Jun. 2021 | 1 | 82–23 | 65.6 | 0 | 0 | ||||
| Febr. 2022 | 9 | 52–123 | 46.7 ± 16.8 | 3 | 0.01 ± 0.01 | ||||
| Finale Ligure | Apr. 2018 | 4 | 44.15817 | 8.36462 | 75–103 | 85.3 ± 13.4 | 0 | 0 | |
| Jun. 2021 | 1 | 75–89 | 79.6 ± 9.8 | 0 | 0 | ||||
| Apr. 2021 | 1 | 75–84 | 83.4 ± 6.7 | 3 | 0.04 | ||||
| Varigotti | Apr. 2018 | 2 | 44.18263 | 8.42620 | 84–108 | 81.2 ± 8.9 | 0 | 0 | |
| Apr. 2021 | 1 | 84–94 | 73 | 0 | 0 | ||||
| Noli Cape | Apr. 2018 | 2 | 44.19635 | 8.42927 | 50–69 | 36.8 ± 16.9 | 0 | 0 | |
| Apr. 2021 | 2 | 50–80 | 23 ± 8.4 | 1 | 0.02 ± 0.02 | ||||
| Gallinara Island | Febr. 2018 | 2 | 44.01300 | 8.23373 | 85–89 | 68.5 ± 13.8 | 0 | 0 | |
| Apr. 2018 | 1 | 32–45 | 73.8 | 0 | 0 | ||||
| Apr. 2021 | 2 | 85–89 | 75.4 ± 9.1 | 0 | 0 | ||||
| Albenga | Febr. 2018 | 3 | 44.02396 | 8.24063 | 58–69 | 62 ± 5.2 | 0 | 0 | |
| Apr. 2021 | 2 | 52–62 | 73.4 ± 8.2 | 1 | 0.01 ± 0.01 | ||||
| Sites, 12 | Transects, 92 | Specimens, 203 | |||||||
| Macro-area A3 | Diano Marina | Aug. 2015 | 3 | 43.88217 | 8.08675 | 47–54 | 98.3 ± 5.8 | 2 | 0.02 ± 0.003 |
| Aug. 2019 | 2 | 47–54 | 95.5 ± 8 | 0 | 0 | ||||
| Febr. 2022 | 2 | 47–54 | 93.7 ± 11.1 | 0 | 0 | ||||
| Ospedaletti | Jun. 2018 | 3 | 43.77670 | 7.72368 | 51–59 | 38.5 ± 7.8 | 0 | 0 | |
| Apr. 2021 | 3 | 51–59 | 33.6 ± 11.2 | 2 | 0.02 ± 0.02 | ||||
| Sanremo Est | Jun. 2018 | 3 | 43.79290 | 7.79271 | 47–78 | 100 | 2 | 0.01 ± 0.01 | |
| Apr. 2021 | 3 | 47–78 | 100 | 0 | 0 | ||||
| Capo Nero | Jun. 2018 | 3 | 43.77934 | 7.75821 | 45–54 | 56.4 ± 12.1 | 0 | 0 | |
| Apr. 2021 | 2 | 45–54 | 65 ± 7.3 | 0 | 0 | ||||
| Sanremo West | Jun. 2018 | 3 | 43.76695 | 7.77113 | 41–66 | 50.3. 8.1 | 1 | 0.01 ± 0.01 | |
| Apr. 2021 | 3 | 41–66 | 85.7 ± 13.4 | 3 | 0.01 ± 0.01 | ||||
| Bussana | Jun. 2018 | 3 | 43.80298 | 7.81507 | 45–59 | 33.7 ± 12.5 | 1 | 0.01 ± 0.01 | |
| Apr. 2021 | 3 | 45–59 | 48.6 ± 9.4 | 0 | 0 | ||||
| Arma di Taggia | Jun. 2018 | 3 | 43.80384 | 7.84114 | 46–59 | 45 ± 6.7 | 1 | 0.01 ± 0.01 | |
| Porto Maurizio | Aug. 2015 | 3 | 43.84858 | 8.01065 | 34–57 | 65.2 ± 6.8 | 0 | 0 | |
| Aug. 2019 | 1 | 34–39 | 68.7 | 0 | 0 | ||||
| Febr. 2022 | 1 | 34–39 | 71 | 0 | 0 | ||||
| Imperia | Jun. 2012 | 1 | 43.9301 | 8.2302 | 101 | 78.3 | 1 | 0.01 | |
| St. Stefano Shoal | Aug. 2015 | 3 | 43.79490 | 7.91910 | 49–92 | 75.6 ± 13.6 | 4 | 0.01 ± 0.01 | |
| Aug. 2019 | 2 | 49–92 | 68.4 ± 9.7 | 10 | 0.08 ± 0.08 | ||||
| Febr. 2022 | 2 | 49–92 | 66.5 ± 8 | 24 | 0.18 ± 0.18 | ||||
| Bordighera Est | Sept. 2016 | 3 | 43.76990 | 7.67639 | 44–71 | 71.3 ± 12.5 | 5 | 0.03 ± 0.02 | |
| Aug. 2019 | 3 | 44–71 | 54.2 ± 7.4 | 10 | 0.06 ± 0.02 | ||||
| Oct. 2021 | 3 | 44–71 | 68.5 ± 12 | 27 | 0.14 ± 0.09 | ||||
| Bordighera West | Sept. 2016 | 3 | 43.77011 | 7.67047 | 40–97 | 69.5 ± 4.3 | 0 | 0 | |
| Aug. 2019 | 1 | 40–67 | 81.6 ± 13.9 | 0 | 0 | ||||
| Oct. 2021 | 1 | 40–67 | 78.3 ± 12.5 | 0 | 0 | ||||
| Capo Mortola | Sept. 2016 | 3 | 43.76952 | 7.56353 | 22–42 | 84.5 ± 6.4 | 0 | 0 | |
| Apr. 2021 | 2 | 22–39 | 78.5 ± 5.3 | 0 | 0 | ||||
| Sites, 13 | Transects, 71 | Specimens, 93 | |||||||
| df | SS | MS | Pseudo-F | P (perm) | |
|---|---|---|---|---|---|
| Presence/Absence | 1 | 39,233 | 39,233 | 22.584 | 0.0001 |
| Macro-Area | 2 | 1962.3 | 981.14 | 0.56478 | 0.7617 |
| Presence/Absence x Macro-area | 2 | 1546.9 | 773.43 | 0.44522 | 0.87 |
| Res | 207 | 3.596 × 105 | 1737.2 | ||
| Total | 212 | 4.0788 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canessa, M.; Betti, F.; Bo, M.; Enrichetti, F.; Toma, M.; Bavestrello, G. Possible Population Growth of Astrospartus mediterraneus (Risso, 1826) (Ophiuroidea, Gorgonocephalidae) in the Mediterranean Sea. Diversity 2023, 15, 122. https://doi.org/10.3390/d15010122
Canessa M, Betti F, Bo M, Enrichetti F, Toma M, Bavestrello G. Possible Population Growth of Astrospartus mediterraneus (Risso, 1826) (Ophiuroidea, Gorgonocephalidae) in the Mediterranean Sea. Diversity. 2023; 15(1):122. https://doi.org/10.3390/d15010122
Chicago/Turabian StyleCanessa, Martina, Federico Betti, Marzia Bo, Francesco Enrichetti, Margherita Toma, and Giorgio Bavestrello. 2023. "Possible Population Growth of Astrospartus mediterraneus (Risso, 1826) (Ophiuroidea, Gorgonocephalidae) in the Mediterranean Sea" Diversity 15, no. 1: 122. https://doi.org/10.3390/d15010122







