Diet of Bottlenose Dolphin, Tursiops truncatus (Montagu, 1821), in the Northwestern Mediterranean Sea
Abstract
1. Introduction
2. Materials and Methods
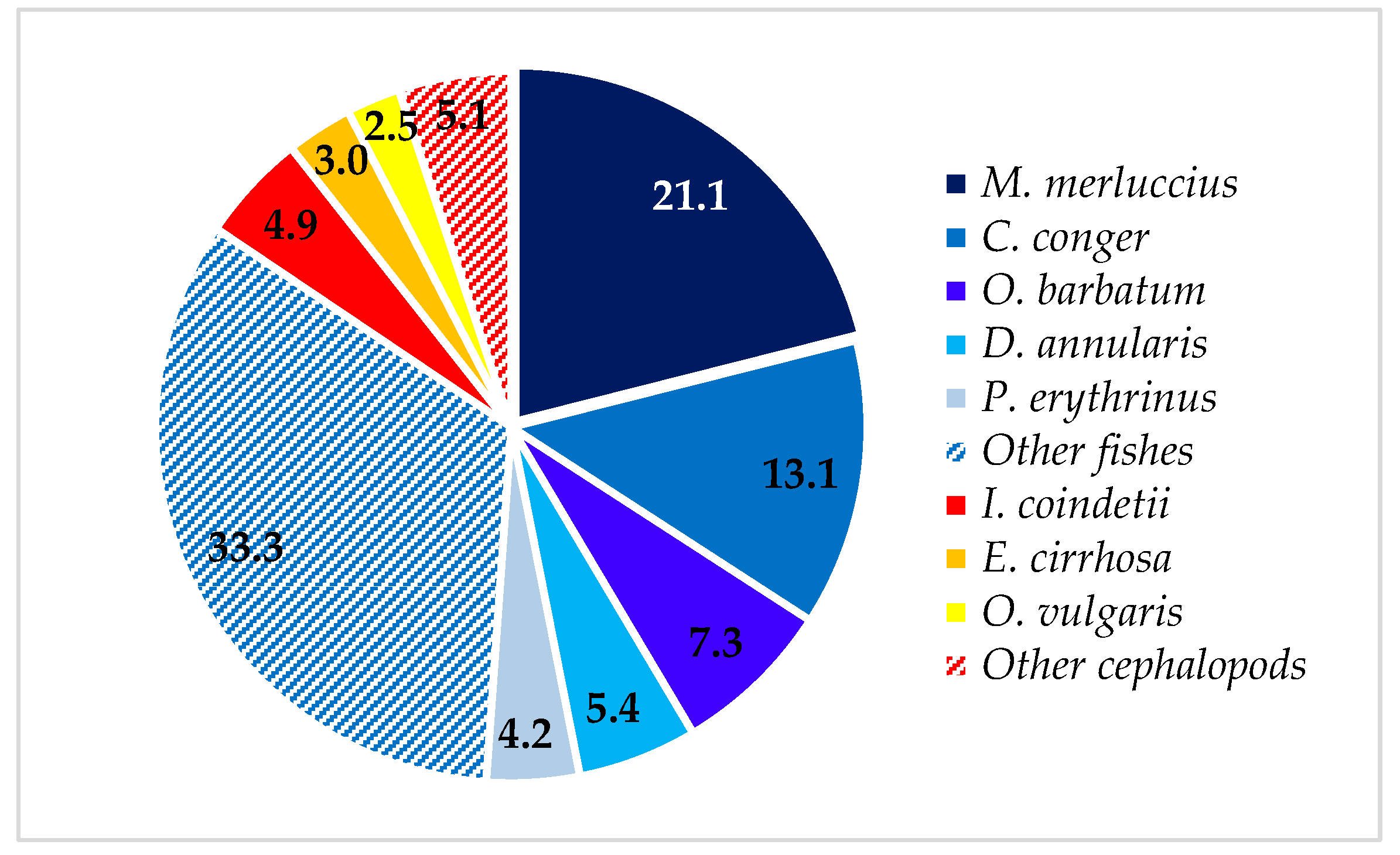
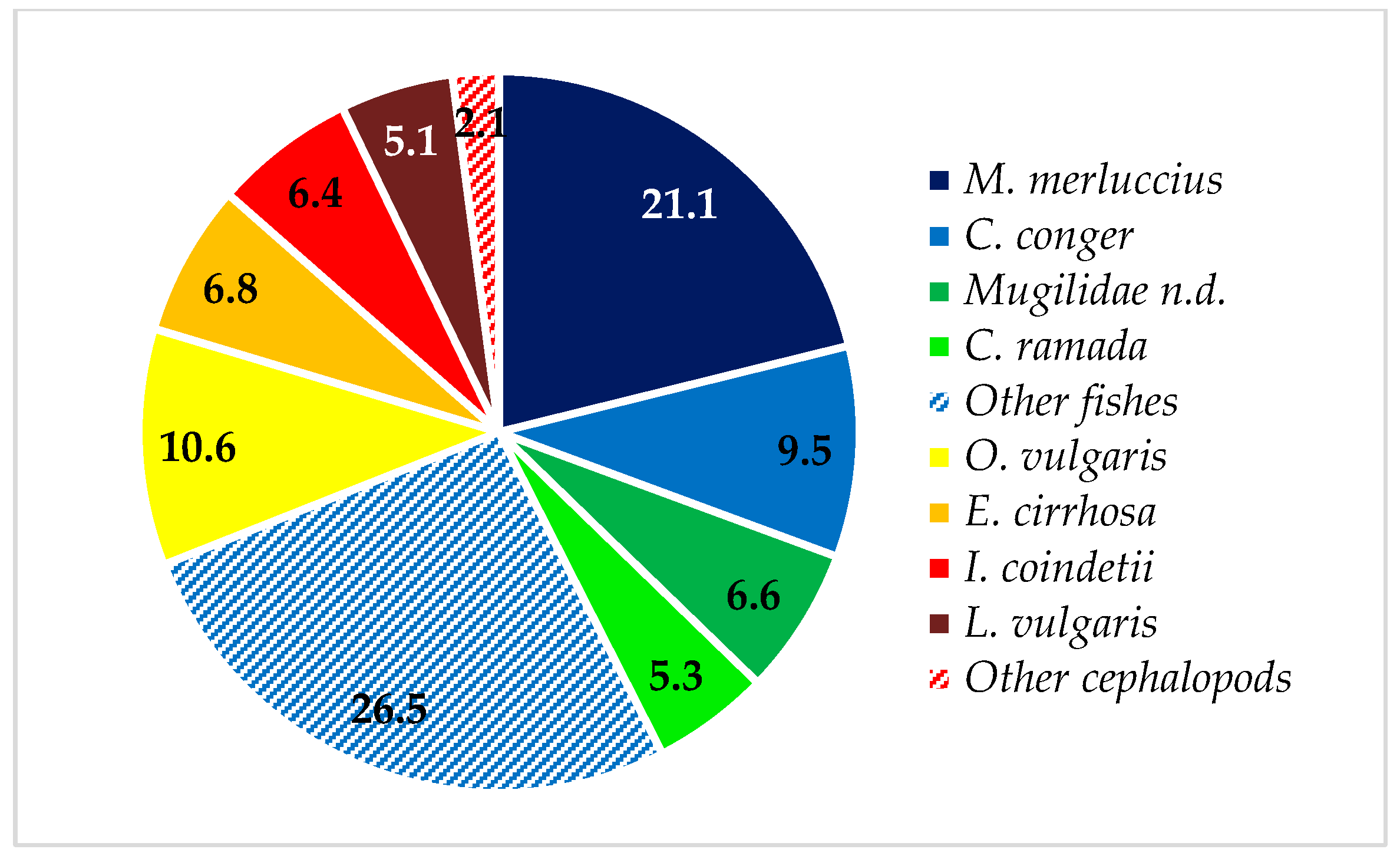
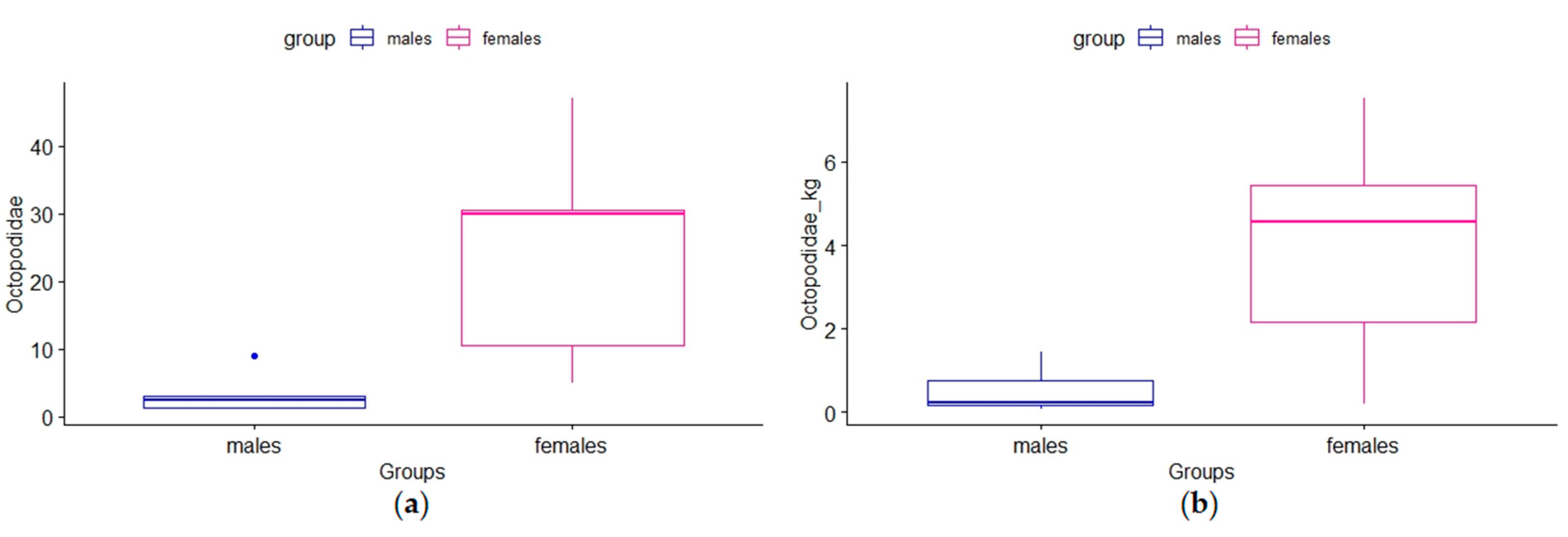
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jefferson, T.A.; Leatherwood, S.; Webber, M.A. FAO Species Identification Guide. Marine Mammals of the World; FAO: Rome, Italy, 1993; pp. 154–155. [Google Scholar]
- Wells, R.S.; Scott, M.D. Common Bottlenose Dolphin: Tursiops truncatus. In Encyclopedia of Marine Mammals, 2nd ed.; Perrin, W.F., Wursig, B., Thewissen, J.G.M., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 249–255. [Google Scholar]
- Natoli, A.; Genov, T.; Kerem, D.; Gonzalvo, J.; Lauriano, G.; Holcer, D.; Labach, H.; Marsili, L.; Mazzariol, S.; Moura, A.E.; et al. Tursiops truncatus (Mediterranean Subpopulation). The IUCN Red List of Threatened Species in 2021. Available online: https://www.iucnredlist.org/species/16369383/215248781 (accessed on 6 October 2022).
- Bearzi, G.; Notarbartolo Di Sciara, G.; Politi, E. Social ecology of bottlenose dolphins in the Kvarneric (northern Adriatic Sea). Mar. Mammal Sci. 1997, 13, 650–668. [Google Scholar] [CrossRef]
- Genov, T.; Kotnjek, P.; Lesjak, J.; Hace, A.; Fortuna, C.M. Bottlenose dolphins (Tursiops truncatus) in Slovenian and adjacent waters (northern Adriatic Sea). Ann. Ser. Hist. Nat. 2008, 18, 227–244. [Google Scholar]
- Marini, C.; Fossa, F.; Paoli, C.; Bellingeri, M.; Gnone, G.; Vassallo, P. Predicting bottlenose dolphin distribution along Liguria coast (northwestern Mediterranean Sea) through different modeling techniques and indirect predictors. J. Environ. Manage. 2015, 150, 9–20. [Google Scholar] [CrossRef]
- ACCOBAMS. Conserving Whales, Dolphins and Porpoises in the Mediterranean Sea, Black Sea and Adjacent Areas: An ACCOBAMS Status Report; Notarbartolo di Sciara, G., Tonay, A.M., Eds.; ACCOBAMS: Monaco, 2021. [Google Scholar]
- Gnone, G.; Bellingeri, M.; Dhermain, F.; Dupraz, F.; Nuti, S.; Bedocchi, D.; Moulins, A.; Rosso, M.; Alessi, J.; Mccrea, R.S.; et al. Distribution, abundance, and movements of the bottlenose dolphin (Tursiops truncatus) in the Pelagos Sanctuary MPA (north-west Mediterranean Sea). Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 372–388. [Google Scholar] [CrossRef]
- Mead, J.G.; Potter, C.W. Natural History of Bottlenose Dolphins Along the Central Atlantic Coast of the United States. In The Bottlenose Dolphin; Leatherwood, S., Reeves, R.R., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 165–195. [Google Scholar] [CrossRef]
- Barros, N.B.; Wells, R.S. Prey and feeding patterns of resident bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. J. Mammal 1998, 79, 1045–1059. [Google Scholar] [CrossRef]
- McCabe, E.J.B.; Gannon, D.P.; Barros, N.B.; Wells, R.S. Prey selection by resident common bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Mar. Biol. 2010, 157, 931–942. [Google Scholar] [CrossRef]
- Bowen, S.R. Diet of Bottlenose Dolphins Tursiops truncatus in the Northwest Florida Panhandle and Foraging. Master’s Thesis, Savannah State University, Savannah, GA, USA, May 2011. [Google Scholar]
- Van Waerebeek, K.; Reyes, J.C.; Read, A.J.; McKinnon, J.S. Preliminary Observations of Bottlenose Dolphins from the Pacific Coast of South America. In The Bottlenose Dolphin; Leatherwood, S., Reeves, R.R., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 143–154. [Google Scholar] [CrossRef]
- García-Godos, I.; Van Waerebeek, K.; Reyes, J.C.; Alfaro-Shigueto, J.; Arias-Schreiber, M. Prey occurrence in the stomach contents of four small cetacean species in Peru. LAJAM 2007, 6, 171–183. [Google Scholar] [CrossRef]
- Milmann, L.; Danilewicz, D.; Machado, R.; Santos, R.A.D.; Ott, P.H. Feeding ecology of the common bottlenose dolphin, Tursiops truncatus, in southern Brazil: Analyzing its prey and the potential overlap with fisheries. Braz. J. Oceanogr. 2016, 64, 415–422. [Google Scholar] [CrossRef]
- Corkeron, P.J.; Bryden, M.M.; Hedstrom, K.E. Feeding by Bottlenose Dolphins in Association with Trawling Operations in Moreton Bay, Australia. In The Bottlenose Dolphin; Leatherwood, S., Reeves, R.R., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 329–336. [Google Scholar] [CrossRef]
- Gibbs, S.E.; Harcourt, R.G.; Kemper, C.M. Niche differentiation of bottlenose dolphin species in South Australia revealed by stable isotopes and stomach contents. Wildl. Res. 2011, 38, 261–270. [Google Scholar] [CrossRef]
- Cockcroft, V.G.; Ross, G.J.B. Food and Feeding of the Indian Ocean Bottlenose Dolphin off Southern Natal, South Africa. In The Bottlenose Dolphin; Leatherwood, S., Reeves, R.R., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 295–308. [Google Scholar] [CrossRef]
- Santos, M.B.; Pierce, G.J.; Reid, R.J.; Patterson, I.A.P.; Ross, H.M.; Mente, E. Stomach contents of bottlenose dolphins (Tursiops truncatus) in Scottish waters. J. Mar. Biol. Assoc. UK 2001, 81, 873–878. [Google Scholar] [CrossRef]
- De Pierrepont, J.F.; Dubois, B.; Desormonts, S.; Santos, M.B.; Robin, J.P. Stomach contents of English Channel cetaceans stranded on the coast of Normandy. J. Mar. Biol. Assoc. UK 2005, 85, 1539–1546. [Google Scholar] [CrossRef]
- Spitz, J.; Rousseau, Y.; Ridoux, V. Diet overlap between harbour porpoise and bottlenose dolphin: An argument in favour of interference competition for food? Estuar. Coast. Shelf Sci. 2006, 70, 259–270. [Google Scholar] [CrossRef]
- Santos, M.B.; Fernández, R.; López, A.; Martínez, J.A.; Pierce, G.J. Variability in the diet of bottlenose dolphin, Tursiops truncatus, in Galician waters, north-western Spain, 1990–2005. J. Mar. Biol. Assoc. UK 2007, 87, 231–241. [Google Scholar] [CrossRef]
- Arronte, J.C.; Valdés, P.; Pérez, C. Diet of the Bottlenose dolphin (Tursiops truncatus) in the central Cantabrian Sea. In Proceedings of the 23rd Annual Conference of the European Cetacean Society, Istanbul, Turkey, 2–4 March 2009. [Google Scholar]
- Hernandez-Milian, G.; Berrow, S.; Santos, M.B.; Reid, D.; Rogan, E. Insights into the trophic ecology of bottlenose dolphins (Tursiops truncatus) in Irish waters. Aquat. Mammal 2015, 41, 226–239. [Google Scholar] [CrossRef]
- Giménez, J.; Marçalo, A.; Ramírez, F.; Verborgh, P.; Gauffier, P.; Esteban, R.; Nicolau, L.; González-Ortegón, E.; Baldó, F.; Vilas, C.; et al. Diet of bottlenose dolphins (Tursiops truncatus) from the Gulf of Cadiz: Insights from stomach content and stable isotope analyses. PLoS ONE 2017, 12, e0184673. [Google Scholar] [CrossRef]
- Fernández, R.; García-Tiscar, S.; Santos, M.B.; López, A.; Martínez-Cedeira, J.A.; Newton, J.; Pierce, G.J. Stable isotope analysis in two sympatric populations of bottlenose dolphins Tursiops truncatus: Evidence of resource partitioning? Mar. Biol. 2011, 158, 1043–1055. [Google Scholar] [CrossRef]
- Gladilina, E.V.; Gol’din, P.E. New prey fishes in diet of black sea bottlenose dolphins, Tursiops truncatus (mammalia, cetacea). Vestn. Zool. 2014, 48, 83–92. [Google Scholar] [CrossRef]
- Voliani, A.; Volpi, C. Stomach content analysis of a stranded individual of Tursiops truncatus. Rapp. Comm. Int. Mer Médit. 1990, 32, 238. [Google Scholar]
- Orsi Relini, L.; Capello, M.; Poggi, R. The stomach content of some bottlenose dolphins (Tursiops truncatus) from the Ligurian Sea. In Proceedings of the 8th Annual Conference of the European Cetacean Society, Montpellier, France, 2–5 March 1994. [Google Scholar]
- Salomon, O.; Blanco, C.; Raga, J.A. Diet of the bottlenose dolphin (Tursiops truncatus) in the Gulf of Valencia (Western Mediterranean). In Proceedings of the Eleventh Annual Conference of the European Cetacean Society, Stralsund, Germany, 10–12 March 1997. [Google Scholar]
- Miokovic, D.; Kovacic, D.; Pribanic, S. Stomach content analysis of one bottlenose dolphin (Tursiops truncatus, Montague 1821) from the Adriatic Sea. Nat. Croat. 1999, 8, 61–65. [Google Scholar]
- Blanco, C.; Salomón, O.; Raga, J.A. Diet of the bottlenose dolphin (Tursiops truncatus) in the western Mediterranean Sea. J. Mar. Biol. Assoc. UK 2001, 81, 1053–1058. [Google Scholar] [CrossRef]
- Scuderi, A.; Voliani, A.; Mancusi, C.; Pedà, C.; Romeo, T. Stomach contents of bottlenose dolphins stranded along the coasts of Tuscany (North Western Mediterranean Sea). In Proceedings of the 25th Annual Conference of the European Cetacean Society, Cádiz, Spain, 21–23 March 2011. [Google Scholar] [CrossRef]
- Scheinin, A.P.; Kerem, D.; Lojen, S.; Liberzon, J.; Spanier, E. Resource partitioning between common bottlenose dolphin (Tursiops truncatus) and the Israeli bottom trawl fishery? Assessment by stomach contents and tissue stable isotopes analysis. J. Mar. Biol. Assoc. UK 2014, 94, 1203–1220. [Google Scholar] [CrossRef]
- Milani, C.B.; Vella, A.; Vidoris, P.; Christidis, A.; Koutrakis, E.; Frantzis, A.; Miliou, A.; Kallianiotis, A. Cetacean stranding and diet analyses in the North Aegean Sea (Greece). J. Mar. Biol. Assoc. UK 2018, 98, 1011–1028. [Google Scholar] [CrossRef]
- Borrell, A.; Vighi, M.; Genov, T.; Giovos, I.; Gonzalvo, J. Feeding ecology of the highly threatened common bottlenose dolphin of the Gulf of Ambracia, Greece, through stable isotope analysis. Mar. Mammal Sci. 2021, 37, 98–110. [Google Scholar] [CrossRef]
- Gonzalvo, J.; Valls, M.; Cardona, L.; Aguilar, A. Factors determining the interaction between common bottlenose dolphins and bottom trawlers off the Balearic Archipelago (western Mediterranean Sea). J. Exp. Mar. Biol. Ecol. 2008, 367, 47–52. [Google Scholar] [CrossRef]
- Pennino, M.G.; Rotta, A.; Pierce, G.J.; Bellido, J.M. Interaction between bottlenose dolphin (Tursiops truncatus) and trammel nets in the Archipelago de La Maddalena, Italy. Hydrobiologia 2015, 747, 69–82. [Google Scholar] [CrossRef]
- Revuelta, O.; Domènech, F.; Fraija-Fernández, N.; Gozalbes, P.; Novillo, O.; Penadés-Suay, J.; Tomás, J. Interaction between bottlenose dolphins (Tursiops truncatus) and artisanal fisheries in the Valencia region (Spanish Mediterranean Sea). Ocean Coast. Manag. 2018, 165, 117–125. [Google Scholar] [CrossRef]
- Díaz López, B. Interactions between Mediterranean bottlenose dolphins (Tursiops truncatus) and gillnets off Sardinia, Italy. ICES J. Mar. Sci. 2006, 63, 944–951. [Google Scholar] [CrossRef]
- Díaz López, B.; Bernal Shirai, J.A. Bottlenose dolphin (Tursiops truncatus) presence and incidental capture in a marine fish farm on the north-eastern coast of Sardinia (Italy). J. Mar. Biol. Assoc. UK 2007, 87, 113–117. [Google Scholar] [CrossRef]
- Neri, A. Confronto della Dieta di Stenella coeruleoalba (Meyen, 1833) e Tursiops truncatus (Montagu, 1821) (Cetartiodactyla, Delphinidae) Negli Esemplari Spiaggiati Lungo le Coste della Toscana. Master’s Thesis, University of Trieste, Trieste, Italy, 26 February 2015. [Google Scholar]
- Pedà, C.; Battaglia, P.; Scuderi, A.; Voliani, A.; Mancusi, C.; Andaloro, F.; Romeo, T. Cephalopod prey in the stomach contents of odontocete cetaceans stranded in the western Mediterranean Sea. Mar. Biol. Res. 2015, 11, 593–602. [Google Scholar] [CrossRef]
- Santuario Pelagos. Available online: https://www.sanctuaire-pelagos.org/it/ (accessed on 10 August 2022).
- Clarke, M.R. A Handbook for the Identification of Cephalopods Beaks; Clarendon Press: Oxford, UK, 1986. [Google Scholar]
- Tuset, V.M.; Lombarte, A.; Assis, C.A. Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Sci. Mar. 2008, 72, 7–198. [Google Scholar] [CrossRef]
- Pinkas, L.; Oliphant, S.; Iverson, I.L.K. Food habits of albacore, bluefin tuna and bonito in California waters. Fish Bull. 1971, 152, 1–105. [Google Scholar]
- Hyslop, E.J. Stomach contents analysis—A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 10 March 2022).
- Viva, C.; Sartor, P.; Bertolini, D.; De Ranieri, S.; Ligas, A. Relationship of otolith length to fish total length in six demersal species from the NW Mediterranean Sea. J. Appl. Ichthyol. 2015, 31, 973–974. [Google Scholar] [CrossRef]
- Giménez, J.; Manjabacas, A.; Tuset, V.M.; Lombarte, A. Relationships between otolith and fish size from Mediterranean and north-eastern Atlantic species to be used in predator-prey studies. J. Fish Biol. 2016, 89, 2195–2202. [Google Scholar] [CrossRef]
- Russo, L. Analisi Comparative delle Caratteristiche Morfologiche degli Otoliti della Specie Pagellus erythrinus nel Mar Ligure e Mar Tirreno Centro-Settentrionale. Master’s Thesis, University of Pisa, Pisa, Italy, 14 December 2021. [Google Scholar]
- Wolff, G.A. Identification and Estimation of Size from the Beaks of 18 Species of Cephalopods from the Pacific Ocean; NOAA Technical Report NMFS 17; U.S. Department of Commerce, NOAA/National Marine Fisheries Service: Washington, DC, USA, 1984; pp. 1–50. [Google Scholar]
- Lu, C.C.; Ickeringill, R. Cephalopod beak identification and biomass estimation techniques: Tools for dietary studies of southern Australian finfishes. Mus. Vic. Sci. Rep. 2002, 6, 1–65. [Google Scholar] [CrossRef]
- De Ranieri, S. Programma Nazionale Italiano per la Raccolta di Dati alieutici 2010. Campionamento Biologico delle Catture; Sezioni C ed E. Rapporto Finale. Final Technical Report. 2011; 261p, unpublished work. (In Italian) [Google Scholar]
- Romeo, T.; Battaglia, P.; Pedà, C.; Perzia, P.; Consoli, P.; Esposito, V.; Andaloro, F. Pelagic cephalopods of the central Mediterranean Sea determined by the analysis of the stomach content of large fish predators. Helgol. Mar. Res. 2012, 66, 295–306. [Google Scholar] [CrossRef]
- Ligas, A.; Mannini, A.; Carpentieri, P.; Mancusi, C.; Sartor, P.; De Ranieri, S. Lenght weigth relationship in demersal species from Ligurian and northern-central Tyrrhenian Sea. Biol. Mar. Mediterr. 2012, 19, 212–213. [Google Scholar]
- De Ranieri, S. Programma Nazionale Italiano per la Raccolta di Dati Alieutici 2012. Campionamento Biologico delle Catture; Sezioni C ed E. Rapporto Finale. Final Technical Report. 2013; 306p, unpublished work. (In Italian) [Google Scholar]
- Maiorano, P.; Sabatella, R.F.; Marzocchi, B.M. (Eds.) Annuario sullo Stato delle Risorse e sulle Strutture Produttive dei Mari Italiani; 2019; 432p, (In Italian). Available online: http://www.nisea.eu/dir/wp-content/uploads/2019/09/Annuario-20142016_2019_08_05 (accessed on 15 June 2021).
- FishBase. World Wide Web Electronic Publication. Available online: www.fishbase.org (accessed on 15 June 2021).
- Ricker, W.E. Linear Regressions in Fishery Research. J. Fish. Res. Board Can. 1973, 30, 409–434. [Google Scholar] [CrossRef]
- Ligas, A. Strengthening Regional Cooperation in the Area of Fisheries Biological Data Collection in the Mediterranean and Black Sea (STREAM); MARE/2016/22 SI2.770115. Final Report. 2019. Available online: https://www.coispa.it/it/progetti/41/stream-strengthening-regional-cooperation-in-the-area-of-fisheries-biological-data-collection-in-the-mediterranean-and-black-sea.html (accessed on 28 August 2021).
- Pierce, G.J.; Santos, M.B.; Learmonth, J.; Mente, E.; Stowasser, G. Methods for dietary studies on marine mammals. In Proceedings of the Investigating the roles of cetaceans in marine ecosystems. In Proceedings of the CIESM Workshop Monographs n°25, Venice, Italy, 28–31 January 2004. [Google Scholar]
- Pierce, G.J.; Boyle, P.R. A review of methods for diet analysis in piscivorous marine mammals. Oceanogr. Mar. Biol. Annu. Rev. 1991, 29, 409–486. [Google Scholar]
- Cresson, P.; Ruitton, S.; Ourgaud, M.; Harmelin-Vivien, M. Contrasting perception of fish trophic level from stomach content and stable isotope analyses: A Mediterranean artificial reef experience. J. Exp. Mar. Biol. Ecol. 2014, 452, 54–62. [Google Scholar] [CrossRef]
- Wijnsma, G.; Pierce, G.J.; Santos, M.B. Assessment of errors in cetacean diet analysis: In vitro digestion of otoliths. J. Mar. Biol. Assoc. UK 1999, 79, 573–575. [Google Scholar] [CrossRef]
- Clarke, M.R. Cephalopods in the diet of odontocetes. In Research on Dolphins; Bryden, M.M., Harrison, R., Eds.; Oxford Science Publications: New York, NY, USA, 1986; pp. 281–321. [Google Scholar]
- Tortonese, E. Osteichthyes (Pesci ossei). Parte II; Calderini: Bologna, Italy, 1975; pp. 402–408. [Google Scholar]
- Matallanas, J.; Riba, G. Aspectos biolo’gicos de Ophidion barbatum Linnaeus, 1758 y O. rochei Muller, 1845 (Pisces, Ophidiidae) de la costa catalana. Investig. Pesquera 1980, 44, 399–406. [Google Scholar]
- Nielsen, J.G. Ophidiidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, C.J., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; Volume 3, pp. 1158–1166. [Google Scholar]
- Casadevall, M.; Matallanas, J.; Carrasson, M.; Muñoz, M. Morphometric, meristic and anatomical differences between Ophidion barbatum L., 1758 and O. rochei Müller, 1845 (Pisces, Ophidiidae). Publ. Espec. Inst. Esp. Oceanogr. 1996, 21, 45–61. [Google Scholar]
- Parmentier, E.; Fontenelle, N.; Fine, M.L.; Vandewalle, P.; Henrist, C. Functional morphology of the sonic apparatus in Ophidion barbatum (Teleostei, Ophidiidae). J. Morphol. 2006, 267, 1461–1468. [Google Scholar] [CrossRef]
- Parmentier, E.; Compère, P.; Casadevall, M.; Fontenelle, N.; Cloots, R.; Henrist, C. The rocker bone: A new kind of mineralised tissue? Cell Tissue Res. 2008, 334, 67–79. [Google Scholar] [CrossRef][Green Version]
- Gannon, D.P.; Barros, N.B.; Nowacek, D.P.; Read, A.J.; Waples, D.M.; Wells, R.S. Prey detection by bottlenose dolphins, Tursiops truncatus: An experimental test of the passive listening hypothesis. Anim. Behav. 2005, 69, 709–720. [Google Scholar] [CrossRef]
- Barros, N.B.; Myrberg, A.A. Prey detection by means of passive listening in bottlenose dolphins (Tursiops truncatus). J. Acoust. Soc. Am. 1987, 82, S65. [Google Scholar] [CrossRef]
- Bartolino, V.; Ottavi, A.; Colloca, F.; Ardizzone, G.D.; Stefánsson, G. Bathymetric preferences of juvenile European hake (Merluccius merluccius). ICES J. Mar. Sci. 2008, 65, 963–969. [Google Scholar] [CrossRef]
- Froese, R.; Garilao, C.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.; Dimarchopoulou, D.; Scarcella, G.; Sampang-Reyes, A. Exploitation and Status of European Stocks. World Wide Web Electronic Publication. Available online: https://europe.oceana.org/reports/exploitation-and-status-european-stocks/ (accessed on 1 September 2022).
- STECF 2019. Stock Assessments: Demersal Stocks in the Western Mediterranean Sea (STECF-19-10); JRC119055; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar] [CrossRef]
- Spedicato, M.T.; Massutí, E.; Mérigot, B.; Tserpes, G.; Jadaud, A.; Relini, G. The MEDITS trawl survey specifications in an ecosystem approach to fishery management. Sci. Mar. 2019, 83, 9–20. [Google Scholar] [CrossRef]
- Levy, A.; Able, K.W.; Grimes, C.B.; Hood, P. Biology of the conger eel Conger oceanicus in the Mid-Atlantic Bight—II. Foods and feeding ecology. Mar. Biol. 1988, 98, 597–600. [Google Scholar] [CrossRef]
- Sartor, P.; (Consorzio per il Centro Interuniversitario di Biologia Marina ed Ecologia Applicata “G. Bacci” (CIBM), Livorno, Italy). Personal communication, 2022.
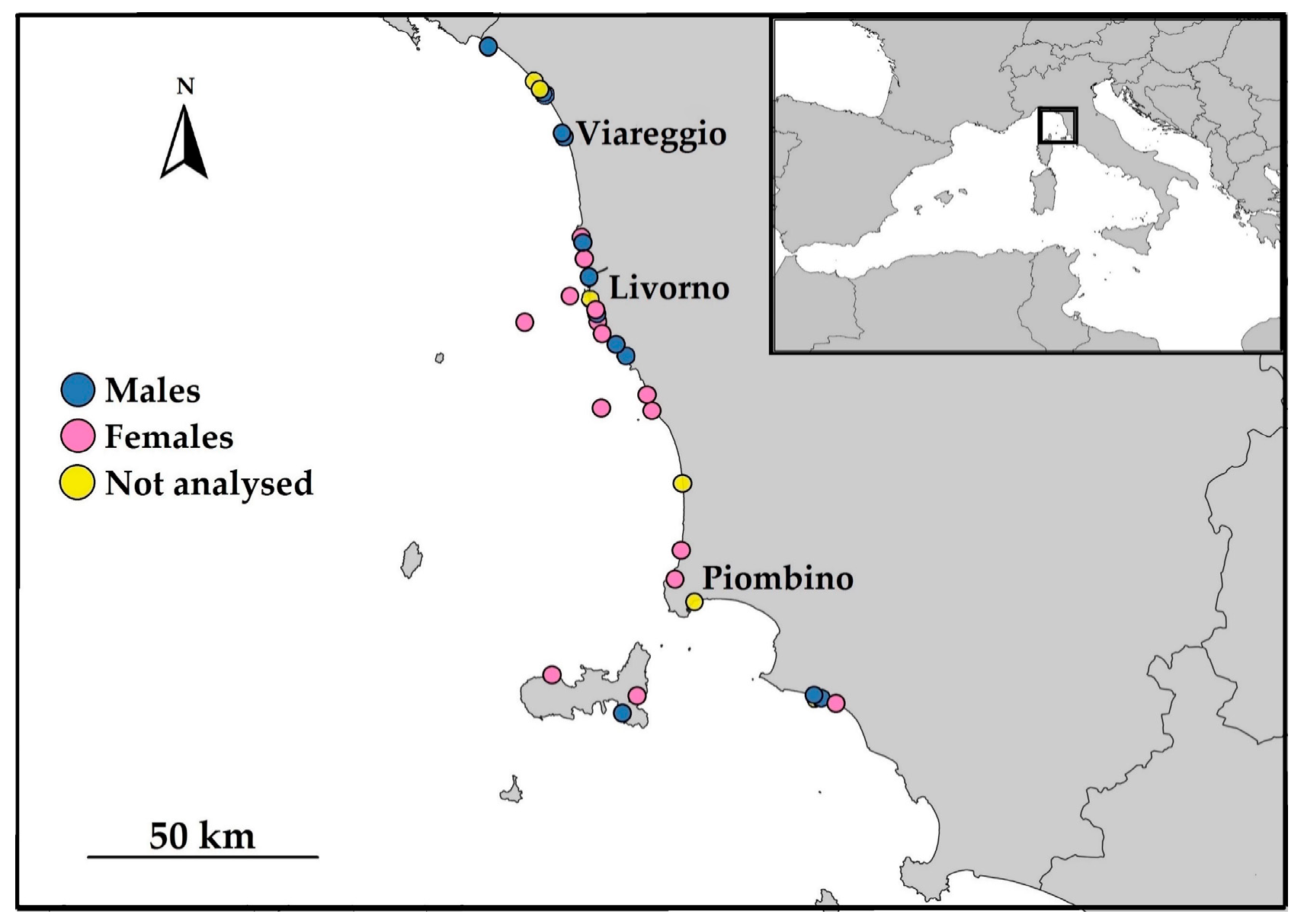
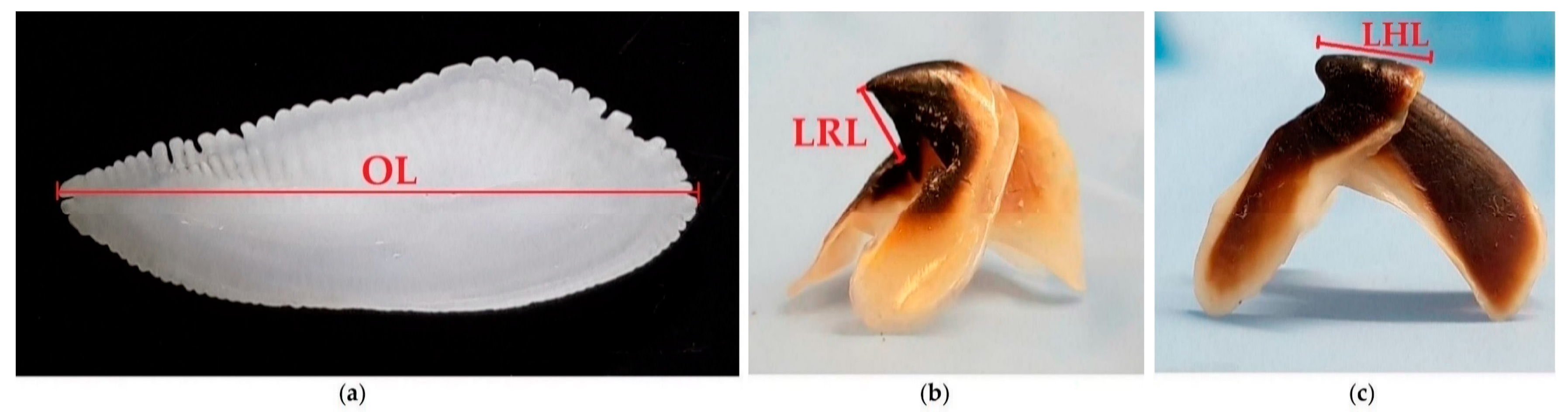


| Code | Date of Collection | TL (cm) | N | Fish Taxa | Cephalopods Taxa |
|---|---|---|---|---|---|
| Males | |||||
| RT46Tt | 5 June 2012 | 315 | 32 | 8 | 5 |
| RT49Tt | 17 September 2012 | 300 | 14 | 2 | 2 |
| RT73Tt | 10 September 2013 | 258 | 246 | 15 | 2 |
| RT83Tt | 21 June 2014 | 294 | 27 | 3 | 1 |
| RT84Tt | 13 August 2014 | 203 | 84 | 12 | 4 |
| RT98Tt | 3 March 2015 | 260 | 76 | 14 | 2 |
| RT99Tt | 3 November 2015 | 250 | 9 | 5 | - |
| RT111Tt | 27 April 2017 | 265 | 57 | 8 | 1 |
| RT112Tt | 28 April 2017 | 230 | 147 | 15 | 2 |
| RT119Tt | 7 March 2018 | 296 | 155 | 17 | 4 |
| RT134Tt | 22 July 2019 | 189 | 11 | 2 | - |
| RT180Tt | 2 May 2021 | 235 | 32 | 6 | 1 |
| RT184Tt | 17 August 2021 | 255 | 135 | 13 | - |
| Females | |||||
| RT5T | 13 March 2008 | 199 | 89 | 13 | 2 |
| RT6T | 14 March 2008 | 175 | 70 | 10 | 1 |
| RT20T | 10 April 2010 | 279 | 222 | 18 | 4 |
| AM9T | 21 May 2010 | 300 | 68 | 12 | 4 |
| AM10T | 7 September 2011 | 280 | 196 | 11 | 4 |
| RT41Tt | 14 March 2012 | 196 | 23 | 9 | 1 |
| RT96Tt | 23 April 2015 | 207 | 102 | 5 | 1 |
| RT100Tt | 18 December 2015 | 250 | 140 | 12 | 6 |
| RT110Tt | 22 April 2017 | 300 | 36 | 6 | 6 |
| RT127Tt | 24 March 2019 | 255 | 299 | 11 | 7 |
| RT135Tt | 23 July 2019 | 250 | 9 | 3 | - |
| RT136Tt | 23 July 2019 | 310 | 3 | 2 | 1 |
| RT138Tt | 26 July 2019 | 270 | 45 | 8 | 1 |
| RT147Tt | 5 June 2020 | 170 | 29 | 2 | 6 |
| RT148Tt | 15 June 2020 | 242 | 64 | 13 | - |
| H | %N | %F | %W | %IRI | TL/DML Range | TW Range | |
|---|---|---|---|---|---|---|---|
| OSTEICHTHYES | |||||||
| Bothidae | 0.19 | 10.71 | 0.04 | 0.02 | |||
| Arnoglossus sp. | B | 0.15 | 7.14 | 0.04 | 0.02 | 7.4–16.1 | 2.98–31.89 |
| Bothus podas (Delaroche, 1809) | B | 0.04 | 3.57 | 0.01 | * | 10.2 | 10.39 |
| Callionymidae | 0.15 | 7.14 | 0.01 | 0.01 | |||
| Callionymus risso Lesueur, 1814 | B | 0.04 | 3.57 | * | * | 5.7 | 1.01 |
| Callionymus sp. Linneaus, 1758 | B | 0.12 | 3.57 | 0.01 | 0.01 | 9.7 | 4.43 |
| Carangidae | 3.76 | 35.71 | 2.65 | 2.22 | |||
| Trachurus mediterraneus (Steindachner, 1868) | D | 2.45 | 28.57 | 2.37 | 1.64 | 8.0–26.2 | 4.04–149.29 |
| Trachurus sp. | D | 1.30 | 10.71 | 0.28 | 0.21 | 7.1–15.7 | 2.84–31.52 |
| Centracanthidae | 1.38 | 32.14 | 0.89 | 0.71 | |||
| Spicara flexuosa Rafinesque, 1810 | D | 0.77 | 25.00 | 0.50 | 0.38 | 12.9–18.1 | 20.99–66.12 |
| Spicara smaris (Linneaus, 1758) | D | 0.61 | 14.29 | 0.39 | 0.17 | 11.3–18.8 | 13.44–57.58 |
| Cepolidae | 0.04 | 3.57 | * | * | |||
| Cepola macrophthalma (Linneaus, 1758) | D | 0.04 | 3.57 | * | * | 15.5 | 2.97 |
| Citharidae | 0.23 | 10.71 | 0.10 | 0.03 | |||
| Citharus linguatula (Linneaus, 1758) | B | 0.23 | 10.71 | 0.10 | 0.04 | 11.4–17.3 | 10.07–36.63 |
| Clupeidae | 3.84 | 35.71 | 0.56 | 0.70 | |||
| Sardina pilchardus (Walbaum, 1792) | P | 3.30 | 10.71 | 0.52 | 0.50 | 10.8 | 9.76 |
| Sardinella aurita Valenciennes, 1847 | P | 0.12 | 7.14 | 0.04 | 0.01 | 10.1–16.4 | 8.27–35.26 |
| Unidentified Clupeidae | P | 0.42 | 17.86 | n.a. | n.a. | n.a. | n.a. |
| Congridae | 13.89 | 96.43 | 9.53 | 20.68 | |||
| Ariosoma balearicum (Delaroche, 1809) | B | 0.42 | 21.43 | n.a. | n.a | n.a. | n.a. |
| Conger conger (Linneaus, 1758) | B | 13.08 | 92.86 | 9.48 | 25.09 | 11.3–66.4 | 1.23–399.40 |
| Gnathophis mystax (Delaroche, 1809) | B | 0.38 | 10.71 | 0.05 | 0.06 | 11.8–23.4 | 1.67–14.42 |
| Engraulidae | 1.65 | 28.57 | 0.23 | 0.53 | |||
| Engraulis encrasicolus (Linneaus, 1758) | P | 1.65 | 28.57 | 0.23 | 0.66 | 8.7–12.8 | 3.86–13.48 |
| Gadidae | 2.19 | 28.57 | 0.80 | 0.84 | |||
| Micromesistius poutassou (Risso, 1827) | D | 0.04 | 3.57 | 0.02 | * | 18.4 | 36.34 |
| Trisopterus capelanus (Lacepède, 1800) | D | 2.15 | 28.57 | 0.78 | 1.01 | 6.3–20.6 | 2.24–95.03 |
| Gobiidae | 6.02 | 64.29 | 0.50 | 3.19 | |||
| Lesueurigobius sp. | B | 0.96 | 32.14 | 0.01 | 0.38 | 2.3–6.9 | 0.08–2.30 |
| Gobius niger Linneaus, 1758 | B | 3.53 | 39.29 | 0.46 | 1.92 | 4.7–13.6 | 1.08–27.29 |
| Gobius spp. | B | 0.31 | 10.71 | 0.03 | 0.04 | 4.3–12.7 | 0.78–22.30 |
| Unidentified Gobiidae | B | 1.23 | 3.57 | n.a. | n.a. | n.a. | n.a. |
| Haemulidae | 0.04 | 3.57 | 0.04 | * | |||
| Pomadasys incisus (Bowdich, 1825) | D | 0.04 | 3.57 | 0.04 | * | ||
| Merlucciidae | 21.06 | 53.57 | 21.15 | 21.76 | |||
| Merluccius merluccius (Linneaus, 1758) | D | 21.06 | 53.57 | 21.15 | 26.92 | 5.7–59.0 | 1.32–1535.40 |
| Moronidae | 0.04 | 3.57 | 0.15 | 0.01 | |||
| Dicentrarchus labrax (Linneaus, 1758) | D | 0.04 | 3.57 | 0.15 | 0.01 | 28.9 | 240.02 |
| Mugilidae | 1.8 | 39.29 | 11.89 | 4.87 | |||
| Chelon ramada (Risso, 1827) | P | 0.58 | 14.29 | 5.28 | 0.97 | 23.1–46.2 | 109.07–860.90 |
| Unidentified Mugilidae | P | 0.81 | 25.00 | 6.61 | 2.14 | 25.4–52.1 | 144.85–1223.92 |
| Mullidae | 2.53 | 28.57 | 1.47 | 1.11 | |||
| Mullus barbatus Linneaus, 1758 | D | 0.04 | 3.57 | 0.07 | * | 21.4 | 112.74 |
| Mullus sp. | D | 2.49 | 25.00 | 1.40 | 1.17 | 5.5–24.6 | 1.97–170.97 |
| Ophidiidae | 7.33 | 46.43 | 2.31 | 4.39 | |||
| Ophidion barbatum Linneaus, 1758 | B | 7.33 | 46.43 | 2.31 | 5.43 | 5.6–26.0 | 0.65–83.75 |
| Phycidae | 0.46 | 3.57 | 0.02 | 0.02 | |||
| Phycis sp. | D | 0.46 | 3.57 | 0.02 | 0.02 | 3.9–10.8 | 0.19–6.61 |
| Sciaenidae | 0.46 | 7.14 | 0.98 | 0.10 | |||
| Umbrina cirrosa (Linneaus, 1758) | D | 0.46 | 7.14 | 0.98 | 0.12 | 18.5–26.0 | 67.21–190.41 |
| Serranidae | 0.50 | 17.86 | 0.19 | 0.12 | |||
| Serranus cabrilla (Linneaus, 1758) | D | 0.04 | 3.57 | 0.05 | * | 20.6 | 77.16 |
| Serranus sp. | D | 0.46 | 14.29 | 0.14 | 0.10 | 6.7–17.3 | 2.68–45.59 |
| Soleidae | 0.12 | 10.71 | 0.19 | 0.02 | |||
| Solea solea (Linneaus, 1758) | B | 0.04 | 3.57 | 0.10 | 0.01 | 26.4 | 154.61 |
| Solea sp. | B | 0.04 | 3.57 | 0.09 | 0.01 | 26.0 | 147.15 |
| Unidentified Soleidae | B | 0.04 | 3.57 | n.a. | n.a. | n.a. | n.a. |
| Sparidae | 11.74 | 75.00 | 13.63 | 18.26 | |||
| Boops boops (Linneaus, 1758) | D | 0.84 | 17.86 | 1.42 | 0.48 | 15.1–27.0 | 32.42–185.60 |
| Dentex dentex (Linneaus, 1758) | D | 0.04 | 3.57 | 0.04 | * | 17.8 | 68.71 |
| Diplodus annularis (Linneaus, 1758) | D | 5.45 | 53.57 | 3.76 | 5.91 | 8.1–20.3 | 7.47–124.43 |
| Diplodus vulgaris (Geoffroy Saint-Hilaire, 1817) | D | 0.19 | 7.14 | 0.22 | 0.03 | 14.6–20.7 | 43.86–127.15 |
| Lithognathus mormyrus (Linneaus, 1758) | D | 0.61 | 3.57 | 3.69 | 0.18 | 24.3–35.3 | 188.79–579.67 |
| Pagellus acarne (Risso, 1827) | D | 0.04 | 3.57 | 0.10 | 0.01 | 23.1 | 161.17 |
| Pagellus erythrinus (Linneaus, 1758) | D | 4.22 | 57.14 | 3.23 | 5.09 | 6.1–22.7 | 3.30–148.70 |
| Sparus aurata Linneaus, 1758 | D | 0.31 | 7.14 | 1.14 | 0.12 | 22.3–32.7 | 149.29–473.20 |
| Spondyliosoma cantharus (Linneaus, 1758) | D | 0.04 | 3.57 | 0.03 | * | 14.6 | 42.21 |
| Sphyraenidae | 0.35 | 10.71 | 1.25 | 0.16 | |||
| Sphyraena sphyraena (Linneaus, 1758) | P | 0.35 | 10.71 | 1.25 | 0.20 | 26.7–37.4 | 113.97–304.49 |
| Synodontidae | 0.04 | 3.57 | 0.04 | * | |||
| Synodus saurus (Linneaus, 1758) | B | 0.04 | 3.57 | 0.04 | * | 20.0 | 62.04 |
| Triglidae | 1.19 | 42.86 | 0.38 | 0.13 | |||
| Chelidonichthys cuculus (Linneaus, 1758) | B | 0.31 | 10.71 | 0.19 | 0.06 | 8.7–23.2 | 5.64–112.43 |
| Chelidonichthys lucerna (Linneaus, 1758) | B | 0.08 | 7.14 | 0.19 | 0.02 | 22.5–28.2 | 102.84–203.54 |
| Unidentified Triglidae | B | 0.81 | 28.57 | n.a. | n.a. | n.a. | n.a. |
| Unidentified Osteichthyes | 3.87 | 60.71 | n.a | n.a | n.a. | n.a. | |
| Total Osteichthyes | 84.43 | 100.00 | 69.00 | 80.40 | |||
| CEPHALOPODA | |||||||
| Argonautidae | 0.04 | 3.57 | * | * | |||
| Argonauta argo Linneaus, 1758 | P | 0.04 | 3.57 | * | * | 5.55 | |
| Enoploteuthidae | 0.04 | 3.57 | 0.01 | * | |||
| Abralia veranyi (Rüppell, 1844) | P | 0.04 | 3.57 | 0.01 | * | 10.32 | |
| Histioteuthidae | 0.27 | 3.57 | 0.07 | 0.01 | |||
| Histioteuthis reversa (Verrill, 1880) | P | 0.27 | 3.57 | 0.07 | 0.01 | 6.29–26.12 | |
| Loliginidae | 2.38 | 39.29 | 5.17 | 2.82 | |||
| Alloteuthis spp. | D | 0.46 | 17.86 | 0.08 | 0.12 | 6.29–17.49 | |
| Loligo vulgaris Lamarck, 1798 | D | 1.84 | 28.57 | 5.08 | 2.31 | 1.8–27.1 | 4.35–402.29 |
| Loligo sp. | D | 0.08 | 3.57 | 0.01 | * | 4.35–14.16 | |
| Octopodidae | 7.02 | 50.00 | 18.95 | 11.41 | |||
| Eledone cirrhosa (Lamarck, 1798) | B | 3.03 | 28.57 | 6.83 | 3.31 | 2.2–14.5 | 11.33–622.02 |
| Eledone moschata (Lamarck, 1798) | B | 1.53 | 25.00 | 1.49 | 0.90 | 5.8–10.9 | 17.04–132.43 |
| Octopus vulgaris Cuvier, 1797 | B | 2.45 | 32.14 | 10.63 | 4.89 | 5.6–15.6 | 59.32–1052.32 |
| Unidentified Octopodidae | B | 0.04 | 3.57 | n.a. | n.a. | n.a. | n.a. |
| Ommastephidae | 4.95 | 53.57 | 6.37 | 5.81 | |||
| Illex coindetii (Vérany, 1839) | D | 4.95 | 53.57 | 6.37 | 7.19 | 6.4–22.1 | 8.95–273.49 |
| Onychoteuthidae | 0.42 | 7.14 | 0.42 | 0.06 | |||
| Ancistroteuthis lichtensteinii (Férussac [in Férussac & d’Orbigny], 1835) | P | 0.27 | 7.14 | 0.30 | 0.05 | 30.44–96.33 | |
| Onychoteuthis banksii (Leach, 1817) | P | 0.15 | 3.57 | 0.12 | 0.01 | 12.73–104.09 | |
| Sepiolidae | 0.35 | 21.43 | * | 0.01 | |||
| Heteroteuthis dispar (Rüppell, 1844) | P | 0.08 | 7.14 | * | 0.01 | 2.81 | |
| Unidentified Sepiolidae | D | 0.27 | 14.29 | n.a. | n.a. | ||
| Unidentified Cephalopoda | 0.08 | 7.14 | n.a. | n.a. | |||
| Total Cephalopoda | 15.57 | 82.14 | 31.00 | 19.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neri, A.; Sartor, P.; Voliani, A.; Mancusi, C.; Marsili, L. Diet of Bottlenose Dolphin, Tursiops truncatus (Montagu, 1821), in the Northwestern Mediterranean Sea. Diversity 2023, 15, 21. https://doi.org/10.3390/d15010021
Neri A, Sartor P, Voliani A, Mancusi C, Marsili L. Diet of Bottlenose Dolphin, Tursiops truncatus (Montagu, 1821), in the Northwestern Mediterranean Sea. Diversity. 2023; 15(1):21. https://doi.org/10.3390/d15010021
Chicago/Turabian StyleNeri, Alessandra, Paolo Sartor, Alessandro Voliani, Cecilia Mancusi, and Letizia Marsili. 2023. "Diet of Bottlenose Dolphin, Tursiops truncatus (Montagu, 1821), in the Northwestern Mediterranean Sea" Diversity 15, no. 1: 21. https://doi.org/10.3390/d15010021
APA StyleNeri, A., Sartor, P., Voliani, A., Mancusi, C., & Marsili, L. (2023). Diet of Bottlenose Dolphin, Tursiops truncatus (Montagu, 1821), in the Northwestern Mediterranean Sea. Diversity, 15(1), 21. https://doi.org/10.3390/d15010021








