After Wildfires and Rewetting: Results of 15+ Years’ Monitoring of Vegetation and Environmental Factors in Cutover Peatland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Region
2.2. Study Objects and Sites
2.3. Climatic Data
2.4. Environment Monitoring
2.5. Vegetation Studies
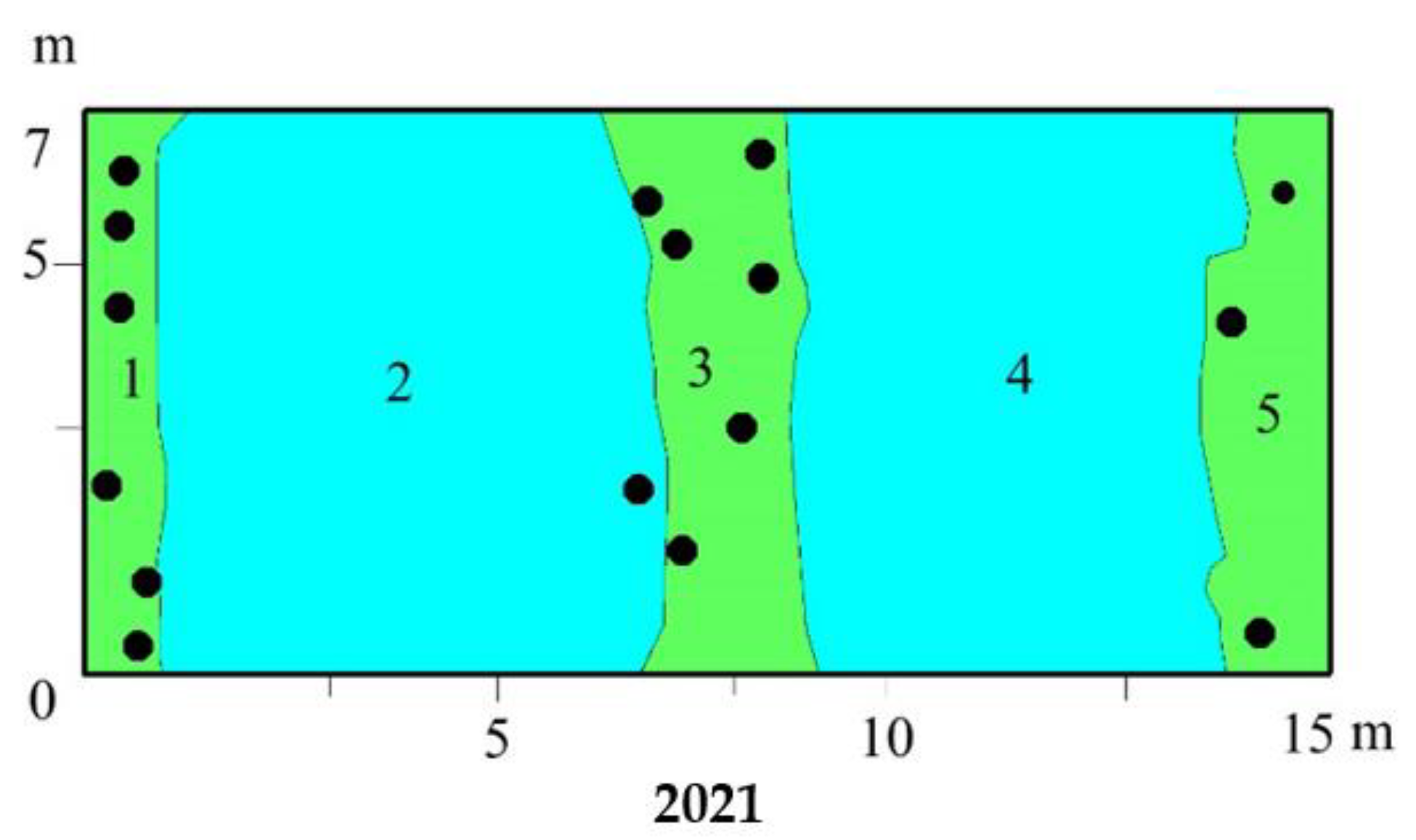
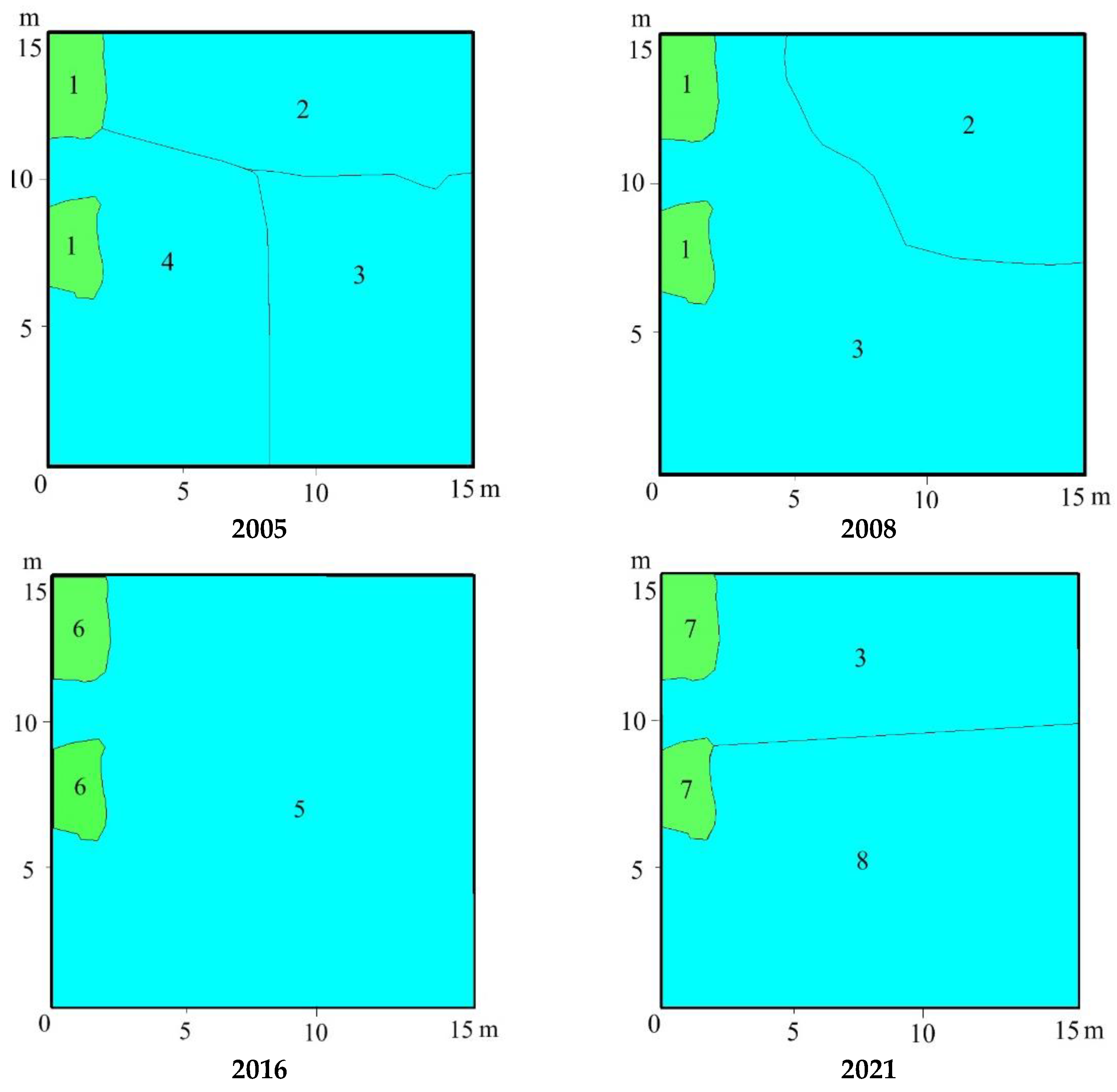
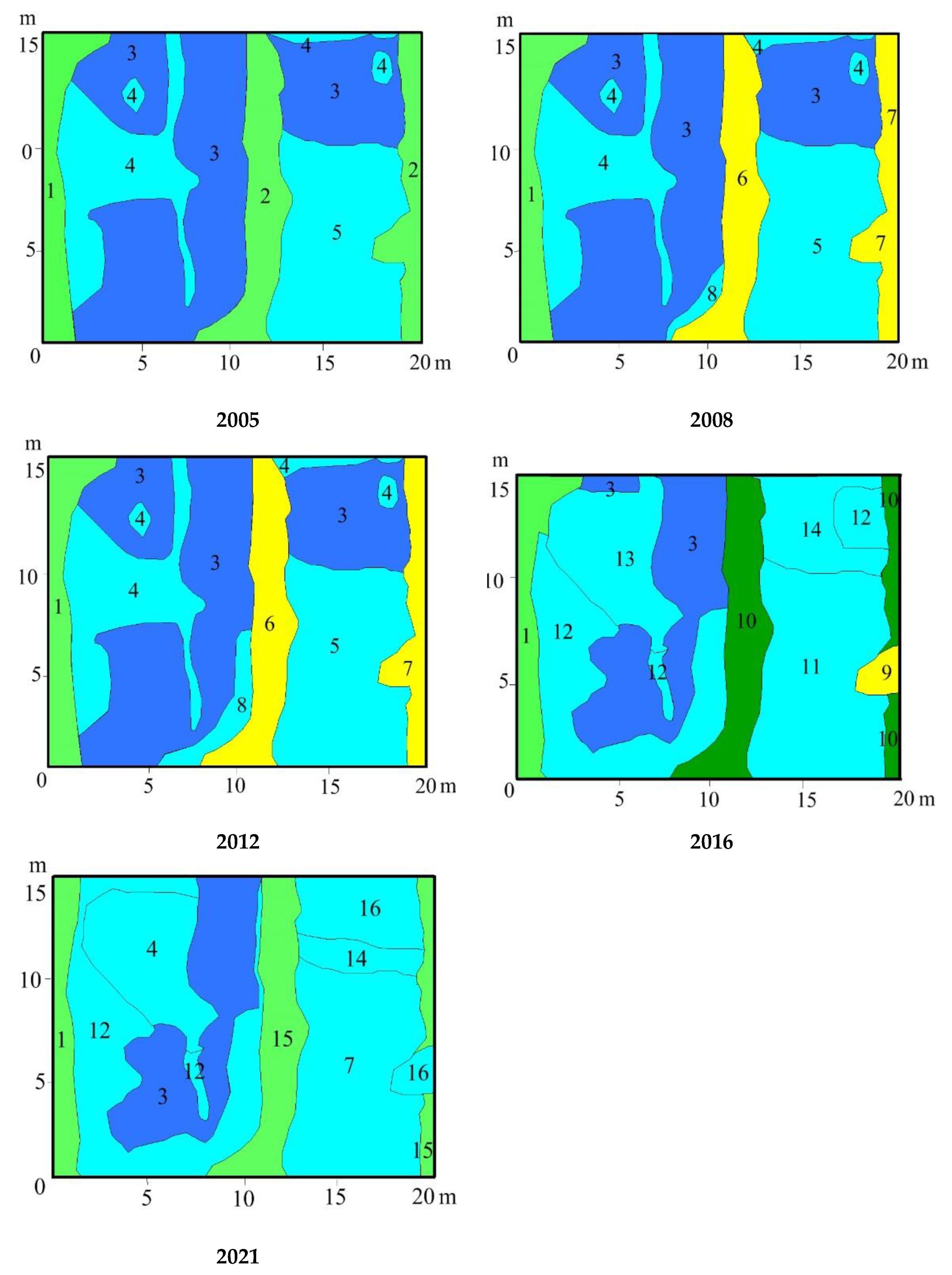
2.6. Sites Characteristics
3. Results
3.1. Climatic Conditions over Study Period
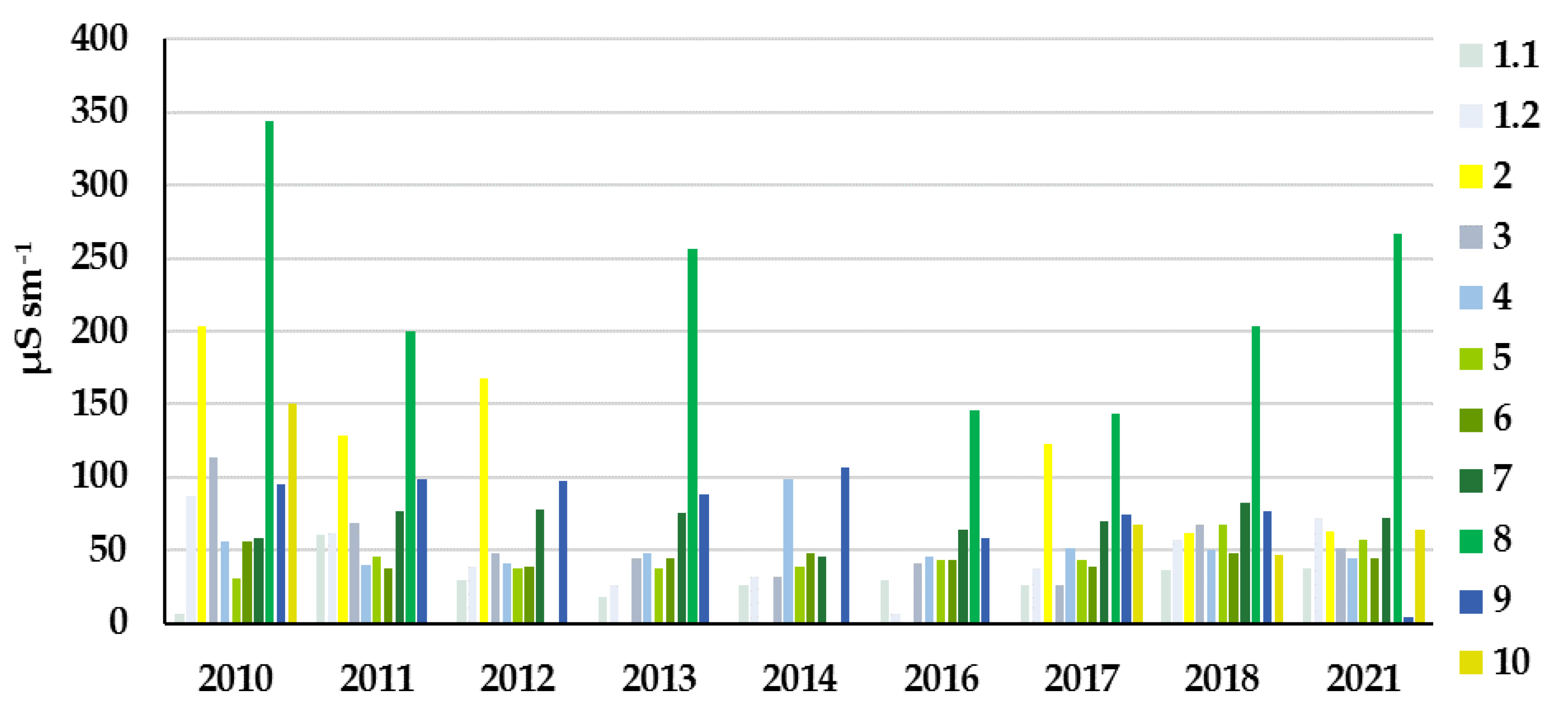
3.2. Study Sites Environmental Conditions
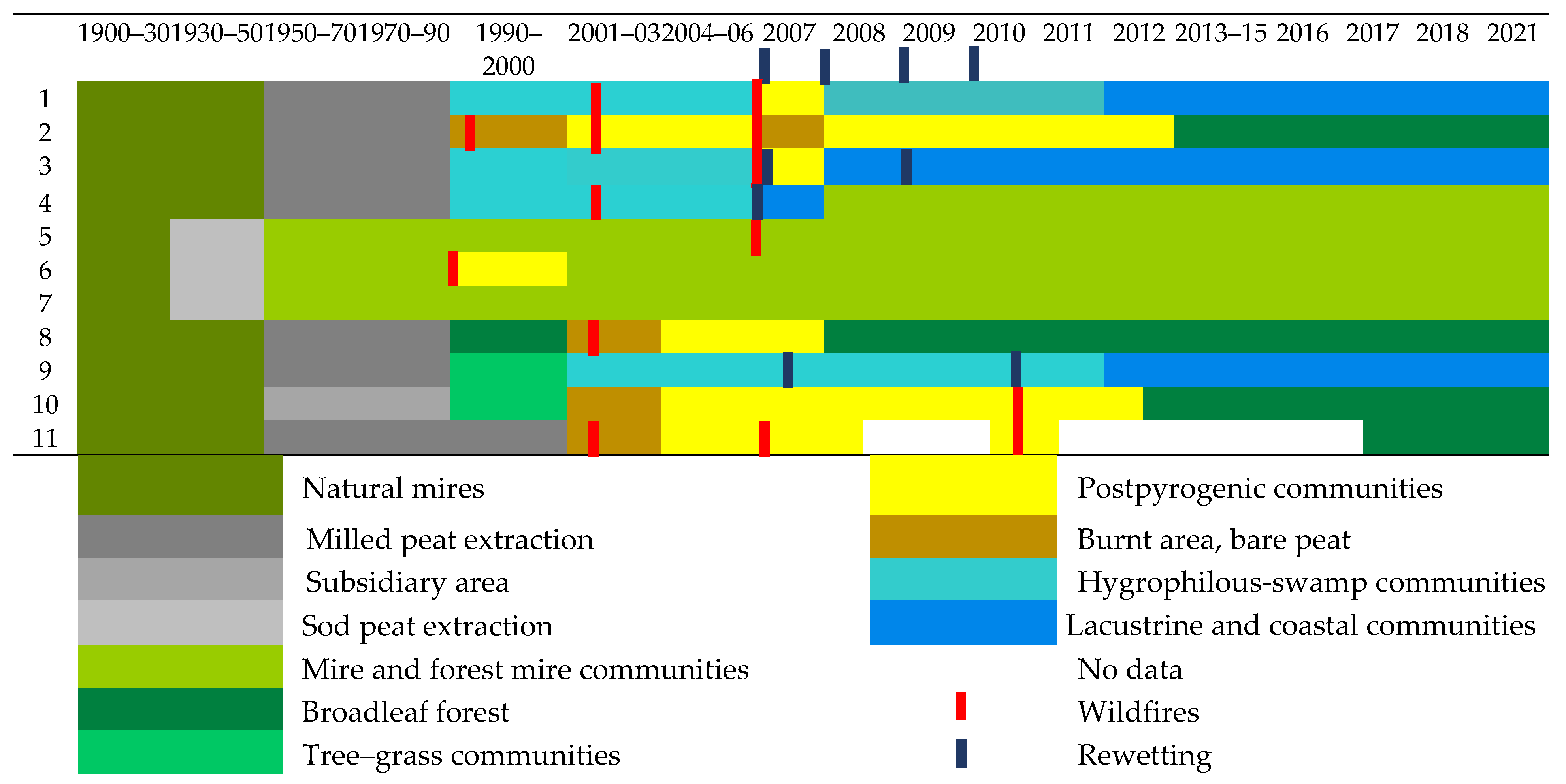
3.3. Vegetation Changes
4. Discussion
4.1. Vegetation Succession after Peat Extraction
4.2. Vegetation Succesion after Wildwires
4.3. Vegetation Changes following Rewetting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parish, F.; Sirin, A.; Charman, D.; Joosten, H.; Minayeva, T.; Silvius, M.; Stringer, L. (Eds.) Assessment on Peatlands, Biodiversity and Climate Change: Main Report; Global Environment Centre: Kuala Lumpur, Malaysia; Wetlands International: Wageningen, The Netherlands, 2008; p. 179. [Google Scholar]
- Joosten, H.; Sirin, A.; Couwenberg, J.; Laine, J.; Smith, P. The role of peatlands in climate regulation. In Peatland Restoration and Ecosystem Services: Science, Policy and Practice; Bonn, A., Allott, T., Evans, M., Joosten, H., Stoneman, R., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 66–79. [Google Scholar] [CrossRef]
- Minayeva, T.Y.; Sirin, A.A. Peatland biodiversity and climate change. Biol. Bull. Rev. 2012, 2, 164–175. [Google Scholar] [CrossRef]
- Joosten, H.; Clarke, D. Wise Use of Mires and Peatlands: Background and Principles Including a Framework for Decision-Making; International Mire Conservation Group/International Peat Society, Saarijärven Offset Oy: Saarijärvi, Finland, 2002; 304p, ISBN 951-97744-8-3. [Google Scholar]
- IPCC 2019. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Calvo Buendia, E., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; in press; Available online: https://www.ipcc.ch/srccl/ (accessed on 15 December 2022).
- Sirin, A.; Minayeva, T.; Vozbrannaya, A.; Bartalev, S. How to avoid peat fires? Sci. Russ. 2011, 2, 13–21. [Google Scholar]
- Wilson, D.; Blain, D.; Couwenberg, J.; Evans, C.D.; Murdiyarso, D.; Page, S.E.; Renou-Wilson, F.; Rieley, J.O.; Sirin, A.; Strack, M.; et al. Greenhouse gas emission factors associated with rewetting of organic soils. Mires Peat 2016, 17, 1–28. [Google Scholar] [CrossRef]
- Günther, A.; Barthelmes, A.; Huth, V.; Joosten, H.; Jurasinski, G.; Koebsch, F.; Couwenberg, J. Prompt rewetting of drained peatlands reduces climate warming despite methane emissions. Nat. Commun. 2020, 11, 1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granath, G.; Moore, P.A.; Lukenbach, M.C.; Waddington, J.M. Mitigating wildfire carbon loss in managed northern peatlands through restoration. Sci. Rep. 2016, 6, 28498. [Google Scholar] [CrossRef] [Green Version]
- Sirin, A.A.; Medvedeva, M.A.; Makarov, D.A.; Maslov, A.A.; Joosten, H. Multispectral satellite based monitoring of land cover change and associated fire reduction after large-scale peatland rewetting following the 2010 peat fires in Moscow region (Russia). Ecol. Eng. 2020, 158, 106044. [Google Scholar] [CrossRef]
- Minayeva, T.Y.; Bragg, O.M.; Sirin, A.A. Towards ecosystem-based restoration of peatland biodiversity. Mires Peat 2017, 19, 1–36. [Google Scholar] [CrossRef]
- Ahmad, S.; Haojie, L.; Günther, A.; Couwenberg, J.; Lennartz, B. Long-term rewetting of degraded peatlands restores hydrological buffer function. Sci. Total Environ. 2020, 749, 141571. [Google Scholar] [CrossRef]
- Bonn, A.; Reed, M.; Bain, C.; Chris, D.E.; Joosten, H.; Farmer, J.; Emmer, I.; Couwenberg, J.; Moxey, A.; Artz, R.; et al. Investing in nature: Developing ecosystem service markets for peatland restoration. Ecosyst. Serv. 2014, 9, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Global Peatland Database. Available online: https://www.greifswaldmoor.de/global-peatland-database-en.html (accessed on 28 August 2021).
- Vompersky, S.E.; Sirin, A.A.; Salnikov, A.A.; Tsyganova, O.P.; Valyaeva, N.A. Estimation of forest cover extent over peatland and paludified shallow peatlands in Russia. Contemp. Probl. Ecol. 2011, 4, 734–741. [Google Scholar] [CrossRef]
- Tanneberger, F.; Tegetmeyer, C.; Busse, S.; Barthelmes, A.; Shumka, S.; Mariné, A.M.; Jenderedjian, K.; Steiner, G.M.; Essl, F.; Etzold, J.; et al. The peatland map of Europe. Mires Peat 2017, 19, 1–17. [Google Scholar] [CrossRef]
- Minayeva, T.; Sirin, A.; Bragg, O. (Eds.) A Quick Scan of Peatlands in Central and Eastern Europe; Wetlands International: Wageningen, The Netherlands, 2009; 132p, Available online: https://www.wetlands.org/publications/a-quick-scan-of-peatlands-in-central-and-eastern-europe/ (accessed on 15 December 2022).
- Sirin, A.; Minayeva, T.; Yurkovskaya, T.; Kuznetsov, O.; Smagin, V.; Fedotov, Y.U. Russian Federation (European part). In Mires and Peatlands of Europe: Status, Distribution and Conservation; Joosten, H., Tanneberger, F., Moen, A., Eds.; Schweizerbart Science Publishers: Stuttgart, Germany, 2017; pp. 589–616. [Google Scholar] [CrossRef]
- Tanneberger, F.; Moen, A.; Barthelmes, A.; Lewis, E.; Miles, L.; Sirin, A.; Tegetmeyer, C.; Joosten, H. Mires in Europe—Regional diversity, condition and protection. Diversity 2021, 13, 381. [Google Scholar] [CrossRef]
- Safronov, A.N.; Fokeeva, E.V.; Rakitin, V.S.; Grechko, E.I.; Shumsky, R.A. Severe wildfires near Moscow, Russia in 2010: Modeling of carbon monoxide pollution and comparisons with observations. Remote Sens. 2014, 7, 395–429. [Google Scholar] [CrossRef] [Green Version]
- Sirin, A.; Medvedeva, M.; Korotkov, V.; Itkin, V.; Minayeva, T.; Ilyasov, D.; Suvorov, G.; Joosten, H. Addressing peatland rewetting in Russian Federation climate reporting. Land 2021, 10, 1200. [Google Scholar] [CrossRef]
- Medvedeva, M.A.; Vozbrannaya, A.E.; Bartalev, S.A.; Sirin, A.A. Ocenka sostojanija zabroshennyh torforazrabotok po mnogospektral’nym sputnikovym izobrazhenijam [Assessment of abandoned peat extraction sites using multispectral satellite imagery] Issled. Zemli Iz Kosm. 2011, 5, 80–88. [Google Scholar]
- Medvedeva, M.A.; Vozbrannaya, A.E.; Sirin, A.A.; Maslov, A.A. Capabilities of multispectral remote-sensing data in an assessment of the status of abandoned fire hazardous and rewetting peat extraction lands. Izv. Atmos. Ocean. Phys. 2017, 53, 1070–1078. [Google Scholar] [CrossRef]
- Sirin, A.; Medvedeva, M.; Maslov, A.; Vozbrannaya, A. Assessing the land and vegetation cover of abandoned fire hazardous and rewetted peatlands: Comparing different multispectral satellite data. Land 2018, 7, 71. [Google Scholar] [CrossRef] [Green Version]
- Abramova, L.I. Formirovanie rastitel’nosti na vyrabotannyh torfjanikah i osnovnye puti ih ispol’zovanija [Vegetation formation on excavated peatlands and the main ways of their use]. Extended abstract of candidate’s thesis (PhD), Moscow State University, Moscow, Russia, 1969; 24p. [Google Scholar]
- Robert, É.C.; Rochefort, L.; Garneau, M. Natural revegetation of two block-cut mined peatlands in eastern Canada. Can. J. Bot 1999, 77, 447–459. [Google Scholar]
- Smagin, V.A. Dinamika zarastanija torfjanyh kar’erov (na primere vyrabotannyh torfjanikov Leningradskoj oblasti) [Dynamics of overgrowth in peat pits (the example of mined out peatlands in the Leningrad region)]. Bot. Zhurnal. 1982, 67, 1112–1117. [Google Scholar]
- Panov, V.V. Bolotoobrazovatel’nyj Process i Torfjanye Resursy. Vosstanovlenie Torfjanyh Bolot [Peatland Formation and Peat Resources. Peatland Restoration]; Izdatel’stvo Tomskogo Gosudarstvennogo Pedagogicheskogo Universiteta: Tomsk, Russia, 2007; 80p. [Google Scholar]
- Murav’eva, L.V.; Tihomirov, O.A.; Markov, M.V. Formirovanie akval’no-territorial’nyh kompleksov vyrabotannyh torfjanyh bolot i ih klassifikacija [Formation of aquatic-territorial complexes of drained peatlands and their classification]. Ychenii Zap. Kazan. Univ. 2010, 152, 102–115. [Google Scholar]
- Grishutkin, O.G. Bolota Mordovii: Landshaftno-Jekologicheskij Analiz, Flora, Posledstvija Antropogennogo Vozdejstvija [Peatlands of Mordovia: Landscape-Ecological Analysis, Flora, Effects of Anthropogenic Impact]; Saransk: Pushta, Russia, 2015; 154p. [Google Scholar]
- Tuittila, E.-S.; Vasander, H.; Laine, J. Impact of Rewetting on the Vegetation of a Cut-Away Peatland. Appl. Veg. Sci. 2000, 3, 205–212. [Google Scholar] [CrossRef]
- Lavoie, C.; Rochefort, L. The natural revegetation of a harvested peatland in sourthern Quebec: A spatial and dendroecological analysis. Ecoscience 1996, 3, 101–111. [Google Scholar] [CrossRef]
- Feldmeyer-Christe, E.; Küchler, M.; Wildi, O. Patterns of early succession on bare peat in a Swiss mire after a bog burst. J. Veg. Sci. 2011, 22, 943–954. [Google Scholar] [CrossRef]
- Priede, A.; Mežaka, A.; Dobkeviča, L.; Grīnberga, L. Spontaneous revegetation of cutaway fens: Can it result in valuable habitats? Mires Peat 2016, 18, 1–14. [Google Scholar] [CrossRef]
- Edom, F.; Keßler, K.; Stegmann, H.; Wendel, D.; Dittrich, I.; Münch, A. Hydrologisches und moorkundliches Gutachten zur Konkretisierung von 20 Erhaltungs- und Entwicklungsmaßnahmen für das Moor Stengelhaide im FFHGebiet "Mothäuser Heide“. Dr. Dittrich & Partner Hydro-Consult GmbH & HYDROTELM—Edom & Stegmann, Bannewitz & Dresden, im Auftrag der LfULG Zwickau, 2009, p. 77.
- Minayeva, T.; Sirin, A.; Stracher, G.B. The Peat Fires of Russia. In Coal and Peat Fires: A Global Perspective; V.2: Photographs and Multimedia Tours; Stracher, G.B., Prakash, A., Sokol, E.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 375–394. ISBN 9780444594129. [Google Scholar]
- Tuittila, E.-S.; Rita, H.; Vasander, H.; Laine, J. Vegetation patterns around Eriophorum vaginatum L. tussocks in a cut-away peatland in southern Finland. Can. J. Bot. 2000, 78, 47–58. [Google Scholar]
- Rochefort, L. Sphagnum—A Keystone Genus in Habitat Restoration. Bryol. 2000, 103, 503–508. [Google Scholar] [CrossRef]
- Rochefort, L.; Quinty, F.; Campeau, S.; Johnson, K.; Master, T. North American approach to the restoration of Sphagnum dominated peatlands. Wetl. Ecol. Manag. 2003, 11, 3–20. [Google Scholar] [CrossRef]
- Vasander, H.; Tuittila, E.-S.; Lode, E.; Lundin, L.; Ilomets, M.; Sallantaus, T.; Heikkilä, R.; Pitkänen, M.-L.; Laine, J. Status and restoration of peatlands in northern Europe. Wetl. Ecol. Manag. 2003, 11, 51–63. [Google Scholar] [CrossRef]
- Kozlov, S.A.; Lundin, L.; Avetov, N.A. Revegetation dynamics after 15 years of rewetting in two extracted peatlands in Sweden. Mires Peat 2016, 18, 1–17. [Google Scholar] [CrossRef]
- Purre, A.-H.; Truus, L.; Ilomets, M. A decade of vegetation development on two revegetated milled peatlands with different trophic status. Mires Peat 2021, 27, 1–16. [Google Scholar] [CrossRef]
- Gorham, E.; Rochefort, L. Peatland restoration: A brief assessment with special reference to Sphagnum bogs. Wetl. Ecol. Manag. 2003, 11, 109–119. [Google Scholar] [CrossRef]
- Frei, S.; Holderegger, R.; Bergamini, A. Thirty years later: How successful was the restoration of a raised bog in the Swiss lateau? Mires Peat 2021, 27, 1–27. [Google Scholar] [CrossRef]
- Hytteborn, H.; Maslov, A.A.; Nazimova, D.I.; Rysin, L.P. Boreal forests of Eurasia. In Coniferous Forests; Andersson, F., Goodall, D.W., Eds.; Ecosystems of the World; Elsevier: Amsterdam, The Netherlands, 2005; Volume 6, pp. 23–29. ISBN 9780444816276. [Google Scholar]
- Moen, A.; Joosten, H.; Tanneberger, F. Mire diversity in Europe: Mire regionality. In Mires and Peatlands of Europe. Status, Distribution and Conservation; Joosten, H., Tanneberger, F., Moen, A., Eds.; Schweizerbart: Stuttgart, Germany, 2017; pp. 97–150. [Google Scholar]
- Antipin, V.; Boychuk, M.; Grabovik, S.; Stoikina, N. Plant cover of natural mires and disturbed peatlands in Meshchera National Park, Russia. In Mires from Pole to Pole; Lindholm, T., Heikkila, R., Eds.; The Finnish environment: Helsinki, Finland, 2013; pp. 247–257. [Google Scholar]
- Available online: https://rp5.ru/ (accessed on 10 November 2022).
- Available online: https://meteoinfo.ru (accessed on 10 November 2022).
- Polevaja Geobotanika, [Field geobotany]. Korchagin, A.A., Lavrenko, E.M., Eds.; Nauka: St. Petersburg/Moskow, Russia, 1964; Volume 3, 500p.
- Polevaja Geobotanika, [Field geobotany]. Korchagin, A.A., Lavrenko, E.M., Eds.; Nauka: St. Petersburg/Moskow, Russia, 1976; Volume 5, 319p.
- Metody Issledovanij Bolotnyh Jekosistem Taezhnoj Zony [Research Methods for Mire Ecosystems in the Taiga Zone]. Kuznetsov, O.L., Ed.; Nauka: Leningrad, Russia, 1991; 128p.
- Galanina, O. Comparative application of two vegetation classification approaches to large-scale mapping of bog vegetation. Suo 2006, 57, 71–79. [Google Scholar]
- Vahromeev, I.V. Opredelitel’ Sosudistyh Rastenij Vladimirskoj Oblasti [Vascular Plant Handbook for the Vladimir Region]; Tranzit-IKS: Vladimir, Russia, 2002; 312p. [Google Scholar]
- Ignatov, M.S.; Ignatova, E.A. Moss Flora of Middle European Russia; Volume 1: Sphagnaceae—Hedwigiaceae; Association of Scientific Publications KMK: St. Petersburg/Moscow, Russia, 2003; pp. 1–608. [Google Scholar]
- Ignatov, M.S.; Ignatova, E.A. Moss Flora of Middle European Russia; Volume 2: Fontinalaceae—Amblystegiaceae; Association of Scientific Publications KMK: St. Petersburg/Moscow, Russia, 2003; pp. 609–944. [Google Scholar]
- Barriopedro, D.; Fischer, E.M.; Luterbacher, J.; Trigo, R.M.; García-Herrera, R. The hot summer of 2010: Redrawing the temperature record map of Europe. Science 2003, 332, 220–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rydin, H.; Jeglum, J.K. The Biology of Peatlands; University Press: Oxford, UK, 2006; 162p. [Google Scholar]
- Blain, D.; Murdiyarso, D.; Couwenberg, J.; Nagata, O.; Renou-Wilson, F.; Sirin, A.; Strack, M.; Tuittila, E.-S.; Wilson, D.; Evans, C.D.; et al. Rewetted organic soils. Chapter 3. In 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands; Hiraishi, T., Krug, T., Tanabe, K., Srivastava, N., Baasansuren, J., Fukuda, M., Troxler, T.G., Eds.; IPCC: Geneva, Switzerland, 2014; p. 41. Available online: https://www.ipcc.ch/publication/2013-supplement-to-the-2006-ipcc-guidelines-for-national-greenhouse-gas-inventories-wetlands/ (accessed on 15 December 2022).
- Thiele, A.; Edom, F.; Liashchynskaya, N. Prediction of vegetation development with and without rewetting. Chapter: Peatlands and climate. In Carbon Credits from Peatland Rewetting; Tanneberger, F., Wichtmann, W., Eds.; Schweizerbart Science Publishers: Stuttgart, Germany, 2011; pp. 42–52. [Google Scholar] [CrossRef]
- Buttler, A.; Warner, B.G.; Grosvernier, P.; Matthey, Y. Vertical Patterns of Testate Amoebae (Protozoa: Rhizopoda) and Peat-Forming Vegetation on Cutover Bogs in the Jura; Switzerland. New Phytol. 1996, 134, 371–382. [Google Scholar] [CrossRef]
- Sirin, A.; Medvedeva, M. Remote Sensing Mapping of Peat-Fire-Burnt Areas: Identification among Other Wildfires. Remote Sens. 2022, 14, 194. [Google Scholar] [CrossRef]
- Glukhova, T.V.; Sirin, A.A. Losses of soil carbon upon a fire on a drained forested raised bog. Eurasian Soil Sci. 2018, 51, 542–549. [Google Scholar] [CrossRef]
- Panov, V.V.; Tsymlyakova, S.S. Spatial structure of fires and burnt areas in the technologically disturbed peat bogs. Proc. Russ. Geogr. Soc. 2013, 145, 80–90. [Google Scholar]
- Pisani, D.; Pazienza, P.; Perrino, E.V.; Caporale, D.; De Lucia, C. The economic valuation of ecosystem services of biodiversity components in protected areas: A review for a framework of analysis for the Gargano National Park. Sustainability 2021, 13, 11726. [Google Scholar] [CrossRef]

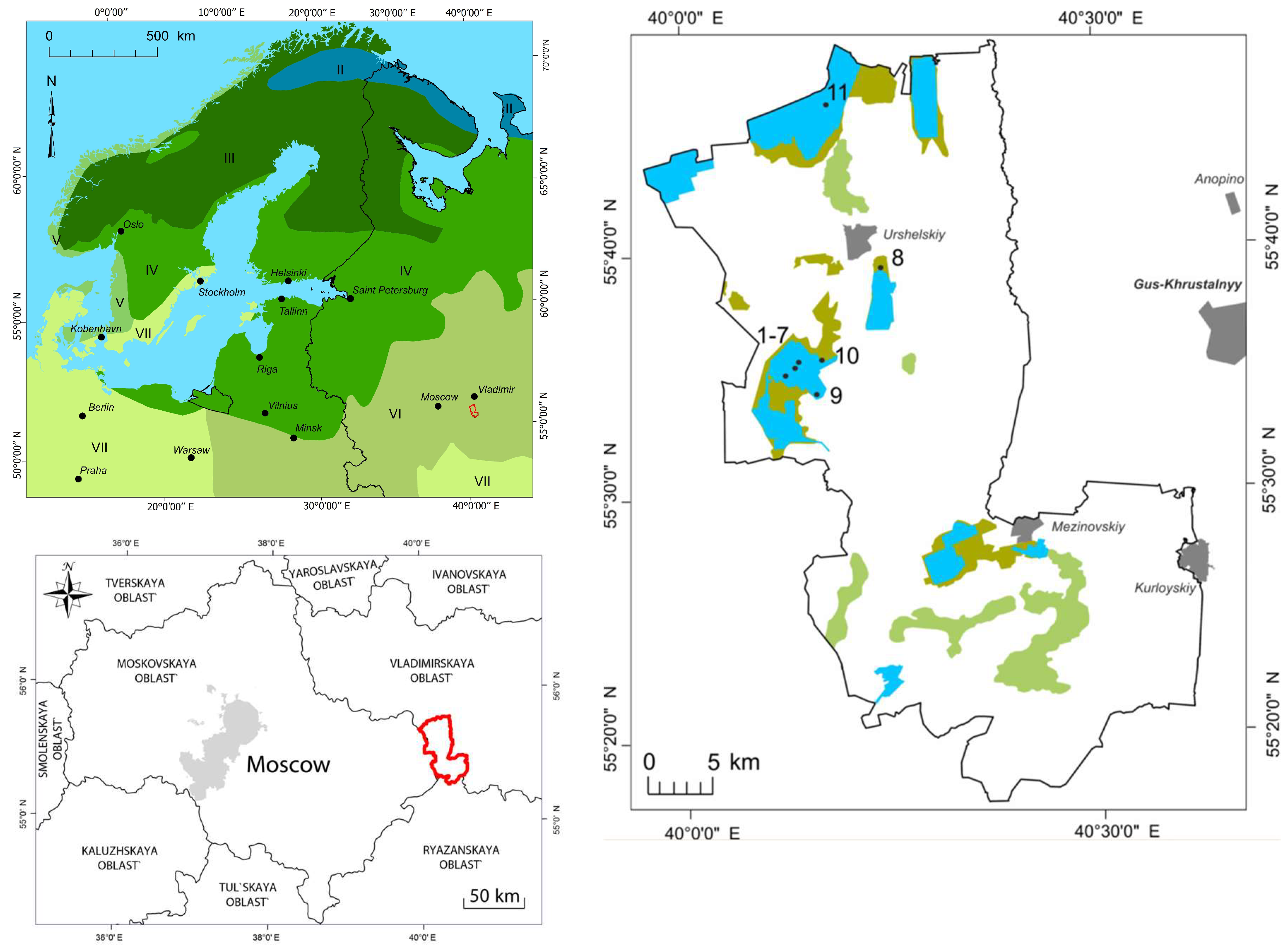




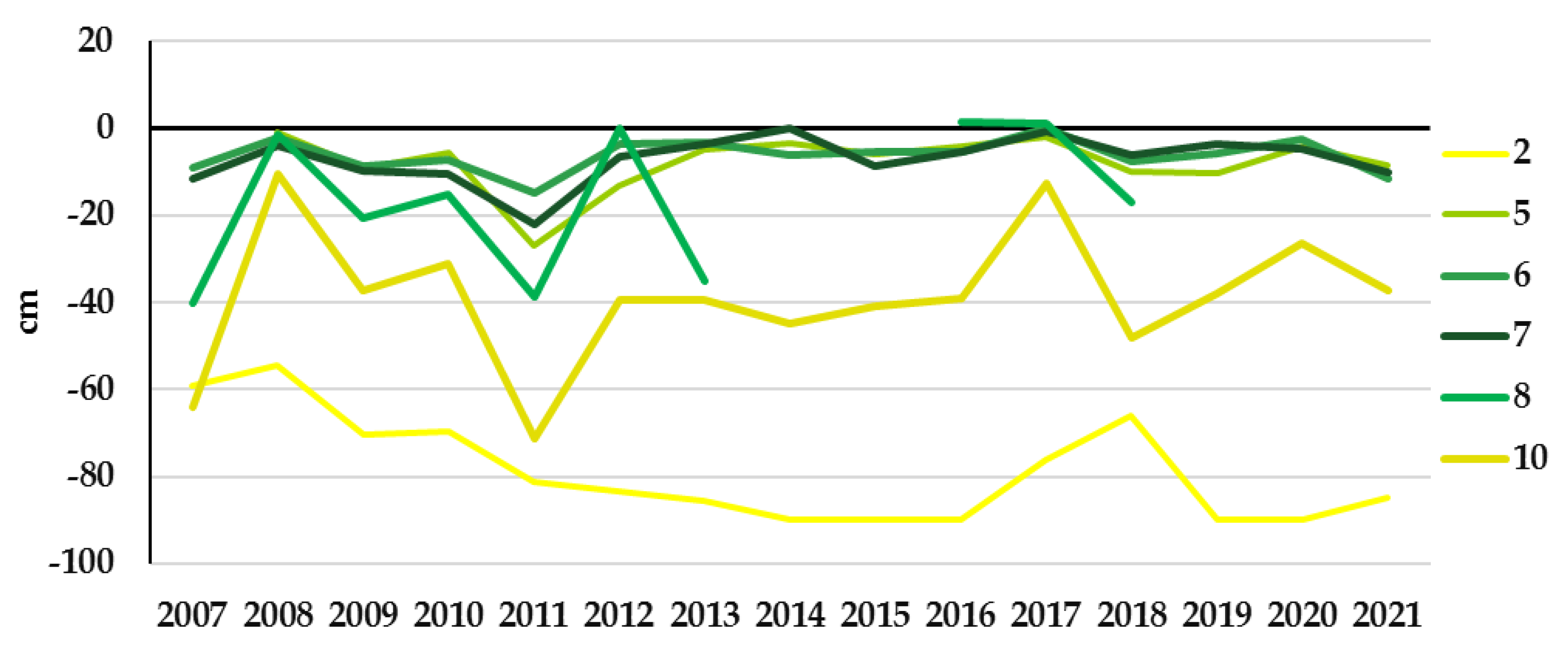
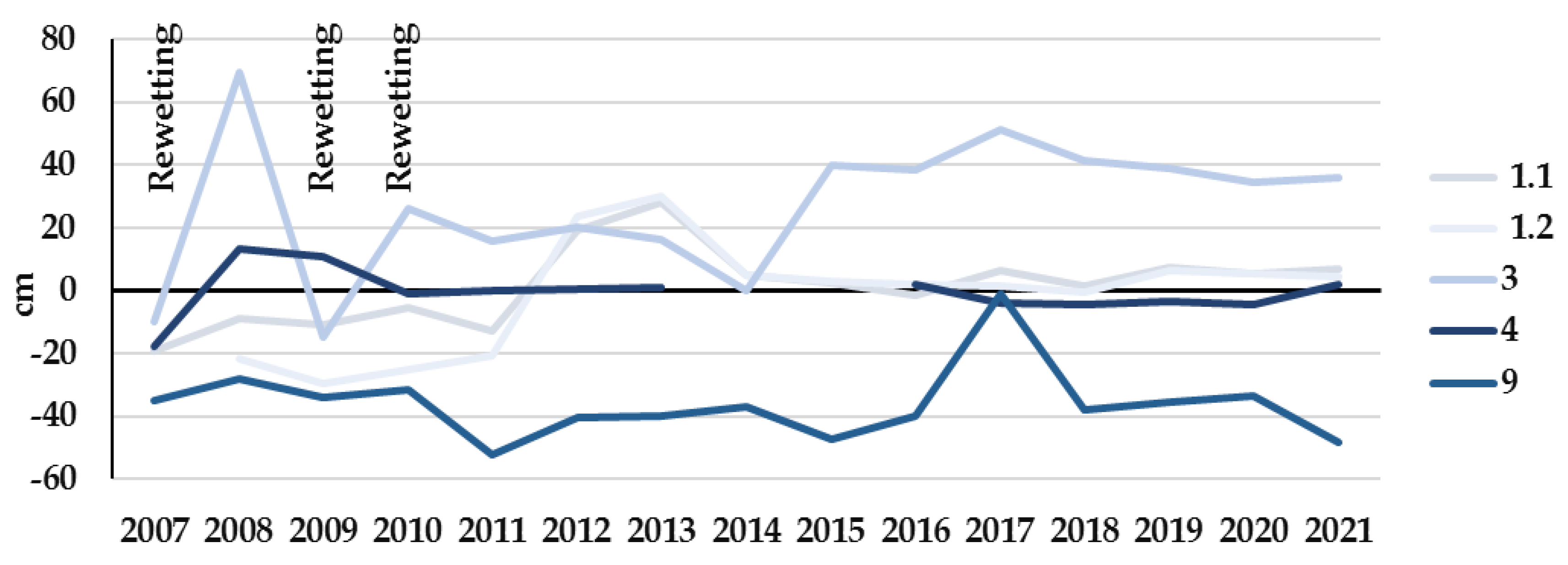
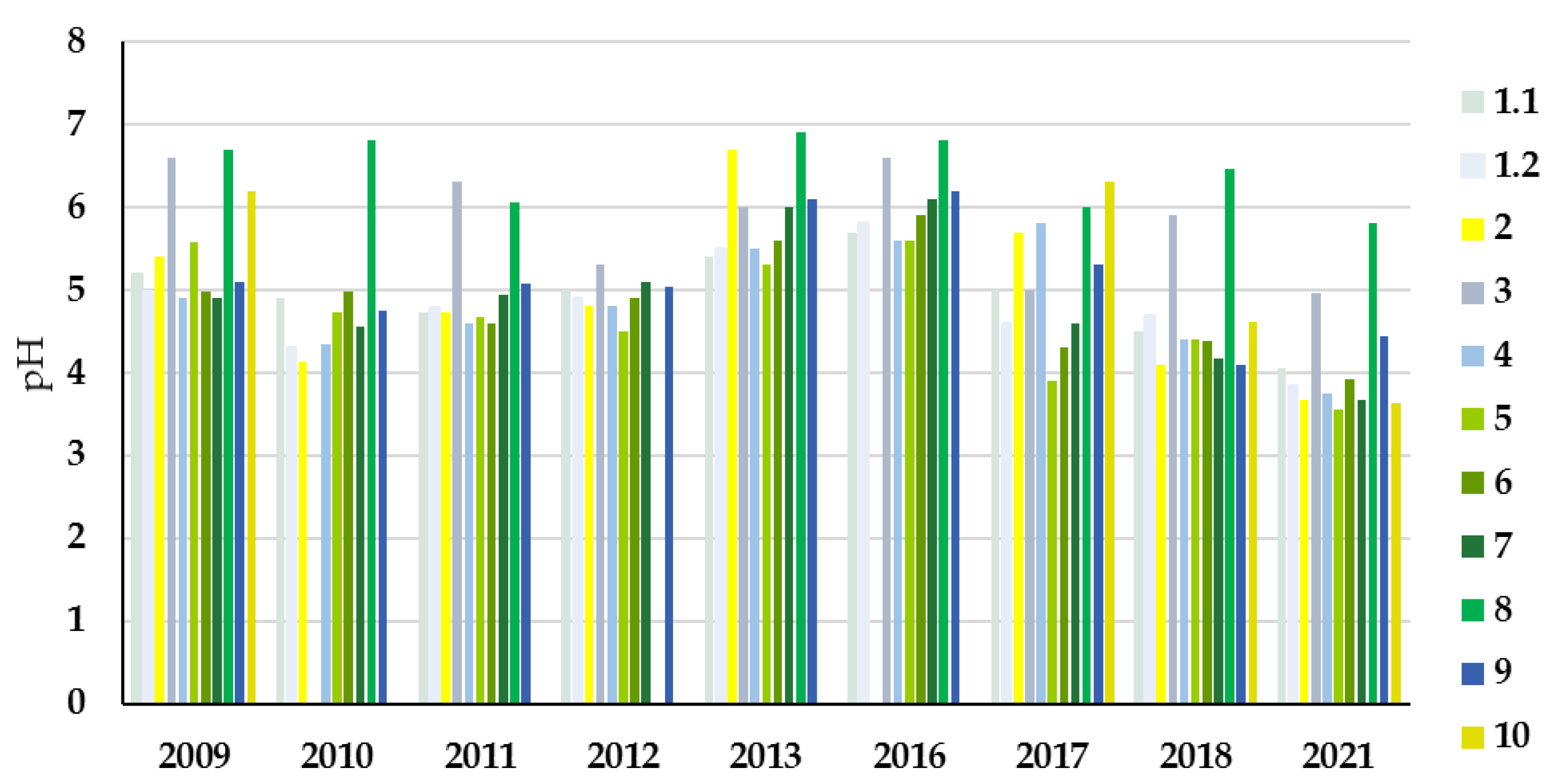
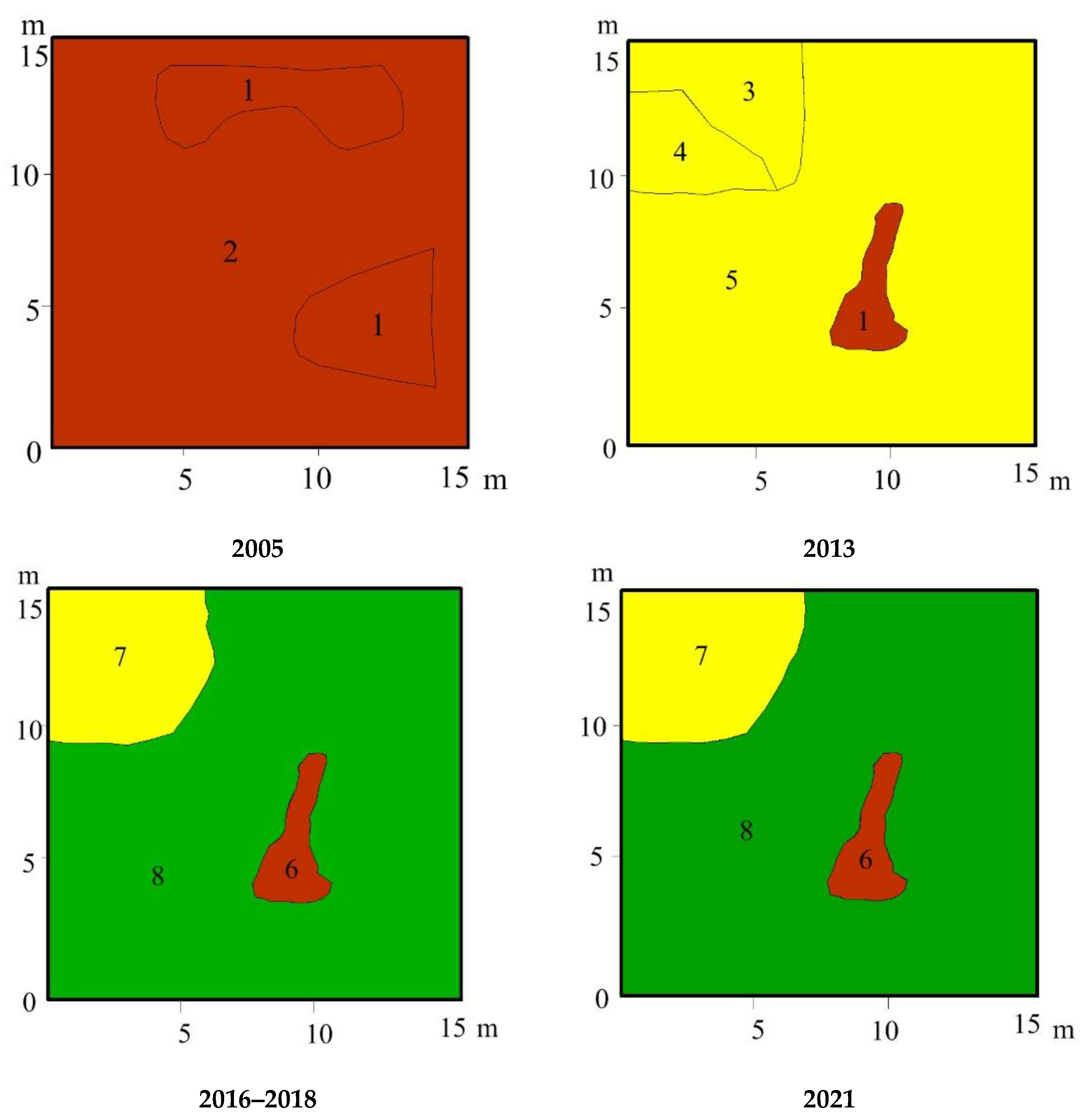
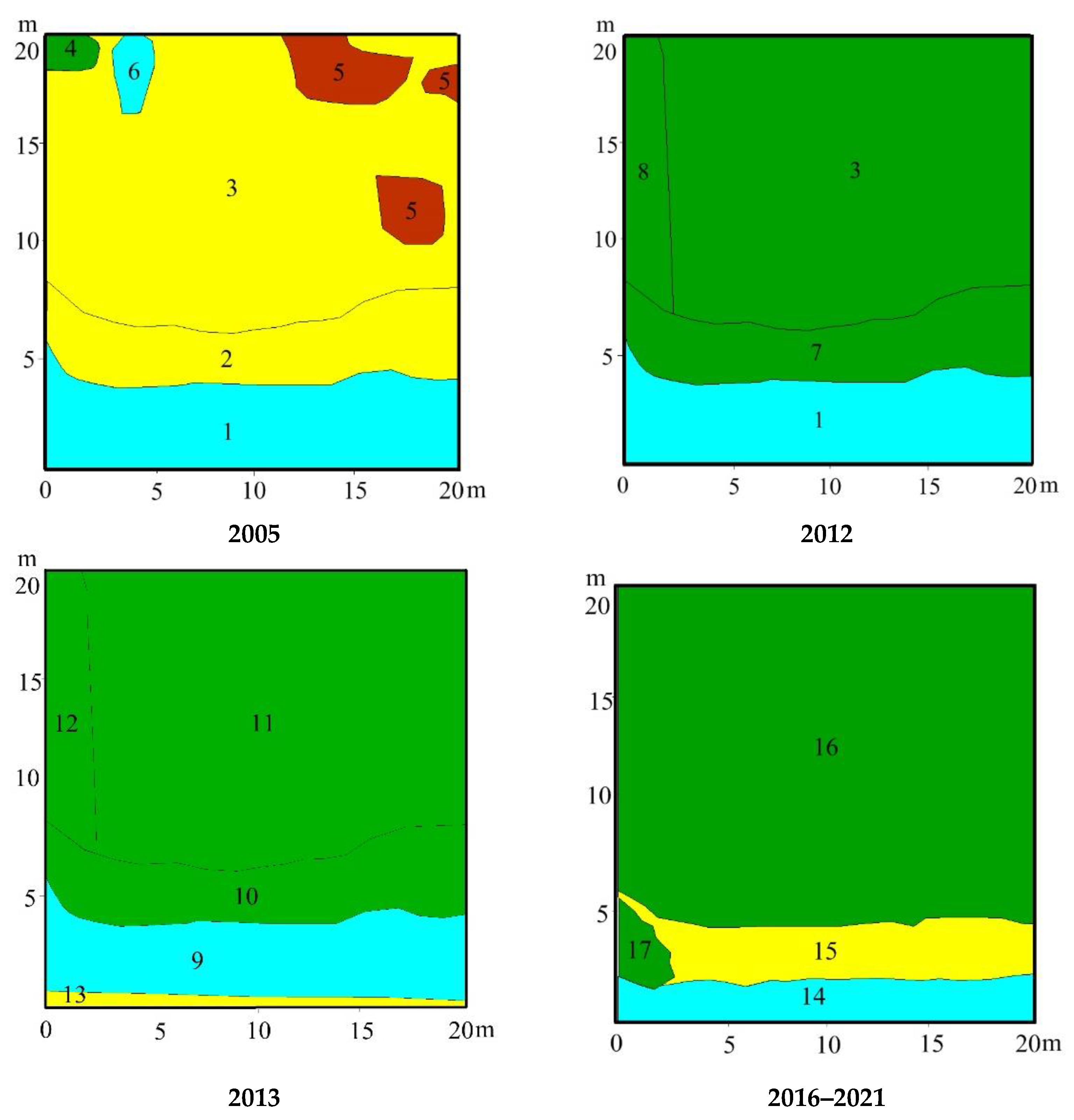
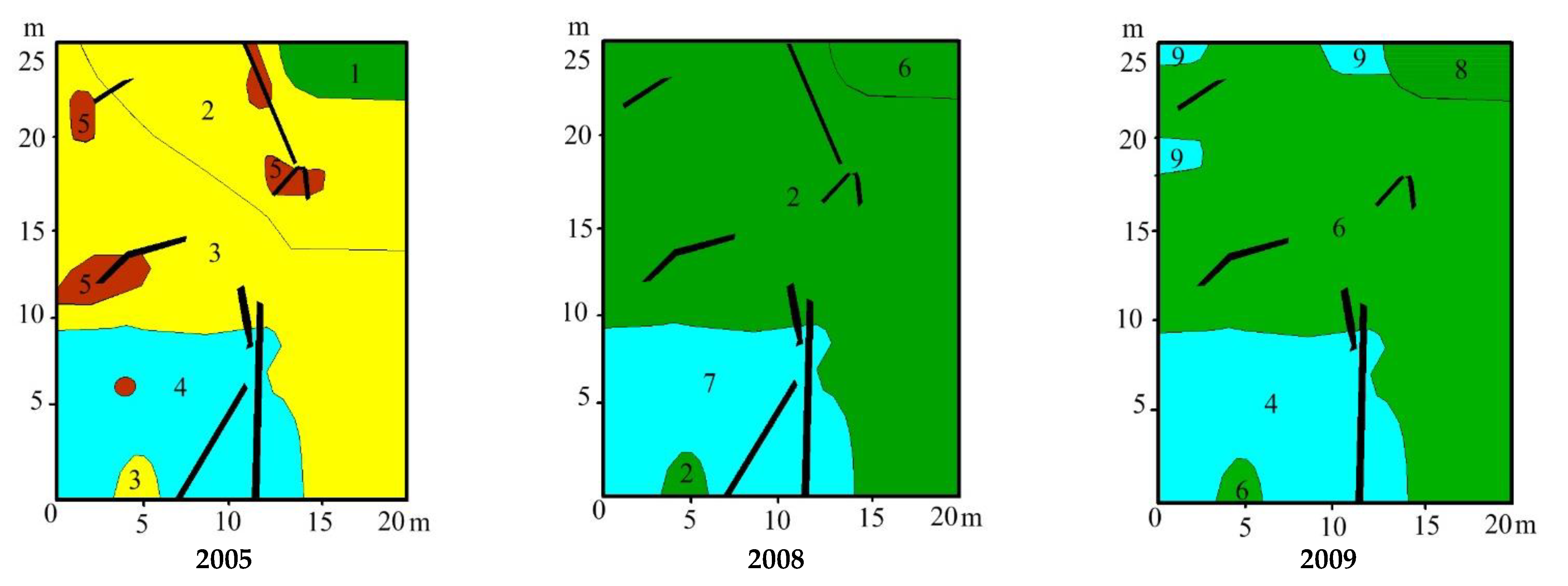
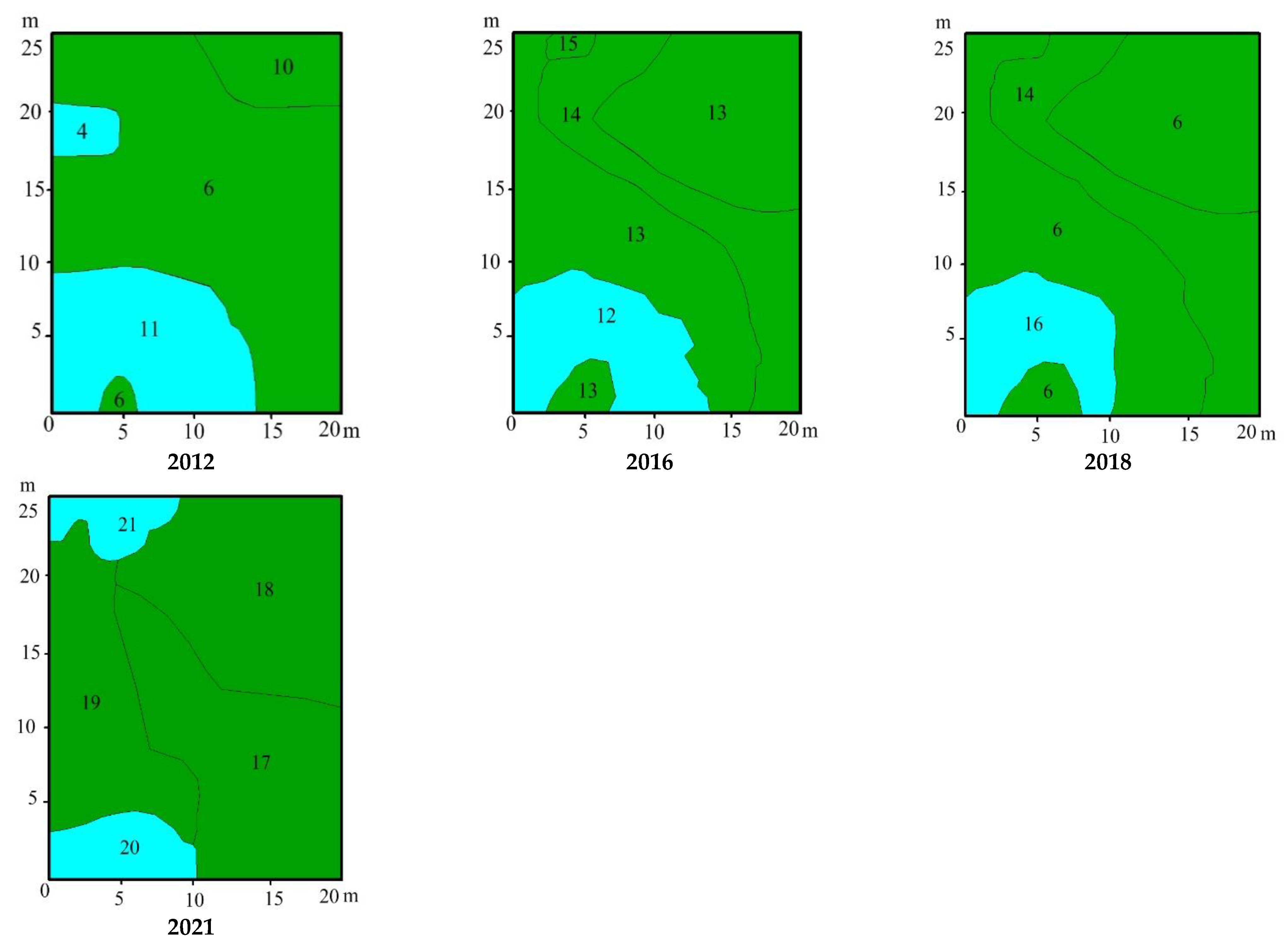
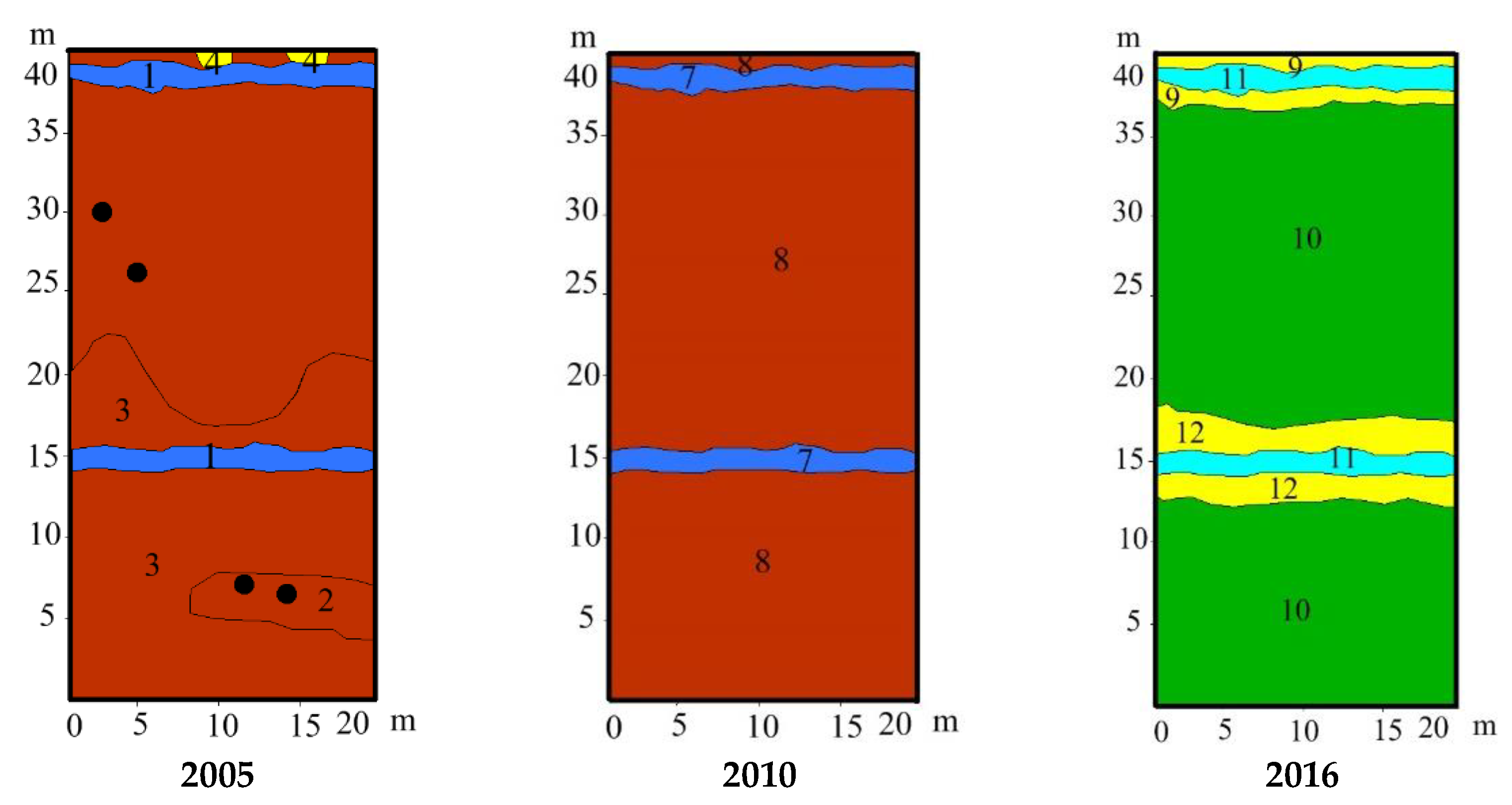


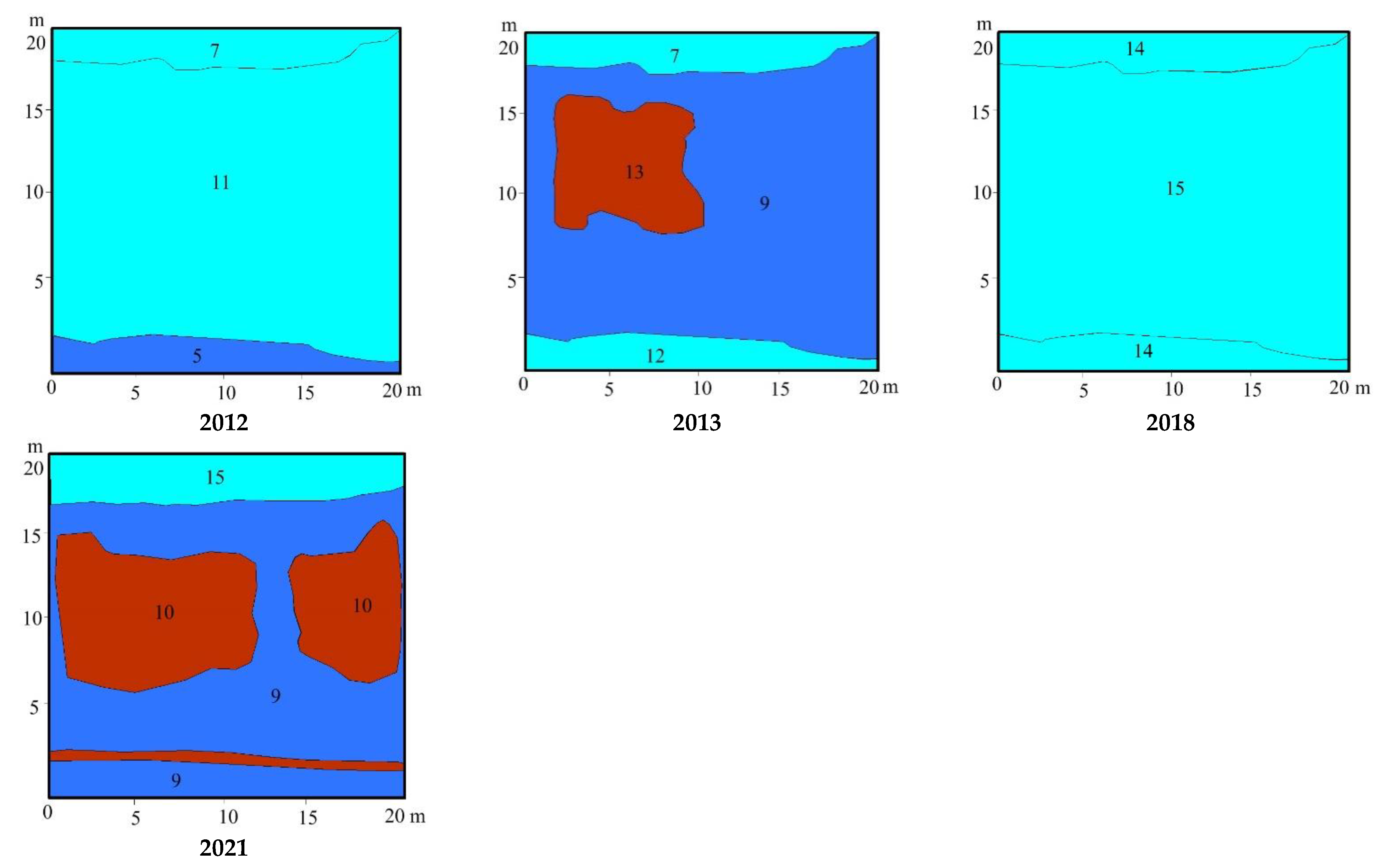
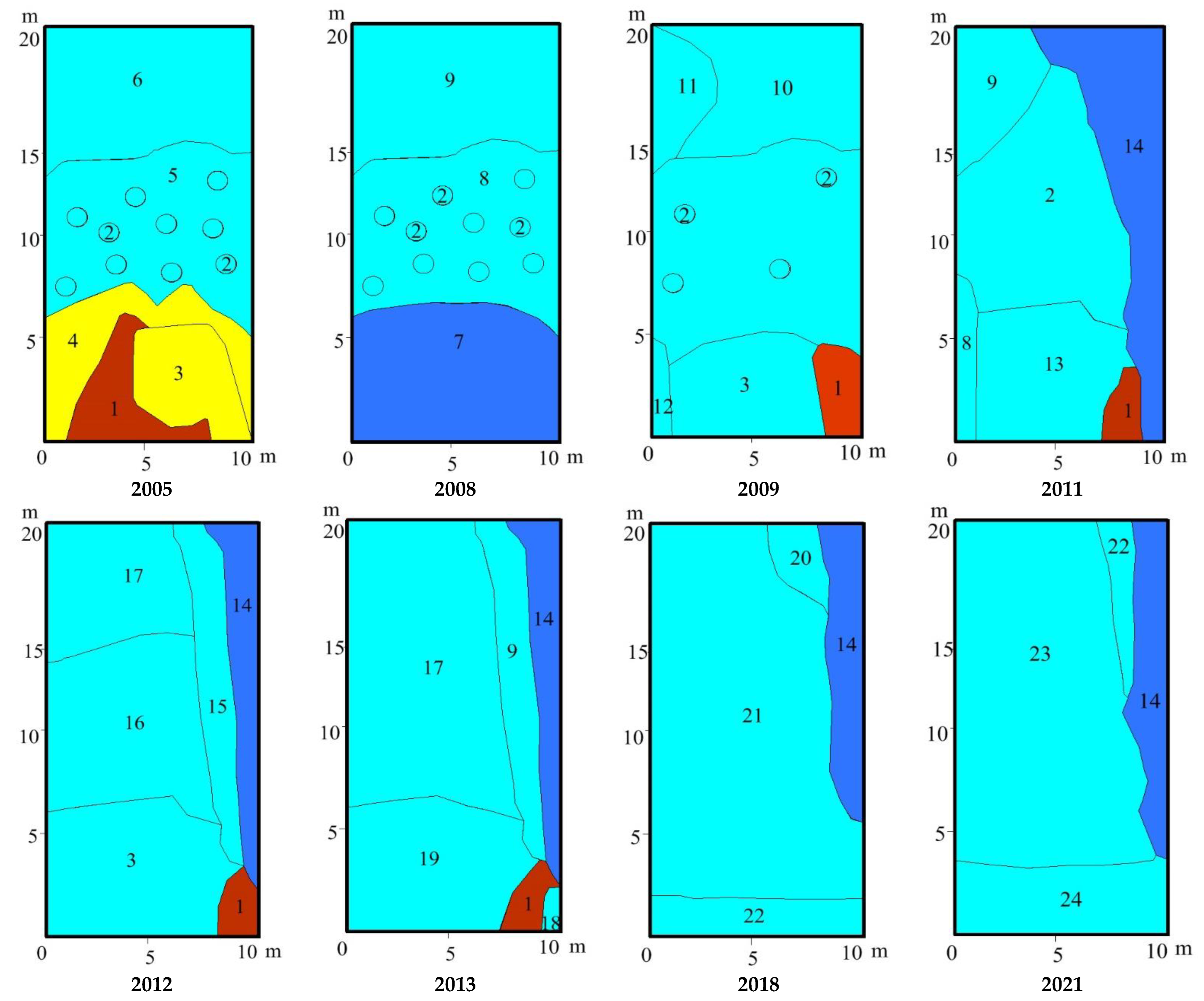
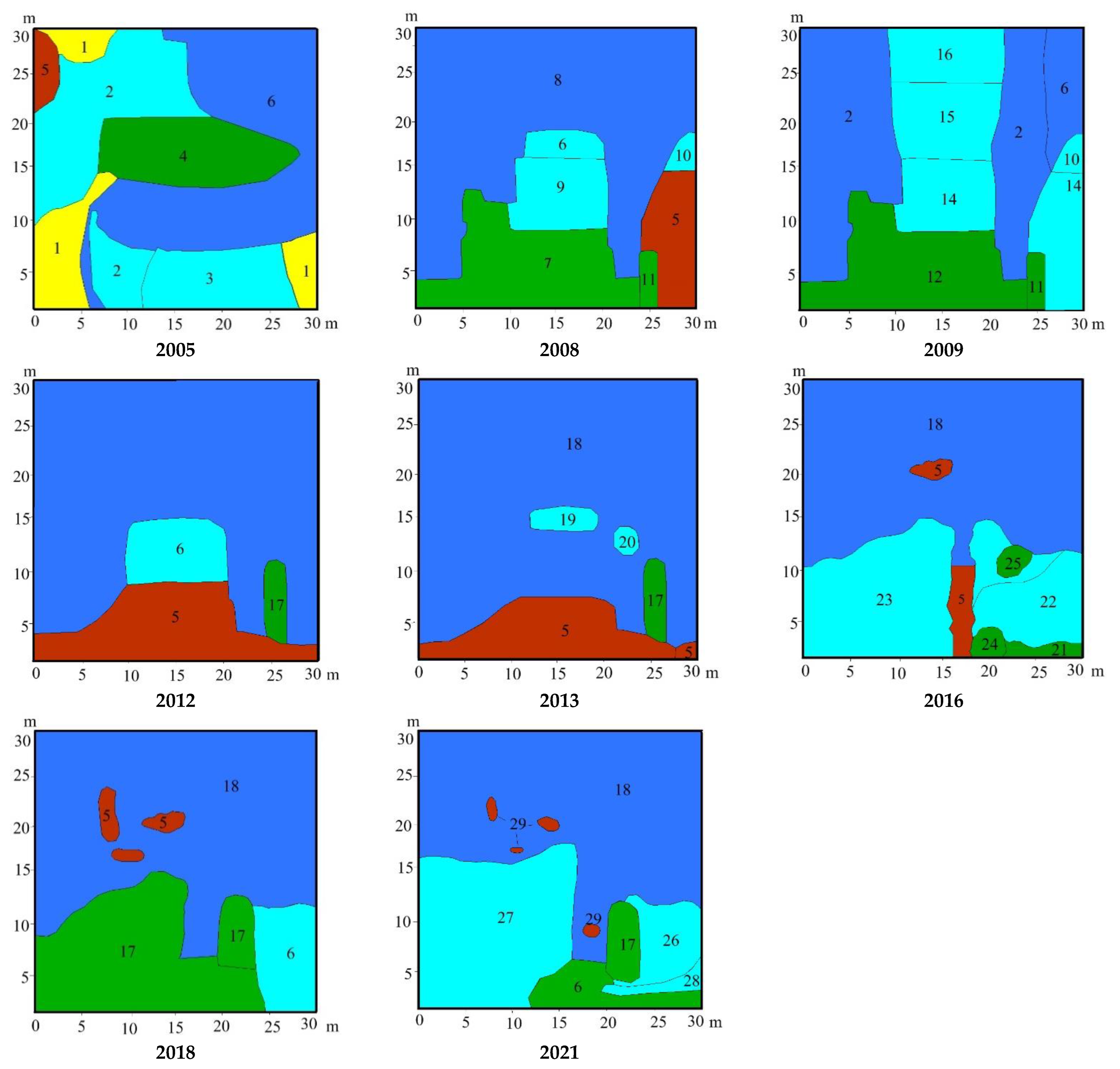
| Peatland | Plot N | Size, m | Orientation | Peat Depth, cm | Peat Type | Coordinates * |
|---|---|---|---|---|---|---|
| Tasinsky | 1 | 20 × 25 | NW | 75 | Eutrophic | 55°35′42.5″ N; 40°08′10.7″ E″ |
| 2 | 15 × 15 | SE | 200 | Oligotrophic | 55°35′35.8″ N; 40°07′56.0″ E″ | |
| 3 | 20 × 20 | NE | 210 | Mesotrophic | 55°35′18.1″ N; 40°07′17.8″ E″ | |
| 4 | 20 × 10 | SW | 90 | Mesotrophic | 55°35′49.4″ N; 40°08′01.4″ E″ | |
| 5 | 20 × 15 | NNW | 300 | Oligotrophic | 55°35′48.9″ N; 40°08′18.4″ E″ | |
| 6 | 15 × 15 | SSE | 250 | Mesotrophic | 55°36′04.4″ N; 40°08′58.4″ E″ | |
| 7 | 15 × 7 | SE | 200 | Eutrophic | 55°36′07.2″ N; 40°09′03.0″ E″ | |
| 9 | 30 × 30 | NNW | 175 | Eutrophic | 55°34′22.8″ N; 40°09′36.9″ E″ | |
| 10 | 20 × 20 | NNW | 20 | Eutrophic | 55°36′06.3″ N; 40°09′45.1″ E″ | |
| Garinsky | 8 | 20 × 25 | S | 30 | Eutrophic | 55°43′52.7″ N; 40°01′21.2″ E″ |
| Ostrovsky | 11 | 20 × 40 | E | 350 | Oligotrophic | 55°46′33.31″ N; 40°11′06.2″ E″ |
| Use | Ecological-Vegetation Complex | Dominant Plant Species | Peat Depth, m | GWL, cm | Plot No. |
|---|---|---|---|---|---|
| Sod peat extraction | Mire | Pinus sylvestris, Vaccinium uliginosum, Chamaedaphne calyculata, Eriophorum vaginatum, Sphagnum fallax | 2.15–3 | Ditch edge −20…−50 Main surface 0–15 Pool > +50 | 5, 6 |
| Forest-mire | P. sylvestris, Ledum palustre, V. uliginosum, Calla palustris, Comarum palustre, Sphagnum riparium | 2 | Ditch edge −40…−70; Main surface 0…−5 | 7 | |
| Peat milled extraction (submerged) | Hygrophilous-quagmire | Eriophorum angustifolium, C. palustris, Carex canescens, Typha latifolia, Sphagnum cuspidatum, S. fallax | 0.75–2.10 | Main surface −10…−50; Ditches +30…+50 | 1, 3, 4 |
| Water and coastal-water | Scirpus sylvaticus, Phragmites australis, Alisma plantago-aquatica | 1.75 | Pool +50…100, Main surface 0... −50 | 9 | |
| Peat milled extraction | Postpyrogenic (peat-mineral soil) | C. canescens, Betula pubescens, Epilobium adenocaulon, T. latifolia, Polytrichum commune | 0.1–0.2 0.75 0.1–0.3 | Ditches and pits +10… +30 Main surface −10…−50 | 8, 10 * |
| Postpyrogenic (raised bog peat) | B. pubescens, V. uliginosum, E. vaginatum, Polytrichum juniperinum | >4 | Main surface −20…−70 | 2, 11 |
| Month Year | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | Annual Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2007 | −12.6 | −15.2 | −4.7 | 5.4 | 12.3 | 18.7 | 16.8 | 17.1 | 12.6 | 5.9 | 1.04 | 0 | 4.77 |
| 2008 | −9.2 | −2.9 | 1.2 | 9.1 | 11.3 | 15.3 | 19.5 | 17.9 | 10.2 | 8.1 | 1.6 | −3.2 | 6.57 |
| 2009 | −7.5 | −6.4 | −1.7 | 3.7 | 13.8 | 17.9 | 18.9 | 15.9 | 13.6 | 5.3 | 0.76 | −8.3 | 5.49 |
| 2010 | −16 | −9.7 | −2.4 | 7.4 | 16.8 | 18.9 | 25.6 | 21.3 | 11.1 | 3.2 | 1.9 | −8.4 | 5.80 |
| 2011 | −9.6 | −13.2 | −3.7 | 4.9 | 14.3 | 18 | 22.7 | 18.8 | 11.1 | 5.8 | −1.9 | −1.7 | 5.45 |
| 2012 | −8.3 | −12.8 | −4.1 | 7.7 | 14.8 | 17.3 | 20.5 | 17.4 | 12.3 | 6.6 | 0.7 | −9.4 | 5.22 |
| 2013 | −9.8 | −5.0 | −7.0 | 5.7 | 16.2 | 19.4 | 19.0 | 18.1 | 10.3 | 5.6 | 2.8 | −2.7 | 6.05 |
| 2014 | −10.1 | −3.5 | 1.3 | 6.0 | 15.8 | 15.5 | 19.8 | 18.4 | 11.4 | 2.4 | −2.7 | −4.5 | 5.81 |
| 2015 | −6.5 | −3.4 | 0.4 | 5.12 | 15 | 18.1 | 18.0 | 16.2 | 13.9 | 3.1 | −0.7 | −1.2 | 6.50 |
| 2016 | −11.3 | −1.3 | −0.4 | 7.8 | 14.3 | 17.6 | 20.4 | 19.5 | 10.0 | 3.9 | −3.3 | −6.8 | 5.86 |
| 2017 | −9.7 | −5.9 | 1.6 | 5.3 | 10.4 | 14.2 | 17.7 | 18.0 | 12.5 | 4.6 | −1.3 | −1.0 | 5.53 |
| 2018 | −6.1 | −11 | −7.1 | 6.5 | 15.3 | 16.6 | 20.3 | 18.6 | 13.2 | 6.0 | −2.3 | −7.4 | 5.21 |
| 2019 | −9.0 | −3.2 | −0.6 | 6.8 | 16.0 | 18.8 | 16.3 | 15.4 | 11.0 | 7.8 | −0.5 | −1.1 | 6.47 |
| 2020 | −1.3 | −2.0 | 3.0 | 4.0 | 11.6 | 17.8 | 19.3 | 16.6 | 12.8 | 7.6 | 0.5 | −7.5 | 6.86 |
| 2021 | −6.9 | −13.7 | −3.0 | 6.8 | 14.7 | 20.2 | 21.8 | 20.0 | 9.4 | 5.5 | 1.1 | −8.5 | 5.61 |
| 1981–2010 | −8.0 | −7.9 | −2.1 | 6.0 | 12.9 | 17 | 19.2 | 16.8 | 10.9 | 4.8 | −2.2 | −6.61 | 5.06 |
| Month Year | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | Annual Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2007 | 38.0 | 41.1 | 36.3 | 44.2 | 86.3 | 27.8 | 119.2 | 90.3 | 56.3 | 66.4 | 99.8 | 54.6 | 760.3 |
| 2008 | 27.7 | 44.3 | 117.0 | 36.1 | 102.0 | 93.5 | 102.0 | 97.7 | 70.7 | 49.3 | 5.5 | 32.6 | 778.4 |
| 2009 | 43.4 | 38.2 | 42.3 | 30.2 | 21.7 | 86.2 | 80 | 80.9 | 31.4 | 153.7 | 58.0 | 49.1 | 715.1 |
| 2010 | 24.3 | 41.6 | 22.7 | 27.4 | 120.0 | 47.2 | 1.0 | 49.2 | 40.4 | 55.3 | 78.9 | 84.3 | 592.3 |
| 2011 | 53.7 | 33.0 | 45.2 | 36.9 | 20.4 | 22.7 | 89.5 | 13.7 | 70.1 | 36.1 | 66.1 | 69.4 | 556.8 |
| 2012 | 73.3 | 31.9 | 41.8 | 89.2 | 41.8 | 139.1 | 33.5 | 57.0 | 0 | 0 | 50.3 | 61.9 | 619.8 |
| 2013 | 33.2 | 21.0 | 77.0 | 46 | 55.8 | 33.8 | 91.1 | 33.1 | 185.2 | 34.9 | 39.4 | 42 | 692.5 |
| 2014 | 41.9 | 26.1 | 18.9 | 15.3 | 33.2 | 130.7 | 24.9 | 68.5 | 16.8 | 61.6 | 20.6 | 71.1 | 529.6 |
| 2015 | 53.5 | 34.8 | 4.7 | 42.6 | 60.5 | 127.7 | 45.3 | 49.7 | 28.7 | 31.8 | 31.4 | 50.8 | 561.5 |
| 2016 | 90.7 | 52.2 | 32.4 | 46.1 | 41.6 | 42.4 | 82.2 | 60.4 | 49.3 | 15.0 | 64.3 | 41.1 | 617.7 |
| 2017 | 31.0 | 34.2 | 42.0 | 37.4 | 54.9 | 80.8 | 96.5 | 36.8 | 64.5 | 89.6 | 55.0 | 88.5 | 711.2 |
| 2018 | 28.9 | 22.9 | 34.6 | 69.8 | 33.9 | 34.8 | 67.6 | 50.4 | 52.5 | 56.3 | 20.7 | 49.6 | 522.0 |
| 2019 | 37.4 | 40.0 | 46.6 | 25.9 | 49.4 | 50.8 | 78 | 89.9 | 42.2 | 84.3 | 26.9 | 30.3 | 601.7 |
| 2020 | 47.3 | 27.1 | 28.1 | 50.2 | 77.4 | 47.6 | 100 | 14.3 | 27.1 | 27.5 | 23.4 | 17.3 | 487.3 |
| 2021 | 76.0 | 60.0 | 33.0 | 94.0 | 73.0 | 36.0 | 14.0 | 49.0 | 91.0 | 30.1 | 81.0 | 72.0 | 709.1 |
| 1981–2010 | 47 | 38 | 34 | 41 | 46 | 81 | 68 | 66 | 58 | 72 | 55 | 56 | 662 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vozbrannaya, A.; Antipin, V.; Sirin, A. After Wildfires and Rewetting: Results of 15+ Years’ Monitoring of Vegetation and Environmental Factors in Cutover Peatland. Diversity 2023, 15, 3. https://doi.org/10.3390/d15010003
Vozbrannaya A, Antipin V, Sirin A. After Wildfires and Rewetting: Results of 15+ Years’ Monitoring of Vegetation and Environmental Factors in Cutover Peatland. Diversity. 2023; 15(1):3. https://doi.org/10.3390/d15010003
Chicago/Turabian StyleVozbrannaya, Anna, Vladimir Antipin, and Andrey Sirin. 2023. "After Wildfires and Rewetting: Results of 15+ Years’ Monitoring of Vegetation and Environmental Factors in Cutover Peatland" Diversity 15, no. 1: 3. https://doi.org/10.3390/d15010003
APA StyleVozbrannaya, A., Antipin, V., & Sirin, A. (2023). After Wildfires and Rewetting: Results of 15+ Years’ Monitoring of Vegetation and Environmental Factors in Cutover Peatland. Diversity, 15(1), 3. https://doi.org/10.3390/d15010003








