The First Data on Harpacticoid Copepod Diversity of the Deep-Water Zone of Lake Baikal (Siberia, Russia)
Abstract
:1. Introduction
2. Materials and Methods
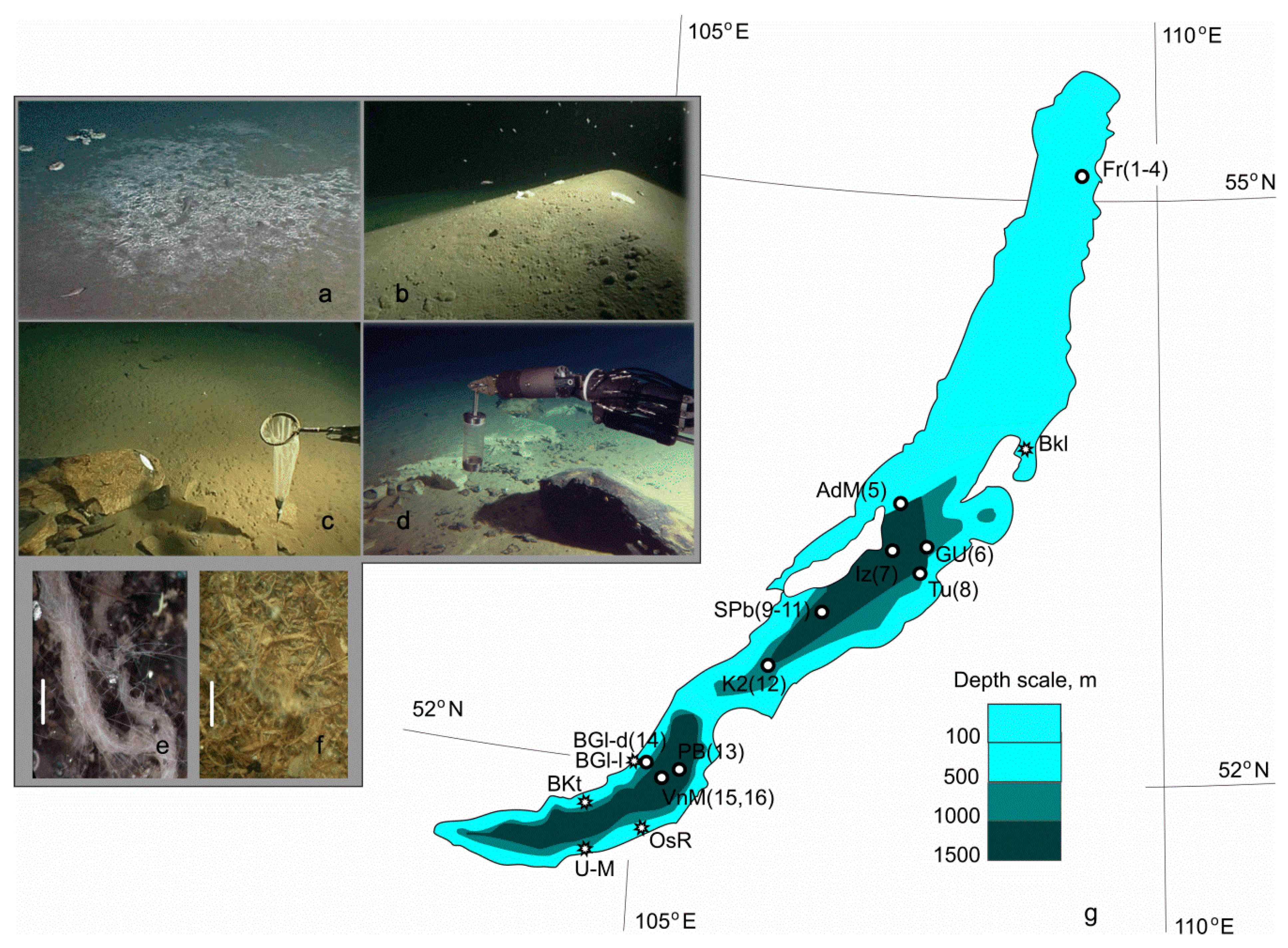
2.1. Study Sites
| No. | Site | Abbreviation | Date | Coordinates | Depth, m | Substrata |
|---|---|---|---|---|---|---|
| Northern Baikal basin | ||||||
| 1 | Frolikha hydrothermal seep # | Fr | 23 July 2010 | 55.517° N, 109.767° E | 409 | grv, spg, fTh |
| 2 | —″— | —″— | 23 July 2010 | —″— | 432 | y_al, slt |
| 3 | —″— | —″— | 25 July 2010 | —″— | 473 | Slt, sd |
| 4 | —″— | —″— | 25 July 2010 | —″— | 482 | br_al, fTh |
| Central Baikal basin | ||||||
| 5 | Akademicheskiy Ridge, mud volcano # | AdM | 8 July 2017 | 53.415° N, 107.875° E | 488 | al, cl |
| 6 | Gorevoy Utes oil–methane seep # | GU | 30 June 2016 | 53.305° N, 108.391° E | 883 | br_al |
| 7 | Near Izhimei Cape | Iz | 30 June 2017 | 53.165° N, 107.993° E | 1632 | dt, fTh, slt, cl |
| 8 | Near Turka settlement | Tu | 18 July 2010 | 52.933° N, 108.117° E | 456 | br_al, dt, fTh |
| 9 | Saint Petersburg methane seep # | SPb | 13 July 2010 | 52.883° N, 107.167° E | 1404 | al, dt, fTh, slt |
| 10 | —″— | —″— | 13 July 2010 | —″— | 1401 | jm, bl_al |
| 11 * | —″— | —″— | 15 July 2010 | —″— | 1396 | al, jm |
| 12 | Selenga region, mud volcano K-2 # | K2 | 2 July 2013 | 52.583° N, 106.767° E | 940 | br_al, dt, cl |
| Southern Baikal basin | ||||||
| 13 | PosolBank methane seep # | PB | 19 June 2010 | 52.038° N 105.846° E | 490–530 | al, fTh, cl |
| 14 | Bolshoe Goloustnoe methane seep # | BGl-d | 17 June 2010 | 51.983° N, 105.367° E | 270 | fTh, slt, cl |
| 15 | Mud Volcano Malenky # | VnM | 20 June 2010 | 51.922° N 105.636° E | 1368 | al, cl, cr |
| 16 | —″— | —″— | 3 July 2015 | —″— | 1393 | al, dt, cr |
| Site | Abbreviation | Date | Depth, m |
|---|---|---|---|
| Near Baklaniy Island | Bkl | 17 August 2017 | 10 |
| Near Bolshoe Goloustnoe Village | BGl-l | 17 June 2010 | 93 |
| Near the River Bolshaya Osinovka mouth | OsR | 18 June 1968 | 45 |
| Utulik-Murina area | U-M | 12 June 1969 | 49 |
| Near Bolshie Koty Village | BKt | 17 October 1969 | 100 |
2.2. Field and Laboratory Methods
2.3. Specimens Examined
2.4. Diversity Analysis
3. Results
3.1. Fauna Composition and Description of Collected Morphological Species
| Taxa | Site (See Table 1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fr | AdM | GU | Iz | Tu | SPb | K2 | PB | BGl-D | VnM | |
| Bryocamptus (Bryocamptus) cf. abyssicola (Borutzky, Okuneva, 1972) | + | + | ||||||||
| B. (B.) cf. sinuatus (Borutzky, Okuneva, 1972) | + | |||||||||
| Bryocamptus sp. 1 | + | |||||||||
| Bryocamptus sp. 2 | + | |||||||||
| Bryocamptus sp. 3 | + | |||||||||
| Bryocamptus sp. 4 | + | |||||||||
| Bryocamptus sp. 5 | + | |||||||||
| Bryocamptus (Rheocamptus) sp. 6 | + | |||||||||
| Bryocamptus sp. 7 | + | |||||||||
| B. (Echinocamptus) smirnovi (Borutzky, 1931) | + | + | + | |||||||
| B. (E.) cf. parvus (Borutzky, 1931) | + | |||||||||
| Bryocamptus sp. 8 | + | + | ||||||||
| Attheyella (Ryloviella) baikalensis Borutzky, 1931 | + | + | + | |||||||
| Moraria (Baikalomoraria) spinulosa Borutzky, Okuneva, 1972 | + | |||||||||
| M. (B.) longicauda Borutzky, 1952 | + | |||||||||
| M. (B.) cf. sinuata Borutzky, 1952 | + | |||||||||
| Moraria sp. 1 | + | + | ||||||||
| Moraria sp. 2 | + | |||||||||
| Moraria sp. 3 | + | |||||||||
| Harpacticoida sp. | + | + | + | + | + | |||||




3.2. Abundance and Diversity of Harpacticoids
| No. | Site Abbreviation (Figure 1; Table 1) | Abundance (ind. m−2) | Harpacticoid Portion in the Meiobenthos (%) | Other Groups’ (Dominant) Portion in the Meiobenthos and Dominant Harpacticoid Species |
|---|---|---|---|---|
| 1 | Fr | 1000 | 1.25 | B. sp. 6 (94% of harpacticoids abundance) |
| 2 | 6635 | 23.00 | ||
| 3 | 4423 | 33.00 | ||
| 4 | 2216 | 6.00 | ||
| 5 | AdM | 121 | <20 | B. cf. abyssicola (100% of harpacticoids abundance) |
| 6 | GU | 909 | n.d. | M. cf. sinuata (100% of harpacticoids abundance) |
| 7 | Iz | n.d. | 11.00 | B. sp. 8 (~50% of harpacticoids abundance) |
| 8 | Tu | 2424 | n.d. | 4 species (25% for each species) |
| 9 | SPb | 19,280 | 38.09 | nematodes (57%), B. sp. 8 (40 % of harpacticoids abundance) |
| 10 | n.d. | n.d. | B. cf. sinuatus (60% of harpacticoids abundance) | |
| 11 | 19,280 | 38.10 | B. smirnovi (96% of harpacticoids abundance) | |
| 12 | K2 | n.d. | n.d. | n.d. |
| 13 | PB | 16,968 | 22.58 | cyclopoids (66%); B. smirnovi, A. baicalensis |
| 14 | BGl-d | 226 | 7.00 | B. cf. abyssicola (100% of harpacticoids abundance) |
| 15 | VnM | 121 | 8.00 | Moraria spinulosa (67 % of harpacticoids abundance) |
| 16 | 121 | 12.50 | nematodes (38%), cyclopoids (31%); B. sp. 4, B. cf. abyssicola (50% for each species) |
4. Discussion
4.1. Fauna Structure
4.2. Quantitative Aspects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherbakov, D.Y.; Kovalenkova, M.V.; Maikova, O.O. Recent results of molecular-phylogenetic studies of endemic invertebrates inhabiting Lake Baikal. Vavilov J. Genet. Breed. 2016, 20, 403–407. (In Russian) [Google Scholar]
- Boxshall, G.A.; Evstigneeva, T.D. The evolution of species flocks of copepods in Lake Baikal: A preliminary analysis. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1994, 44, 235–245. [Google Scholar]
- Okuneva, G.L.; Evstigneeva, T.D. Harpacticoida. In Index of Animal Species Inhabiting Lake Baikal and Its Catchment Area; Timoshkin, O.A., Ed.; Nauka: Novosibirsk, Russia, 2001; Volume 1, pp. 468–490. (In Russian) [Google Scholar]
- Takhteev, V.V. Trends in the evolution of Baikal amphipods and evolutionary parallels with some marine malacostracan faunas. Adv. Ecol. Res. 2000, 31, 197–220. [Google Scholar]
- Khlystov, O.M.; Khabuev, A.V.; Minami, H.; Hachikubo, A.; Krylov, A.A. Gas hydrates in Lake Baikal. Limnol. Freshw. Biol. 2018, 1, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Zemskaya, T.I.; Sitnikova, T.Y.; Pogodaeva, T.V.; Mekhanikova, I.V.; Naumova, T.V.; Shubenkova, O.V.; Chernitsina, S.M.; Kotsar, O.V.; Khlystov, O.M. Faunal communities at sites of gas- and oil-bearing fluids in Lake Baikal. Geo.-Mar. Lett. 2012, 32, 437–451. [Google Scholar] [CrossRef]
- Levin, L.A.; Baco, A.R.; Bowden, D.A.; Colaco, A.; Cordes, E.E.; Cunha, M.R.; Demopoulos, A.W.J.; Gobin, J.; Grupe, B.M.; Le, J.; et al. Hydrothermal Vents and Methane Seeps: Rethinking the Sphere of Influence. Front. Mar. Sci. 2016, 3, 72. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.A.; Gulin, M.B. Ecology of meiobenthos inhabiting the local biotopes of gas seeps in coastal waters of Crimea: Taxonomic composition and distribution in bottom sediments. J. Sib. Fed. Univ. Biol. 2020, 13, 410–423. (In Russian) [Google Scholar] [CrossRef]
- Sitnikova, T.Y.; Naumova, T.V.; Mekhanikova, I.V.; Kiyashko, S.I.; Kalmychkov, G.V.; Karanovic, I.; Zakharenko, A.S.; Bukin, Y.S.; Khabuev, A.V.; Ivanov, V.G.; et al. Sluggish methane discharge and biological traits of benthic invertebrates in Lake Baikal. Hydrobiologia 2022, 849, 1947–1968. [Google Scholar] [CrossRef]
- Naumova, T.V.; Sitnikova, T.Y.; Gagarin, V.G. The species composition and distribution of free-living nematodes (Nematoda) in an area of natural oil and gas seeps in Lake Baikal. Inland Water Biol. 2012, 5, 161–168. [Google Scholar] [CrossRef]
- Okuneva, G.L. Harpacticoids of Lake Baikal; Irkutsk University Press: Irkutsk, Russia, 1989; p. 152. (In Russian) [Google Scholar]
- Kochanova, E.; Mayor, T.; Väinölä, R. Cryptic diversification of harpacticoid copepod Harpacticella inopinata in Lake Baikal. In e-Abstract Booklet of International Conference on Copepoda. 2022, p. 75. Available online: https://e-icoc-2022.com/election/election/ (accessed on 20 November 2022).
- Kozhov, M. Lake Baikal and Its Life; W. Junk: The Hague, The Netherlands, 1963; Volume 11, p. 344. [Google Scholar]
- Kuznetsov, A.P.; Strizhev, V.P.; Kuzin, V.S.; Fialkov, V.A.; Yastrebov, V.S. New in Lake Baikal nature. Community based on bacterial chemosynthesis. Izv. Akad. Nauk SSR Biol. 1991, 5, 766–772. (In Russian) [Google Scholar]
- Shanks, W.C.; Callender, E. Thermal springs in Lake Baikal. Geology 1992, 20, 495–497. [Google Scholar] [CrossRef]
- Sitnikova, T.Y.; Zemskaya, T.I.; Chernitsyna, S.M.; Likhoshway, A.V.; Klimenkov, I.V.; Naumova, T.V. Structure of biocenosis formed on bitumen mounds in the abyssal zone of Lake Baikal. Russ. J. Ecolog. 2015, 46, 292–298. [Google Scholar] [CrossRef]
- Sitnikova, T.Y.; Sideleva, V.G.; Kiyashko, S.I.; Zemskaya, T.I.; Mekhanikova, I.V.; Khlystov, O.M.; Khal’zov, I.A. A Comparative Analysis of Marcroinvertebrate and Fish Communities Associated with Methane and Oil-Methane Seeps in the Abissal Area of Lake Baikal. Uspekhi Sovrem. Biol. 2017, 137, 373–386. (In Russian) [Google Scholar]
- Borutsky, E.V. The Fresh-Water and Brack-Water Harpacticoids of the U.S.S.R. In Keys to Determination of Fresh-Water Organisms of the U.S.S.R., Fresh-Water Fauna 3; Institute of Ichtiology Press: Leningrad, Russia, 1931; p. 245. (In Russian) [Google Scholar]
- Borutsky, E.V. Crustaceans Freshwater Harpacticoids. In Fauna of USSR, Crustacea 3; AN USSR: Moscow-Leningrad, Russia, 1952; p. 425. (In Russian) [Google Scholar]
- Borutsky, E.V.; Okuneva, G.L. New species of Harpacticoida (Copepoda) from South Baikal. III. Russ. Zool. Z. 1972, 51, 1147–1164. (In Russian) [Google Scholar]
- Borutsky, E.V.; Okuneva, G.L. New species of Copepoda Harpacticoida from Southern Baikal. Communication 2. Bull. Soc. Nat. Moscow 1972, 77, 60–69. (In Russian) [Google Scholar]
- Huys, R.; Boxshall, G.A. Copepod Evolution; The Ray Society: London, UK, 1991; p. 468. [Google Scholar]
- Gotelli, N.J.; Colwell, R.K. Estimating species richness. In Biological Diversity: Frontiers in Measurement and Assessment; Magurran, A.E., Mc Gill, B.J., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 39–54. [Google Scholar]
- Kaygorodova, I.A. Deep-water Fauna of Oligochaeta (Annelida, Clitellata) Near hydrothermal spring of the Frolikha Bay, Northern Baikal (East Siberia, Russia). J. Sib. Fed. Univ. Biol. 2011, 4, 117–132. (In Russian) [Google Scholar]
- Lee, W.; Huys, R. New Aegisthidae (Copepoda: Harpacticoida) from western Pacific cold seeps and hydrothermal vents. Zool. J. Linn. Soc. 2001, 131, 249. [Google Scholar]
- Kottmann, J.; Kihara, T.C.; Glatzel, T.; Veit-Köhler, G. A new species of Wellsopsyllus (Copepoda, Harpacticoida, Paramesochridae) from the deep Southern Oceanand remarks on its biogeography. Helgol. Mar. Res. 2013, 67, 33–48. [Google Scholar] [CrossRef]
- Park, E.-O.; Rohal, M.; Lee, W. A New Deep-Sea Enhydrosoma (Copepoda, Harpacticoida, Cletodidae) from the Northern Gulf of Mexico. Taxonomy 2021, 1, 23. [Google Scholar] [CrossRef]
- Mathiske, A.; Thistle, D.; Gheerardin, H.; Veit-Köhler, G. Deep sea without limits—Four new closely related species of Emertonia Wilson,1932 (Copepoda: Harpacticoida: Paramesochridae) show characters with a world-wide distribution. Zootaxa 2021, 5051, 443–486. [Google Scholar] [CrossRef]
- Heptner, M.V.; Ivanenko, V.N. Copepoda (Crustacea) of hydrothermal ecosystems of the World Ocean. Arthropoda Selecta 2002, 11, 117–134. [Google Scholar]
- Nakasugi, F.; Shimanaga, M.; Nomaki, H.; Watanabe, H.K.; Kitahashi, T.; Motomura, Y.; Iseda, K. Simple harpacticoid composition observed at deep hydrothermal vent sites on sea knoll calderas in the North-west Pacific. J. Mar. Biolog. Assoc. 2022, 101, 947–956. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fefilova, E.B.; Sitnikova, T.Y.; Novikov, A.A. The First Data on Harpacticoid Copepod Diversity of the Deep-Water Zone of Lake Baikal (Siberia, Russia). Diversity 2023, 15, 94. https://doi.org/10.3390/d15010094
Fefilova EB, Sitnikova TY, Novikov AA. The First Data on Harpacticoid Copepod Diversity of the Deep-Water Zone of Lake Baikal (Siberia, Russia). Diversity. 2023; 15(1):94. https://doi.org/10.3390/d15010094
Chicago/Turabian StyleFefilova, Elena B., Tatyana Y. Sitnikova, and Aleksandr A. Novikov. 2023. "The First Data on Harpacticoid Copepod Diversity of the Deep-Water Zone of Lake Baikal (Siberia, Russia)" Diversity 15, no. 1: 94. https://doi.org/10.3390/d15010094
APA StyleFefilova, E. B., Sitnikova, T. Y., & Novikov, A. A. (2023). The First Data on Harpacticoid Copepod Diversity of the Deep-Water Zone of Lake Baikal (Siberia, Russia). Diversity, 15(1), 94. https://doi.org/10.3390/d15010094






