First Report of Diseases and Compromised Health Conditions on Hard Corals around Rodrigues Island, Southwest Indian Ocean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Disease and CHC Prevalence on Hard Corals
2.3. Benthic Cover Assessment
2.4. Statistical Analysis
3. Results
3.1. Coral Community Structure
3.2. Coral Disease Occurrence and Prevalence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harvell, D.; Jordán-Dahlgren, E.; Merkel, S.; Rosenberg, E.; Raymundo, L.; Smith, G.; Weil, E.; Willis, B. Coral Disease, Environmental Drivers, and the Balance between Coral and Microbial Associates. Oceanography 2007, 20, 172–195. [Google Scholar] [CrossRef]
- Weil, E. Coral Reef Diseases in the Wider Caribbean. In Coral Health and Disease; Rosenberg, E., Loya, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 35–68. ISBN 978-3-642-05863-9. [Google Scholar]
- Weil, E.; Smith, G.; Gil-Agudelo, D.L. Status and Progress in Coral Reef Disease Research. Dis. Aquat. Organ. 2006, 69, 1–7. [Google Scholar] [CrossRef]
- Woodley, C.M.; Downs, C.A.; Bruckner, A.W.; Porter, J.W.; Galloway, S.B. Diseases of Coral; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 978-0-8138-2411-6. [Google Scholar]
- Harvell, C.D.; Kim, K.; Burkholder, J.M.; Colwell, R.R.; Epstein, P.R.; Grimes, D.J.; Hofmann, E.E.; Lipp, E.K.; Osterhaus, A.D.M.E.; Overstreet, R.M.; et al. Emerging Marine Diseases-Climate Links and Anthropogenic Factors. Science 1999, 285, 1505–1510. [Google Scholar] [CrossRef]
- Green, E.; Bruckner, A. The Significance of Coral Disease Epizootiology for Coral Reef Conservation. Biol. Conserv. 2000, 96, 347–361. [Google Scholar] [CrossRef]
- Cramer, K.L.; Jackson, J.B.C.; Donovan, M.K.; Greenstein, B.J.; Korpanty, C.A.; Cook, G.M.; Pandolfi, J.M. Widespread Loss of Caribbean Acroporid Corals Was Underway before Coral Bleaching and Disease Outbreaks. Sci. Adv. 2020, 6, eaax9395. [Google Scholar] [CrossRef] [PubMed]
- Aronson, R.; Precht, W.; Aronson, R.B.; Precht, W.F. White-Band Disease and the Changing Face of Caribbean Coral Reefs. Hydrobiologia 2001, 460, 25–38. [Google Scholar] [CrossRef]
- Cramer, K.L.; Donovan, M.K.; Jackson, J.B.C.; Greenstein, B.J.; Korpanty, C.A.; Cook, G.M.; Pandolfi, J.M. The Transformation of Caribbean Coral Communities since Humans. Ecol. Evol. 2021, 11, 10098–10118. [Google Scholar] [CrossRef]
- Raymundo, L.J.; Rosell, K.B.; Reboton, C.T.; Kaczmarsky, L. Coral Diseases on Philippine Reefs: Genus Porites Is a Dominant Host. Dis. Aquat. Organ. 2005, 64, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Bourne, D.G.; Willis, B.L. Dynamics of Seasonal Outbreaks of Black Band Disease in an Assemblage of Montipora Species at Pelorus Island (Great Barrier Reef, Australia). Proc. Biol. Sci. 2009, 276, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Aeby, G.S.; Williams, G.J.; Franklin, E.C.; Kenyon, J.; Cox, E.F.; Coles, S.; Work, T.M. Patterns of Coral Disease across the Hawaiian Archipelago: Relating Disease to Environment. PLoS ONE 2011, 6, e20370. [Google Scholar] [CrossRef]
- Weil, E.; Irikawa, A.; Casareto, B.; Suzuki, Y. Extended Geographic Distribution of Several Indo-Pacific Coral Reef Diseases. Dis. Aquat. Org. 2012, 98, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Das, R.R.; Sreeraj, C.R.; Mohan, G.; Simon, N.T.; Ramachandran, P.; Ramachandran, R.; Krishnan, P.; Kumar, D.S.V. Evidence of Coral Diseases, Phase Shift, and Stressors in the Atolls of Lakshadweep Islands, Arabian Sea—With Geographical Notes on Their Occurrence within the Indian EEZ and Contiguous International Waters. Diversity 2023, 15, 382. [Google Scholar] [CrossRef]
- Bharath, M.S.; Chandran, R.; Aeby, G.S.; Senthilkumaran, R.; Ramkumaran, K.; Thanappan, V.P.P.; Chaudhury, N.R.; Satyanarayana, C. First Report of Yellow-Banded Tissue Loss Disease on Coral Reefs Outside the Arabian/Persian Gulf. Dis. Aquat. Org. 2023, 153, 1–8. [Google Scholar] [CrossRef]
- Burke, S.; Pottier, P.; Lagisz, M.; Macartney, E.L.; Ainsworth, T.; Drobniak, S.M.; Nakagawa, S. The Impact of Rising Temperatures on the Prevalence of Coral Diseases and Its Predictability: A Global Meta-Analysis. Ecol. Lett. 2023, 26, 1466–1481. [Google Scholar] [CrossRef] [PubMed]
- Aeby, G.S.; Shore, A.; Jensen, T.; Ziegler, M.; Work, T.; Voolstra, C.R. A Comparative Baseline of Coral Disease in Three Regions along the Saudi Arabian Coast of the Central Red Sea. PLoS ONE 2021, 16, e0246854. [Google Scholar] [CrossRef]
- Myers, R.L.; Raymundo, L.J. Coral Disease in Micronesian Reefs: A Link between Disease Prevalence and Host Abundance. Dis. Aquat. Organ. 2009, 87, 97–104. [Google Scholar] [CrossRef]
- Mohamed, A.; Sweet, M. Current Knowledge of Coral Diseases Present Within the Red Sea. In Oceanographic and Biological Aspects of the Red Sea. Springer Oceanography; Springer: Cham, Switzerland, 2019; pp. 387–400. ISBN 978-3-319-99416-1. [Google Scholar]
- Hazraty-Kari, S.; Tavakoli-Kolour, P.; Das, R.R.; Farhadi, M.; Barkhordari-Ahmadi, A.; Yahyavi, M.; Rezai, H. Baseline Assessment of Coral Diseases in an Environmentally Extreme Environment of the Northern Persian Gulf. Mar. Pollut. Bull. 2021, 171, 112707. [Google Scholar] [CrossRef]
- Beeden, R.; Willis, B.L.; Raymundo, L.; Page, C.; Weil, E. Underwater Cards for Assessing Coral Health on Indo-Pacific Reefs. Coral Reef Targeted Research and Capacity Building for Management Program; Currie Communications: Melbourne, VIC, Australia, 2008. [Google Scholar]
- Weil, E.; Hooten, A.J. Underwater Cards for Assessing Coral Health on Caribbean Coral Reefs; GEF-CRTR-Currie Communications: Melbourne, VIC, Australia, 2008. [Google Scholar]
- Aldyza, N. Afkar Analisis Genus Dan Penyakit Karang Di Perairan Pulau Tuan Kecamatan Peukan Bada Kabupaten Aceh Besar. BIOTIK J. Ilm. Biol. Teknol. Dan Kependidikan 2017, 3, 107–115. [Google Scholar] [CrossRef]
- Hadi, T.A. Impacts of sedimentation on stony corals. Oseana 2017, 42, 45–58. [Google Scholar] [CrossRef]
- Box, S.J.; Mumby, P.J. Effect of Macroalgal Competition on Growth and Survival of Juvenile Caribbean Corals. Mar. Ecol. Prog. Ser. 2007, 342, 139–149. [Google Scholar] [CrossRef]
- Yucharoen, M. Pink Pigmentation Response during Recovery Period after Coral Bleaching. Master’s Thesis, Shizuoka University, Shizuoka, Japan, 2016. [Google Scholar]
- Jogee, S.Y.; Jeetun, S.; Ricot, M.; Taleb-Hossenkhan, N.; Mattan-Moorgawa, S.; Kaullysing, D.; Riemann, P.; Blanc, L.; Casareto, B.E.; Suzuki, Y.; et al. Photo-Physiology of Healthy-Looking and Diseased/Health-Compromised Hard Corals from Mauritius Island, Western Indian Ocean. Indo Pac. J. Ocean. Life 2023, 7, 27–37. [Google Scholar] [CrossRef]
- McClanahan, T.; Munbodhe, V.; Naggea, J.; Muthiga, N.; Bhagooli, R. Rare coral and reef fish species status, possible extinctions, and associated environmental perceptions in Mauritius. Conserv. Sci. Pract. 2021, 3, e527. [Google Scholar] [CrossRef]
- Bhagooli, R.; Mattan-Moorgawa, S.; Kaullysing, D.; Taleb-Hossenkhan, N. A first field report of coral diseases around Mauritius Island, Western Indian Ocean. West. Indian Ocean. J. Mar. Sci. 2017, 1/2, 71–72. [Google Scholar]
- Mattan-Moorgawa, S.M.; Kaullysing, D.; Taleb-Hossenkhan, N.; Rughooputh, S.D.D.V.; Bhagooli, R. Photophysiology of in hospite zooxanthellae in diseased and non-diseased scleractinian corals from Belle Mare, Mauritius. WIOJMS 2017, 1/2, 1–12. [Google Scholar]
- Bhagooli, R.; Jogee, S.; Kaullysing, D.; Ramah, S. First report of White Syndrome on branching Acropora at Saya de Malha, Mascarene Plateau. West. Indian Ocean. J. Mar. Sci. 2021, 2, 189–192. [Google Scholar] [CrossRef]
- Sheridan, C.; Baele, J.M.; Kushmaro, A.; Fréjaville, Y.; Eeckhaut, I. Terrestrial Runoff Influences White Syndrome Prevalence in SW Madagascar. Mar. Environ. Res. 2014, 101, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Montano, S.; Strona, G.; Seveso, D.; Galli, P. First Report of Coral Diseases in the Republic of Maldives. Dis. Aquat. Org. 2012, 101, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Montano, S.; Strona, G.; Seveso, D.; Maggioni, D.; Galli, P. Widespread Occurrence of Coral Diseases in the Central Maldives. Mar. Freshw. Res. 2015, 67, 1253–1262. [Google Scholar] [CrossRef]
- Sheppard, C.R.C.; Ateweberhan, M.; Bowen, B.W.; Carr, P.; Chen, C.A.; Clubbe, C.; Craig, M.T.; Ebinghaus, R.; Eble, J.; Fitzsimmons, N.; et al. Reefs and Islands of the Chagos Archipelago, Indian Ocean: Why It Is the World’s Largest No-Take Marine Protected Area. Aquat. Conserv. 2012, 22, 232–261. [Google Scholar] [CrossRef]
- McClanahan, T.; Weil, E.; Maina, J. Strong Relationship between Coral Bleaching and Growth Anomalies in Massive Porites. Glob. Change Biol. 2009, 15, 1804–1816. [Google Scholar] [CrossRef]
- Séré, M.; Chabanet, P.; Turquet, J.; Quod, J.-P.; Schleyer, M. Identification and Prevalence of Coral Diseases Observed on Three Western Indian Ocean (WIO) Coral Reefs. Dis. Aquat. Org. 2015, 114, 249–261. [Google Scholar] [CrossRef]
- Bhagooli, R.; Mattan-Moorgawa, S.; Kaullysing, D.; Chumun, P.K.; Klaus, R.; Munbodhe, V. Status and sustainability of reefs and shorelines of the Republic of Mauritius. In Sustainable Development Goals; Parsad, R., Ed.; Star Publication Pvt. Ltd.: New Delhi, India, 2021; pp. 142–177. [Google Scholar]
- Fenner, D.; Clark, T.H.; Turner, J.R.; Chapman, B. A checklist of the corals of the island state of Rodrigues, Mauritius. J. Nat. Hist. 2004, 38, 3091–3102. [Google Scholar] [CrossRef]
- Jouval, F.; Latreille, A.C.; Bureau, S.; Adjeroud, M.; Penin, L. Multiscale Variability in Coral Recruitment in the Mascarene Islands: From Centimetric to Geographical Scale. PLoS ONE 2019, 14, e0214163. [Google Scholar] [CrossRef] [PubMed]
- Hardman, E.R.; Meunier, S.; Turner, J.R.; Lynch, T.L.; Klaus, R. The extent of coral bleaching in Rodrigues. J. Nat. Hist. 2004, 38, 3077–3089. [Google Scholar] [CrossRef]
- Klaus, R. Assessing the impact of the 2015–2016 coral bleaching in Rodrigues (Republic of Mauritius). In Proceedings of the Western Indian Ocean Marine Science Association Symposium (WIOMSA) 2017, Dar es Salaam, Tanzania, 30 October–4 November 2017. [Google Scholar]
- Turner, J.; Klaus, R. Coral Reefs of the Mascarenes, Western Indian Ocean: One Contribution of 24 to a Discussion Meeting “Atmosphere-Ocean-Ecology Dynamics in the Western Indian Ocean”. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2005, 363, 229–250. [Google Scholar] [CrossRef]
- Bhagooli, R.; Kaullysing, D. Seas of Mauritius, Chapter 16. In World Seas: An Environmental Evaluation Volume II: The Indian Ocean to the Pacific, 2nd ed.; Sheppard CCR; Elsevier: Dordrecht, The Netherlands, 2019; pp. 253–277. [Google Scholar]
- Pasnin, O.; Klaus, R.; Attwood, C. Marine Systematic Conservation Plan for Rodrigues Island: Western Indian Ocean. Ocean. Coast. Manag. 2016, 130, 213–220. [Google Scholar] [CrossRef]
- Montaggioni, L. Coral reefs and quaternary shorelines in the Mascarene archipelago, Indian Ocean. In Proceedings of the Second International Coral Reef Symposium, Brisbane, Australia, 22 June–2 July 1974; Volume 1, pp. 579–593. [Google Scholar]
- Faure, G. Etude comparative des récifs coralliens de l’archipel des Mascareignes (Océan Indien). In Biologie Marine et Exploitation des Ressources de l’Océan Indien Occidental; ORSTOM: Paris, France, 1976; pp. 153–177. [Google Scholar]
- Montaggioni, L.F.; Faure, G. Récifs Coralliens des Mascareignes: Océan Indien; Université française de l’Océan indien, Centre universitaire de la Réunion, 1980. Available online: https://www.eaureunion.fr/fileadmin/user_upload/Etudes/ETUDE_01127.PDF (accessed on 12 October 2023).
- Veron, J.E.N. Corals of the World; Australian Institute of Marine Science: Townsville, QLD, Australia, 2000; pp. 1–3.
- English, S.; Wilkinson, C.; Baker, V. Survey Manual for Tropical Marine Resources; Australian Institute of Marine Science: Cape Cleveland, QLD, Australia, 1997; No. 333.952 S9.
- Thinesh, T.; Gilbert, M.; Patterson, E. Coral Disease Prevalence in Mandapam Group of Islands, Gulf of Mannar, Southeastern India. Indian J. Geo-Mar. Sci. 2009, 38, 444–450. [Google Scholar]
- Thinesh, T.; Mathews, G.; Edward, J.K.P. Coral Disease Prevalence in the Palk Bay, Southeastern India—with Special Emphasis to Black Band. Indian J. Geo-Mar. Sci. 2011, 40, 813–820. [Google Scholar]
- Thangaradjou, T.; Machendiranathan, M.; Ranith, R.; Senthilnathan, L.; Sasamal, S.K.; Choudhury, S.B. Coral Disease Prevalence in Gulf of Mannar and Lakshadweep Islands. Indian J. Geo-Mar. Sci. 2016, 45, 1755–1762. [Google Scholar]
- Clark, T.H. The Status of the Coral Reefs in Rodrigues, Report to the Shoals of Capricorn Programme. 2001; 27p.
- Aeby, G.S.; Callahan, S.; Cox, E.F.; Runyon, C.; Smith, A.; Stanton, F.G.; Ushijima, B.; Work, T.M. Emerging Coral Diseases in Kāne’ohe Bay, O’ahu, Hawai’i (USA): Two Major Disease Outbreaks of Acute Montipora White Syndrome. Dis. Aquat. Organ. 2016, 119, 189–198. [Google Scholar] [CrossRef]
- Bruno, J.F.; Selig, E.R.; Casey, K.S.; Page, C.A.; Willis, B.L.; Harvell, C.D.; Sweatman, H.; Melendy, A.M. Thermal Stress and Coral Cover as Drivers of Coral Disease Outbreaks. PLoS Biol. 2007, 5, e124. [Google Scholar] [CrossRef]
- Sussman, M.; Willis, B.L.; Victor, S.; Bourne, D.G. Coral Pathogens Identified for White Syndrome (WS) Epizootics in the Indo-Pacific. PLoS ONE 2008, 3, e2393. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.B.; True, J.D.; Piromvaragorn, S.; Willis, B.L. Scuba Diving Damage and Intensity of Tourist Activities Increases Coral Disease Prevalence. Biol. Conserv. 2014, 178, 88–96. [Google Scholar] [CrossRef]
- Greene, A.; Donahue, M.J.; Caldwell, J.M.; Heron, S.F.; Geiger, E.; Raymundo, L.J. Coral Disease Time Series Highlight Size-Dependent Risk and Other Drivers of White Syndrome in a Multi-Species Model. Front. Mar. Sci. 2020, 7, 601469. [Google Scholar] [CrossRef]
- Richardson, L.; Smith, G.; Ritchie, K.; Carlton, R. Integrating Microbiological, Microsensor, Molecular, and Physiologic Techniques in the Study of Coral Disease Pathogenesis. Hydrobiologia 2001, 460, 71–89. [Google Scholar] [CrossRef]
- Page, C.; Willis, B. Distribution, Host Range and Large-Scale Spatial Variability in Black Band Disease Prevalence on the Great Barrier Reef, Australia. Dis. Aquat. Org. 2006, 69, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.C.; Halas, J.C.; McCarty, H.B. Calicoblastic Neoplasms in Acropora Palmata, with a Review of Reports on Anomalies of Growth and Form in Corals. J. Natl. Cancer Inst. 1986, 76, 895–912. [Google Scholar]
- Richardson, L.L. Black Band Disease. In Coral Health and Disease; Rosenberg, E., Loya, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 325–336. ISBN 978-3-662-06414-6. [Google Scholar]
- Edmunds, P. Extent and effect of black band disease on a carribean reef. Coral Reefs 1991, 10, 161–165. [Google Scholar] [CrossRef]
- Kuta, K.G.; Richardson, L. Ecological Aspects of Black Band Disease of Corals: Relationships between Disease Incidence and Environmental Factors. Coral Reefs 2002, 21, 393–398. [Google Scholar] [CrossRef]
- Zvuloni, A.; Artzy-Randrup, Y.; Stone, L.; Kramarsky-Winter, E.; Barkan, R.; Loya, Y. Spatio-Temporal Transmission Patterns of Black-Band Disease in a Coral Community. PLoS ONE 2009, 4, e4993. [Google Scholar] [CrossRef]
- Aeby, G.S.; Work, T.M.; Runyon, C.M.; Shore-Maggio, A.; Ushijima, B.; Videau, P.; Beurmann, S.; Callahan, S.M. First Record of Black Band Disease in the Hawaiian Archipelago: Response, Outbreak Status, Virulence, and a Method of Treatment. PLoS ONE 2015, 10, e0120853. [Google Scholar] [CrossRef]
- Pollock, F.J.; Lamb, J.B.; Field, S.N.; Heron, S.F.; Schaffelke, B.; Shedrawi, G.; Bourne, D.G.; Willis, B.L. Sediment and Turbidity Associated with Offshore Dredging Increase Coral Disease Prevalence on Nearby Reefs. PLoS ONE 2014, 9, e102498. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, J.; Jones, R.; Page, C.; Marnane, M.; Lestang, P.; Elsdon, T. No Effect of Dredging on the Prevalence of Coral Disease Detected during a Large Dredging Program. Mar. Pollut. Bull. 2019, 140, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Page, C.A.; Baker, D.M.; Harvell, C.D.; Golbuu, Y.; Raymundo, L.; Neale, S.J.; Rosell, K.B.; Rypien, K.L.; Andras, J.P.; Willis, B.L. Influence of Marine Reserves on Coral Disease Prevalence. Dis. Aquat. Org. 2009, 87, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Raymundo, L.J.; Halford, A.R.; Maypa, A.P.; Kerr, A.M. Functionally Diverse Reef-Fish Communities Ameliorate Coral Disease. Proc. Natl. Acad. Sci. USA 2009, 106, 17067–17070. [Google Scholar] [CrossRef]
- Cróquer, A.; Weil, E. Changes in Caribbean Coral Disease Prevalence after the 2005 Bleaching Event. Dis. Aquat. Org. 2009, 87, 33–43. [Google Scholar] [CrossRef]
- Borger, J.L.; Steiner, S.C.C. The Spatial and Temporal Dynamics of Coral Diseases in Dominica, West Indies. Bull. Mar. Sci. 2005, 77, 137–154. [Google Scholar]
- Selig, E.R.; Drew Harvell, C.; Bruno, J.F.; Willis, B.L.; Page, C.A.; Casey, K.S.; Sweatman, H. Analyzing the Relationship Between Ocean Temperature Anomalies and Coral Disease Outbreaks at Broad Spatial Scales. In Coral Reefs and Climate Change: Science and Management; American Geophysical Union (AGU): Washington, DC, USA, 2006; pp. 111–128. ISBN 978-1-118-66615-9. [Google Scholar]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate Change, Human Impacts, and the Resilience of Coral Reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, J.-P.A.; Frisch, A.J. Coral Disease in the Indian Ocean: Taxonomic Susceptibility, Spatial Distribution and the Role of Host Density on the Prevalence of White Syndrome. Dis. Aquat. Org. 2010, 89, 1–8. [Google Scholar] [CrossRef]
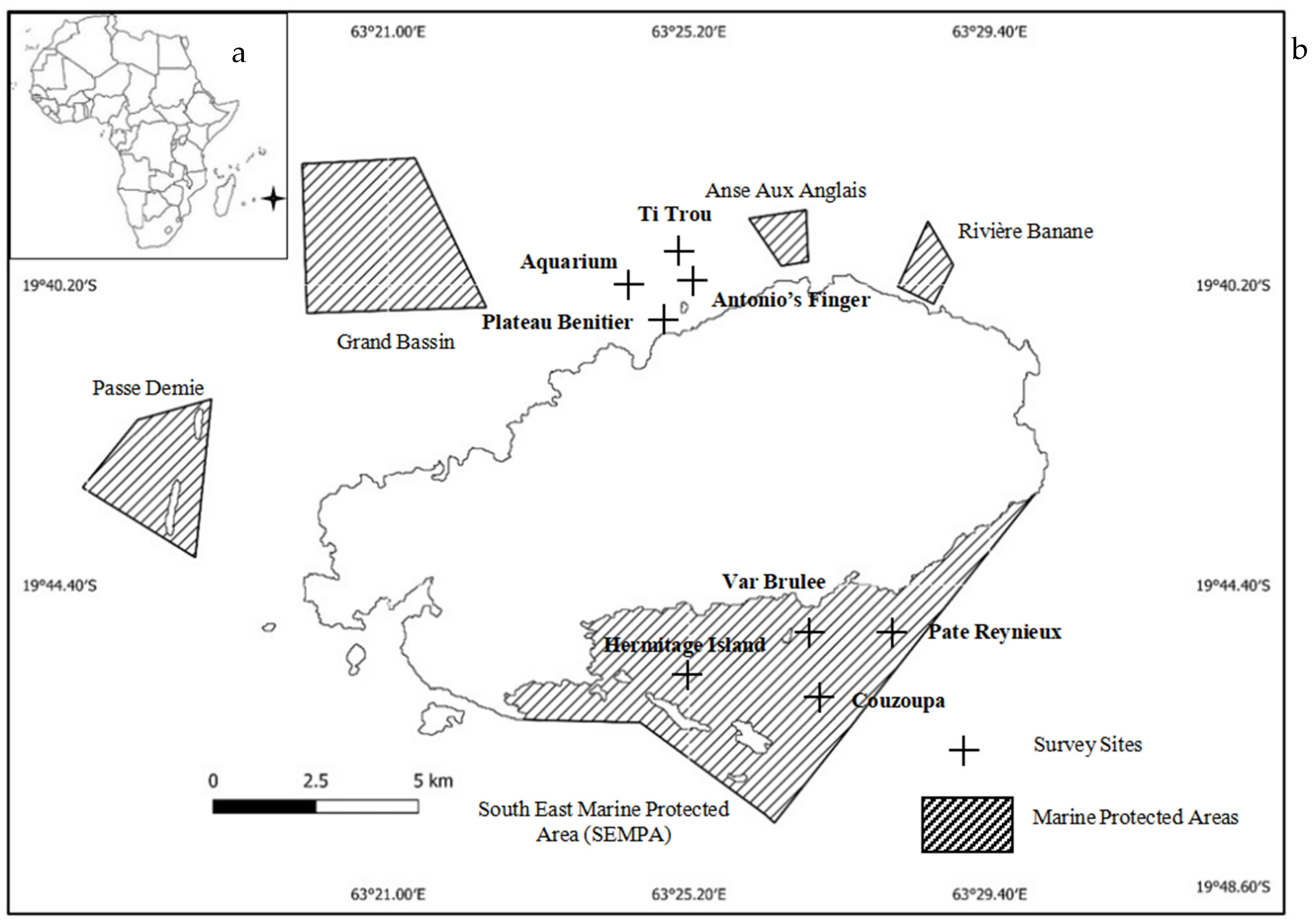




| Site | Geographic Location | Depth (m) | Depth Category (D1–D4) | Protected/Non-Protected |
|---|---|---|---|---|
| Plateau Benitier | 19°40′11.60″ S, 63°25′44.90″ E | 6–9 | D3 | Non-protected |
| Ti Trou | 19°40′0.00″ S, 63°26′14.00″ E | 6–9 | D3 | Non-protected |
| Aquarium | 19°40′21.0″ S, 63°28′36.0″ E | 6–9 | D3 | Non-protected |
| Antonio’s Finger | 19°40′11.4″ S, 63°25′47.4″ E | 6–9 | D3 | Non-protected |
| Couzoupa | 19°45′32.4″ S, 63°27′48.5″ E | 9–12 | D4 | Protected |
| Pate Reynieux | 19°44′44.00″ S, 63°28′43.00″ E | 1–3 | D1 | Protected |
| Hermitage Island | 19°44′59.0″ S, 63°20′40.0″ E | 1–3 | D1 | Protected |
| Var Brule | 19°44′44.00″ S, 63°28′43.00″ E | 1–3 | D1 | Protected |
| Genus | n | Disease/CHC Observed | Number of Affected Colonies | Mean Overall Prevalence (%) ± SE | |
|---|---|---|---|---|---|
| Disease/CHC-Specific | Taxon-Specific | ||||
| Pocillopora | 62 | - | - | - | - |
| Branching Acropora | 248 | - | - | - | - |
| Montipora | 281 | WS | 8 | 0.90 ± 0.34 | 4.67 ± 3.72 |
| WP | 1 | 0.38 ± 0.27 | 0.23 ± 0.23 | ||
| BBD | 1 | 0.023 ± 0.023 | 0.13 ± 0.13 | ||
| Gardineroseris | 2 | GA | 1 | 0.03 ± 0.16 | 4.17 ± 4.17 |
| Pachyseris | 4 | - | - | - | - |
| Pavona | 248 | - | - | - | - |
| Fungia | 262 | PP | 97 | 3.63 ± 1.33 | 15.92 ± 5.65 |
| Goniopora | 3 | - | - | - | - |
| Massive Porites | 17 | PP | 1 | 3.63 ± 1.33 | 4.17 ± 4.17 |
| GA | (1) | - | - | ||
| PLS | (1) | - | - | ||
| Echinopora | 36 | - | - | - | - |
| Goniastrea | 36 | WS | 2 | 0.90 ± 0.34 | 1.89 ± 1.31 |
| Favites | 81 | - | - | - | - |
| Dipsastrea | 23 | - | - | - | - |
| Platygyra | 189 | - | - | - | - |
| Lobophyllia | 5 | - | - | - | - |
| Millepora | 238 | WP | 10 | 0.38 ± 0.27 | 1.10 ± 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jogee, S.Y.; Gopalsing, S.; Jeetun, S.; Ricot, M.; Taleb-Hossenkhan, N.; Mattan-Moorgawa, S.; Kaullysing, D.; Wijayanti, D.P.; Casareto, B.E.; Suzuki, Y.; et al. First Report of Diseases and Compromised Health Conditions on Hard Corals around Rodrigues Island, Southwest Indian Ocean. Diversity 2023, 15, 1086. https://doi.org/10.3390/d15101086
Jogee SY, Gopalsing S, Jeetun S, Ricot M, Taleb-Hossenkhan N, Mattan-Moorgawa S, Kaullysing D, Wijayanti DP, Casareto BE, Suzuki Y, et al. First Report of Diseases and Compromised Health Conditions on Hard Corals around Rodrigues Island, Southwest Indian Ocean. Diversity. 2023; 15(10):1086. https://doi.org/10.3390/d15101086
Chicago/Turabian StyleJogee, Shakeel Yavan, Shivam Gopalsing, Sruti Jeetun, Melanie Ricot, Nawsheen Taleb-Hossenkhan, Sushma Mattan-Moorgawa, Deepeeka Kaullysing, Diah Permata Wijayanti, Beatriz Estela Casareto, Yoshimi Suzuki, and et al. 2023. "First Report of Diseases and Compromised Health Conditions on Hard Corals around Rodrigues Island, Southwest Indian Ocean" Diversity 15, no. 10: 1086. https://doi.org/10.3390/d15101086






