The Phylogenetic Relationships of Australian Species within Charopidae (Gastropoda: Punctoidea)
Abstract
:1. Introduction
2. Materials and Methods
2.1. 28S rDNA D1 Data Collection
2.2. Dataset Compilation
| Species | Registration Number | 28S rRNA Type | GenBank Accession |
|---|---|---|---|
| Barringtonica montana Shea, Colgan & Stanisic, 2012 | C.472876 | A | OR471375 |
| Barringtonica polblue Shea, Colgan & Stanisic, 2012 | C.309166 | B | OR471374 |
| Cancellocochlea coolongolook Shea, Colgan & Stanisic, 2012 | C.466292 | A | OR471402 |
| Cancellocochlea coolongolook | C.466293 | A | OR471373 |
| Cancellocochlea Shea, Colgan & Stanisic, 2012 cf. coolongolook | MO80164 | A | OR471377 |
| Charopidae NN14 | C.463618 | B | OR471392 |
| Charopidae NN23 | C.463582 | A | OR471384 |
| Comboynea boorganna Shea, Colgan & Stanisic 2012 | C.163287 | A | OR471376 |
| Cumberlandica impressa (Hedley, 1924) | C.457178 | A | OR471381 |
| Cumberlandica impressa | C.462479 | A | OR471379 |
| Cumberlandica impressa | C.462481 | A | OR471372 |
| Cumberlandica impressa | C.462483 | A | OR471380 |
| Cumberlandica impressa | C.463083 | A | OR471389 |
| Cumberlandica impressa | C.463909 | A | OR471383 |
| Decoriropa lirata (Cox, 1864) | C.466867 | C | OR471404 |
| Diphyoropa saturni (Cox, 1864) | C.462488 | A | OR471366 |
| Diphyoropa saturni | C.462489 | A | OR471391 |
| Diphyoropa saturni | C.462495 | A | OR471367 |
| Diphyoropa saturni | C.462525 | A | OR471368 |
| Diphyoropa saturni | C.475921 | A | OR471365 |
| Elsothera sericatula (Pfeiffer, 1849) | C.462523 | A | OR471369 |
| Elsothera sericatula | C.463085 | A | OR471370 |
| Gyrocochlea vinitincta Hedley, 1924 | C.466294 | B | OR471393 |
| Macphersonea canalis (Stanisic, 2010) | C.463585 | D | OR471386 |
| Macrophallikoropa belli (Cox, 1864) | C.465686 | E | OR471403 |
| Macrophallikoropa belli | C.465866 | E | OR471362 |
| Macrophallikoropa Hyman & Stanisic, 2005, sp. | C.466865 | F | OR471401 |
| Nautiliropa omicron (Pfeiifer, 1852) | C.466862_G87 | B | OR471394 |
| Nautiliropa omicron | C.466862_G88 | B | OR471395 |
| Ngairea corticicola (Cox, 1866) | C.466863 | G | OR471397 |
| Planorbacochlea hawkesburyana Stanisic, 2010 | C.462434 | B | OR471382 |
| Planorbacochlea hawkesburyana | C.462496 | B | OR471396 |
| Planorbacochlea hawkesburyana | C.462508 | B | OR471390 |
| Planorbacochlea hawkesburyana | C.462831 | B | OR471405 |
| Planorbacochlea nambucca Shea, Colgan & Stanisic, 2012 | MO44862 | B | OR471378 |
| Planorbacochlea parriwiensis Shea, Colgan & Stanisic, 2012 | C.462478 | A | OR471363 |
| Planorbacochlea parriwiensis | C.462509 | A | OR471364 |
| Planorbacochlea planorbis (Hedley, 1924) | C.309923 | B | OR471371 |
| Planorbacochlea planorbis | C.463729 | B | OR471388 |
| Planorbacochlea Shea, Colgan & Stanisic, 2012 sp. “Port Macquarie” | MO59761 | B | OR471398 |
| Planorbacochlea yessabahensis Shea, Colgan & Stanisic, 2012 | C.168684 | A | OR471387 |
| Planorbacochlea yessabahensis | C.466289 | A | OR471361 |
| Richmondaropa prava (Hedley, 1924) | C.466861 | B | OR471385 |
| Setomedea seticostata (Hedley, 1924) | C.466864_G92 | H | OR471399 |
| Setomedea seticostata | C.466864_G93 | H | OR471400 |
2.3. Data Analysis
2.3.1. Scanning Electron Microscopy
2.3.2. Genetic Analyses
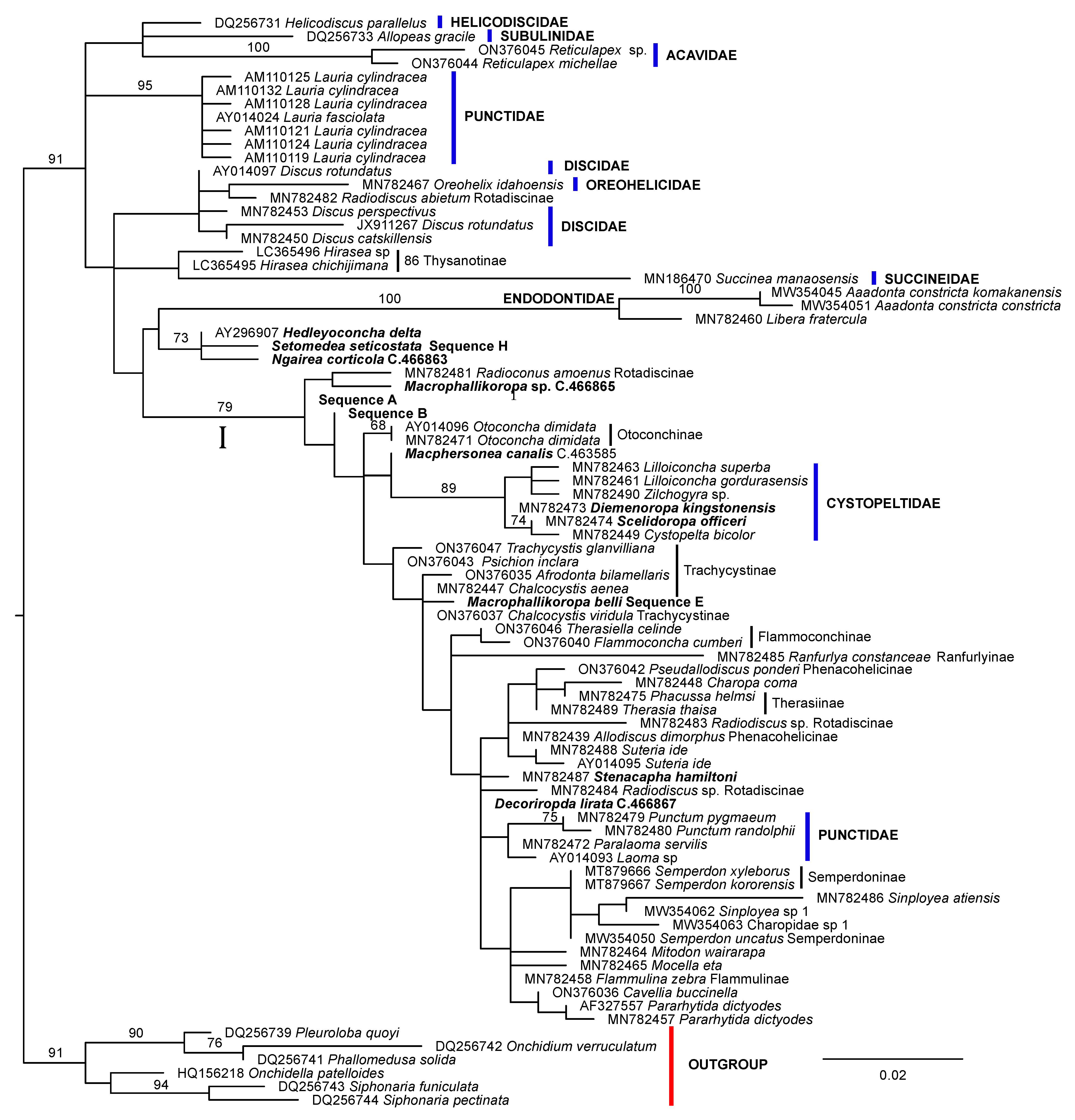
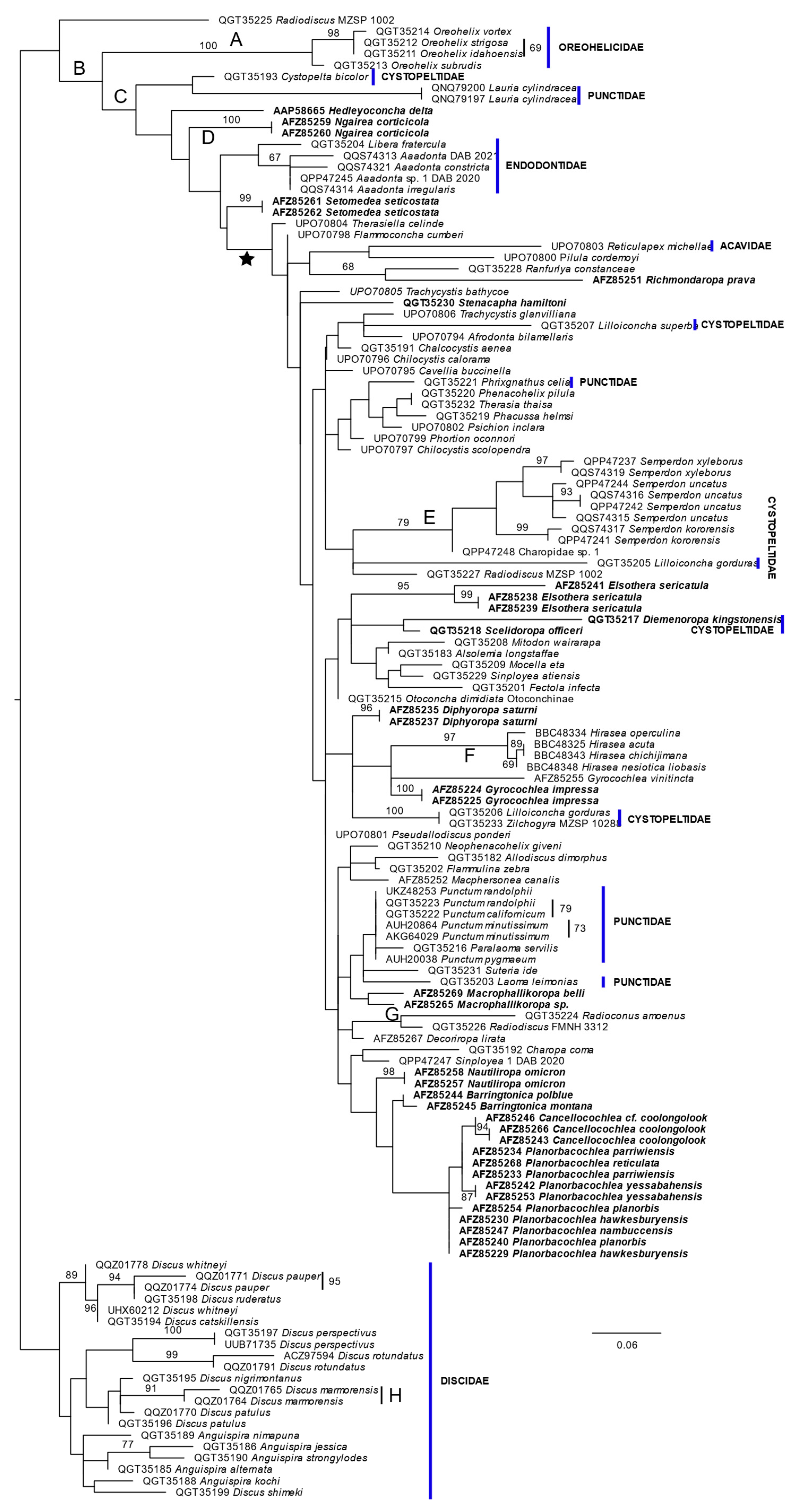
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emberton, K.C.; Pearce, T.A.; Kasigwa, P.F.; Tattersfield, P.; Habibu, Z. High diversity and regional endemism in land snails of eastern Tanzania. Biodivers. Conserv. 1997, 6, 1123–2236. [Google Scholar] [CrossRef]
- Herbert, D.; Kilburn, D. Field Guide to the Land Snails and Slugs of Eastern South Africa; Natal Museum: Pietermaritzburg, South Africa, 2004. [Google Scholar]
- Hedley, C. Observations on the Charopidae. Proc. Linn. Soc. N.S.W. 1892, 7, 157–169. [Google Scholar]
- Hedley, C. Some notes on Australian land shells. Aust. Zool. 1924, 3, 215–221. [Google Scholar]
- Kershaw, R.C. Studies on Australian Charopidae. Part 2. Some genera. Vic. Nat. 1955, 72, 28–30. [Google Scholar]
- Kershaw, R.C. Studies on Australian Charopidae. Part 3. Planate genera. Vic. Nat. 1956, 72, 137–143. [Google Scholar]
- Stanisic, J. Studies on the Charopidae of tropical and subtropical Australia. 1. Oreokera: A primitive genus from the high mountains of North Queensland (Mollusca: Pulmonata: Charopidae). J. Malacol. Soc. Aust. 1987, 8, 1–21. [Google Scholar]
- Stanisic, J. Systematics and biogeography of eastern Australian Charopidae (Mollusca, Pulmonata) from subtropical rainforests. Mem. Queensl. Mus. 1990, 30, 1–241. [Google Scholar]
- Smith, B.J.; Kershaw, R.C. Description of three Tasmanian charopid genera (Pulmonata: Mollusca) with notes on their type species. Rec. Queen Vic. Mus. Art Gall. 1985, 88, 1–14. [Google Scholar]
- Stanisic, J. Danielleilona gen. nov., from the Wet Tropics, northeastern Queensland (Pulmonata: Charopidae). Mem. Queensl. Mus. 1993, 34, 11–20. [Google Scholar]
- Stanisic, J. Lenwebbia paluma sp. nov., from the Wet Tropics, northeastern Queensland (Pulmonata: Charopidae). Mem. Queensl. Mus. 1993, 34, 21–26. [Google Scholar]
- Hyman, I.T.; Stanisic, J. New charopid landsnails, chiefly from limestone outcrops in eastern New South Wales (Eupulmonata: Charopidae). Mem. Queensl. Mus. 2005, 50, 219–302. [Google Scholar]
- Stanisic, J.; Shea, M.; Potter, D.; Griffiths, O. Australian Land Snails Volume 1: A Field Guide to Eastern Australian Species; Bioculture Press: Senneville, Mauritius, 2010. [Google Scholar]
- Climo, F.M. Classification of New Zealand Arionacea. Nat. Mus. N. Z. Rec. 1981, 2, 9–15. [Google Scholar]
- Barker, G.M. The character of the New Zealand land snail fauna and communities: Some evolutionary and ecological perspectives. Rec. W. A. Mus. 2005, 68, 53–102. [Google Scholar] [CrossRef]
- Marshall, B.A.; Barker, G.M. A revision of the New Zealand landsnails referred to Allodiscus Pilsbry, 1892 and Pseudallodiscus Climo, 1971, with the introduction of three new genera (Mollusca: Gastropoda: Charopidae). Tuhinga 2008, 19, 57–167. [Google Scholar]
- Muratov, I.V.; Abdou, A.; Bouchet, P. Charopid land snails (Gastropoda Pulmonata Charopidae) from Mayotte, Comores: Alive and well. Trop. Zool. 2005, 18, 171–208. [Google Scholar] [CrossRef]
- Iredale, T. The land Mollusca of Lord Howe Island. Aust. Zool. 1944, 10, 299–334. [Google Scholar]
- Iredale, T. The land Mollusca of Norfolk Island. Aust. Zool. 1945, 11, 46–71. [Google Scholar]
- Solem, A. Endodontoid Land Snails from Pacific Islands (Mollusca: Pulmonata: Sigmurethra). Part II. Families Punctidae and Charopidae, Zoogeography; Field Museum: Chicago, IL, USA, 1983. [Google Scholar]
- Pawlowska-Banasiak, E. Andrefrancia Solem, 1960 (Gastropoda: Pulmonata: Charopidae)—A systematic revision. Folia Malacol. 2008, 16, 101–195. [Google Scholar] [CrossRef]
- Slapcinsky, J. A new species of Paryphantopsis (Gastropoda: Pulmonata: Charopidae) from Crater Mountain, Simbu (Chimbu) Province, Papua New Guinea. Nautilus 2009, 123, 53–58. [Google Scholar]
- Brook, F.J. Coastal landsnail fauna of Rarotonga, Cook Islands: Systematics, diversity, biogeography, faunal history, and environmental influences. Tuhinga 2010, 21, 161–252. [Google Scholar]
- Pugh, P.J.A.; Lewis Smith, R.I. Notodiscus (Charopidae) on South Georgia: Some implications of shell size, shell shape, and site isolation in a singular sub-Antarctic land snail. Antarct. Sci. 2011, 23, 442–448. [Google Scholar] [CrossRef]
- Schileyko, A.A. A redescription of Ruthvenia biciliata (L. Pfeiffer, 1855), with revised generic diagnosis for Ruthvenia Gude, 1911 (Gastropoda: Pulmonata: Charopidae). Ann. Nat. Mus. Wien Ser. B 2010, 111, 13–18. [Google Scholar]
- Hausdorf, B. The genus Lillioconcha in Colombia (Gastropoda: Charopidae). J. Nat. Hist. 2005, 39, 2795–2808. [Google Scholar] [CrossRef]
- Cadiz, F.J.; Gallardo, C.S. Morphological and anatomical features of Flammulina festiva Scott, 1970 (Stylommatophora; Charopidae) from southern Chile, with notes on its natural history. Gayana 2008, 72, 1–8. [Google Scholar]
- Miquel, S.E.; Cadiz Lorca, F.J. Araucocharopa gallardoi gen. et sp. n. de Charopidae (Mollusca: Gastropoda: Stylommatophora) del sur de Chile. Rev. Mus. Argent. Cienc. Nat. 2008, 10, 329–339. [Google Scholar] [CrossRef]
- Delhey, V.K.; Piza, J.; Burela, S. Zilchogyra franzi Weyrauch, 1965 (Gastropoda: Charopidae), a minute landsnail rediscovered in Sierra de la Ventana (Southern Pampas, Argentina). Zootaxa 2010, 2450, 61–64. [Google Scholar] [CrossRef]
- Agudo-Padron, A.I. Threatened freshwater and terrestrial molluscs (Mollusca, Gastropoda et Bivalvia) of Santa Catarina State, Southern Brazil: Check list and evaluation of regional threats. Biodivers. J. 2011, 2, 59–66. [Google Scholar]
- Cunha, C.M.; Salvador, R.B.; Simone, L.R.L. The terrestrial microgastropods of Trindade Island, Brazil. Spixiana 2015, 38, 139–143. [Google Scholar]
- Pérez, A.M.; López, A. Listado de la malacofauna continental (Mollusca: Gastropoda) del Pacífico de Nicaragua. Rev. Biol. Trop. 2003, 51 (Suppl. S3), 405–451. [Google Scholar]
- Correa Sandoval, A. Zoogeografía de los gastrópodos terrestres de la región oriental de San Luis Potosí, México. Rev. Biol. Trop. 1999, 47, 493–502. [Google Scholar] [CrossRef]
- Ramirez Perez, M.C.; Hausdorf, B. Low abundance but high land snail diversity in montane rainforest on the western slope of the Andes in Ecuador. J. Mollusc. Stud. 2022, 88, eyab048. [Google Scholar] [CrossRef]
- Branson, B.A. Radiodiscus hubrichti (Pulmonata: Endodontidae) new species from the Olympic Peninsula, Washington. Nautilus 1975, 89, 47–48. [Google Scholar]
- Nekola, J.C. Overview of the North American terrestrial gastropod fauna. Am. Malacol. Bull. 2014, 32, 225–235. [Google Scholar] [CrossRef]
- Herbert, D.G. Revision of the aperturally dentate Charopidae (Gastropoda: Stylommatophora) of southern Africa—Genus Afrodonta s. lat., with description of five new genera, twelve new species and one new subspecies. Eur. J. Taxon. 2020, 629, 1–55. [Google Scholar] [CrossRef]
- Holcroft, L. A revision of Gyrocochlea-grade Charopidae from mid-eastern Queensland and redescription and generic reassignment of three Gyrocochlea-grade species (Eupulmonata: Charopidae). Mem. Queensl. Mus. Nat. 2018, 61, 1–28. [Google Scholar] [CrossRef]
- Holcroft, L. A revision of Charopidae with a finely cancellate protoconch sculpture from mid-eastern Queensland (Eupulmonata: Charopidae). Mem. Queensl. Mus. Nat. 2018, 61, 83–107. [Google Scholar] [CrossRef]
- Holcroft, L.; Stanisic, J. Thirteen new charopid land snails from mid-eastern Queensland rainforests (Gastropoda: Eupulmonata: Charopidae). Mem. Queensl. Mus. Nat. 2018, 61, 155–186. [Google Scholar] [CrossRef]
- Stanisic, J. Two new species of pinwheel snail from contrasting Queensland habitats and a redescription and generic re-assignment of Gyrocochlea myora Stanisic, 2010 (Gastropoda: Eupulmonata: Charopidae). Mem. Queensl. Mus. 2016, 60, 1–12. [Google Scholar] [CrossRef]
- Stanisic, J. New species and genera of pinwheel snails from the brigalow lands of south central Queensland (Gastropoda: Eupulmonata: Charopidae). Mem. Queensl. Mus. Nat. 2020, 62, 157–185. [Google Scholar]
- Stanisic, J. A new genus and three new species of pinwheel snails from Queensland and New South Wales (Gastropoda: Eupulmonata: Charopidae). Mem. Queensl. Mus. Nat. 2022, 64, 1–11. [Google Scholar] [CrossRef]
- Vermeulen, J.J.; Marzuki, M.E. ‘Charopa’ lafargei (Gastropoda, Pulmonata, Charopidae), a new, presumed narrowly endemic species from Peninsular Malaysia. Basteria 2014, 78, 31–34. [Google Scholar]
- Sutcharit, C.; Pholyotha, A. Hidden lineages in the mountains: The genus Glyptaulax Gude, 1914 and Maelamaodiscus gen. nov. (Heterobranchia: Stylommatophora: Charopidae and Ariophantidae) with description of two new species from western Thailand. Trop. Nat. Hist. 2023, 7, 123–138. [Google Scholar]
- Miquel, S.E.; Barker, G.M. New Charopidae from Chilean—Argentine Patagonia (Mollusca: Gastropoda: Stylommatophora). Arch. Mollusk. 2009, 138, 53–61. [Google Scholar] [CrossRef]
- Miquel, S.E.; Araya, J.F. A new Charopidae from Chile and Argentina, Stephacharopa calderaensis n. gen. and n. sp., with remarks on the taxonomy of the genus Stephadiscus Hylton Scott 1981 (Mollusca: Gastropoda Pulmonata). Arch. Molluskenk. 2013, 142, 227–235. [Google Scholar] [CrossRef]
- Araya, J.F.; Miquel, S.E. A new Stephacharopa (Gastropoda: Punctoidea: Charopidae) From Paposo, Northern Chile. J. Conch. 2018, 43, 129–133. [Google Scholar]
- Miquel, S.E.; Araya, J.F. Re-description of genitalia of Stephacharopa and description of a new Peruvian genus (Gastropoda, Stylommatophora, Charopidae). Spixiana 2022, 45, 1–7. [Google Scholar]
- Salvador, R.B.; Cavallari, D.C.; Simone, L.R.L. Taxonomical study on a sample of land snails from Alto Ribeira State Park (Sao Paulo, Brazil), with description of a new species. Arch. Molluskenk. 2016, 145, 59–68. [Google Scholar] [CrossRef]
- Salvador, R.B.; Charles, L.; Simone, L.R.L.; Maestrati, P. Terrestrial gastropods from Pedra Talhada Biological Reserve, Alagoas state, Brazil, with the description of a new species of Radiodiscus (Gastropoda: Charopidae). Arch. Molluskenk. 2018, 147, 101–128. [Google Scholar] [CrossRef]
- Miquel, S.E.; Araya, J.F. New records of terrestrial molluscs of the Juan Fernandez Archipelago (Chile), with the description of a new genus and species of Charopidae (Gastropoda: Stylommatophora). Arch. Molluskenk. 2015, 144, 155–167. [Google Scholar] [CrossRef]
- Rutherford, M.G. A new species of Radiodiscus (Gastropoda: Eupulmonata: Charopidae) from Trinidad and Tobago. Arch. Molluskenk. 2020, 149, 67–74. [Google Scholar] [CrossRef]
- Salvador, R.B.; Brook, F.J.; Shepherd, L.D.; Kennedy, M. Molecular phylogenetic analysis of Punctoidea (Gastropoda, Stylommatophora). Zoosyst. Evol. 2020, 96, 397–410. [Google Scholar] [CrossRef]
- Salvador, R.B. Phylogenetic Position of African punctoid snails (Stylommatophora, Punctoidea, Trachycystinae). Taxonomy 2022, 2, 227–235. [Google Scholar] [CrossRef]
- Holcroft, L. Protoconch sculpture as a taxonomic tool in Australian charopid systematics (Gastropoda: Eupulmonata: Charopidae). Mollusc. Res. 2018, 38, 258–273. [Google Scholar] [CrossRef]
- Shea, M.; Colgan, D.J.; Stanisic, J. Systematics of the landsnail genus Gyrocochlea and relatives (Mollusca: Charopidae). Zootaxa 2012, 3585, 1–109. [Google Scholar] [CrossRef]
- Wade, C.M.; Mordan, P.B.; Naggs, F. Evolutionary relationships among the Pulmonate land snails and slugs (Pulmonata, Stylommatophora). Biol. J. Linn. Soc. 2006, 87, 593–610. [Google Scholar] [CrossRef]
- McArthur, A.G.; Koop, B.F. Partial 28S rDNA sequences and the antiquity of the hydrothermal vent endemic gastropods. Mol. Phylogenet. Evol. 1999, 13, 255–274. [Google Scholar] [CrossRef]
- Colgan, D.J.; Ponder, W.F.; Beacham, E.; Macaranas, J. Molecular phylogenetics of Caenogastropoda (gastropoda: Mollusca). Mol. Phylogenet. Evol. 2007, 42, 717–737. [Google Scholar] [CrossRef]
- Colgan, D.J.; Ponder, W.F.; Beacham, E.; Macaranas, J.M. Molecular phylogenetic studies of Gastropoda based on six gene segments representing coding or non-coding and mitochondrial or nuclear DNA. Mollusc. Res. 2003, 23, 123–148. [Google Scholar] [CrossRef]
- Bullis, D.A.; Rundell, R.J. Molecular phylogenetics and premating isolation in the punctoid land snails of Belau (Republic of Palau, Oceania). Zool. Script. 2021, 50, 555–570. [Google Scholar] [CrossRef]
- Schileyko, A.A. Treatise on recent terrestrial pulmonate molluscs. Part 7. Endodontidae, Thyrophorellidae, Charopidae. Ruthenica 2001, 2, 881–1034. [Google Scholar]
- MolluscaBase. Charopidae F. W. Hutton, 1884. 2023. Available online: https://www.molluscabase.org/aphia.php?p=taxdetails&id=816165 (accessed on 4 September 2023).
- Baker, R.J.; Solari, S.; Cirranello, A.; Simmons, N.B. Higher level classification of phyllostomid bats with a summary of DNA synapomorphies. Acta Chiropt. 2016, 18, 1–38. [Google Scholar] [CrossRef]
- Hütter, T.; Ganser, M.H.; Kocher, M.; Halkic, M.; Agatha, S.; Augsten, N. DeSignate: Detecting signature characters in gene sequence alignments for taxon diagnoses. BMC Bioinform. 2020, 21, 151. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Wada, S.; Mori, H.; Uchida, S.; Saito, T.; Chiba, S. Genetic and morphometric rediscovery of an extinct land snail on oceanic islands. J. Mollusc. Stud. 2018, 84, 148–156. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL X Windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl. Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Holznagel, W.E.; Colgan, D.J.; Lydeard, C. Pulmonate phylogeny based on 28S rRNA gene sequences: A framework for discussing habitat transitions and character transformation. Mol. Phylogenet. Evol. 2010, 57, 1017–1025. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov (2010); Gateway Computing Environments Workshop: New Orleans, LA, USA, 2010; pp. 1–8. [Google Scholar]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 312–313. [Google Scholar] [CrossRef]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web-servers. Syst. Biol. 2008, 75, 758–771. [Google Scholar] [CrossRef]
- Pattengale, N.D.; Alipour, M.; Bininda-Emonds, O.R.P.; Moret, B.M.E.; Stamatakis, A. How many bootstrap replicates are necessary? J. Computat. Biol. 2010, 17, 337–354. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J. Tracer 1.3. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 31 August 2023).
- Swofford, D.L. PAUP: Phylogenetic Analysis Using Parsimony; Version 4.0; Laboratory of Molecular Systematics, Smithsonian Institution: Washington, DC, USA, 2003. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [PubMed]
- Bui, Q.M.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar]
- Diep, T.H.; Chernomor, O.; von Haeseler, A.; Bui, Q.M.; Le, S.V. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar]
- Kalyaanamoorthy, S.; Bui, Q.M.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Meth. 2017, 14, 587–589. [Google Scholar]
- Chernomor, O.; von Haeseler, A.; Bui, Q.M. Terrace aware data structure for phylogenomic inference fromsupermatrices. Syst. Biol. 2016, 65, 997–1008. [Google Scholar]
- Kishino, H.; Hasegawa, M. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in hominoidea. J. Mol. Evol. 1989, 29, 170–179. [Google Scholar]
- Shimodaira, H. An approximately unbiased test of phylogenetic tree selection. Syst. Biol. 2002, 51, 492–508. [Google Scholar] [CrossRef]
- Ueshima, R.; Kurozumi, T. Anatomical features of Hirasea (ss) diplomphalus and taxonomic position of the genus Hirasea Pilsbry, 1902 (Pulmonata: Sigmurethra). Venus 1988, 47, 261–270. [Google Scholar]
- MolluscaBase. Hirasea Pilsbry, 1902. 2021. Available online: https://molluscabase.org/aphia.php?p=taxdetails&id=996012 (accessed on 4 September 2023).
- Iredale, T. Guide to the land shells of New South Wales, part IV. Aust. Nat. 1942, 11, 33–40. [Google Scholar]
- Bellosi, E.S.; Miquel, S.E.; Kay, R.F.; Madden, R.H. Un calcrete de edad Mustersense de Patagonia central con los primeros Charopidae (Gastropoda) del Eoceno: Significado paleoclimático. Ameghiniana 2002, 39, 465–477. [Google Scholar]
- Miquel, S.E.; Bellosi, E.S. Un nuevo microgastrópodo terrestre (Charopidae) del Eoceno de Patagonia central, Argentina. Ameghiniana 2004, 41, 111–114. [Google Scholar]
- Miquel, S.E.; Bellosi, E.S. Microgasterópodos terrestres (Charopidae) del Eoceno medio de Gran Barranca (Patagonia Central, Argentina). Ameghiniana 2007, 44, 121–131. [Google Scholar]
- Miquel, S.E.; Ramirez, R. First records of actual and fossil Stephadiscus outside Patagonia, and description of a new Amazonian species (Mollusca: Pulmonata: Charopidae). Arch. Molluskenk. 2011, 140, 49–56. [Google Scholar]
- Rodriguez, P.E.; Miquel, S.E.; Tauber, A.A.; Krapovickas, J.M. First record of land gastropods of the family Charopidae in the Early to Middle Miocene from Santa Cruz Province, Southern Patagonia, Argentina (Gastropoda Pulmonata: Stylommatophora: Charopidae). Arch. Molluskenk. 2012, 141, 57–66. [Google Scholar] [CrossRef]
- Salvador, R.B.; Simone, L.R.L. Taxonomic revision of the fossil pulmonate mollusks of Itaborai Basin (Paleocene), Brazil. Pap. Avul. Zool. 2013, 53, 5–46. [Google Scholar] [CrossRef]
- Miquel, S.E.; Rodriguez, P.E. A novel late Early Miocene assemblage of terrestrial gastropods from Santa Cruz (Patagonia, Argentina). J. Paleont. 2015, 89, 748–761. [Google Scholar]
- Turazzini, G.F.; Miquel, S.E. A terrestrial gastropod community from the early Pliocene (Neogene) of Mendoza, Argentina, with description of a new species of Radiodiscus Pilsbry and Ferriss, 1906 (Mollusca: Pulmonata: Charopidae). Ameghiniana 2014, 51, 396–404. [Google Scholar]
- Salvador, R.B.; Cabrera, F.; Martínez, S.; Miquel, S.E.; Simone, L.R.L.; Cunha, C.M. Annotated catalogue of the fossil Hygrophila and Eupulmonata (Mollusca: Gastropoda) from South America (Cretaceous–Neogene). Jb. Geol. Paläont. Abh. 2018, 289, 249–280. [Google Scholar] [CrossRef]
- Marshall, B.A.; Worthy, T.H. Miocene land snails (Mollusca: Gastropoda: Pulmonata) from palaeolake Manuherikia, southern New Zealand. J. Roy. Soc. N. Z. 2017, 47, 294–318. [Google Scholar]
- Ladd, H.S. Fossil land shells from western Pacific atolls. J. Paleontol. 1958, 32, 183–198. [Google Scholar]
- Kadolsky, D. A remarkable non-marine mollusc fauna of early Eocene age from a fissure infill in Karsdorf quarry (Sachsen-Anhalt, Germany). Geol. Saxonica 2020, 65–66, 31–76. [Google Scholar]
- Wägele, J.-W.; Rödding, F. A priori estimation of phylogenetic information conserved in aligned sequences. Mol. Phylogenet Evol. 1998, 9, 358–365. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colgan, D.J.; Stanisic, J. The Phylogenetic Relationships of Australian Species within Charopidae (Gastropoda: Punctoidea). Diversity 2023, 15, 1124. https://doi.org/10.3390/d15111124
Colgan DJ, Stanisic J. The Phylogenetic Relationships of Australian Species within Charopidae (Gastropoda: Punctoidea). Diversity. 2023; 15(11):1124. https://doi.org/10.3390/d15111124
Chicago/Turabian StyleColgan, Donald James, and John Stanisic. 2023. "The Phylogenetic Relationships of Australian Species within Charopidae (Gastropoda: Punctoidea)" Diversity 15, no. 11: 1124. https://doi.org/10.3390/d15111124
APA StyleColgan, D. J., & Stanisic, J. (2023). The Phylogenetic Relationships of Australian Species within Charopidae (Gastropoda: Punctoidea). Diversity, 15(11), 1124. https://doi.org/10.3390/d15111124






