Decapods of the Southern Tip of South America and the Marine Protected Area Namuncurá–Burdwood Bank: A Nearshore–Offshore Comparison
Abstract
1. Introduction
2. Materials and Methods
2.1. Decapod Records
2.2. Species Identification
2.3. Statistical Analyses
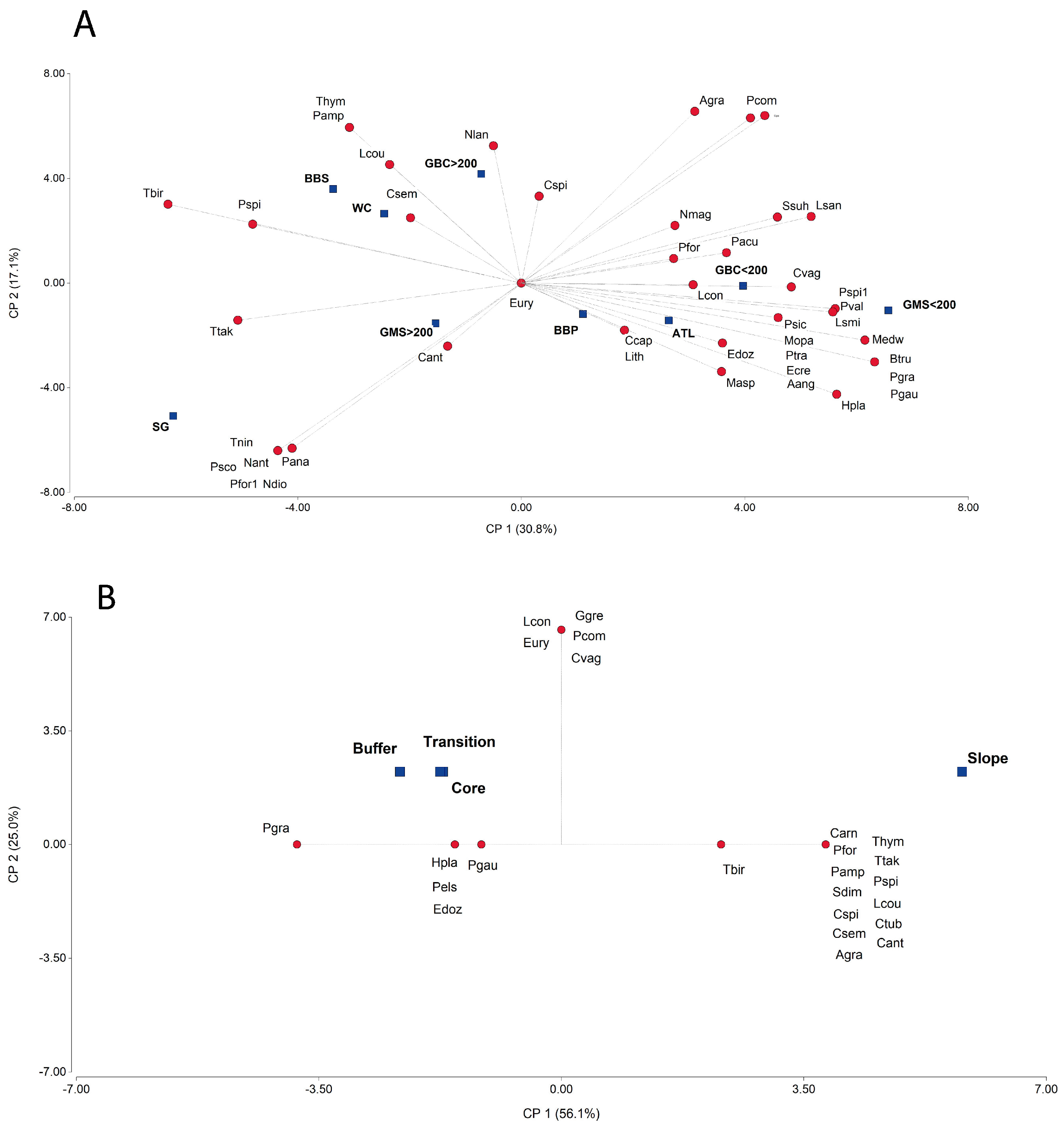
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sampling Zone | Average Similarity | Species | Contribution (%) |
|---|---|---|---|
| Atlantic | 20.59 | Eurypodius spp. | 39.73 |
| Peltarion spinulosum | 19.56 | ||
| Burdwood Bank plateau | 37.95 | Lithodes confundens | 34.60 |
| Eurypodius spp. | 26.96 | ||
| Burdwood Bank slope | 9.08 | Thymops birsteini | 20.74 |
| Chorismus tuberculatus | 14.89 | ||
| Lithodes confundens | 12.60 | ||
| Eurypodius spp. | 10.21 | ||
| Western Channel | 20.85 | Eurypodius spp. | 59.58 |
| Pagurus comptus | 16.44 | ||
| “Great” Beagle Channel < 200m | 12.31 | Peltarion spinulosum | 16.33 |
| Eurypodius spp. | 13.78 | ||
| Grimothea gregaria | 13.15 | ||
| Austropandalus grayi | 10.99 | ||
| “Great” Beagle Channel > 200m | 15.90 | Campylonotus semistriatus | 39.44 |
| Peltarion spinulosum | 29.61 | ||
| “Great” Magellan Strait < 200m | 15.49 | Halicarcinus planatus | 52.06 |
| “Great” Magellan Strait > 200m | 2.78 | Halicarcinus planatus | 100.00 |
| South Georgia | 19.10 | Paralomis spinosissima | 51.51 |
| ATL | BBP | BBS | WC | GBC < 200 | GBC > 200 | GMS < 200 | GMS > 200 | SG | |
|---|---|---|---|---|---|---|---|---|---|
| ATL | - | 78.23 | 91.82 | 82.31 | 86.77 | 89.77 | 86.97 | 92.35 | 99.23 |
| BBP | - | - | 87.52 | 82.17 | 87.36 | 93.72 | 89.24 | 93.94 | 99.29 |
| BBS | - | - | - | 88.72 | 94.76 | 93.82 | 95.48 | 95.48 | 96.03 |
| WC | - | - | - | - | 88.50 | 91.08 | 89.86 | 92.84 | 98.68 |
| GBC < 200 | - | - | - | - | - | 88.56 | 89.20 | 93.20 | 99.43 |
| GBC > 200 | - | - | - | - | - | - | 91.19 | 92.19 | 98.23 |
| GMS < 200 | - | - | - | - | - | - | - | 88.45 | 99.53 |
| GMS > 200 | - | - | - | - | - | - | - | - | 98.90 |
| SG | - | - | - | - | - | - | - | - | - |
| Sampling Zone | Average Sismilarity | Species | Contribution (%) |
|---|---|---|---|
| MPA N−BB buffer | 49.38 | Eurypodius spp. | 34.80 |
| Grimothea gregaria | 23.68 | ||
| MPA N−BB core | 48.73 | Lithodes confundens | 37.76 |
| Eurypodius spp. | 21.43 | ||
| MPA N−BB transition | 30.36 | Lithodes confundens | 45.23 |
| Eurypodius spp. | 23.16 | ||
| BB slope | 9.08 | Thymops birsteini | 20.74 |
| Chorismus tuberculatus | 14.89 | ||
| Lithodes confundens | 12.60 | ||
| Eurypodius spp. | 10.21 |
| Buffer | Core | Transition | Slope | |
|---|---|---|---|---|
| Buffer | - | 48.03 | 60.65 | 85.90 |
| Core | - | - | 59.96 | 85.11 |
| Transition | - | - | - | 88.50 |
| Slope | - | - | - | - |
| Decapod Distribution According to: | Decapod Records in the Area: | Endemic of | |||
|---|---|---|---|---|---|
| Species | Boschi (2000) [6] | Spivak et al. (2019) [7] | Fueguia Province [72] | Burdwood Bank | Fueguia Province |
| Acanthephyra pelagica | - | Both | Both | - | - |
| Acanthocyclus albatrossis | - | Both | Both | - | - |
| Anacalliax argentiniensis | Atlantic | Atlantic | Atlantic | - | Yes |
| Artemesia longinaris | Atlantic | - | - | - | - |
| Austropandalus grayi | Both | Both | Both | Yes | - |
| Bathicaris brasiliensis | Atlantic | Atlantic | - | - | - |
| Bellia picta | Pacific | - | - | - | - |
| Betaeus truncatus | Both | Both | Both | - | Yes |
| Campylonotus arntzianus | - | Atlantic | Atlantic | Yes | - |
| Campylonotus semistriatus | Both | Both | Both | Yes | Yes |
| Campylonotus vagans | Both | Both | Both | Yes | - |
| Chaecon notialis | Atlantic | - | - | - | Yes |
| Chorismus antarcticus | Both | Atlantic | Both | Yes | Yes |
| Chorismus tuberculatus | Atlantic | Atlantic | Atlantic | Yes | - |
| Coenobita compressus | Pacific | - | - | - | - |
| Coenophtalmus tridentatus | Atlantic | - | - | - | - |
| Corystoides chilensis | Both | - | - | - | - |
| Cyrtograpsus affinis | Atlantic | - | - | - | - |
| Cyrtograpsus altimanus | Atlantic | - | - | - | - |
| Cyrtograpsus angulatus | Both | Atlantic | Both | - | - |
| Eualus dozei | - | Both | Both | Yes | - |
| Eurypanopeus crenatus | Pacific | - | Pacific | - | - |
| Eurypodius latreillii/Eurypodius sp. | Both | - | Both | Yes | - |
| Eusergestes antarcticus | Both | Atlantic | Atlantic | - | - |
| Gomeza serrata | Pacific | - | Pacific | - | - |
| Grimothea gregaria (as Munida gregaria) | Both | Both | Both | Yes | - |
| Grimothea spinosa (as Munida spinosa) | Atlantic | Both | Both | Yes | - |
| Halicarcinus planatus | Both | Both | Both | Yes | - |
| Hemigrapsus crenulatus | Pacific | - | Pacific | - | - |
| Homalapsis plana | Pacific | - | Pacific | - | - |
| Inachoides microrhynchus | Pacific | - | - | - | - |
| Latreutes antiborialis | Pacific | - | - | - | - |
| Lebbeus antarcticus | - | Both | Both | - | - |
| Leucippa pentagona | Both | - | - | - | - |
| Leucosia planata | Atlantic | - | - | - | Yes |
| Leurocyclus tuberculosus | Both | Atlantic | - | - | - |
| Libidoclaea granaria | Both | Both | Both | - | - |
| Libidoclaea smithi | Pacific | - | Pacific | - | - |
| Liopetrolisthes mitra | Pacific | - | - | - | - |
| Liopetrolisthes patagonicus | - | Pacific | Pacific | - | - |
| Lithodes confundens | Both | Both | Both | Yes | Yes |
| Lithodes couesi | - | - | Atlantic | Yes | - |
| Lithodes santolla | Both | Both | Both | - | Yes |
| Lithodes turkayi | Both | Both | Both | - | - |
| Metacarcinus edwadsi | Pacific | Pacific | Pacific | - | - |
| Munidospsis aspera | Pacific | - | Pacific | - | - |
| Nauticaris magellanica | Both | Both | Both | - | Yes |
| Nematocarcinus lanceopes | - | Both | Both | - | - |
| Nematocarcinus longirostris | - | Atlantic | Atlantic | - | - |
| Neolithodes diomedeae | - | Both | Both | - | - |
| Notiax brachyophtalma | Both | Both | Both | - | Yes |
| Notiax santarita | - | Both | Both | - | - |
| Notocrangon antarcticus | Both | Both | Both | - | Yes |
| Ovalipes trimaculatus | Both | - | - | - | - |
| Paguristes weddelli | Pacific | - | Pacific | - | - |
| Pagurus comptus | Both | Both | Both | Yes | - |
| Pagurus forceps | - | Both | Both | Yes | - |
| Pandalopsis ampla | Both | Both | Both | Yes | - |
| Paralomis anamerae | Atlantic | - | Atlantic | - | Yes |
| Paralomis formosa | Atlantic | Atlantic | Atlantic | - | - |
| Paralomis granulosa | Both | Both | Both | Yes | Yes |
| Paralomis spinosissima | Atlantic | Atlantic | Atlantic | Yes | Yes |
| Paralomis tuberipes | Pacific | - | Pacific | - | Yes |
| Pasiphaea acutifrons | Both | Both | Both | - | - |
| Pasiphaea dofleni | Pacific | Both | Both | - | Yes |
| Pasiphaea rathbunae | - | Atlantic | Atlantic | - | - |
| Pasiphaea scotia | - | Atlantic | Atlantic | - | - |
| Peltarion spinulosum | Both | Both | Both | Yes | - |
| Pentacheles validus | - | Both | Both | - | - |
| Petalidium foliaceum | - | Atlantic | Atlantic | - | - |
| Petrolisthes laevigatus | Pacific | - | - | - | - |
| Petrolisthes violaceous | Pacific | - | - | - | - |
| Pilumnoides hassleri | Atlantic | - | Atlantic | - | - |
| Pilumnoides perlatus | Pacific | - | Pacific | - | - |
| Pinaxodes chilensis | Both | Pacific | Pacific | - | - |
| Pinnixa valdiviensis | Pacific | Both | Both | - | - |
| Pinnotherelia laevigata | Pacific | - | Pacific | - | - |
| Pisoides edwardsii | Pacific | - | Pacific | - | - |
| Planes cyaneus | Pacific | - | Pacific | - | - |
| Pleoticus muelleri | Atlantic | Atlantic | - | - | - |
| Propagurus gaudichaudii | Both | Both | Both | Yes | - |
| Pseudocorystes sicarius | Pacific | - | Pacific | - | - |
| Rochinia gracilipes | Pacific | Atlantic | Both | - | - |
| Romaleon setosum (as Cancer setosus) | Pacific | - | - | - | - |
| Sergia potens | Atlantic | - | Atlantic | - | - |
| Stereomastis suhmi | Pacific | Both | Both | - | Yes |
| Sympagurus dimorphus | Both | Both | Both | Yes | - |
| Synalpheus spinifrons | Pacific | - | Pacific | - | - |
| Taliepus dentatus | Pacific | - | - | - | - |
| Thymops birsteini | Both | Both | Both | Yes | - |
| Thymops takedai | - | Atlantic | Atlantic | Yes | - |
| Thymopsis ninlenta | - | Atlantic | Atlantic | - | - |
| Upogebia australis | - | Both | Both | - | - |
| Uroptychus parvulus | Pacific | - | Pacific | - | Yes |
References
- Clarke, A.; Johnston, N.M. Antarctic marine benthic diversity. In Oceanography and Marine Biology, An Annual Review; Gordon, J.D.M., Atkinson, R.J.A., Gibson, R.N., Eds.; CRC Press: Boca Raton, FL, USA, 2003; Volume 41, pp. 55–57. [Google Scholar]
- Arntz, W.E. The Magellan-Antarctic connection: Links and frontiers at southern high latitudes. Summary review. Sci. Mar. 2005, 69, 359–368. [Google Scholar] [CrossRef]
- Hellberg, M.E.; Aronson, R.B.; Smith, K.E.; Duhon, M.I.; Ahyong, S.T.; Lovrich, G.A.; Thatje, S.; McClintock, J.B. Population expansion of an Antarctic king crab? Front. Biogeogr. 2019, 11, e43165. [Google Scholar] [CrossRef]
- Arntz, W.E.; Thatje, S.; Gerdes, D.; Gili, J.-M.; Gutt, J.; Jacob, U.; Montiel, A.; Orejas, C.; Teixidó, N. The Antarctic-Magellan connection: Macrobenthos ecology on the shelf and upper slope, a progress report. Sci. Mar. 2005, 69, 237–269. [Google Scholar] [CrossRef]
- Gorny, M. On the biogeography and ecology of the Southern Ocean decapod fauna. Sci. Mar. 1999, 63, 367–382. [Google Scholar] [CrossRef]
- Boschi, E.E. Biodiversity of Marine Decapod Brachyurans of the Americas. J. Crustac. Biol. 2000, 20, 337–342. [Google Scholar] [CrossRef]
- Spivak, E.D.; Farías, N.E.; Ocampo, E.H.; Lovrich, G.A.; Luppi, T.A. Annotated catalogue and bibliography of marine and estuarine shrimps, lobsters, crabs and their allies (Crustacea: Decapoda) of Argentina and Uruguay (Southwestern Atlantic Ocean). Frente Marit. 2019, 26, 164. [Google Scholar]
- Thomson, M.R.A. Geological and palaeoenvironmental history of the Scotia Sea region as a basis for biological interpretation. Deep Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 1467–1487. [Google Scholar] [CrossRef]
- van de Lagemaat, S.H.A.; Swart, M.L.A.; Vaes, B.; Kosters, M.E.; Boschman, L.M.; Burton-Johnson, A.; Bijl, P.K.; Spakman, W.; van Hinsbergen, D.J.J. Subduction initiation in the Scotia Sea region and opening of the Drake Passage: When and why? Earth-Sci. Rev. 2021, 215, 103551. [Google Scholar] [CrossRef]
- Lovrich, G.A.; Romero, M.C.; Tapella, F.; Thatje, S. Distribution, reproductive and energetic conditions of decapod crustaceans along the Scotia Arc (Southern Ocean). Sci. Mar. 2005, 69, 183–193. [Google Scholar] [CrossRef]
- Tombesi, M.L.; Rabuffetti, F.; Lovrich, G.A. Las áreas marinas protegidas en la Argentina: Área Marina Protegida Namuncurá-Banco Burdwood. Lupa Colecc. Fueguina Divulg. Cient. 2020, 16, 2–7. Available online: http://id.caicyt.gov.ar/ark:/s27967360/no7kfy3zg (accessed on 3 January 2021).
- Matano, R.P.; Palma, E.D.; Combes, V. The Burdwood Bank Circulation. J. Geophys. Res. Ocean. 2019, 124, 6904–6926. [Google Scholar] [CrossRef]
- García Alonso, V.A.; Brown, D.; Martín, J.; Pájaro, M.; Capitanio, F.L. Seasonal patterns of Patagonian sprat Sprattus fuegensis early life stages in an open sea Sub-Antarctic Marine Protected Area. Polar Biol. 2018, 41, 2167–2179. [Google Scholar] [CrossRef]
- Guinder, V.A.; Malits, A.; Ferronato, C.; Krock, B.; Garzón-Cardona, J.; Martínez, A. Microbial plankton configuration in the epipelagic realm from the Beagle Channel to the Burdwood Bank, a Marine Protected Area in Sub-Antarctic waters. PLoS ONE 2020, 15, e0233156. [Google Scholar] [CrossRef]
- Bértola, G. Patrón Espacial de la Estructura de la Comunidad Planctónica Unicelular del Área Marina Protegida Namuncurá-Banco Burdwood e Inmediaciones, con Énfasis en las Diatomeas del Género Rhizosolenia. Licence Degree Thesis, Universidad de Buenos Aires, Buenos Aires, Argentina, 2018. [Google Scholar]
- Spinelli, M.L.; Malits, A.; García Alonso, V.A.; Martín, J.; Capitanio, F.L. Spatial gradients of spring zooplankton assemblages at the open ocean sub-Antarctic Namuncurá Marine Protected Area/Burdwood Bank, SW Atlantic Ocean. J. Mar. Syst. 2020, 210, 103398. [Google Scholar] [CrossRef]
- Riccialdelli, L.; Becker, Y.A.; Fioramonti, N.E.; Torres, M.; Bruno, D.O.; Raya Rey, A.; Fernández, D.A. Trophic structure of southern marine ecosystems: A comparative isotopic analysis from the Beagle Channel to the oceanic Burdwood Bank area under a wasp-waist assumption. Mar. Ecol. Prog. Ser. 2020, 655, 1–27. [Google Scholar] [CrossRef]
- Fischer, L.; Covatti Ale, M.; Deli Antoni, M.; Díaz de Astarloa, J.M.; Delpiani, G. Feeding ecology of the longtail southern cod, Patagonotothen ramsayi (Regan, 1913) (Notothenioidei) in the Marine Protected Area Namuncurá-Burdwood Bank, Argentina. Polar Biol. 2022, 45, 1483–1494. [Google Scholar] [CrossRef]
- Rossi, S.; Bramanti, L.; Gori, A.; Orejas, C. Animal Forests of the World: An Overview. In Marine Animal Forests; Rossi, S., Bramanti, L., Gori, A., Orejas, C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–28. [Google Scholar] [CrossRef]
- Schejter, L.; Albano, M. Benthic communities at the marine protected area Namuncurá/Burdwood bank, SW Atlantic Ocean: Detection of vulnerable marine ecosystems and contributions to the assessment of the rezoning process. Polar Biol. 2021, 44, 2023–2037. [Google Scholar] [CrossRef]
- Schejter, L.; Bremec, C.S. Stony corals (Anthozoa: Scleractinia) of Burdwood Bank and neighbouring areas, SW Atlantic Ocean. Sci. Mar. 2019, 83, 247–260. [Google Scholar] [CrossRef]
- Berke, S.K. Functional Groups of Ecosystem Engineers: A Proposed Classification with Comments on Current Issues. Integr. Comp. Biol. 2010, 50, 147–157. [Google Scholar] [CrossRef]
- CCAMLR (Comisión Para la Conservación de los Recursos Vivos Marinos Antárticos). Medida de Conservación 91-03. Protección de la Plataforma Sur de las Islas Orcadas del Sur. Available online: https://www.ccamlr.org/es/node/74906 (accessed on 23 January 2023).
- Jones, C.D.; Lockhart, S.J. Detecting vulnerable marine ecosystems in the Southern Ocean using research trawls and underwater imagery. Mar. Policy 2011, 35, 732–736. [Google Scholar] [CrossRef]
- Pallas, A.; Garcia-Calvo, B.; Corgos, A.; Bernardez, C.; Freire, J. Distribution and habitat use patterns of benthic decapod crustaceans in shallow waters: A comparative approach. Mar. Ecol. Prog. Ser. 2006, 324, 173–184. [Google Scholar] [CrossRef]
- Doti, B.L.; Chiesa, I.L.; Roccatagliata, D. Biodiversity of Isopoda and Cumacea (Peracarida, Crustacea) from the Marine Protected Area Namuncurá-Burdwood Bank, South-West Atlantic. Polar Biol. 2020, 43, 1519–1534. [Google Scholar] [CrossRef]
- Bremec, C.; Elías, R.; Calla, S.; Genzano, G.; Puente-Tapia, A.; Schejter, L. Polychaetes from Burdwood Bank:“Namuncurá I” Marine Protected Area and slope, SW Atlantic Ocean. Rev. Biol. Trop. 2019, 67, 119–135. [Google Scholar] [CrossRef]
- Moyano, G.H.I. Scotia Arc bryozoans from the LAMPOS expedition: A narrow bridge between two different faunas. Sci. Mar. 2005, 69, 103–112. [Google Scholar] [CrossRef]
- Fraysse, C.; Calcagno, J.; Pérez, A.F. Asteroidea of the southern tip of South America, including Namuncurá Marine Protected Area at Burdwood Bank and Tierra del Fuego Province, Argentina. Polar Biol. 2018, 41, 2423–2433. [Google Scholar] [CrossRef]
- Teso, V.; Urteaga, D.; Pastorino, G. Assemblages of certain benthic molluscs along the southwestern Atlantic: From subtidal to deep sea. BMC Ecol. 2019, 19, 49. [Google Scholar] [CrossRef]
- Güller, M.; Zelaya, D.G. New insigths into the diversity of rissoids from sub-antarctic and antarctic waters (Gastropoda: Rissooidea). Polar Biol. 2017, 40, 1923–1937. [Google Scholar] [CrossRef]
- Roccatagliata, D. Informe Sumario. Campana Antártica de Verano CAV 3ra. etapa Ushuaia—Mar del Plata. 19 de marzo—04 abril de 2013. Buque Oceanográfico ARA “Puerto Deseado” (Technical Report); CONICET: Buenos Aires, Argentina, 2013; p. 66. Available online: https://proyectosinv.conicet.gov.ar/wp-content/uploads/sites/6/Campa%C3%B1a-Antartica-de-Verano-2012-2013-Puerto-Deseado.pdf (accessed on 20 January 2021).
- Roccatagliata, D. Campaña al AMP Namuncurá—Banco Burdwood: Bentos (Technical Report); CONICET: Buenos Aires, Argentina, 2016; p. 274. Available online: https://argentina.gob.ar/sites/default/files/06._amp_namuncura_banco_burdwood_-_abril_2016.pdf (accessed on 20 January 2021).
- Schejter, L. Informe de Campaña: Banco Burdwood—Buque Oceanográfico ARA “Puerto Deseado”—PD BB Abr 17 (Technical Report); APN: Buenos Aires, Argentina, 2017; p. 296. Available online: https://proyectosinv.conicet.gov.ar/wp-content/uploads/sites/6/Informe-de-Campa%C3%B1a-Puerto-Deseado-Banco-Burdwood-2017.pdf (accessed on 21 January 2021).
- Lovrich, G.A. Estudios Biológicos en Plataforma Patagónica Austral. Informe de la Campaña Oceanográfica a Bordo del BO Puerto Deseado. Puerto Madryn 30NOV–Ushuaia 16DIC 2009 (Technical Report); CONICET: Buenos Aires, Argentina, 2010; p. 131. Available online: https://proyectosinv.conicet.gov.ar/wp-content/uploads/sites/6/Informe-Campa%C3%B1a-Puerto-Deseado-CADIC-2009.pdf (accessed on 20 January 2021).
- Lovrich, G.A. Patagonia Austral. Informe de la Campaña Oceanográfica a Bordo del BO Puerto Deseado. Ushuaia 27MAR-14ABR 2012 (Technical Report); CONICET: Buenos Aires, Argentina, 2012; p. 150. Available online: https://proyectosinv.conicet.gov.ar/wp-content/uploads/sites/6/Informe-Campa%C3%B1a-BOPD-Patagonia-Austral-2012.pdf (accessed on 21 January 2021).
- Tapella, F.; Romero, M.C.; Lovrich, G.A.; Chizzini, A. Life history of the galatheid crab Munida subrugosa in subantarctic waters of the Beagle Channel, Argentina. In Crabs in Cold Water Regions: Biology, Management, And Economics; Paul, A.J., Dawe, E.G., Elner, R., Jamieson, G.S., Kruse, G.H., Otto, R.S., Sainte-Marie, B., Shirley, T.C., Woodby, D., Eds.; University of Alaska Sea Grant: Fairbanks, AK, USA, 2002; pp. 114–124. [Google Scholar]
- Pérez-Barros, P.; Tapella, F.; Romero, M.C.; Calcagno, J.A.; Lovrich, G.A. Benthic decapod crustaceans associated with captures of Munida spp. (Decapoda: Anomura) in the Beagle Channel, Argentina. Sci. Mar. 2004, 68, 237–246. [Google Scholar] [CrossRef]
- Arntz, W.E.; Brey, T. The Expedition ANTARKTIS XIX/5 (LAMPOS) of RV” Polarstern” in 2002. Ber. Polarforsch. 2003, 462, 124. [Google Scholar]
- Arntz, W.E.; Gorny, M.; Soto, R.; Lardies, M.A.; Retamal, M.; Wehrtmann, I.S. Species composition and distribution of decapod crustaceans in the waters off Patagonia and Tierra del Fuego, South America. Sci. Mar. 1999, 63, 303–314. [Google Scholar] [CrossRef]
- GBIF. The Global Biodiversity Information Facility: 2021. Available online: https://www.gbif.org/occurrence/download/0155588-200613084148143 (accessed on 14 January 2021).
- OBIS (Ocean Biodiversity Information System). Intergovernmental Oceanographic Commission of UNESCO. 2021. Available online: www.obis.org (accessed on 13 January 2021).
- Boschi, E.E.; Fischbach, C.E.; Iorio, M.I. Catálogo ilustrado de los crustáceos estomatópodos y decápodos marinos de Argentina. Frente Marit. 1992, 10, 7–94. [Google Scholar]
- Holthuis, L.B. A General Revision of the Palaemonidae (Crustacea Decapod Natantia) of the Americas. II. The Subfamily Palaemonidae; Allan Hancock Foundation Publications of the University of Southern California Occasional Papers; The University of Southern California Press: Los Angeles, CA, USA, 1952; p. 396. [Google Scholar]
- Holthuis, L.B. Biological results of the University of Miami deep-sea expeditions. 106. The lobsters of the superfamily Nephropidea of the Atlantic Ocean (Crustacea: Decapoda). Bull. Mar. Sci. 1974, 24, 723–884. [Google Scholar]
- Ahyong, S.T.; Webber, W.R.; Chan, T.-Y. Thymops takedai, a new species of deepwater lobster from the Southwest Atlantic Ocean with additional records of ‘thymopine’ lobsters (Decapoda, Nephropidae). In Studies on Eumalacostraca: A Homage to Masatsune Takeda; Komatsu, H., Okuno, J., Fukuoka, K., Eds.; Brill: Leiden, The Netherlands, 2012; pp. 49–61. [Google Scholar] [CrossRef]
- Retamal, M.A.; Arana, P.M. Descripción y distribución de cinco crustáceos decápodos recolectados en aguas profundas en torno a las islas Robinson Crusoe y Santa Clara (Archipiélago de Juan Fernández, Chile). Investig. Mar. 2000, 28, 149–163. [Google Scholar] [CrossRef]
- Meyer, R.; Weis, A.; Melzer, R.R. Decapoda of southern Chile: DNA barcoding and integrative taxonomy with focus on the genera Acanthocyclus and Eurypodius. Syst. Biodivers. 2013, 11, 389–404. [Google Scholar] [CrossRef]
- Machordom, A.; Ahyong, S.T.; Andreakis, N.; Baba, K.; Buckley, D.; García-Jiménez, R.; McCallum, A.W.; Rodríguez-Flores, P.C.; Macpherson, E. Deconstructing the crustacean squat lobster genus Munida to reconstruct the evolutionary history and systematics of the family Munididae (Decapoda, Anomura, Galatheoidea). Invertebr. Syst. 2022, 36, 926–970. [Google Scholar] [CrossRef]
- Macpherson, E. Revision of the family Lithodidae Samouelle, 1819 (Crustacea, Decapoda, Anomura) in the Atlantic Ocean. Monogr. De Zool. Mar. 1988, 2, 9–153. [Google Scholar]
- Pérez-Barros, P.; Confalonieri, V.A.; Paschke, K.; Lovrich, G.A. Incongruence between molecular and morphological characters in the southern king crabs Lithodes santolla and Lithodes confundens (Decapoda: Anomura). Polar Biol. 2015, 38, 2097–2107. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. User Manual/Tutorial; Primer-E Ltd.: Plymouth, UK, 2006; p. 93. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. An approach to statistical analysis and interpretation. Chang. Mar. Communities 1994, 2, 117–143. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat, Versión 2017; Universidad Nacional de Córdoba: Córdoba, Argentina, 2017.
- Vinuesa, J.H.; Lovrich, G.A.; Tapella, F. New localities for Crustacea Decapoda in the Magellan region, southern South America. Sci. Mar. 1999, 63, 321–323. [Google Scholar] [CrossRef]
- Thatje, S. Campylonotus arntzianus, a new species of the Campylonotidae (Crustacea: Decapoda: Caridea) from the Scotia Sea (Antarctica). Polar Biol. 2003, 26, 242–248. [Google Scholar] [CrossRef]
- Torti, M.R.; Boschi, E.E. Decapodos Caridea del genero Campylonotus Bate, 1888. Physis Secc. A 1973, 32, 65–84. [Google Scholar]
- Nye, V.; Copley, J.T.; Linse, K. A new species of Eualus Thallwitz, 1892 and new record of Lebbeus antarcticus (Hale, 1941) (Crustacea: Decapoda: Caridea: Hippolytidae) from the Scotia Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2013, 92, 145–156. [Google Scholar] [CrossRef]
- Anosov, S.E.; Spiridonov, V.A.; Neretina, T.V.; Uryupova, E.F.; Schepetov, D. King crabs of the western Atlantic sector of Antarctic and adjacent areas: New records, molecular barcode data and distribution (Crustacea: Decapoda: Lithodidae). Polar Biol. 2015, 38, 231–249. [Google Scholar] [CrossRef]
- Stevens, B.G.; Lovrich, G.A. King crabs of the world: Species and distributions. In King Crabs of the World: Biology and Fisheries Management; Stevens, B.G., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–29. [Google Scholar]
- Petraroia, A. Estudio del Estatus Taxonómico, la Posición Filogenética y la Diversidad Genética de la Langostilla Munida spinosa (Crustacea: Decapoda: Munididae). Licence Degree Thesis, Universidad de Buenos Aires, Buenos Aires, Argentina, 2014. [Google Scholar]
- Raupach, M.J.; Thatje, S.; Dambach, J.; Rehm, P.; Misof, B.; Leese, F. Genetic homogeneity and circum-Antarctic distribution of two benthic shrimp species of the Southern Ocean, Chorismus antarcticus and Nematocarcinus lanceopes. Mar. Biol. 2010, 157, 1783–1797. [Google Scholar] [CrossRef][Green Version]
- Mantelatto, F.L.; Pardo, L.M.; Pileggi, L.G.; Felder, D.L. Taxonomic re-examination of the hermit crab species Pagurus forceps and Pagurus comptus (Decapoda: Paguridae) by molecular analysis. Zootaxa 2009, 2133, 20–32. [Google Scholar] [CrossRef]
- López Abellan, L.J.; Balguerías, E. On the presence of Paralomis spinosissima and Paralomis formosa in catches taken during the spanish survey Antartida 8611. CCAMLR Sci. 1994, 1, 165–173. [Google Scholar]
- Basher, Z.; Bowden, D.A.; Costello, M.J. Diversity and Distribution of Deep-Sea Shrimps in the Ross Sea Region of Antarctica. PLoS ONE 2014, 9, e103195. [Google Scholar] [CrossRef]
- Farias, N.E.; Ocampo, E.H.; Luppi, T.A. On the presence of the deep-sea blind lobster Stereomastis suhmi (Decapoda: Polychelidae) in Southwestern Atlantic waters and its circum-Antarctic distribution. N. Z. J. Zool. 2015, 42, 119–125. [Google Scholar] [CrossRef]
- Holthuis, L.B. FAO Species Catalogue, Vol. 13: Marine Lobsters of the World. An Annotated and Illustrated Catalogue of Species of Interest to Fisheries Known to Date; FAO: Rome, Italy, 1991; pp. 1–292. [Google Scholar]
- Laptikhovsky, V.; Reyes, P.R. Distribution and reproductive biology of a subantarctic deep-sea lobster, the Patagonian lobsterette Thymops birsteini (Zarenkov and Semenov, 1972) (Decapoda, Astacidea, Nephropidae). J. Nat. Hist. 2009, 43, 35–46. [Google Scholar] [CrossRef]
- Brun, A.A.; Griotti, M.; Roig-Juñent, S.A.; Acha, M.E. Biogeographical patterns and areas of endemism for the Magellan region based on the distribution of crustacean species (Amphipoda, Copepoda, and Euphausiacea). Polar Biol. 2020, 43, 237–250. [Google Scholar] [CrossRef]
- Boschi, E.E.; Gavio, M.A. On the distribution of decapod crustaceans from the Magellan Biogeographic Province and the Antarctic region. Sci. Mar. 2005, 69, 195–200. [Google Scholar] [CrossRef]
- Briggs, J.C. Marine Zoogeography; McGraw-Hill: New York, NY, USA, 1974; pp. 1–475. [Google Scholar]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. Bioscience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Briggs, J.C.; Bowen, B.W. A realignment of marine biogeographic provinces with particular reference to fish distributions. J. Biogeogr. 2012, 39, 12–30. [Google Scholar] [CrossRef]
- Soberón, J.; Peterson, A.T. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodiv. Inf. 2005, 2, 1–10. [Google Scholar] [CrossRef]
- Dayton, P.K. Ecology of kelp communities. Annu. Rev. Ecol. Syst. 1985, 16, 215–245. [Google Scholar] [CrossRef]
- Diez, M.J.; Lovrich, G.A. Reproductive biology of the crab Halicarcinus planatus (Brachyura, Hymenosomatidae) in sub-Antarctic waters. Polar Biol. 2010, 33, 389–401. [Google Scholar] [CrossRef]
- Gaylord, B.; Nickols, K.J.; Jurgens, L. Roles of transport and mixing processes in kelp forest ecology. J. Exp. Biol. 2012, 215, 997–1007. [Google Scholar] [CrossRef]
- Miller, R.J.; Lafferty, K.D.; Lamy, T.; Kui, L.; Rassweiler, A.; Reed, D.C. Giant kelp, Macrocystis pyrifera, increases faunal diversity through physical engineering. Proc. R. Soc. B. 2018, 285, 20172571. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Kwak, S.N.; Riedel, R. Crustacean decapod assemblage associated with seagrass (Zostera marina) beds in Southern waters of Korea. Diversity 2020, 12, 89. [Google Scholar] [CrossRef]
- Costa, G.; Bavestrello, G.; Canese, S.; Canessa, M.; Mazzoli, C.; Montagna, P.; Puce, S.; Schiaparelli, S.; Bertolino, M. Sponges associated with stylasterid thanatocoenosis (Cnidaria, Hydrozoa) from the deep Ross Sea (Southern Ocean). Polar Biol. 2022, 45, 703–718. [Google Scholar] [CrossRef]
- Schejter, L.; Genzano, G.; Gaitán, E.; Perez, C.D.; Bremec, C.S. Benthic communities in the Southwest Atlantic Ocean: Conservation value of animal forests at the Burdwood Bank slope. Aquat. Conserv. Mar. Freshwat. Ecosyst. 2020, 30, 426–439. [Google Scholar] [CrossRef]
- Schejter, L.; Martín de Nascimento, J.; Lovrich, G.A. Unveiling the submarine landscape of the Namuncurá marine protected area, Burdwood Bank, SW Atlantic Ocean. Pan-Am. J. Aquat. Sci. 2017, 12, 248–253. [Google Scholar]
- Pérez-Barros, P.; Albano, M.; Diez, M.J.; Lovrich, G.A. Pole to pole: The deep-sea king crab Lithodes couesi (Decapoda: Lithodidae) in the Burdwood Bank, Southwestern Atlantic Ocean. Polar Biol. 2020, 43, 81–86. [Google Scholar] [CrossRef]
- De Clippele, L.H.; Buhl-Mortensen, P.; Buhl-Mortensen, L. Fauna associated with cold water gorgonians and sea pens. Cont. Shelf Res. 2015, 105, 67–78. [Google Scholar] [CrossRef]
- Bracken-Grissom, H.; Widder, E.; Johnsen, S.; Messing, C.; Frank, T. Decapod diversity associated with deep-sea octocorals in the Gulf of Mexico. Crustaceana 2018, 91, 1267–1275. [Google Scholar] [CrossRef]
- Krieger, K.J.; Wing, B.L. Megafauna associations with deepwater corals (Primnoa spp.) in the Gulf of Alaska. Hydrobiologia 2002, 471, 83–90. [Google Scholar] [CrossRef]
- Klompmaker, A.A.; Jakobsen, S.L.; Lauridsen, B.W. Evolution of body size, vision, and biodiversity of coral-associated organisms: Evidence from fossil crustaceans in cold-water coral and tropical coral ecosystems. BMC Evol. Biol. 2016, 16, 132. [Google Scholar] [CrossRef]
- De Clippele, L.H.; Huvenne, V.A.; Molodtsova, T.N.; Roberts, J.M. The diversity and ecological role of non-scleractinian corals (Antipatharia and Alcyonacea) on scleractinian cold-water coral mounds. Front. Mar. Sci. 2019, 6, 184. [Google Scholar] [CrossRef]
- Thomson, A.I.; Archer, F.I.; Coleman, M.A.; Gajardo, G.; Goodall-Copestake, W.P.; Hoban, S.; Laikre, L.; Miller, A.D.; O’Brien, D.; Pérez-Espona, S.; et al. Charting a course for genetic diversity in the UN Decade of Ocean Science. Evol. Appl. 2021, 14, 1497–1518. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.D.; Matano, R.P.; Combes, V. Circulation and cross-shelf exchanges in the Malvinas Islands Shelf region. Prog. Oceanogr. 2021, 198, 102666. [Google Scholar] [CrossRef]
- Frey, D.I.; Piola, A.R.; Krechik, V.A.; Fofanov, D.V.; Morozov, E.G.; Silvestrova, K.P.; Tarakanov, R.Y.; Gladyshev, S.V. Direct measurements of the Malvinas Current velocity structure. J. Geophys. Res. Oceans. 2021, 126, e2020JC016727. [Google Scholar] [CrossRef]
- Pereira, E. Sistemática Filogenética y Biogeografía de Isópodos Valvifera (Crustacea: Peracarida) de la Plataforma Continental y Talud de Argentina. Licence Degree Thesis, Universidad de Buenos Aires, Buenos Aires, Argentina, 2022. [Google Scholar]
- Flores, J.N.; Penchaszadeh, P.E.; Brogger, M.I. Heart urchins from the depths: Corparva lyrida gen. et sp. nov. (Palaeotropidae), and new records for the southwestern Atlantic Ocean. Rev. Biol. Trop. 2021, 69, 14–33. [Google Scholar] [CrossRef]
- GBIF. The Global Biodiversity Information Facility: 2023. Available online: https://www.gbif.org/occurrence/download/0270431-220831081235567 (accessed on 2 February 2023).
- Moon, K.L.; Chown, S.L.; Fraser, C.I. Reconsidering connectivity in the sub-Antarctic. Biol. Rev. 2017, 92, 2164–2181. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.I.; Morrison, A.K.; Hogg, A.M.; Macaya, E.C.; van Sebille, E.; Ryan, P.G.; Padovan, A.; Jack, C.; Valdivia, N.; Waters, J.M. Antarctica’s ecological isolation will be broken by storm-driven dispersal and warming. Nat. Clim. Chang. 2018, 8, 704–708. [Google Scholar] [CrossRef]
- Thornhill, D.J.; Mahon, A.R.; Norenburg, J.L.; Halanych, K.M. Open-ocean barriers to dispersal: A test case with the Antarctic Polar Front and the ribbon worm Parborlasia corrugatus (Nemertea: Lineidae). Mol. Ecol. 2008, 17, 5104–5117. [Google Scholar] [CrossRef]
- González-Wevar, C.A.; Segovia, N.I.; Rosenfeld, S.; Maturana, C.S.; Jeldres, V.; Pinochet, R.; Saucède, T.; Morley, S.A.; Brickle, P.; Wilson, N.G.; et al. Seven snail species hidden in one: Biogeographic diversity in an apparently widespread periwinkle in the Southern Ocean. J. Biogeogr. 2022, 49, 1521–1534. [Google Scholar] [CrossRef]
- Thatje, S. Effects of Capability for Dispersal on the Evolution of Diversity in Antarctic Benthos. Integr. Comp. Biol. 2012, 52, 470–482. [Google Scholar] [CrossRef]
- Matano, R.P.; Combes, V.; Young, E.F.; Meredith, M.P. Modeling the Impact of Ocean Circulation on Chlorophyll Blooms Around South Georgia, Southern Ocean. J. Geophys. Res. Ocean. 2020, 125, e2020JC016391. [Google Scholar] [CrossRef]
- Thatje, S.; Fuentes, V. First record of anomuran and brachyuran larvae (Crustacea: Decapoda) from Antarctic waters. Polar Biol. 2003, 26, 279–282. [Google Scholar] [CrossRef]
- Frederich, M.; Sartoris, F.J.; Pörtner, H.O. Distribution patterns of decapod crustaceans in polar areas: A result of magnesium regulation? In Ecological Studies in the Antarctic Sea Ice Zone: Results of EASIZ Midterm Symposium; Arntz, W.E., Clarke, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 246–250. [Google Scholar] [CrossRef]
- Lovrich, G.A.; Perroni, M.; Vinuesa, J.H.; Tapella, F.; Chizzini, A.; Romero, M.C. Occurrence of Lithodes confundens (Decapoda: Anomura) in the intertidal of the Southwestern Atlantic. J. Crustac. Biol. 2002, 22, 894–902. [Google Scholar] [CrossRef][Green Version]
- Sotelano, M.P.; Gowland Sainz, M.F.; Diez, M.J.; Lovrich, G.A. Distribution of Lithodes confundens Macpherson, 1988 (Decapoda, Anomura) along the Atlantic continental shelf of southern South America. Crustaceana 2013, 86, 246–252. [Google Scholar] [CrossRef]
- Ludt, W.B. Missing in the Middle: A Review of Equatorially Disjunct Marine Taxa. Front. Mar. Sci. 2021, 8, 660984. [Google Scholar] [CrossRef]
- Pinsky, M.L.; Palumbi, S.R. Meta-analysis reveals lower genetic diversity in overfished populations. Mol. Ecol. 2014, 23, 29–39. [Google Scholar] [CrossRef]
- Allendorf, F.W.; England, P.R.; Luikart, G.; Ritchie, P.A.; Ryman, N. Genetic effects of harvest on wild animal populations. Trends Ecol. Evol. 2008, 23, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Yorisue, T.; Iguchi, A.; Yasuda, N.; Yoshioka, Y.; Sato, T.; Fujita, Y. Evaluating the effect of overharvesting on genetic diversity and genetic population structure of the coconut crab. Sci. Rep. 2020, 10, 10026. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). Fisheries and Aquaculture. Available online: https://www.fao.org/fishery/es/aqspecies/search (accessed on 15 February 2023).
- Halpern, B.S.; Lester, S.E.; McLeod, K.L. Placing marine protected areas onto the ecosystem-based management seascape. Proc. Natl. Acad. Sci. USA 2010, 107, 18312–18317. [Google Scholar] [CrossRef]
- Berkström, C.; Wennerström, L.; Bergström, U. Ecological connectivity of the marine protected area network in the Baltic Sea, Kattegat and Skagerrak: Current knowledge and management needs. Ambio 2022, 51, 1485–1503. [Google Scholar] [CrossRef]
- Guihou, K.; Piola, A.R.; Palma, E.D.; Chidichimo, M.P. Dynamical connections between large marine ecosystems of austral South America based on numerical simulations. Ocean Sci. 2020, 16, 271–290. [Google Scholar] [CrossRef]

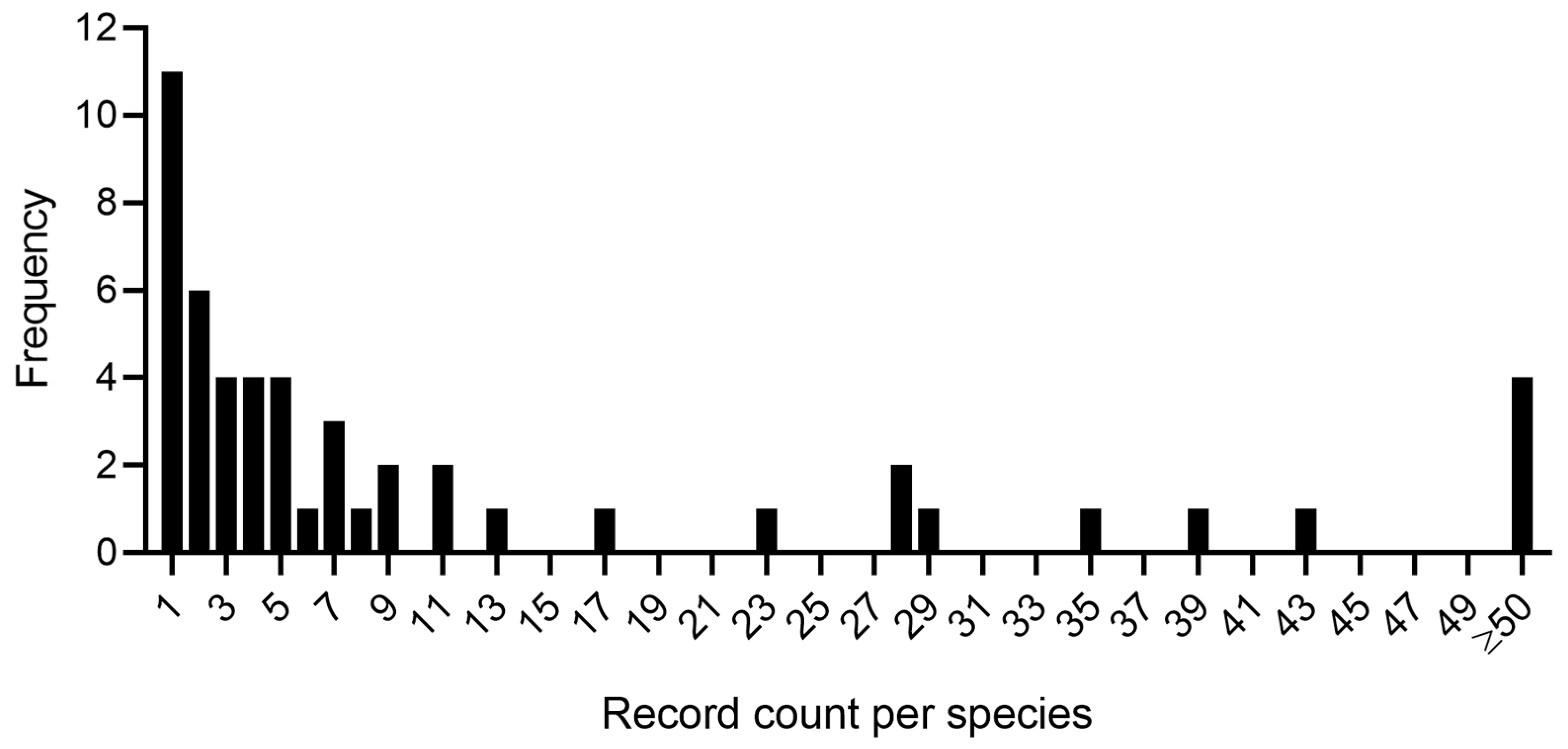
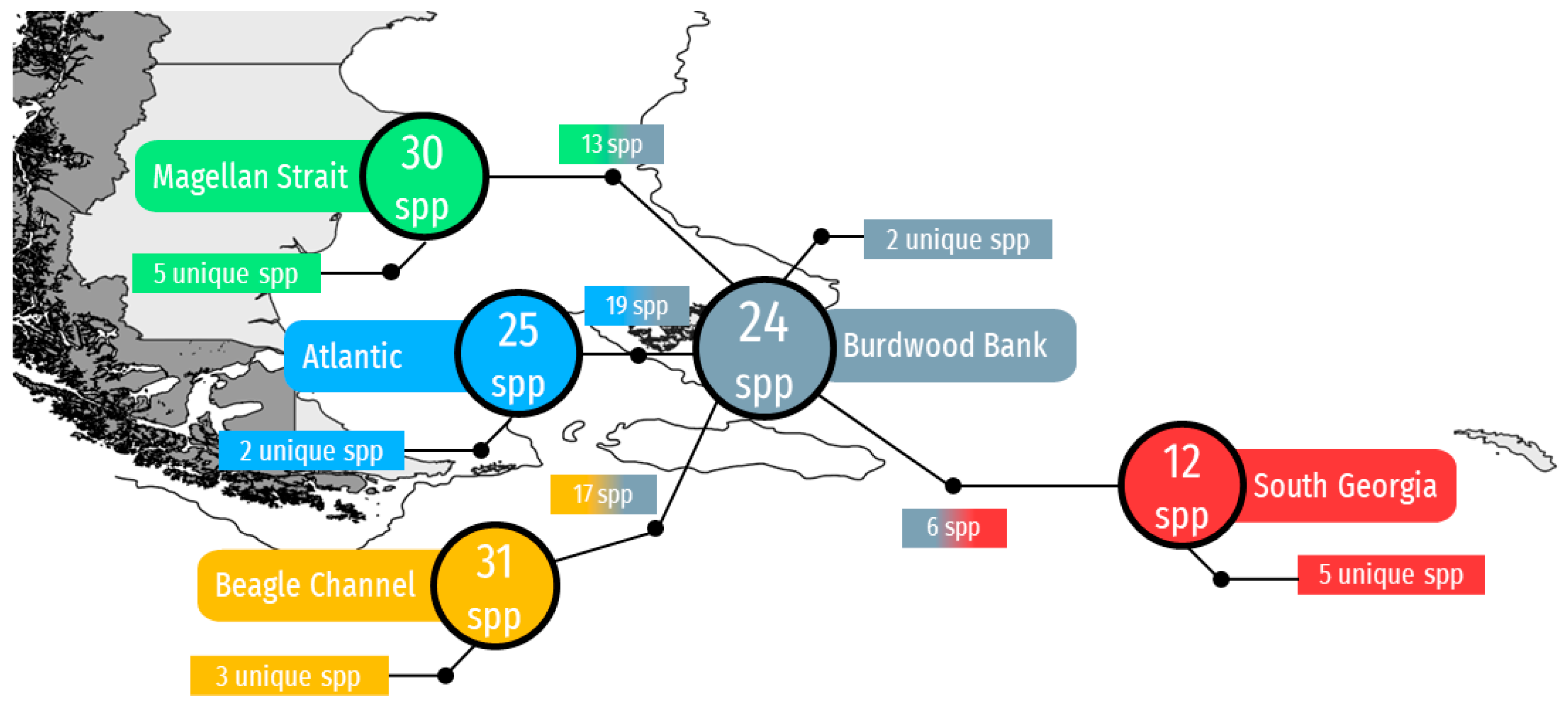
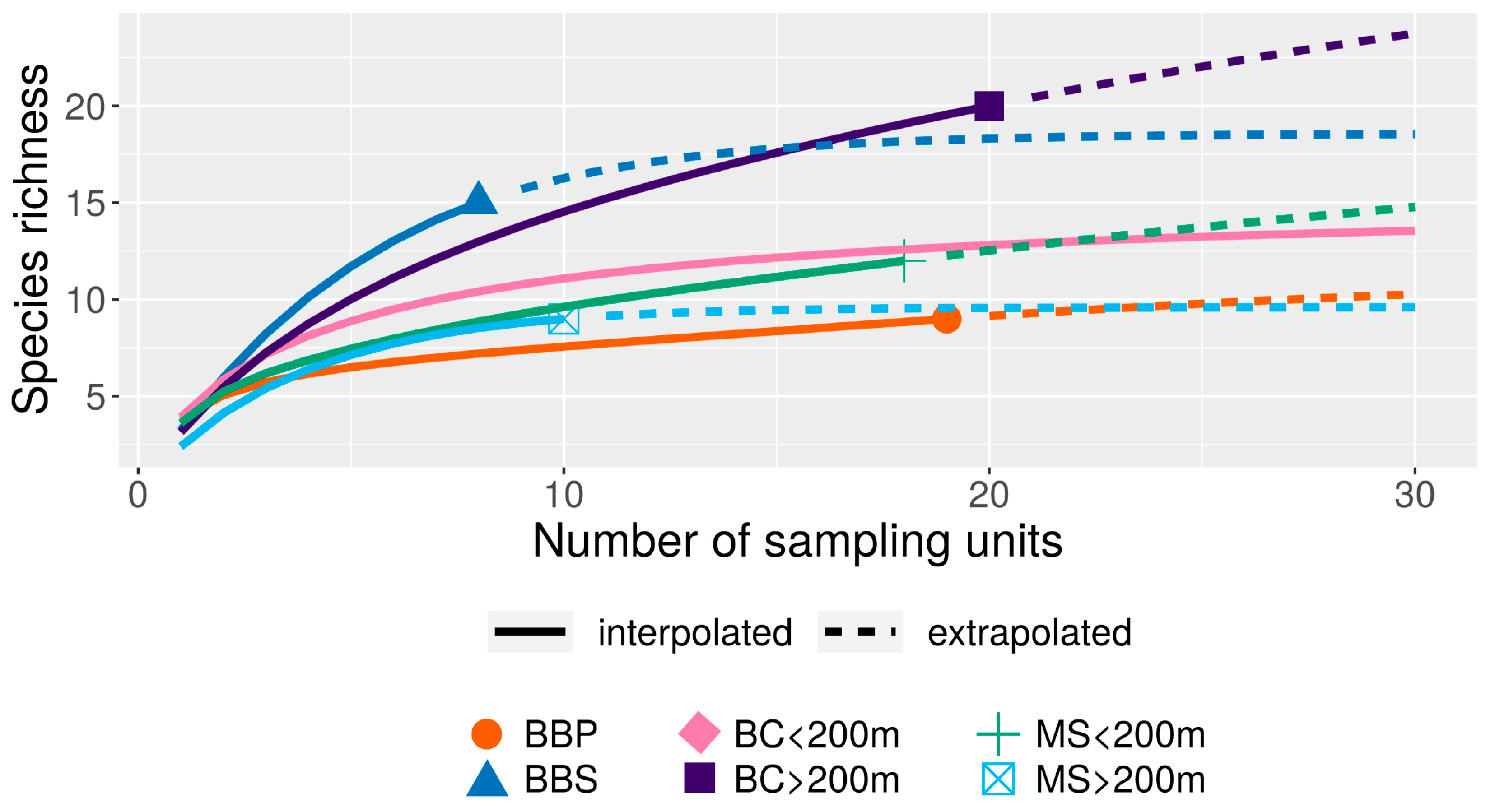
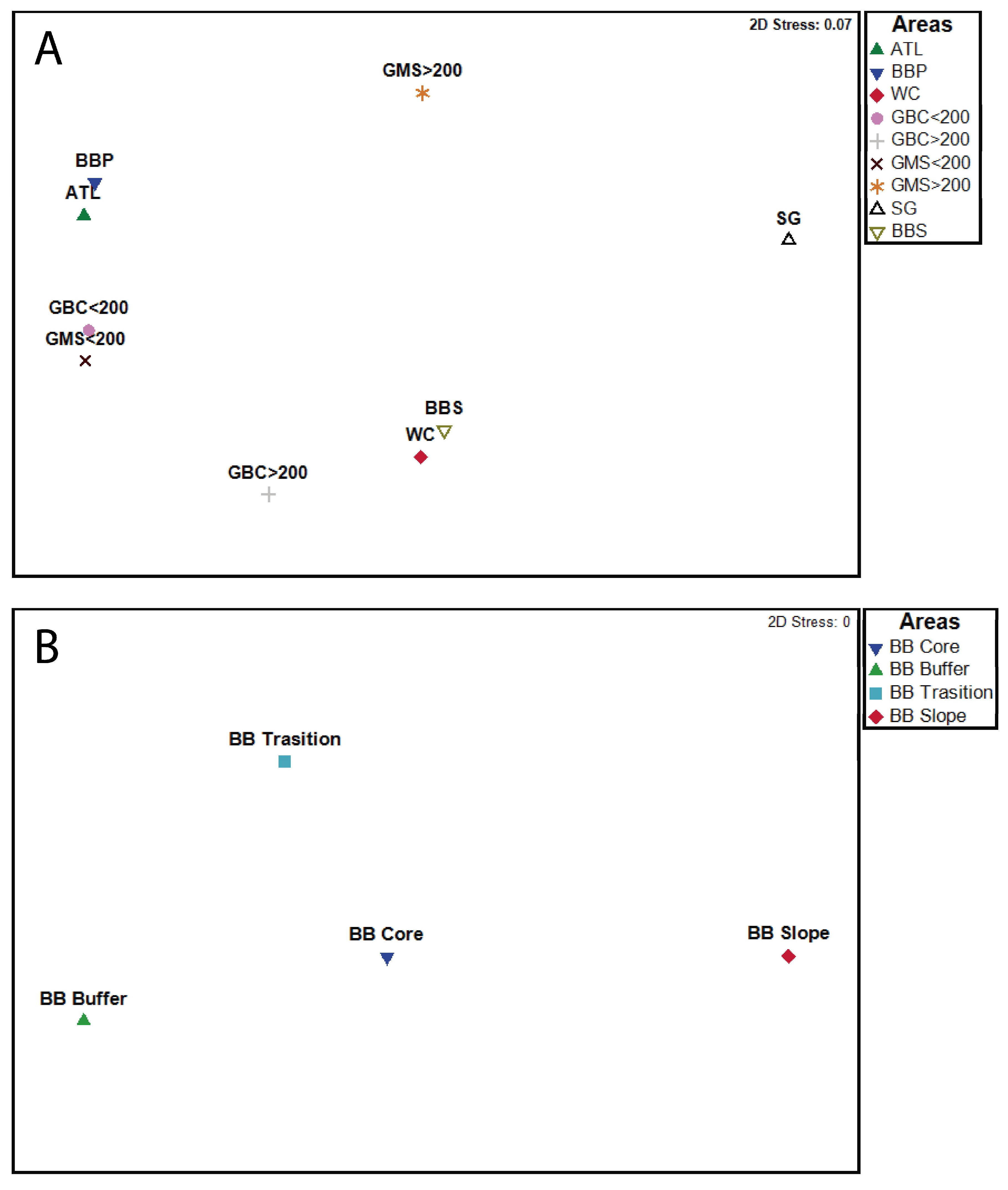

| Depth Range (m) | Records | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Bibliography | This Study | ATL | BBP | BBS | WC | GBC <200 | GBC >200 | GMS <200 | GMS >200 | SG | Total |
| Acanthocyclus albatrossis | 0–2 1,2 | - | 1 | 1 | 2 | |||||||
| Allopetrolisthes angulosus | 0–20 2 | - | 1 | 1 | ||||||||
| Austropandalus grayi | 24–414 2,3 | 4–330 | 4 | 1 | 3 | 13 | 2 | 5 | 28 | |||
| Betaeus truncatus | 0–107 2,3 | 17–208 | 1 | 7 | 1 | 9 | ||||||
| Campylonotus arntzianus | 475–589 4 | 415–785 | 2 | 2 | ||||||||
| Campylonotus capensis | 140–1300 4 | 133 | 1 | 1 | ||||||||
| Campylonotus semistriatus | 30–2086 3,5 | 4–785 | 3 | 2 | 8 | 6 | 7 | 1 | 1 | 28 | ||
| Campylonotus vagans | 18–506 2,3 | 15–209 | 6 | 16 | 2 | 10 | 1 | 35 | ||||
| Chorismus antarcticus | 15–915 3,6 | - | 4 | 1 | 1 | 1 | 7 | |||||
| Chorismus tuberculatus | 400–815 3,6 | 392–642 | 4 | 2 | 1 | 7 | ||||||
| Curtonida spinosa | 100–1250 6,12 | 767–785 | 4 | 1 | 5 | |||||||
| Eualus dozei | 13–385 2,3 | 17–165 | 1 | 1 | 7 | 9 | ||||||
| Eurypanopeus crenatus | 2–40 2 | - | 1 | 1 | ||||||||
| Eurypodius spp. | 8–1507 2,3 | 4–516 | 29 | 20 | 5 | 9 | 16 | 2 | 17 | 1 | 1 | 100 |
| Grimothea gregaria | 0–1095 2 | 4–294 | 14 | 17 | 5 | 3 | 16 | 3 | 18 | 1 | 77 | |
| Halicarcinus planatus | 0–270 2 | 23–208 | 7 | 1 | 7 | 26 | 2 | 43 | ||||
| Lebbeus antarcticus | 450–2598 7 | - | 1 | 1 | ||||||||
| Libidoclaea granaria | 30–450 2,6 | 60–263 | 1 | 1 | 2 | 4 | ||||||
| Libidoclaea smithii | 18–2060 2 | 205–210 | 1 | 4 | 5 | |||||||
| Lithodes confundens | 0–775 8,9 | 40–642 | 10 | 23 | 5 | 1 | 39 | |||||
| Lithodes couesi | 221–1200 10 | 608 | 1 | 1 | ||||||||
| Lithodes santolla | 5–700 6 | 15208 | 6 | 1 | 8 | 1 | 6 | 23 | ||||
| Lithodes sp. | – | 122 | 1 | 1 | ||||||||
| Lithodes turkayi | 70–1410 9,11 | 205–230 | 1 | 1 | ||||||||
| Metacarcinus edwardsii | 0–40 2 | - | 1 | 1 | 2 | |||||||
| Munidopsis aspera | 100–2800 2 | - | 1 | 4 | 1 | 6 | ||||||
| Munidopsis opalescens | 700–1000 2 | - | 1 | 1 | ||||||||
| Nauticaris magellanica | 1–746 2,3 | 15–208 | 1 | 8 | 2 | 11 | ||||||
| Nematocarcinus lanceopes | 550–4000 13 | - | 3 | 3 | ||||||||
| Neolithodes diomedeae | 640–2450 11 | - | 1 | 1 | 2 | |||||||
| Notocrangon antarcticus | 250–1500 2,6 | - | 8 | 8 | ||||||||
| Pagurus comptus | 2–400 3,14 | 7–460 | 20 | 7 | 4 | 5 | 12 | 2 | 13 | 63 | ||
| Pagurus forceps | 1–660 15 | - | 1 | 1 | 1 | 3 | ||||||
| Pandalopsis ampla | 130–1250 3,6 | 483–785 | 1 | 3 | 4 | |||||||
| Paralomis anamerae | 130–1250 3,11 | - | 1 | 1 | ||||||||
| Paralomis formosa | 320–2075 11,16 | - | 7 | 7 | ||||||||
| Paralomis granulosa | 2–568 2,3 | 15–191 | 6 | 8 | 10 | 5 | 29 | |||||
| Paralomis spinosissima | 160–812 9,16 | 607–785 | 3 | 1 | 13 | 17 | ||||||
| Pasiphaea acutifrons | 110–1550 2,6 | 38–205 | 6 | 3 | 3 | 1 | 13 | |||||
| Pasiphaea dofleini | 110–653 2,3 | - | 1 | 1 | 1 | 3 | ||||||
| Pasiphaea scotiae | 1000–3200 17 | - | 2 | 2 | ||||||||
| Peltarion spinulosum | 5–1138 2,3 | 4–263 | 21 | 1 | 15 | 5 | 16 | 1 | 59 | |||
| Pinnixa transversalis | 1–5 2 | - | 1 | 1 | ||||||||
| Pinnixa valdiviensis | 0–10 2 | - | 1 | 1 | 2 | |||||||
| Propagurus gaudichaudii | 0–746 3,6 | - | 2 | 1 | 1 | 1 | 5 | |||||
| Pseudocorystes sicarius | 5–100 2,3 | - | 1 | 1 | ||||||||
| Stereomastis suhmi | 200–2200 18 | - | 1 | 2 | 1 | 4 | ||||||
| Sympagurus dimorphus | 70–750 3,6 | 263–294 | 1 | 1 | 1 | 3 | ||||||
| Thymops birsteini | 122–2516 19,20 | 209–642 | 5 | 3 | 1 | 1 | 1 | 11 | ||||
| Thymops sp. | – | 415–785 | 3 | 1 | 4 | |||||||
| Thymops takedai | 220–1720 21 | - | 1 | 1 | 2 | |||||||
| Thymopsis nilenta | 220–2886 21,22 | - | 5 | 5 | ||||||||
| Total records | 136 | 95 | 52 | 33 | 153 | 39 | 141 | 10 | 42 | 701 | ||
| Species count | 19 | 10 | 19 | 11 | 23 | 19 | 27 | 9 | 12 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Salvatore, P.; Albano, M.J.; Diez, M.J.; Tapella, F.; Pérez-Barros, P.; Lovrich, G.A. Decapods of the Southern Tip of South America and the Marine Protected Area Namuncurá–Burdwood Bank: A Nearshore–Offshore Comparison. Diversity 2023, 15, 1143. https://doi.org/10.3390/d15111143
Di Salvatore P, Albano MJ, Diez MJ, Tapella F, Pérez-Barros P, Lovrich GA. Decapods of the Southern Tip of South America and the Marine Protected Area Namuncurá–Burdwood Bank: A Nearshore–Offshore Comparison. Diversity. 2023; 15(11):1143. https://doi.org/10.3390/d15111143
Chicago/Turabian StyleDi Salvatore, Pablo, Mariano J. Albano, Mariano J. Diez, Federico Tapella, Patricia Pérez-Barros, and Gustavo A. Lovrich. 2023. "Decapods of the Southern Tip of South America and the Marine Protected Area Namuncurá–Burdwood Bank: A Nearshore–Offshore Comparison" Diversity 15, no. 11: 1143. https://doi.org/10.3390/d15111143
APA StyleDi Salvatore, P., Albano, M. J., Diez, M. J., Tapella, F., Pérez-Barros, P., & Lovrich, G. A. (2023). Decapods of the Southern Tip of South America and the Marine Protected Area Namuncurá–Burdwood Bank: A Nearshore–Offshore Comparison. Diversity, 15(11), 1143. https://doi.org/10.3390/d15111143







