5.1.4. Description of Xenorophus simplicidens sp. nov.
The cranial description of
Xenorophus simplicidens is restricted to features or regions that differ from
Xenorophus sloanii; for a comprehensive description of the skull of
Xenorophus, see the description of
Xenorophus sloanii below. Much of the dorsal surface of the skull of the holotype (CCNHM 8720) is obscured by matrix and postcranial bones (
Figure 2), and parts of the palate are highly fractured (
Figure 3); the description is supplemented with observations from the paratype and referred specimens (
Figure 4,
Figure 5,
Figure 6 and
Figure 7), including the braincase (ChM PV 4266, 4822, 4823), rostrum and palate (ChM PV 4823), and basicranium (ChM PV 4266, 4823).
Ontogenetic Status: Specimens were assigned to the growth classes of Perrin [
83] based, chiefly, on tooth development, skull suture closure, and closure of vertebral epiphyseal sutures. Additional information, such as nasal length, was also considered.
Class I, II, and III: No specimens of Xenorophus simplicidens are assignable to either class I (fetus) or II (neonate).
Class IV: The holotype (CCNHM 8720) and referred specimen ChM PV 4822 represent Class IV, based on slightly smaller size than Class V specimens, no open sutures nor obliterated sutures, more than 75% of the vertebral column with fused epiphyses (CCNHM 8720), and relatively short nasal bones (ChM PV 4822), and light tooth wear (CCNHM 8720).
Class V and VI: Two specimens (ChM PV 4266, 4823) are assigned to class V based on their slightly larger size, all sutures being either strongly mortised (complex zigzag sutures with numerous parallel ridges and grooves, e.g., maxillofrontal, premaxilla–maxilla, nasofrontal, frontoparietal, occipital–parietal sutures) or obliterated, longer nasals than ChM PV 4822, and most vertebral epiphyses fused (though only thoracic and cervical vertebrae are represented in ChM PV 4266). ChM PV 4266 has the most well-developed nuchal crests of any specimen of Xenorophus simplicidens, and may represent either Class V or VI.
Rostrum: The ventral surface of the holotype skull (CCNHM 8720) is well-exposed and slightly fractured (
Figure 2;
Table 1). In CCNHM 8720, the rostrum has a Rostral Proportion Index (RPI) of 2.78; in ChM PV 4823, the slightly shorter rostrum has an RPI of 2.41. This proportionally shorter and wider rostrum is perhaps a result of incorrect reassembly of ChM PV 4823 as it possesses an anomalously wide ventral gap between the maxillae (
Figure 4 and
Figure 5); if this gap of 21–22 mm is subtracted, RPI is recalculated at 2.8. In CCNHM 8720, the premaxilla is incomplete but bears partial alveoli for I2 and I3. The maxilla of CCNHM 8720 is complete and preserves empty alveoli for C1 through PC3 and left PC9; all other maxillary teeth (left PC4–8, right PC4–9) are preserved in situ.
In CCNHM 8720, the premaxillae contact ventromedially along the anterior 140 mm of the rostrum, and the anterior 175 mm of the rostrum in the more completely preserved paratype (ChM PV 4823). A narrow, approximately 100 mm long vomerine window may have been present in CCNHM 8720, but is unclear owing to crushing; the vomer is incompletely preserved in ChM PV 4823, but the window appears to have been at least 175 mm long. The palatal process of the premaxilla is long, narrow, and bears a longitudinal groove; it extends approximately 130 mm posterior to the C1 in CCNHM 8720.
The posterior two thirds of the palate is ventrally flat, as in Xenorophus sloanii. A shallow furrow is present about 15 mm medial to PC5–3 and may be continuous posteriorly with the greater palatine foramen. On the left side, there is at least one greater palatine foramen opening at the position of PC7–8; on the right side, there is one large foramen with a long sulcus (positioned 12–13 mm anterior to the left greater palatine foramen) and one additional posterior foramen with a shorter sulcus, positioned slightly lateral to the primary foramen and at the level of PC9. These sulci open anteriorly.
In ChM PV 4823, the rostrum deviates 3.2° to the left (
Figure 5), as in most specimens of
Xenorophus sloanii (see below). In CCNHM 8720, the rostrum deviates 6.8° to the right, caused by diagenetic distortion. Indeed, deviation to the left was probably the case, given that the distance between the posteriormost tooth (PC9) and the postglenoid process is approximately 1 cm shorter on the left versus the right side in both ChM PV 4823 and CCNHM 8720, paralleling rostral and mandibular asymmetry in
Xenorophus sloanii (e.g., CCNHM 168, 104, ChM PV 7677). In ChM PV 4823, the entire rostrum is twisted counterclockwise in anterior view by 6.3°.
Dorsally, the premaxilla has parallel lateral margins in the paratype (ChM PV 4823) and does not widen anteriorly (
Figure 5). In lateral view, the rostrum is deepest at the level of PC1, and the rostrum bears a lightly sinuous ventral profile (
Figure 6). Along the anterior third of the rostrum, the premaxilla faces dorsolaterally, but along the posterior 2/3, the premaxilla faces dorsally. The premaxillary sac fossa is positioned approximately 3 cm anterior to the antorbital notch, and there are two premaxillary foramina on the left side. The larger premaxillary foramen is positioned 118 mm anterior to the nasals, and the smaller is positioned 30 mm further anterior; both open posterodorsally. There is at least one right premaxillary foramen, the broken margin of which is positioned approximately 1 cm anterior to the primary left premaxillary foramen. The premaxillary sac fossae are narrow and face anteromedially and somewhat anterodorsally, and anteriorly transition into a subtle furrow that appears to be the posteromedial sulcus; this sulcus extends toward the posteriormost premaxillary foramen. Anteromedial to the premaxillary sac fossae, the medial edges of the premaxilla rise toward the midline and likely contacted each other as in other
Xenorophus specimens; their separation in ChM PV 4823 is likely an artifact of reconstruction (see above). These dorsomedial ridges measured at least 110 mm in length and extended anterior to the anteriormost premaxillary foramen. These ridges are incomplete but clearly rotated to the left on both sides, resulting in a transversely deeper and trough-like premaxillary sac fossa on the left. On the left side, a poorly defined anteromedial sulcus flanks the dorsomedial ridge of the premaxilla. In addition to the paratype, both referred skulls ChM PV 4266 and 4822 possess a rounded, versus V-shaped (as in
X. sloanii), anterior margin of the bony nares.
The antorbital fossae are clearly developed on both sides of the rostrum in the paratype (ChM PV 4823;
Figure 4), but fracturing of the lateral edge of the maxilla precludes assessment of whether the degree of excavation of the fossae is asymmetrical in width and excavation (e.g., as in
Xenorophus sloanii). However, the antorbital fossae are asymmetrical in length, measuring approximately 92 mm and 113 mm long on the left and right sides (respectively); this differs from
Xenorophus sloanii, where the left antorbital fossa is longer than the right. Medial to the alveoli for PC8–9, the maxilla was excavated to a depth only a few mm in thickness by the fossae on both sides (2.2 mm on the left, 2.4 mm on the right). On the right side, there are at least three dorsal infraorbital foramina present dorsal to the infraorbital canal, the roof of which has broken away. The infraorbital canal is situated ventromedially within the fossa and the foramina dorsal to it are on the medial wall of the fossa; additionally, there is one laterally directed foramen emanating from the premaxilla–maxilla suture at the level of PC9. On the left side, the fossa appears to bear infraorbital foramina coalesced into a fenestra, though likely artificially expanded by fracturing. Each fossa is bilobate, with a low transverse ridge at the level of PC8 dividing it into a shallow oval-shaped trough posteriorly and a small circular fossa anteriorly. On the left side, a low and laterally dissipating transverse ridge is positioned posteromedially, partially dividing the posterior oval-shaped trough into two fossae. The primary dorsal infraorbital foramen is large, anteriorly facing, approximately circular, and interrupts a ‘stepped’ profile of the maxilla between the horizontal floor of the antorbital fossa and the subhorizontal ascending process of the maxilla overlying the frontal and premaxilla. On the right side, a secondary infraorbital foramen is positioned ventrolaterally to the primary foramen and along the posterolateral margin of the antorbital fossa; a longitudinal sulcus emanates from this foramen and extends anteriorly along the lateral edge of the fossa.
The antorbital notch appears nearly semicircular on the left side in ChM PV 4823 (
Figure 5), but this is likely owing to loss of the maxilla and jugal within the notch; on the right side, the antorbital notch appears to have a straight and transverse posterior margin and a forms a right angle with the base of the rostrum, similar to
Xenorophus sloanii specimens CCNHM 1077 and CCNHM 168, but differing from the nearly semicircular notch in CCNHM 104 and the
Xenorophus sloanii holotype.
Facial/Interorbital Region: The nasals are well preserved in the paratype specimen and referred skulls (ChM PV 4266 and 4822); they are relatively short (
Figure 5; 37–47 mm in length) compared to adult specimens of
Xenorophus sloanii (66–79 mm;
Table 1). ChM PV 4266 bears an anterior median cleft between the nasals, giving them an M-shaped anterior margin, but such a cleft is missing in ChM PV 4823 and 4822, where the nasals have a simple anteriorly convex margin. The nasals are widest anteriorly. The nasals are slightly separated posteromedially by an anterior median wedge of the frontal, which gives the posterior edge of the nasals a W-shape similar to some specimens of
Xenorophus sloanii (e.g., CCNHM 168); the condition in ChM PV 4822 is unclear, and this specimen seems to have an irregular but approximately transverse frontonasal suture.
Lateral to the nares and anterior to the anterior margin of the nasals, the premaxilla is transversely narrow and dorsoventrally anteriorly positioned nares (e.g.,
Simocetus,
Ashleycetus,
Olympicetus, other Xenorophidae); this crest rises in height posteriorly toward the nasals (
Figure 7). There is no laterally overhanging crest in ontogenetically mature specimens such as ChM PV 4823 and 4266, unlike
Xenorophus sloanii (e.g., CCNHM 1077).
In ChM PV 4266 (
Figure 5), there is a large anterior median interparietal (AMI; [
92]) that is dorsally exposed, measuring approximately 41 mm long and 17 mm wide. The AMI contacts the posterior edge of the nasals and nearly completely separates the left and right frontals at the midline. The sutures of the AMI seem vertical and deep, suggesting that this element is dorsoventrally thick, as in other Xenorophidae [
92].
Anterior Basicranium: The palatines are crushed but appear to be widest anteriorly and taper posteriorly into a sub-cylindrical shape; the ventrolateral surface of the palatines bear low posteromedially trending ridges (
Figure 3 and
Figure 6). The pterygoid is poorly preserved, but the pterygoid–palatine suture is somewhat mortised and overall has a posteromedial orientation and meets the midline about halfway between the choanae and the posteriormost maxillopalatine suture. In addition to the vomerine window present on the rostral portion of the palate, a 63 mm long and 15 mm wide posterior exposure of the vomer is present between the posterior palatines and pterygoids as in
Xenorophus sloanii. The exposure of the vomer in ChM PV 4823 appears to be anteroposteriorly continuous from the anterior rostrum to the choanae, though this is perhaps best interpreted as a result of incorrect assembly of the rostrum with an artificial gap between the left and right maxillae (see above). The medial margin of the pterygoid suggests a narrow gap was present at the anterior margin of the choanae between the pterygoid and the vomer (as in
Albertocetus meffordorum). The posterior edge of the pterygoid is posterolaterally oriented. The posterior lamina of the pterygoid underlaps the basisphenoid and extends posteriorly to the level of the falciform process of the squamosal.
Vertex and Dorsal Braincase: The intertemporal constriction is narrow but anteroposteriorly short (
Figure 5;
Table 1). The median parietal suture is at the midline and not asymmetrical in any specimen, unlike some adult specimens of
Xenorophus sloanii and
Albertocetus meffordorum (see below; [
1]). There is only a low sagittal crest in subadult specimen ChM PV 4822, but not in the adult specimens (ChM PV 4266, 4823), unlike the low sagittal crest in
Xenorophus sloanii. Instead, there are low anterolaterally diverging temporal ridges defining a triangular, flat dorsal surface. Where the ridges converge posteriorly at the vertex, the intertemporal constriction is rounded in cross-section in the adult specimens; in old adult specimens of
Xenorophus sloanii, the sagittal crest is only present immediately anterior to the vertex where the temporal lines converge. The frontoparietal suture forms a shallow V-shape and converges posteromedially in ChM PV 4823 and possibly ChM PV 4266; in this latter specimen, the suture is completely remodeled, but a low ridge seems to demarcate the position. This suture is more approximately transverse in ChM PV 4822.
The vertex (defined as the apex of the supraoccipital) is elevated far above the parietals in all specimens (
Figure 7). The apex of the occipital shield is triangular and pointed. The occipital shield of the most mature specimen, ChM PV 4266, bears short anteroposteriorly aligned ridges for neck muscle attachments. The occipital shield (
Figure 8) in all specimens is shallowly transversely concave; flattest in ChM PV 4822, slightly more concave in ChM PV 4823 and CCNHM 8720, and most concave in ChM PV 4266. In dorsal view, the nuchal crest is sinuous in ChM PV 4823 (slightly anterolaterally concave in its anterior half, and laterally convex posteriorly), and the nuchal crest is continuously convex in ChM PV 4266. In all specimens, the nuchal crest is dorsoventrally deep and in lateral view, forms a posterodorsally convex arc; the crest is lowest in ChM PV 4822 and highest in ChM PV 4266 (
Figure 8). In lateral view, the frontoparietal suture ascends the anterior wall of the braincase posterodorsally along its ventral two-thirds, and in the dorsal third, trends anterodorsally.
Posterior Basicranium and Squamosal: The basioccipital of the holotype is flattened taphonomically but exhibits basioccipital crests diverging at a 44° angle and measuring about 19 mm in maximum thickness, about 6.2% of bizygomatic width (
Figure 3 and
Figure 4). Other specimens (ChM PV 4266, 4822, 4823) similarly possess basioccipital crests diverging between a 43 and 47° angle (
Figure 6).
Despite crushing of the holotype skull, the periotic fossa and cranial hiatus are well-preserved (
Figure 3 and
Figure 4). The falciform process is straight and trends posterolaterally toward the deeply excavated periotic fossa. The fossa includes a deep bowl-shaped fossa named the suprameatal pit [
10], positioned dorsolateral to the spiny process (broken); this bowl-shaped fossa bears minute spurs. It is separated posteriorly by a transverse ridge from a posterior shelf-like fossa for the posterior process of the periotic. Ventromedially, a rugose laminated sheet of the alisphenoid covers (underlaps) the squamosal medial to the suprameatal pit and forms the medial rim of the pit. This inflated region of the alisphenoid appears to have contacted the suprameatal fossa of the periotic, or at least the superior process, which loosely contacts the alisphenoid.
The lateral tuberosity of the periotic fits into a shallow fossa immediately anterior to the spiny process of the squamosal. The spiny process is broken but articulates with the hiatus epitympanicus of the periotic. When the periotic is placed into articulation, there is a slight gap between the base of the anterior process of the periotic and the falciform process, as in other Xenorophidae. A large gap is present dorsal to the superior process of the periotic and the roof of the bowl-shaped fossa within the periotic fossa, perhaps as great as 10 mm.
The zygomatic process is similar in length to
Xenorophus sloanii, but its tip is abraded; as preserved, it tapers transversely and terminates at the level of the supraoccipital apex but likely met the postorbital process, as in
X. sloanii (
Figure 3,
Figure 4,
Figure 5 and
Figure 6). In lateral view, the zygomatic process is roughly rectangular. The squamosal prominence is low and less prominent than in
Xenorophus sloanii (e.g., CCNHM 168, 1077). The sternomastoid fossae are similar to
Xenorophus sloanii; each consists of a series of oval fossae separated by three to four low anteroposterior ridges in all specimens. These specimens also exhibit a deeply incised furrow along the anterior margin of the fossa (
Figure 7); a shallow furrow is only developed in some specimens of
Xenorophus sloanii (e.g., CCNHM 168).
In posterior view, the occiput is less trefoil-shaped, owing to exoccipitals that extend less laterally than in
Xenorophus sloanii (
Figure 8). In ChM PV 4266, the foramen magnum bears a deep median cleft dorsally, as in some
Xenorophus sloanii (e.g., CCNHM 168). The jugular notch is transversely wide and semicircular in all specimens, as opposed to the narrower and more acute notch in
Xenorophus sloanii.
Periotic: The left periotic of CCNHM 8720 is well-preserved but missing part of the anterior process; both periotics are preserved in ChM PV 4266 and ChM PV 4823, though the left periotic of the former is fractured and both periotics of the latter are missing the pars cochlearis (
Figure 9,
Figure 10,
Figure 11 and
Figure 12;
Table 2). The periotic is nearly identical to
Xenorophus sloanii (see below) and
Albertocetus meffordorum [
1], though larger than the latter; it shares with these taxa relatively small size, and a proportionally large pars cochlearis (
Figure 9). In addition, these periotics share the following combination of features unique to Xenorophidae (but not synapomorphies of the entire clade): transversely narrow and bladelike anterior process; dorsoventrally deep hatchet-shaped anterior process with flat anterior margin, prominent spine-like anterodorsal angle; long, bladelike lateral tuberosity (synapomorphy of Xenorophidae); transversely rounded and widened superior process; small suprameatal fossa formed as deeply excavated pit; long posterior cochlear crest; small, flat, quadrate posterior bullar facet (
Figure 9,
Figure 10,
Figure 11 and
Figure 12).
The anterior process is best-preserved in ChM PV 4823 (
Figure 12 and
Figure 13); the anterior and ventral margins meet at a right angle, and the anterior margin is straight and vertically oriented. The anterodorsal angle is a long triangular spur, giving the anterior process a hatchet shape like
Xenorophus sloanii (ChM PV 7677, CCNHM 1077),
Albertocetus meffordorum, and
Cotylocara macei. The lateral tuberosity is longest in the most mature specimen, ChM PV 4266, and shorter in ChM PV 4823.
The pars cochlearis is subrectangular in CCNHM 8720 owing to a dorsoventrally shallow flange at its anteromedial corner (
Figure 10). In ChM PV 4266 the pars cochlearis lacks such a flange and is more hemispherical in shape. Though broken, an intermediate condition seems to have been present in the left periotic of ChM PV 4823, which possesses a small flange along the broken anterior base of the pars cochlearis. The posterior cochlear crest is long and shelf-like in CCNHM 8720, paralleling the facial crest; it curves posterolaterally at its apex. CCNHM 8720 possesses a tubercle dorsal to the fenestra rotunda; owing to breakage, it is unclear if this was present in ChM PV 4266.
The suprameatal fossa is variable within
Xenorophus simplicidens (
Figure 10). It is developed as a deep pit in CCNHM 8720 and ChM PV 4266; this pit is nearly circular with steep walls in the former, and somewhat larger in the latter with a deep posterior fissure lateral to the aperture for the vestibular aqueduct. In ChM PV 4823, the suprameatal fossa is transversely wider and much longer, in addition to a deep posterior groove as in ChM PV 4266. In ChM PV 4266, the fossa is kidney-shaped and wraps around the circular opening of the facial canal, separated by a narrow ridge.
The superior process is greatly expanded and transversely rounded in all specimens, but most extremely so in CCNHM 8720; it is less expanded in ChM PV 4823 (
Figure 10 and
Figure 12). The dorsal margin of the superior process is anteriorly concave in lateral view and broadly convex posteriorly; an obvious posterodorsal angle is not present in ChM PV 4823 or 4266, but there is a slight bluntly triangular posterodorsal angle in CCNHM 8720. A single posteroexternal foramen is present on the lateral surface, on the base of the posterior process (
Figure 12). The posterior surface of the posterior process is rugose for an articular surface with the postmeatic process of the squamosal. The posterior bullar facet is quadrate in all specimens, is transversely flat and slightly longitudinally convex at the posterolateral end, and bears posterolaterally oriented ridges (
Figure 9).
Tympanic bulla: The left and right bullae of the holotype (CCNHM 8720) and paratype (ChM PV 4823) are well-preserved; the left bulla of ChM PV 4266 is fragmentary (
Figure 13,
Figure 14,
Figure 15,
Figure 16 and
Figure 17;
Table 3). The bulla is similar in size and overall proportions to
Xenorophus sloanii (see below) but all bullae of
Xenorophus simplicidens critically differ in possessing a deeply incised transverse sulcus positioned at about the midpoint of the involucrum (
Figure 13 and
Figure 15). This sulcus is overlapped by a swollen ridge of the posterior involucrum on the dorsal and medial side. In CCNHM 8720, the involucrum immediately anterior to the transverse sulcus is rugose and bears many minute finger-like nodules. The ventral margin of the bulla in lateral view is nearly straight (
Figure 12), similar to some specimens of
Xenorophus sloanii (CCNHM 1077, ChM PV 5022). The cavum tympani is divided into anterior and posterior portions by a transverse ridge at mid-length (
Figure 15). At the anterior end of the cavum tympani, the eustachian outlet is developed as a triangular anteroventrally directed trough.
In dorsal view, the bulla narrows anteriorly to the lateral furrow, while the posterior lobe is subrectangular and the anterior lobe is subtriangular (
Figure 15). Ventrally, the median furrow is short and restricted to the posterior third of the ventral surface (
Figure 16); it is interrupted by a low convex prominence, as in Basilosauridae. The elliptical foramen, posterior process, and pedicles (
Figure 17) are better preserved in CCNHM 8720 and ChM PV 4823 than in any specimen of
Xenorophus sloanii or other previously reported xenorophids. The elliptical foramen is small, 3 mm in diameter, and elevated dorsally on the posterior surface, placed at the level of the inner posterior pedicle (
Figure 17). The elliptical foramen is planoconvex with a straight, lateral margin and a convex medial margin. The inner posterior pedicle is an inflated conical tubercle; the outer posterior pedicle is broken but developed as a narrow vertical flange.
The posterior process is posterolaterally oriented and bears a small trapezoidal posterior facet with longitudinal grooves (
Figure 13 and
Figure 15); the facet deepens posteriorly and has a sinusoidal longitudinal profile (concave posteriorly and convex anteriorly, when viewed on edge in anterodorsal or posteroventral view). The posterior process bears a deep longitudinal trough dorsally. The posterior end of the posterior process has a deep transverse groove ventrally on the lower third of the process; the upper two-thirds bear a rugose articular surface for the postmeatic process of the squamosal. Anteriorly, there is a concave fossa on the posterior process just dorsal to the elliptical foramen.
Upper Dentition: The holotype rostrum preserves right PC4–9 and left PC4–8 within their alveoli; empty alveoli for I2–3, C1, PC1–3, and left PC9 are present (
Figure 3,
Figure 4,
Figure 18 and
Figure 19;
Table 4); alveoli for I1 are not preserved owing to incompleteness. Twelve isolated anterior teeth are present along with the isolated left PC9 (
Figure 18 and
Figure 19). The isolated anterior teeth have roots that are oval to bilobate in cross-section and conical to triangular unicuspid crowns; their position is uncertain, owing to near-uniform root curvature in xenorophid upper and lower teeth. However, three teeth are possible lowers and differ from the remaining nine unicuspid teeth in having slightly more lingually curved crowns and somewhat more striated enamel basally on the lingual side of the crown.
I2 through PC2 alveoli are oval in shape (
Figure 19). The presumed upper unicuspid teeth can be arranged into an anteroposterior series based upon crown diameter, and represent most positions between I1 and PC3, though most are not assignable to an exact position owing to their similarity (
Figure 19). Left and right PC3 are clearly identifiable based on their anteroposteriorly elongate and somewhat bilobate roots, matching the identically shaped alveolus for PC3. The PC3 crown lacks cingula and has a triangular and slightly anteroposteriorly longer than wide crown, smooth and sharp mesial and distal carinae, a single distal cuspule at the crown base, and faint labial apicobasal striations and somewhat stronger lingual striations (
Figure 18). The bilobate root bears a shallow labial sulcus and a faint lingual sulcus.
PC4–5 have similar crowns to PC3; these crowns are triangular in lingual/labial view, slightly anteroposteriorly longer than PC3, with a single basal cuspule on the distal carina (
Figure 19). The crowns appear subtriangular in occlusal view. There are faint apicobasal striations labially, and slightly stronger lingual striations. The root is clearly double-rooted, with a larger and transversely thicker posterior root, especially in PC5. This larger posterior root corresponds to the posterolingual swelling of the crown.
PC6–7 have slightly anteroposteriorly longer crowns. Both possess a distal accessory cusp larger than those on PC4–5; PC6 lacks a mesial cusp, but a single mesial accessory cusp is present in PC7 (
Figure 19). Lingual striations are slightly stronger on PC6–7 than in PC 4–5, and the labial face is less striated. Both teeth are double-rooted, with a greatly inflated posterior root. There is a faint labial cingulum on PC7.
PC8–9 are the most complex upper teeth; they possess one or more mesial cusps, with the apical-most cusp positioned high and near the principal cusp (
Figure 19). Additional basal cusps may have been present but destroyed by tooth wear. These teeth are also the only teeth in the dentition to possess a third “demi root” on the lingual side, in between the anterior and posterior root lobes. This demi root is small and likely did not project beyond the isthmus between the two root lobes. The left PC9 is loose, and less worn than the right PC9 and PC8. It possesses two apically positioned mesial cusps and three distal cusps. It bears striated enamel anteriorly that transitions into slightly nodular enamel posteriorly on the lingual side. The labial side of PC9 bears some basal striations along with a very low but continuous labial cingulum. Apically, the labial surface is smoother. The root lobes of PC9 curve posteriorly and are equal in size and roughly conical.
The upper dentition of
Xenorophus simplicidens is asymmetrical, with many teeth shifted further anterior to their counterparts on the opposite side (
Figure 19). While some positions on the left side (PC4–6) are 2 mm further anterior than the right PC4–6, the manner in which the rostrum has been reconstructed—perhaps forcing the rostrum to be symmetrical—has resulted in the left premaxilla extending 6 mm further anterior to the right premaxilla. This would suggest that the right teeth would instead be positioned up to 4 mm further anterior to those on the left, unlike the condition in
Xenorophus sloanii (e.g., USNM 11049, CCNHM 168; see below). More complete specimens of
Xenorophus simplicidens with three dimensionally preserved and undistorted rostra are needed to further evaluate dental asymmetry.
Mandible: The nearly complete right mandible of CCNHM 8720 is the only one preserved for
Xenorophus simplicidens (
Figure 20;
Table 5). This mandible is missing only the anterior tip, part of the “pan bone”, part of the mandibular condyle, and the angular process. The horizontal ramus is nearly rectilinear but deepens in height posteriorly, with a shallowly and continuously concave dorsal margin between pc4 and the coronoid apex (
Figure 20B,C); as a result, the posterior dentition (pc4–8) is elevated, with each tooth slightly higher than the preceding one. The horizontal ramus is roughly rectangular in cross-section and somewhat transversely flattened (
Figure 20D); the anterior tip and the i1 alveolus are missing, but the i2 alveolus is partially preserved; i3, c1, and pc1 alveoli are present but poorly preserved owing to crushing. The symphyseal surface is poorly preserved but appears to have extended posteriorly to the level of pc2. At least four mental foramina are present, approximately 1–2 mm in diameter with anteroposteriorly short sulci; they are positioned 10 mm below the alveolar margin at pc4, 5, and 6. The ventral margin of the mandible is straight anteriorly and slightly concave in its posterior half where the ascending ramus deepens.
Embrasure pits are present in between teeth from pc2 through pc8. The pits between pc2, pc3, and pc4 are positioned slightly lateral to the toothrow and, from pc5 through pc8, the pits are positioned in the middle of the mandible. Lateral to the pc7–8, the lateral edge of the mandible is raised into a ridge and leads into the base of the coronoid process. This ridge is much higher than the alveolar margin on the medial side of the mandible and the last embrasure pit. This ridge obscures the root and base of the crown of pc8 in lateral view.
The coronoid process is subtriangular and symmetrical with a rounded apex (
Figure 20B,C); the coronoid is nearly vertical but slightly medially deflected at the apex. The mandibular foramen is cavernous and has an evenly concave/arcuate margin; the anterior edge is slightly further anterior to the apex of the coronoid process. The pan bone is slightly thinner than in
Xenorophus sloanii, being 2.3–2.5 mm in thickness anterior to the mandibular condyle. The mandibular condyle is incomplete, but the ventral portion suggests that it was quite transversely narrow, posteriorly convex, and medially excavated by the mandibular fossa. In lateral view, the posterior margin of the mandible ventral to the condyle is steeply anteroventrally oriented, suggesting a posteriorly concave margin above the angular process; it is unclear how far posteriorly the angular process extended.
Lower Dentition: Three aforementioned unicuspid teeth preserved ex situ represent uncertain loci, except for one nearly straight tooth with a relatively small (12.5 mm long) and slightly procumbent crown, likely representing the i1 (
Figure 20A;
Table 6) based on comparison with Basilosauridae and other stem odontocetes (
Ankylorhiza,
Otekaikea,
Waipatia) that possess a procumbent first incisor. A slightly larger unicuspid tooth represents i2, i3, or c1. The c1 alveolus is approximately circular. A tooth with an anteroposteriorly broader root with oval cross-section and more triangular crown likely represents pc1; this tooth has a nearly smooth labial surface with faint striations and slightly stronger lingual striations. Carinae are poorly developed in the teeth representing i2, i3, or c1, and faint mesial and distal carinae are only present along the apical third of the crown; slightly stronger carinae are present in pc1.
The pc2 has a triangular crown with a smooth labial surface and faint apicobasal striations and sharp mesial and distal carinae (
Figure 20E). Discontinuous apicobasal striations are more pronounced lingually; rougher striations form a poorly developed lingual cingulum with lightly nodular enamel. The root bears a vertical sulcus labially and lingually; CT data confirm that the root lobes are fused into an isthmus in their proximal half and diverge within the mandible. A single mesial basal cuspule is present. pc3 has a similar crown that is slightly broader anteroposteriorly, with similar ornamentation and a poorly developed cingulum. This is the anteriormost tooth with clearly divided root lobes, though pc3 through pc8 have an isthmus visible at the alveolar margin, indicating that the root lobes diverge internally.
Pc4 is anteroposteriorly broader and higher than pc3, with sharp mesial and distal carinae, slightly stronger labial apicobasal striations, and much stronger lingual striations on the basal two-thirds of the crown (
Figure 20E). There is one mesial cusp at mid-crown height, and three distal cusps: one just below the principal cusp, and the other two in the basal half. There is a near-continuous but weakly developed lingual cingulum consisting of a narrow basal band of rugose enamel; a discontinuous labial cingulum is also poorly developed, but confined to the mesial and distal ends of the crown.
Posterior cheek teeth pc5–7 are similar to one another in morphology and are the largest mandibular teeth; all are double-rooted (
Figure 20E). The triangular multicuspate crowns bear faint labial striations and strong lingual striations on the basal two-thirds of the crown, as well as sharp carinae interrupted only by accessory cusps. Mesial accessory cusps are uncertain in pc5, but there are four distal accessory cusps; pc6 has two mesial cusps along the apical half of the carina, and possibly three–four distal cusps (uncertainty owing to tooth wear); and pc7 has two large mesial cusps on the apical half of the carina and some basal mesial cuspules where the poorly developed cingulum meets the carina, as well as possibly up to four distal cusps which are mostly destroyed by a distal wear facet. pc5–7 all bear a weak lingual cingulum formed from a band of nodular enamel and a slight labial cingulum, again confined mesially and distally. The crown of pc8 is slightly anteroposteriorly narrower than pc7 and bears three prominent mesial cusps, a cuspule near the principal cusp, and at least one distal cusp; more were likely present but removed by tooth wear. The apicalmost distal cusp is separated from the principal cusp by a deep notch. pc8 has the strongest lingual cingulum in the dentition, forming a narrow shelf; otherwise it shares discontinuous apicobasal lingual striations and faint labial striations with more anteriorly positioned teeth (
Figure 20E).
Starting with pc5, each tooth is successively slightly elevated relative to the preceding tooth; along with this, the diastemata become narrower (
Figure 20B,C). The widest diastemata are between pc2 and pc3 (24.5 mm), whereas the narrowest diastema is between pc7–8 and measures only 7.4 mm.
Tooth Wear: Tooth wear is minimal on the upper anterior teeth (
Figure 18), and wear is greatest on the posterior postcanines (
Figure 19). The teeth on the right are generally worn more extremely than teeth on the left side. A few upper anterior teeth have minute apical wear facets with a small circle of dentine exposed (?PC2 and ?PC3). PC4 has a larger apical wear facet, but lacks wear on either carina. PC5 is the anteriormost tooth with mesial and distal wear facets (3 mm from crown base). It bears a 4–5 mm long mesial wear facet; on the left, the single mesial cuspule bears a facet. PC5 has a slightly larger apical wear facet. The distal facets are small and located along the carina, 2–3 mm long, and 2–2.5 mm from the crown base. PC6 has the largest apical wear facet, a somewhat larger mesial wear facet than PC5, and a similarly small distal wear facet.
The right PC7 has a slightly smaller mesial wear facet than PC6, and lacks apical wear (
Figure 19). A large mesial wear facet is present that extends posterobasally onto the lingual surface of the crown to within 1 mm of the crown base, along the anterior third of the crown, and along the basal half of the crown and carina. The distal wear facet is large and placed along the distal edge of the accessory cusp. An irregular and horizontal 5.8 mm long wear facet just above the crown base and along the posterolingual margin of the tooth is present.
PC8 has a minute apical wear facet on the right side whereas the left is unworn (
Figure 19). The mesial wear facet is large, shallowly concave, and has removed 80% of the apicobasal length of the mesial carina. The distal wear facet has removed accessory cusps and is continuous on the right PC8 with a cingular wear facet basally. On the left PC9, this cingular wear facet (as in PC7) is separate from the distal wear facet.
The left and right PC9 lack apical wear facets but possess large mesial wear facets (
Figure 19). On the right side, this facet has removed approximately 50% of the apicobasal length of the carina. On the left side, there is a separate facet on the mesial accessory cusps. The distal carina and accessory cusps are unworn.
Tooth wear is less extreme in the lower dentition (
Figure 20A,E), and none of the complete teeth possess an apical wear facet on the principal cusp. Mesial wear facets are sporadic and confined to individual accessory cusps throughout the dentition; distal wear facets on pc2–5 are similarly only on accessory cusps. Distal wear on pc6–8 consists of single continuous facets along the lingual side of the distal carina and cusps. In pc6, it extends from the apicalmost cusp to the base of the crown. In pc7–8, the facets are similar but extend onto the posterolabial side of the tooth crown.
Cervical Vertebrae: The atlas vertebra (total width: 134 mm) is complete but partially obscured in the holotype (CCNHM 8720;
Figure 2 and
Figure 3). Even so, it has large, dorsally positioned transverse processes with a rectangular outline, like some
Albertocetus meffordorum (CCNHM 303, Boessenecker et al., 2017B), but differing from specimens of
Xenorophus sloanii that possess a bifurcated transverse process with two tubercles (CCNHM 1077) and
Echovenator sandersi. The atlas bears a low neural spine, a prominent hypapophysis, and an oval outline of the occipital articular facets; the shape of the neural canal is unclear owing to obscuring matrix. The atlas of ChM PV 4266 is similar but has a proportionally larger neural canal, a slightly higher neural spine, and transversely narrower facets for the occipital condyles and axis relative to specimens of
Xenorophus sloanii. The axis is only preserved in the holotype (CCNHM 8720), and the posterior side is exposed in relief (
Figure 2). The posterior face of the centrum is subtriangular and ventrally pointed but lacks a clear hypapophysis. In posterior view, the transverse process is rectangular and transversely longer than in
Xenorophus sloanii. The neural spine is wide, subrectangular, and transversely widens dorsally; it further bears a bifurcated apex, as in
Xenorophus sloanii. The neural canal is more circular than in
Xenorophus sloanii.
A mid-cervical, C3 or C4, is preserved overlying the skull vertex of CCNHM 8720; it has a subtriangular centrum and does not differ from
Xenorophus sloanii. A partial C3 or C4 is preserved in ChM PV 4266 and it differs from the holotype in having a round centrum. A partial C6 is preserved in CCNHM 8720, and a more complete C6 is preserved in ChM PV 4266 (
Figure 21). It bears a long and ventrolaterally projecting parapophysis with a small dorsolateral tubercle partially defining the lateral margin of the vertebrarterial canal.
Thoracic Vertebrae: Eight or nine thoracic vertebrae are preserved in CCNHM 8720; they measure 58–65 mm in centrum width and approximately 43–49 mm in centrum depth (
Figure 2,
Figure 3 and
Figure 21). Like the remaining vertebrae of CCNHM 8720, these vertebrae are smaller than their counterparts in adults of
Xenorophus sloanii (CCNHM 104, 168, 1077, ChM PV 5022). Two vertebrae with laterally protruding tuberosities, with costal facets and facets for the tubercles of the ribs, correspond to posterior-mid thoracics, perhaps T5–8. One vertebra, likely T10, has a short ventrolaterally extending transverse process with a blunt apex for the articulation of what is probably the last rib. T10 resembles lumbar vertebrae but lacks a ventral median keel.
Lumbar Vertebrae: Nine lumbar vertebrae are preserved (
Figure 2,
Figure 3 and
Figure 21). Owing to their exposure in relief, they cannot be arranged into an anteroposterior series; they measure between 68 and 73 mm in centrum width, 48 and 61 mm in centrum length, and 67 and 69 mm in centrum depth. Neural spines and transverse processes are preserved in some and are quite long (spines up to 155 mm and transverse processes up to 100 mm). Neural spines anteroposteriorly widen dorsally in lateral view, and transverse processes are rectangular in dorsal outline. The neural canal diameter ranges from 29 mm wide in the anterior lumbars to 11 mm wide in posterior lumbars. No caudals or posteriormost lumbar with hemal facets are preserved. All exposed lumbars of CCNHM 8720 possess ventral median keels.
Ribs: Several ribs are preserved in relief and ex situ in CCNHM 8720 (
Figure 2 and
Figure 3). They are poorly preserved but most are anteroposteriorly flattened and bear a subrectangular cross-section, transitioning to a rhomboid or lenticular cross-section distally. The ribs are narrow and do not thicken distally.
Xenorophus sloanii Kellogg, 1923 [
8]
Holotype: USNM 11049, partial juvenile cranium including maxillary part of the rostrum, orbital region, frontals, and some postcanine teeth (left and right PC6–8), collected sometime prior to 1908 by unknown collectors, presumably quarry workers, from Ashley Formation exposures in a marl pit near Woodstock Station (Charleston County, South Carolina) owned by the Ingleside Mining Company. The specimen resided in the collection of the pit’s superintendent, who gave the specimen to Sloan, who later donated the specimen to USNM for study by Kellogg [
8].
Referred Specimens: CCNHM 104, a partial skeleton including a nearly complete cranium, left and right mandibles, four cervical vertebrae, four thoracic vertebrae, two lumbar vertebrae, and one caudal vertebra, collected summer 2006 by P. Bailey from the vicinity of Summerville, Dorchester County, South Carolina; CCNHM 168, a partial skeleton including nearly complete cranium, left and right mandibles, thyrohyal, seven cervical vertebrae, four thoracic vertebrae, three lumbar vertebrae, and ten ribs, collected May or June 1997 by W. Hillenius and Dana Cope from the vicinity of Summerville, Dorchester County, South Carolina; CCNHM 1077, nearly complete skeleton including a partial cranium lacking much of the rostrum, partial mandibles, seven cervical vertebrae, ten thoracic vertebrae, ten lumbar vertebrae, and six caudal vertebrae, collected by Steven Miller and M. Havenstein in 2002 from the vicinity of Crowfield Plantation subdivision in Goose Creek, Berkeley County, South Carolina; CCNHM 5995, partial skull with associated caudal vertebra, collected by Craig Garrison in June 2015 in the vicinity of Summerville, Dorchester County, South Carolina; ChM PV 5022, partial skeleton including partial cranium lacking anterior rostrum, seven cervical vertebrae, seven thoracic vertebrae, ten lumbar vertebrae, three caudal vertebrae, and at least six ribs, collected 17–21 May 1991 by Albert Sanders, Jonathan Geisler, Bricky Way, Betsi Nemeth, and Aaron Stokes, collected just south of Crowfield Plantation subdivision, Goose Creek, Berkeley County, South Carolina; ChM PV 7677, a nearly complete skull with one cervical and three associated thoracic vertebrae, collected August 1986 by S. Miller, S. Faust, and V. McCollum from the vicinity of Crowfield Plantation subdivision, Goose Creek, Berkeley County, South Carolina; ChM PV 5020, associated left and right partial mandibles, collected March 1987 by B. Albright from spoils at Wando Terminal, Charleston County, South Carolina; ChM PV 5709, left partial mandible and isolated rib, collected 26 March 1985 by S. Faust and students in the vicinity of Summerville, Dorchester County, South Carolina.
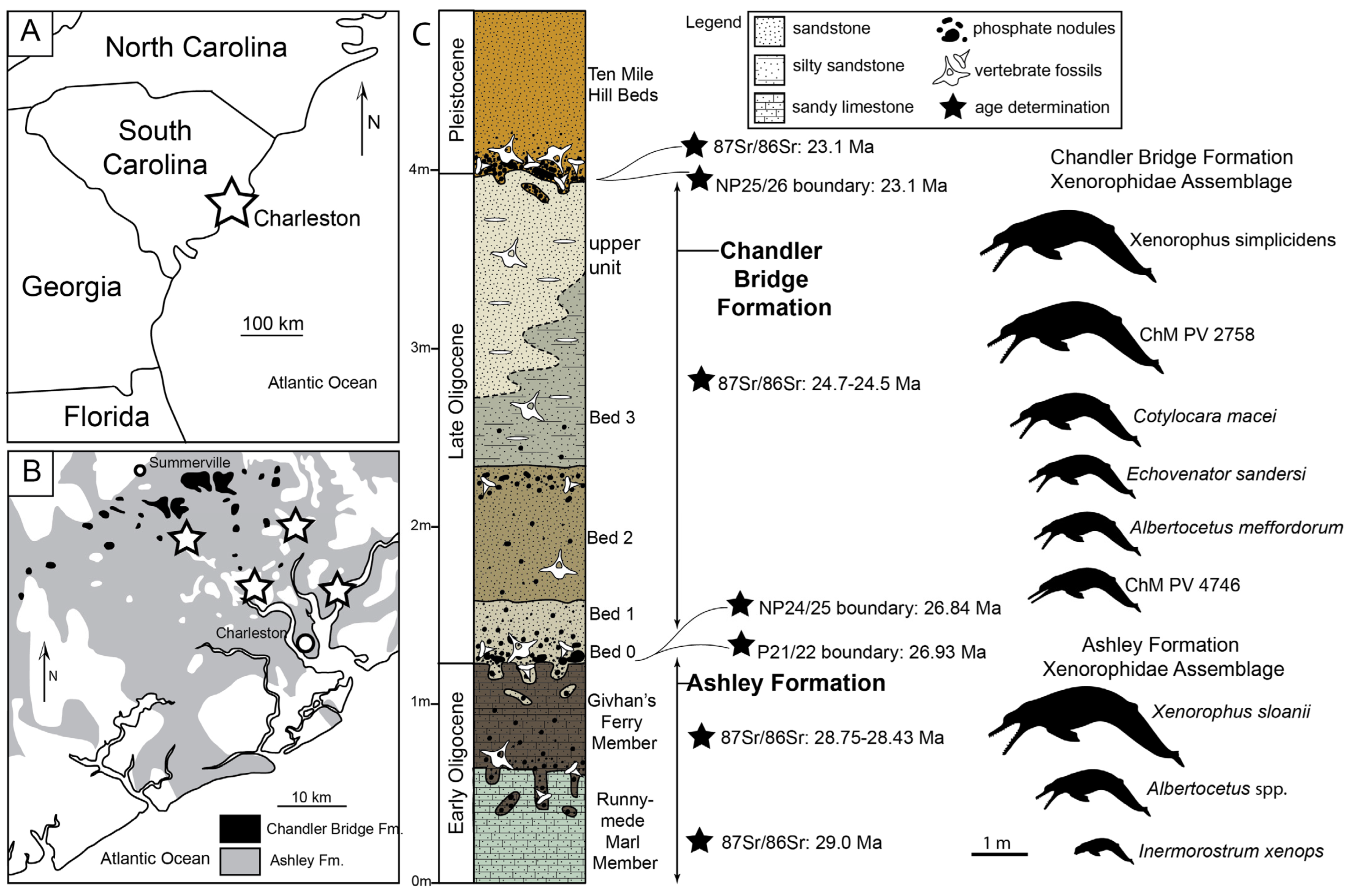
Locality and Age: All known specimens of
Xenorophus sloanii were collected from the lower Oligocene Ashley Formation in the Charleston Embayment, ranging in age from 30.0 to 28.0 Ma [
26].
Amended Diagnosis of Species: A large xenorophid dolphin with approximate adult condylobasal length of 74 cm and bizygomatic width of 29–30 cm and differing from Xenorophus simplicidens in possessing anteroposteriorly longer nasals (22–29% of bizygomatic width vs. 16–19% in X. simplicidens), nasal process of premaxilla possessing lateral overhanging crest adjacent to bony nares in adult specimens, left antorbital fossa longer than right (right antorbital anteroposteriorly longer than left in Xenorophus simplicidens), longer supraorbital process (distance from antorbital notch to posterior margin supraorbital process 43% of bizygomatic width vs. 34% in X. simplicidens), nasals that broaden posteriorly and approximate or exceed nares width, left and right palatines separated by anteroposteriorly short median triangular exposure of maxilla, paroccipital processes extending posterior to occipital condyles, shorter median furrow on the tympanic bulla, more teeth with accessory denticles (on PC5–9 as opposed to PC7–9 in X. simplicidens), more accessory denticles per tooth (five distal cusps vs. three in X. simplicidens; three–four mesial cusps vs. one–three in X. simplicidens), and more rugose enamel throughout the dentition, with nodular enamel present on PC5–9 (only present on PC7–9 in X. simplicidens and less rugose than in X. sloanii).
5.1.5. Description of Xenorophus sloanii
The description of the skull, mandibles, and dentition is based on the most completely preserved skull, CCNHM 168, but is supplemented by CCNHM 104, 1077, 5995, ChM PV 5020, 5022, and 7677, in addition to the holotype (USNM 11049). Where polymorphisms exist among specimens, they are noted; if a statement in the description does not refer to a particular specimen, it is a feature that is identical in all specimens that preserve that structure. Descriptions of the tympanic bullae are based chiefly on CCNHM 168, CCNHM 104, CCNHM 1077, and ChM PV 5022. Descriptions of the periotic are based chiefly on CCNHM 104, CCNHM 1077, ChM PV 5022, and CCNHM 7677. Descriptions of the postcrania are based (in descending order of importance) on CCNHM 1077, ChM PV 5022, CCNHM 168, and CCNHM 104.
Ontogenetic Status: In all specimens the following sutures remain open or unfused (i.e., a suture line that is not obliterated or completely fused/invisible): maxilla–premaxilla, squamosal–parietal, parietal–occipital, squamosal–occipital, maxilla–frontal, median nasal, median parietal, and frontonasal suture (the latter appears obliterated in CCNHM 104, similar to the
Echovenator holotype; [
3]). In CCNHM 1077, the median frontal suture is nearly completely fused and the frontonasal suture is barely visible. In CCNHM 1077, the median parietal suture is sinuous and fine. In contrast, the median parietal suture in CCNHM 104 is straight and open (a fissure several mm wide dorsally), although this may be an artifact of preservation and reassembly.
Tooth wear varies between specimens. The holotype bears no visible wear facets; the entire mesial and distal edges of all cheek teeth in CCNHM 104 are obliterated by large wear facets along with the complete loss of LC1 and LPC1 crowns and most of LI2. In CCNHM 168, there are small mesial and distal wear facets on PC7–8 and, in CCNHM 1077, there are wear facets on the mesial edge only of B1–2 (=PC8–10 or so, depending upon tooth count). Similar patterns of tooth wear exist on the lower dentition of each specimen. Tooth wear does not appear to correlate perfectly with body size as subadult specimen CCNHM 104 has rather extreme tooth wear and incomplete cranial suture closure, whereas the largest specimens (CCNHM 1077, CCNHM 168, and ChM 5022) possess more completely closed sutures, more strongly developed crests, and less strongly developed tooth wear (yet, more than in the holotype specimen). If extreme tooth wear in CCNHM 104 is the cause of unusual behavior in this individual (see 6.5. Feeding Ecology of Xenorophus), tooth wear otherwise seems to correspond to ontogenetic status with juveniles (class III) lacking tooth wear, subadults (class IV) possessing minor tooth wear, and adults (class V and VI) possessing more extreme wear including large shearing facets.
Specimens were assigned to the growth classes of Perrin [
83] based chiefly on tooth development, skull suture closure, and closure of vertebral epiphyseal sutures. Additional information, such as nasal length, was also considered.
Class I and II: No specimens of Xenorophus sloanii are assignable to either class I (fetus) or II (neonate).
Class III: The holotype specimen (USNM 11049) and CCNHM 5995 (
Figure 22,
Figure 23,
Figure 24 and
Figure 25) were assigned to Class III of Perrin [
83] owing to the following combination of features (some preserved only in one specimen): small size, unworn teeth, relatively short nasals, cranial sutures either open or lightly sutured, but not strongly mortised, and, in the case of CCNHM 5995, completely unfused vertebral epiphyses.
Class IV: two specimens (ChM PV 7677, CCNHM 104;
Figure 24,
Figure 25,
Figure 26 and
Figure 27) represent Class IV, based on slightly larger size than Class III, no open sutures (except the median parietal suture in CCNHM 104) nor obliterated sutures, more than 75% of the vertebral column with fused epiphyses (mostly open in ChM PV 7677), and relatively short nasal bones. While ChM PV 7677 exhibits unworn teeth, the teeth of CCNHM 104 record the heaviest wear of any specimen of
Xenorophus—few teeth have any carinae preserved, and some anterior teeth (LI2, LC1, LPC1) are completely missing the crown. Vertebrae of these specimens have a mixture of fused and open epiphyseal sutures.
Class V: two specimens (CCNHM 168, CCNHM 1077;
Figure 24,
Figure 25,
Figure 26,
Figure 27,
Figure 28 and
Figure 29) are assigned to class V based on large size, all sutures being either strongly mortised or obliterated, long nasals, and most—but not all—vertebral epiphyses fused. CCNHM 1077 is perhaps the most mature specimen based upon skull size, nuchal crest height, and cranial suture development—but still has a few vertebrae with unfused epiphyses. If preserved from a skull only, his specimen would have likely been assigned to class VI.
Class VI: Only one specimen (ChM PV 5022;
Figure 24,
Figure 25,
Figure 28 and
Figure 29) is assigned to class VI—based on large size, long nasals, mostly obliterated (and some mortised) skull sutures, and completely fused epiphyses throughout the vertebral column.
Other features such as the development of the paranaris crest, nasal length, nuchal crests, length of the supraoccipital, development of the sternomastoid fossa, and surface texture of the occipital condyles are ontogenetically informative in Xenorophidae and other early Odontoceti, yet not part of Perrin’s [
83] framework. In Class III–V, the paranaris crest is vertical, yet in Class VI, the crest has a slight lateral overhang resembling the condition in
Cotylocara macei and more mature (but unpublished) specimens of
Echovenator (CCNHM 217, 219). The nasal bones are relatively short in Class III (31–34 mm, USNM 11049), are similar to somewhat longer in class IV (28–31 mm, ChM PV 7677; 48+ mm, CCNHM 104), and are longest in class V and VI (66–79 mm;
Figure 30 and
Figure 31). The nuchal crests are vertically higher in successively more mature specimens (
Figure 25), with relatively low crests in Class III, slightly higher crests in Class IV, and much higher crests in Class V and VI. Along with dorsal growth of the nuchal crests, the supraoccipital lengthens during ontogeny resulting in a longer distance from the foramen magnum to the supraoccipital apex (
Figure 30 and
Figure 31). In Class III and IV, the length is 107–117 mm (CCNHM 104, ChM PV 7677), and longer in Class V and VI (123–128 mm, CCNHM 1077, ChM PV 5022). An exception to this is Class V specimen CCNHM 168 (113 mm), owing to a deep dorsal notch in the margin of the foramen magnum. Concomitant with anterodorsal growth of the supraoccipital, the anteroposterior length of the parietal decreases slightly during late postnatal ontogeny as it is overridden by the supraoccipital, with the longest length present in Class IV specimens like ChM PV 7677 (55.6 mm), and generally shorter lengths in class V and VI specimens (34–42 mm; CCNHM 168, CCNHM 1077; note that these specimens are similar in bizygomatic skull width;
Figure 31). A much shorter length in Class III individual CCNHM 5995 is due to the smaller size of the skull. In Class III specimen CCNHM 5995, the occipital condyles bear porous rather than smooth finished bone surfaces. The sternomastoid fossa is shallow, less rugose, and anteroposteriorly shorter in Class IV (CCNHM 104, ChM PV 7677) than Class V and VI (CCNHM 168, CCNHM 1077, ChM PV 5022).
Rostrum and Palate: The rostrum is relatively elongate, with RPI values in the range of 2.44–2.62 (Max: CCNHM 104; min: ChM PV 7677;
Figure 22,
Figure 23,
Figure 26,
Figure 27 and
Figure 30;
Table 7). In CCNHM 168, the anterior 90 mm of the rostrum is formed by the premaxilla only. The premaxillae do not taper anteriorly in dorsal view but have a smoothly convex anterolateral margin rather than a triangular apex of the premaxilla. There is a slight bulging around tooth roots giving the premaxilla a subtle ‘scalloped’ edge, which continues along the anterior half of the maxilla. In dorsal view the rostrum is triangular but has a sinuous lateral margin; the rostrum widens greatly in its posterior third and then narrows precipitously at the antorbital notch, making the posterior 10 cm of the maxilla markedly laterally convex.
The rostrum in all specimens of
Xenorophus sloanii diverges from the sagittal plane within the braincase and interorbital region by approximately 1.5–4.7° to the left side (determined in dorsal and ventral view, measured in ImageJ based on the osteological midline of the rostrum and braincase, with an angle vertex placed at about least interorbital width;
Figure 22,
Figure 23,
Figure 26,
Figure 27 and
Figure 30). It is least divergent in ChM PV 7677 (0.9°), somewhat more divergent in CCNHM 168 (2.3°) and most divergent in CCNHM 104 (4.7°) and the holotype (~4.0°). In lateral view the rostrum is nearly straight with a slightly sinuous ventral margin, the palatal margin becoming slightly ventrally convex anteriorly and concave posteriorly. The rostrum is slightly downturned relative to the basicranial stem; in CCNHM 168 and CCNHM 104, it descends anteroventrally 22° from the horizontal plane, and only 14° in ChM PV 7677, though this may be due to diagenetic distortion near the vertex and it was likely closer to or exceeding 20°. Lastly, the rostrum is also twisted along its longitudinal axis (counterclockwise in anterior view) by 11° in CCNHM 168 (
Figure 32).
In dorsal view the premaxilla has a nearly straight lateral edge (
Figure 22,
Figure 26 and
Figure 30). The lateral edges of the premaxillae are nearly parallel along the posterior half of the rostrum, but widen gradually and diverge along the anterior half. The premaxillae are completely separated along their entire length by a continuous and parallel-sided mesorostral groove, measuring about 5–10 mm wide. The groove is artificially widened (by about 10–11 mm) in CCNHM 104 by a combination of diagenetic deformation and difficulty of reassembling the deformed rostrum. The premaxilla–maxilla suture is formed as a narrow fissure, though not positioned within a deep longitudinal furrow as in many other odontocetes. The lateral surface of the rostrum is nearly planar, giving it an approximately triangular cross section along the anterior two-thirds of the rostrum. The anteriormost part of the rostrum is slightly wider than deep, and nearly an equilateral triangle in cross section, and becomes relatively wider and shallower posteriorly.
Embrasure pits are present on the maxilla but not the premaxilla (
Figure 27). Shallow pits with clusters of minute foramina occur on the ventrolateral edge from between C1–PC1 to just anterior to PC4; deep embrasure pits are present posterior to PC4 (8.7 mm deep) and end anterior to PC7. Embrasure pits are largely laterally positioned on the maxilla anterior to PC4, but from just posterior to PC4 to just anterior to PC7, the embrasure pits shift medially so they are in line with the toothrow. No embrasure pits are present behind PC7, contrasting with the deep posterior embrasure pits on the mandible (see below). From medial to lateral, a longitudinal ridge and groove are present on the palatal surface of the maxilla, running from the anterior tip to the level of PC3, just medial to the teeth. We interpret this as a homolog of the alveolar groove in later diverging odontocetes. In the holotype, the diastemata are narrow and the embrasure pits small and shallow, positioned between PC2–6 (
Figure 23). In ChM PV 7677, the right maxilla is complete but C1–P3 and their corresponding alveoli are not present (
Figure 23 and
Figure 33). Instead, shallow vascularized pits are present; while the lateral edge of the left maxilla is damaged, these embrasure pits are less excavated than in other specimens of
Xenorophus sloanii (e.g., CCNHM 104, 168). The C1–P3 alveoli are replaced with shallow vascularized pits with punctate bone texture, with a deeper pit with numerous small foramina present at the approximate position of the former PC2 alveolus. The left maxilla has all the alveoli preserved as in other specimens, indicating this is a pathology on the right side. Likewise, shallow embrasure pits are present on the right maxilla of ChM PV 7677 between PC1–4, as in other specimens.
Ventrally, the maxilla bears a tongue-like sheet that extends anteromedial to C1 (
Figure 23 and
Figure 27). Medial to this is a fissure-like premaxilla–maxilla suture on the palate, which continues posteriorly. The premaxilla has an anteroposteriorly long and transversely narrow splint-like palatal exposure that tapers posteriorly and terminates against the vomer; in CCNHM 168, it terminates 165 mm posterior to the anterior edge of C1. The premaxilla bears longitudinal grooves ventrally. A lanceolate exposure of vomer attains a length of 270 mm on the palate of CCNHM 168 and a maximum width of 10.5 mm; it is asymmetrical, with a trapezoidal outline, and its exposure is shifted approximately 5 mm to the left side so that the right maxilla–vomer suture is closer to the midline than the left maxilla–vomer suture. Accordingly, at the level of PC5, the vomer is 21 mm from right PC5 and 17 mm from left PC5. The vomer is more symmetrical and narrow in juveniles like the holotype (USNM 11049;
Figure 23,
Figure 27 and
Figure 34). The anterior palatal exposure of the vomer is smallest in juveniles, measuring 61% of antorbital rostrum width in the holotype (USNM 11049), 100–110% in subadults ChM PV 7677 and CCNHM 104, and 191% in adult CCNHM 168 (
Figure 34). A posterior palatal exposure of the vomer is present between the palatines and pterygoids, and is longest in juvenile specimens like the holotype (USNM 11049) where it separates the palatines along at least 75% of their length; in ontogenetically mature specimens like CCNHM 1077, the vomerine exposure is restricted far posteriorly and the palatines contact each other for at least 80% of their length (
Figure 23,
Figure 27,
Figure 29, and
Figure 34).
The palate is nearly completely flattened transversely in cross-section in most specimens but slightly more convex in CCNHM 1077. The greater palatine foramina open at the level of PC8 on the maxilla and close to the midline (~18 mm from midline) and are confluent with anteriorly widening trough-like sulci with well-defined edges for ~55 mm (
Figure 27 and
Figure 29). Anteriorly, the sulci become diffuse (but palpable) and continue anteriorly to the level of PC2. The foramina are asymmetrically positioned with the left positioned further posterior to the right by 11 mm in CCNHM 168 and 20 mm in ChM PV 7677.
The maxilla–palatine suture is ‘M’-shaped with wide lobate posterior median wedge of maxilla penetrating between palatines, which extend anteriorly to the position of the posteriormost tooth (
Figure 23,
Figure 27,
Figure 29 and
Figure 34). Like the greater palatine foramina, the right palatine extends further anterior to the left. This style of asymmetry with an anteriorly positioned right palatine is consistently developed in all skulls of
Xenorophus sloanii as well as
Albertocetus-group specimens. Amongst adult specimens, the right palatine is shifted anteriorly relative to the left as little as 8 mm (ChM PV 7677), 19 mm in subadult CCNHM 104, 17.4 mm in adult CCNHM 168, and as much as 26 mm in CCNHM 1077; in the holotype (USNM 11049), it is only 6 mm anterior. In addition to the asymmetrical anterior margin, the median palatine suture is asymmetrical and deviated to the left of the midline in all specimens where the palatines are complete (CCNHM 168, 1077).
The pterygoid is most completely preserved in CCNHM 1077 (
Figure 29); it is plate-like and bears a small (15 mm long) triangular hamulus that terminates near the posterior margin of the temporal fossa. The lateral side of the pterygoid bears longitudinal striations. The pterygoid–palatine suture begins dorsally at the level of the postorbital process and descends ventromedially. The pterygoid articulates with the squamosal 10 mm anteroventral to the foramen ovale and this suture continues anterior at least 30 mm from the foramen.
In lateral view, the palate appears slightly ventrally concave because the palatines conform to the basicranial stem and the 22° deflected rostrum (
Figure 24). The palatines are damaged in most specimens with broken lateral edges but where preserved, appear to become more transversely convex posteriorly (
Figure 23,
Figure 27 and
Figure 29). In CCNHM 1077, the palatines are more completely preserved and form a transversely convex, sub-cylindrical shape anterior to the ventral part of the bony nares. The palatines in this specimen bear a low crest emanating medial to the antorbital notch and converge posteromedially towards the medial pterygo-palatine suture (
Figure 29).
The left and right premaxillae bear elongate, transversely narrow premaxillary sac fossae (
Figure 22,
Figure 26 and
Figure 28), which, in CCNHM 168, measure approximately 100 mm long and 20 mm at the widest. The premaxillary sac fossae are asymmetrical, with the left fossa somewhat wider, placed further anteriorly, and more deeply concave than the right fossa. A poorly developed anteromedial sulcus is present in CCNHM 168 but more strongly entrenched in CCNHM 104. About 30–60 mm anterior to the nares, the premaxillae form median ridges which are closely appressed to one another, closing the mesorostral canal. In CCNHM 168, this ridge is high and twisted so that the mesorostral groove is tilted to the left of the sagittal plane; this is associated with the more deeply concave left premaxillary sac fossa.
The premaxillary foramina (
Figure 22,
Figure 26 and
Figure 28) are present at the level of PC7 in CCNHM 168; in this specimen, three are present on left and two are present on the right. They measure 3–5 mm in diameter, except for the posterior right foramen which is 8.2 mm. Most open dorsally, but the anteriormost foramina open slightly anterodorsally. In CCNHM 1077, there is a large right premaxillary foramen with a narrow, oval foramen just posterior to it. Two premaxillary foramina are present on the left, and they are offset anteriorly relative to their counterparts on the right; the smaller anterior and larger posterior left premaxillary foramina are positioned about 15 mm anterior to and 10 mm posterior to the anteriormost right premaxillary foramen (respectively). In CCNHM 104, the premaxilla here is broken, but there are bilateral large posterior left and right foramina at the same level; there is an additional foramen on the right positioned 4 cm anterior. In ChM PV 5022, there is a large single premaxillary foramen on the left and two on the right; the left premaxillary foramen is shifted slightly further anterior to the large posterior foramen on the right. In ChM PV 7677, there is similarly only a single large premaxillary foramen, but on the right, there is only a single small foramen located somewhat further posterior. The premaxillae are too fractured in the holotype to evaluate. In CCNHM 168, a shallow posterolateral sulcus extends posterolaterally 50 mm from the anteriormost premaxillary foramina and sits adjacent to a longitudinal sharp ridge leading to the dorsolateral edge of the nares.
A shallowly excavated antorbital fossa is present on the base of the maxilla and also excavates the antorbital region, forming a steep, transverse, vertical, surface that separates the rostral part of the maxilla from its ascending process (
Figure 22,
Figure 26,
Figure 28,
Figure 32, and
Figure 35). The antorbital fossa is shallower in ontogenetically immature specimens (ChM PV 7677). The antorbital fossae are consistently longer on the left than on the right, differing from the condition in
Xenorophus simplicidens (see above). Furthermore, the left antorbital fossa is more deeply excavated on the left than on the right; the minimum depth of the maxilla lateral to the fossa on the left side is 59% of the depth on the right in CCNHM 104 and 72% the depth of the right side in CCNHM 168. The pattern and number of dorsal infraorbital foramina are variable and obscured by breakage in these specimens. At least two separate small foramina are present on the left side of CCNHM 168, along with a cavernous space for the infraorbital canal exposed by surficial bone breakage, with unclear margins. In CCNHM 168, a cluster of four dorsal infraorbital foramina are present on the right side, with two small foramina opening dorsally to dorsolaterally (8.5 and 5 mm wide, respectively) and two large (10–12 mm wide) anteriorly opening foramina anteroventral to these. In CCNHM 104, there is one small (3 mm wide) posterodorsal foramen opening dorsally and three larger (9–15 mm wide) foramina opening (from anterior to posterior) anteriorly, posterodorsally, and dorsolaterally. All dorsal infraorbital foramina are located in the posterior half of the antorbital fossa. In CCNHM 1077, the dorsal infraorbital foramina are asymmetrical and coalesced into a 20–30 mm fenestra on the left side with an additional foramen 15 mm further anteriorly; three separate foramina are present on the right side (
Figure 35), one large dorsally opening foramen medial to the antorbital notch, a smaller dorsally opening foramen positioned further dorsally, and a large anteriorly positioned and anteriorly opening foramen 20 mm further anterior. The foramina on the left are similarly coalesced into a large fenestra in the holotype and ChM PV 7677; a similar condition may be present in ChM PV 5022, though obscured by damage. The medial wall of the antorbital fossa (
Figure 35) is vertical in some individuals (CCNHM 104) and subvertical in others (CCNHM 168). In summary, the dorsal infraorbital foramina are consistently coalesced on the left side and appear to be separated on the right.
Facial Region: In dorsal view, the bony nares are oval-shaped with the posterior margin transversely oriented and straight, truncated by the nasals (
Figure 22,
Figure 26 and
Figure 28;
Table 7). The nasal passages are approximately vertical, and confluent anteriorly with the mesorostral groove. The “frontal shield” is approximately twice as wide as long (~240 × 110 mm in CCNHM 168) and includes portions of the maxilla, frontal, lacrimal, and premaxilla. Medially, it is horizontal, but slopes gradually ventrolaterally towards the orbital margin.
Medially, a flat subrectangular table is formed by the nasals, frontal, and nasal processes of the premaxillae just anterior to the vertex (here defined as the parietal–occipital contact at the midline;
Figure 22,
Figure 26 and
Figure 28). The combined width of the nasals is about twice as long as their greatest length; there is a shallow median furrow anteriorly with a short median fissure that continues posteriorly as the internasal suture in adult specimens (CCNHM 1077, ChM PV 5022), whereas they are transversely flat in younger specimens (USNM 11049, ChM PV 7677, CCNHM 104). The nasals are anteroventrally excavated with concave oval fossae facing anteroventrally, roofing over the anterodorsal part of the narial passage. The nasals are slightly longitudinally arched in some specimens (CCNHM 168) and flat in others (CCNHM 104). The lateral edges of the nasals are nearly parallel but slightly converge posteriorly in the holotype; in other specimens they are parallel (CCNHM 104, 168; ChM PV 5022, 7677) and widen slightly posteriorly in the largest individual (CCNHM 1077).
In larger specimens, there is a triangular (CCNHM 1077) or tongue-shaped (CCNHM 168) median wedge of the frontal which extends anteriorly between the nasals (
Figure 26 and
Figure 28). No such wedge is present in the holotype, and, instead, the left and right nasals converge posteriorly to a point at the midline. In CCNHM 168, the nasals bear a posterior splint that is separated laterally from the nasal process of the premaxilla by a triangular prong of the frontal, whereas in CCNHM 1077, posterior splints are not evident, and the nasofrontal suture is partially obliterated; this suture is at least partially obliterated in CCNHM 104 as well, though breakage and putty obscure the suture. A shorter, more blunt splint appears on the right nasal of ChM PV 5022 with a lateral wedge of the frontal. ChM PV 7677 highlights a transitional morphology between CCNHM 168 and the holotype: a shallow median frontal triangle is present, short and blunt posterior splints of the nasals are present, and a shallow lateral wedge of the frontal is also present.
In some specimens, the nasals are asymmetrical (
Figure 22,
Figure 26,
Figure 28,
Figure 30 and
Figure 31;
Table 7), with the left slightly narrower than the right (CCNHM 168, 88% of right; CCNHM 1077, 92% of right); in all others they are approximately symmetrical (CCNHM 104, ChM PV 5022, 7677, USNM 11049). In ontogenetically immature specimens, the nasals are anteroposteriorly shorter (holotype USNM 11049, ChM PV 7677, CCNHM 104).
Immediately anterior to the nasals, the premaxilla nasal process is laterally abutted by the ascending maxilla. The two bones together form a vertical longitudinal paranaris crest lateral to the nares; dorsally, the crest curls over laterally, forming a slight overhang in larger individuals (CCNHM 1077, present but broken in ChM PV 5022;
Figure 32), but not as extreme as in
Cotylocara macei. This ridge continues posteriorly along the premaxilla–maxilla suture to the level of the posterior half of the nasals. A posteriorly widening wedge-shaped strip of premaxilla is exposed dorsally and laterally along the entire length of the nasal (
Figure 22,
Figure 26,
Figure 28, and
Figure 31), as in other xenorophids. Where preserved well (e.g., the holotype, CCNHM 1077, ChM PV 5022), this exposure of the premaxilla widens gradually until the level of the posteriormost nasals, where they widen abruptly, doubling in width to over 35 mm. The posterior edge of the nasal process of the premaxilla dorsally meets the medial part of the frontal. This triangular dorsal exposure of the premaxilla separates the frontal and nasal from the ascending process of the maxilla.
The ascending process of the maxilla is fan-shaped with a convex posterior margin (although a posterolateral corner is formed in ChM PV 7677), and paralleled by a convex posterior edge of the supraorbital process of the frontal; this curved strip behind the maxilla extends about 15–17 mm more posteriorly (
Figure 22,
Figure 26,
Figure 28, and
Figure 31). The posteromedial part of the maxilla and frontal are damaged in most specimens, but well-preserved in CCNHM 1077 and ChM PV 7677; a shallow transverse trough is developed on the dorsally exposed part of the frontal, parallel with the posterior margin. In some of the larger specimens (CCNHM 1077, ChM PV 5022), there is a low, laterally positioned maxillary ridge along the maxilla–lacrimal suture.
The lacrimal is greatly enlarged with a large, triangular, dorsal exposure where it has overlapped the preorbital process and the anterolateral part of the supraorbital process of the frontal (
Figure 22,
Figure 26,
Figure 28 and
Figure 35). The lacrimal is shaped as a concavo-convex lensoidal element in lateral view, convex dorsally and thickened anteriorly to form the antorbital process and curling ventrally to underlap the preorbital process of the frontal. Posteriorly, the lacrimal thins dorsoventrally into a sheet. The transverse width of the exposure (on one side) measures about 22% of the interorbital width in CCNHM 168. Anteriorly, a steep face on the anterior side of the lacrimal contributes to the posterior margin of the antorbital fossa; this surface is ventral to a sharp ridge that continues posteromedially.
Only the proximal part of the jugal is preserved (e.g., CCNHM 1077; partial in ChM PV 5022 and CCNHM 168) where it is “hammer” or T-shaped in ventral view; it bears a transversely expanded anterior end that articulates with the lacrimal and maxilla and quickly transitions into a transversely narrow, dorsoventrally thin and strap-like bone (
Figure 24,
Figure 29, and
Figure 36). In specimens where the jugal is missing, a clear transverse rectangular articular facet is present on the lacrimal, indicating the two were not fused as in later diverging odontocetes even later into ontogeny (e.g., CCNHM 168). A clear suture is present in even adult specimens where the jugal is preserved, such as CCNHM 1077.
The frontal is exposed laterally along the orbital margin as a thin strip lateral to the lacrimal (
Figure 22,
Figure 26 and
Figure 28). The lacrimal never reaches the lateral edge of the orbital margin and is separated by an anteriorly narrowing wedge of exposed frontal. The orbit is ventrally concave in lateral view and posteriorly defined by a large, subrectangular, and posteroventrally oriented postorbital process (
Figure 24). The orbit diameter measures approximately 23–26% of bizygomatic width, with subadult specimen ChM PV 7677 having the proportionally smallest (22.5%) and adult specimen ChM PV 5022 having the largest (28%). Orbital angles (
sensu [
93]) range from 73 to 86°, with extremely anteriorly tilted orbits present in the juvenile holotype (73.6°), slightly less anteriorly tilted orbits in subadults and adults (e.g., 78.5° in ChM PV 7677, 79.7° in CCNHM 104), and nearly laterally facing orbits in adults (82.5° in CCNHM 168, and an average of 81.3° on the distorted orbits of ChM PV 5022). One outlier is adult specimen CCNHM 1077, which has a low angle and anteromedially shifted orbits (75°); this is largely driven by the more laterally extending postorbital processes, perhaps caused by diagenetic crushing. A lunate fossa for the masseter origin emarginates the posterolateral edge of the postorbital process. The frontal groove is shallowly concave, approximately horizontal, and bordered posteriorly by a low postorbital ridge. No diploic foramina are present. Posterior to the postorbital ridge, the ventral surface of the composite supraorbital process is flat to shallowly concave. Posterior to the postorbital process, the frontal bears a ridge that parallels the edge of the supraorbital process and bears grooves for the ascending process of the maxilla; though incomplete in CCNHM 168, a complete ascending process is preserved in CCNHM 1077, ChM PV 5022, and nearly complete in CCNHM 104. A well-developed frontal window (
Figure 37; [
5]) exposes the premaxilla (medially) and maxilla (laterally). Judging from this exposure and fractured specimens, the nasal process of the premaxilla is osteosclerotic, inflated, and forms the core of the supraorbital process medially and thins laterally. The premaxilla posteriorly terminates within the frontal window and the maxilla occupies the remainder of the window. Within the window, the premaxilla–maxilla suture is anterolaterally directed and planar. The posterior margin of the supraorbital process is developed as a thin sheet of frontal encircling the posterior edge of the frontal window; this is damaged in most specimens (including the holotype) but is smoothly convex in CCNHM 1077 and formed as a posterolateral corner in ChM PV 7677 (
Figure 22,
Figure 23,
Figure 28 and
Figure 29).
Vertex and Dorsal Braincase: An intertemporal constriction is formed by the parietals that contact at the midline, separating the frontals from the occipital (
Figure 22,
Figure 23,
Figure 26,
Figure 27,
Figure 28,
Figure 29 and
Figure 30;
Table 7). An anteroposteriorly short sagittal crest is present and deviates to the left in most adult specimens (CCNHM 168: 9°; ChM PV 5022: 6–12°; CCNHM 1077: 4°), although it is approximately anteroposteriorly aligned in juveniles CCNHM 5995 and subadults ChM PV 7677 and CCNHM 104. The intertemporal constriction is quite narrow and measures about 20% of bizygomatic width in all specimens.
In dorsal view, the braincase is roughly triangular and bears a dorsoventrally blunt subtemporal crest anteroventrally, formed by the squamosal and alisphenoid (
Figure 22,
Figure 23,
Figure 26,
Figure 27,
Figure 28,
Figure 29,
Figure 30 and
Figure 36). Most of the lateral side of the braincase is formed by the parietal and is slightly convex (
Figure 24 and
Figure 36). The frontal is exposed anteriorly on the lateral side of the braincase, but is hidden in dorsal view by the supraorbital process (
Figure 36). It underlaps the nasal process of the premaxilla medially and thins towards the frontal window. The frontoparietal suture is nearly transverse and nearly closed in all specimens (CCNHM 168, CCNHM 1077, CCNHM 5995, ChM PV 5022, ChM PV 7677) with a well-developed and slightly anteroposteriorly mortised suture. The suture is closed but less mortised in CCNHM 5995; this suggests that the posterior end of the frontals in the holotype (USNM 11049) is fractured rather than representing a naturally open suture (
Figure 22).
The occipital shield is triangular and bears a pointed apex, forming about a 95–100° angle in dorsal view (
Figure 22,
Figure 25,
Figure 26,
Figure 28 and
Figure 30;
Table 7). The shield is steep and plunges 50–60° from the roughly horizontal plane of the nasals and frontals. A distinct attachment scar for some of the neck muscles (rectus capitis, obliquus capitis superior, and/or semispinalis) is present as a pitted band parallel to the dorsolateral edge of the nuchal crests. There is no external occipital crest; instead, the dorsal half of the shield is shallowly transversely concave. The nuchal crests are highly elevated and, in lateral view, they are posteriorly convex and overhang the exoccipital posteroventrally. In some specimens (CCNHM 104, 1077), the nuchal crests extend further posteriorly than the occipital condyles; in others, they approach but do not pass the condyles (ChM PV 5022, 7677; CCNHM 168). Although difficult to quantify, they are more strongly developed and elevated above the vertex in absolutely larger individuals such as CCNHM 168, 1077, and ChM PV 5022.
Deep, subhorizontal, and reniform dorsal condyloid fossae are present in most specimens (
Figure 25); they are shallower in CCNHM 104. In CCNHM 168, the left fossa bears an 11 mm wide, circular fenestra of probable pathologic or developmental origin in the occipital; a similar 7.6 mm wide fenestra is present in the occipital within the right fossa. The condyles are set out on a short but well-defined neck that is salient in dorsal and ventral view. The combined width of the condyles is slightly greater than their height; they encircle a circular foramen magnum (CCNHM 104, CCNHM 1077, ChM PV 7677), although in CCNHM 168 the foramen is teardrop-shaped with a triangular dorsal cleft.
The occipital shield is trefoil-shaped with a posterior and ventrolaterally flaring exoccipital (
Figure 25). The exoccipital is quadrate and flat posteriorly, and bears a ventrally convex paroccipital process with a medial point in some specimens (CCNHM 168, ChM PV 7677) and a much longer sharply triangular prong in others (CCNHM 1077, ChM PV 5022).
Basicranium—Exoccipital and Basioccipital: In ventral view, the outline of the basioccipital is triangular, narrow anteriorly and widening posteriorly at the basioccipital crest (
Figure 23,
Figure 27,
Figure 29,
Figure 38 and
Figure 39;
Table 7). The basioccipital crests (
Figure 23,
Figure 27,
Figure 29, and
Figure 38) are oriented ventrolaterally and are transversely thickened (14–21 mm; 5–7% of bizygomatic width) to a similar degree as in basilosaurids (2.5–7% of bizygomatic width) and thicker than the plate-like crest in crown odontocetes (typically 0.9–3.5% of bizygomatic width, with some extinct outliers with thicknesses from 4 to 5%, including
Pomatodelphis and
Kampholophos). The apex of the crest is rugose in adults (CCNHM 168, 1077; ChM PV 5022) and smooth and punctate in subadults CCNHM 104 and ChM PV 7677. The crest transitions anteriorly into a plate-like pharyngeal crest. The basioccipital crest in some specimens (CCNHM 168, 1077) is bifid with a transverse cleft about 10 mm anterior to the jugular notch dividing the crest into a large primary crest and a small secondary posterior crest. This cleft is a possible autapomorphy of
Xenorophus. A shallower cleft is present in
Echovenator and
Cotylocara but absent in all known specimens of
Albertocetus. An approximately transverse, posteriorly concave crescent-shaped scar for the insertion of the rectus capitis ventralis is present medial to the basioccipital crest. This scar terminates laterally at the apex of the primary basioccipital crest. A deeply incised jugular notch is developed and, in CCNHM 168, is 20 mm deep (relative to the apex of the secondary basioccipital crest). The paroccipital process extends further ventrally than the basioccipital crest with a triangular medial spur (
Figure 25) as in
Albertocetus and
Echovenator. The medial edge of the paroccipital process is concave. A deeply excavated subcircular paroccipital concavity is present on the anterior face of the process and is floored with rugose bone (
Figure 38 and
Figure 39). No obvious ventral condyloid fossa are present. Lateral to the condyles the entire occipital forms a horizontal trough; in lateral view, the exoccipital and paroccipital processes are oriented posteroventrally and this trough is overhung by the posterior apex of the nuchal crest.
Squamosal and Auditory Region (Excluding Tympanoperiotics): The zygomatic process is elongate, transversely narrow, and crescent-shaped in lateral view with a concave anteroventral margin (
Figure 24;
Table 7). The zygomatic apex tapers anteriorly and is triangular in lateral view. The supramastoid crest is transversely sharp along the anterior 20 mm of the zygomatic, and the ventral edge is sharp. The zygomatic process is rotated longitudinally so that the lateral side faces slightly dorsolaterally (
Figure 26 and
Figure 28), broadly analogous to the condition in eomysticetid baleen whales. The squamosal fossa is deep and developed as a short parasagittal trough; the posterior end of the supramastoid crest terminates in a small knob-like squamosal prominence (
Figure 24), developed more strongly in larger specimens (CCNHM 168, 1077, and ChM PV 5022) and weaker in CCNHM 104. The squamosal prominence is present in Basilosauridae but positioned further anteriorly, and a similar squamosal prominence is widespread among toothed mysticetes like the Aetiocetidae; such a prominence is otherwise unknown in stem odontocetes.
The sternomastoid fossa is large, pitted, and lunate (
Figure 24 and
Figure 25); like
Albertocetus, the sternomastoid fossa does not extend onto the lateral part of the exoccipital as it does in
Echovenator and
Cotylocara. In CCNHM 1077, it is not developed as a fossa but rather a rugose but flat scar. The sternomastoid fossae of CCNHM 168 are asymmetrical (29.5 mm long, 66 mm deep on left vs. 35.1 mm long and 72 mm deep on right). The fossa is similarly shallow in ChM PV 7677. The medial surface of the zygomatic process is shallowly concave with a longitudinal trough. Posteriorly, the glenoid fossa is also shallowly concave and nearly flat anteriorly, equidimensional and quadrate in ventral view (
Figure 38). A parasagittally oriented shallow trough delimits the medial edge of the glenoid fossa; this trough is not easily visible, but palpable. This trough may be the homolog of the tympanosquamosal recess in later diverging odontocetes. The postglenoid process projects ventrally and has a parabolic outline in posterior view (
Figure 25); it extends ventrally to about the level of the ventral margin of the bulla.
The falciform process is broken in most specimens but appears to have been a triangular transversely narrow sheet that conformed to the outer lip of the bulla (
Figure 23,
Figure 27,
Figure 29 and
Figure 38). Anteriorly, the falciform is perforated by the external foramen ovale, which is about 10 mm wide in most specimens (
Figure 38). Anteromedially, the squamosal forms a delicate thin sheet overlapping the greatly enlarged and pachyostotic alisphenoid. The alisphenoid is typically completely covered by a thin lamina of the squamosal in well-preserved large specimens (CCNHM 168, 1077) but, in others, the squamosal is flaked off, exposing a highly rugose external surface of the alisphenoid (e.g., CCNHM 104,
Figure 27).
The periotic fossa is exposed well in CCNHM 104, 1077, and ChM PV 7677 (
Figure 23 and
Figure 27), but obscured by damage in ChM PV 5022 and by the bulla in CCNHM 168 (
Figure 38). The fossa consists of a deep circular pit medial to the spiny process; the anterior partition of the fossa is damaged in CCNHM 104. Medial to and on the ventral side of the spiny process is a rectangular pit for the sigmoid process of the bulla. Posterior to the periotic fossa, a shallowly excavated “deep fossa” is present on the anterior side of the paroccipital process, immediately posterior to the stylomastoid fossa of the periotic and extending ventrally as a vertical trough.
The external acoustic meatus is developed as an anteroposteriorly narrow, acutely V-shaped trough (
Figure 24). The post-tympanic process is a low transverse ridge that merges with the posterior process of the bulla evenly and without an obvious suture (
Figure 38 and
Figure 39). A shallow transverse trough is present between the post-tympanic ridge and the paroccipital process.
The pterygoid sinus fossa is deeply excavated (
Figure 27,
Figure 29 and
Figure 38). The lateral wall of this fossa is formed by the pterygoid but is missing in most specimens, except for CCNHM 1077 and ChM PV 5022. The fossa is medially walled off by the basisphenoid and pharyngeal crest. It extends anteriorly to the level of the postorbital process. Where exposed (CCNHM 104, 168, ChM PV 7677), the dorsal part of the fossa is smoothly concave.
Anterior Basicranium: The internal choanae are nearly horizontal and tube-like, rather than steeply inclined or vertical as in many extant odontocetes. A median nasal septum extends posteriorly to the level of the posterior edge of the temporal fossa. The posterior end of the vomer is at the level of the anterior edge of the bulla. The basisphenoid vomer suture is straight and parasagittal.
Part of the lateral lamina of the pterygoid is preserved in CCNHM 1077; despite crushing, it seems to have been evenly convex in cross section along its entire length. The alisphenoid and possibly pterygoid seem to have formed the ventral edge of the temporal fossa; the alisphenoid is exposed ventral to the parietal in CCNHM 1077 and ChM PV 5022 as a dorsoventrally shallow strip.
Periotic: The periotic is preserved in several specimens (
Figure 39,
Figure 40,
Figure 41,
Figure 42,
Figure 43 and
Figure 44;
Table 8); it is loose in CCNHM 104, ChM PV 7677, and 5022, and articulated (but visible) in CCNHM 1077, and articulated but obscured by the bulla in CCNHM 168. Overall, the periotic is quite similar to and slightly larger in absolute size (e.g., anteroposterior length) than
Albertocetus meffordorum. They are best preserved in ChM PV 5022 and 7677.
The anterior process has a similar hatchet shape and a transversely narrow triangular profile in ventral view, and a lenticular cross section (
Figure 40,
Figure 41,
Figure 42 and
Figure 43). The anterior process has a straight, vertical anterior margin with a spine-like anterodorsal angle (
Figure 42 and
Figure 43). The lateral tuberosity is large, triangular, blade-like, and overlaps the squamosal just anterior to the pit for the sigmoid process (
Figure 39 and
Figure 40). A deeply excavated mallear fossa is present on the posteromedial side of the lateral tuberosity; anterolateral to the lateral tuberosity is a small, table-like accessory process in all specimens (ChM PV 5022, 7677, and CCNHM 104). A much less prominent tubercle is present in
Albertocetus meffordorum (CCNHM 303). A shallow transverse trough on the lateral tuberosity leads to a tubercle on the anterior rim of the mallear fossa, also present in simocetid-grade odontocetes (e.g., cf.
Olympicetus, [
51]). It is not clear if an accessory ossicle was present, and the anterior bullar facet is not clearly divided from the fovea epitubaria (articular surface for the accessory ossicle). No xenorophid specimen is preserved with an accessory ossicle; numerous acid-prepared simocetid-grade odontocetes in CCNHM collections from the Pysht Formation of Washington possess accessory ossicles (CCNHM 8714, 8715, 8751) and their presence is expected in Xenorophidae. Well-preserved periotic and bulla were found in articulation in a juvenile cf.
Albertocetus (CCNHM 1838), and when in articulation, there is a gap between the outer lip of the bulla and the fovea epitubaria of the periotic, yet no ossicle was present in this space; perhaps the ossicle is unfused or only delicately connected to the outer lip of the bulla in Xenorophidae, as it is in simocetid-grade odontocetes.
In ChM PV 7677, a distinct groove for the tensor tympani insertion is present along the anterior margin of the pars cochlearis (
Figure 42). A shallow anterolaterally directed sulcus trends toward the anterodorsal angle which bears two spurs: one just anterodorsal to the lateral tuberosity and another anterior to this and separated by an anterodorsally short sulcus branch; these spurs are larger on the left side.
The pars cochlearis is proportionally large (51–54% of periotic length) and has a subrectangular outline in ventral view in some specimens (CCNHM 104, ChM PV 7677) and a rounded profile in others (CCNHM 1077, ChM PV 5022;
Figure 40;
Table 8). The anteromedial margin is corner-like and forms a 100° angle in CCNHM 104 (
Figure 40). A low longitudinal ridge is present on the ventral surface of the pars cochlearis, just medial to the fenestra ovalis; this ridge is similar to but less extreme than the basilosaurid condition. The fenestra rotunda is small and subtriangular, but lacks a dorsal fissure; a low tubercle is present dorsal to the fenestra. In ChM PV 7677, the fenestra rotunda is circular and 2.3 mm in diameter. The caudal tympanic process is broken in CCNHM 104, but in ChM PV 7677 it is intact; it is long, posterolaterally deflected, and separated from the facial crest by a 1.5 mm gap.
No specimen of
Xenorophus has yet been scanned using MicroCT and the complete morphology of the bony labyrinth has not yet been studied. However, breakage of the right pars cochlearis of ChM PV 5022 has revealed an endocast consisting of approximately two-thirds of the entire cochlea (
Figure 44). The cochlear endocast preserves a large basal turn with a deep sulcus corresponding to the secondary bony lamina; the basal half of the first turn is separated from the second turn of the cochlea by a relatively wide gap, indicating a loosely coiled cochlea. The spiral-shaped bone in between the cochlear turns indicates that the cochlea had approximately two turns (similar to
Echovenator); further, the second turn and apical half of the first turn are more tightly coiled (
Figure 44).
In dorsal view, the internal acoustic meatus is large and oval, and dominated by a large, funnel-shaped spiral cribriform tract (
Figure 41). A transversely narrow oval and small foramen singulare is positioned lateral to the spiral cribriform tract and separated from it by a low, sharp crest. This crest is slightly lower than the similarly sharp crista transversa in CCNHM 104 and ChM PV 5022, but higher than the crista transversa in ChM PV 7677. The facial canal opens anterodorsally with a teardrop-shaped outline, narrowing anteriorly towards the fissure-like hiatus fallopii. The internal acoustic meatus is separated from the deeply excavated, pit-like suprameatal fossa by a low transversely sharp crest. The suprameatal fossa is transversely narrow, oval, and very deep; it is anteroposteriorly short and less than half the anteroposterior length of the pars cochlearis (
Figure 41), like
Albertocetus and unlike the longer and larger but shallower fossa in
Echovenator and
Cotylocara. Faint radiating ridges emanate from the margins of the fossa in CCNHM 104 and ChM PV 7677, and are more strongly developed in ChM PV 5022. The superior ridge lies lateral to the fossa but bears a saddle-shaped concavity on the dorsal margin in lateral view at about the level of the pars cochlearis (
Figure 41 and
Figure 42). The ridge rises dorsally anteriorly and posteriorly to the anterodorsal angle and broadly convex posterodorsal angle (=tegmen tympani of [
10,
94]).
The apertures for the cochlear and vestibular aqueducts are both small though the former is slightly smaller; they are equally sized in ChM PV 7677 (
Figure 41). The entire posterior side of the pars cochlearis faces posterodorsally. The posterior process is short with a rectangular, flat posterior bullar facet. Longitudinal ridges of the posterior bullar facet are absent in CCNHM 104, but in ChM PV 7677, there are two strong ridges on the left and one on the right posterior process (
Figure 40). An oval-shaped flat surface that articulates with the post-tympanic process is present posterolaterally on the periotic. In ChM PV 7677, this surface is somewhat rugose with dorsally oriented spurs. ChM PV 7677 also possesses an anterolaterally directed articular process, rising about 2.5 mm and 7–8 mm in length. A smaller tubercle is present instead in ChM PV 5022. An identical structure is present in
Albertocetus (CCNHM 303), but not described by Boessenecker et al. [
1]. During removal of the right periotic of CCNHM 303, the articular process remained embedded in the skull and could not be removed (Boessenecker, pers. obs.), similar to removing periotics in crania of extant
Platanista (R.E. Fordyce, pers. comm.). A small pore-like posteroexternal foramen is present between the posterior process and posterodorsal angle (
Figure 43).
When in articulation with the skull, there is a narrow gap with the exoccipital formed by the deep fossa (
Figure 39) of Geisler et al. [
4]. In specimens where the periotic is free, the posterolateral edge of the periotic articulates tightly with the medial side of the post-tympanic ridge. The periotic otherwise only articulates along its lateral edge with the squamosal and along most of the anterior process (
Figure 45). The dorsal part of the superior process appears to not contact the squamosal (
Figure 45), which is deeply excavated in CCNHM 104 and ChM PV 7677. However, the degree of contact in ontogenetically more mature specimens (ChM PV 5022) is unclear owing to adhering matrix. In CCNHM 1077, the elongate spur-like anterodorsal angle articulates tightly with the alisphenoid. The anterior process is complete, unlike CCNHM 104, and is transversely narrow and bladelike in ventral view. The lateral surface of the periotic appears to have had extensive contact with the squamosal and alisphenoid. This suggests that the periotic is likely much more tightly articulated with the basicranium in adult xenorophids than in later odontocetes (
Figure 45), and perhaps similar to the condition in basilosaurids. In medial view, the anterior process is rectangular and bears a deep tensor tympani groove at the junction with the pars cochlearis.
Tympanic Bulla: The tympanic bullae of CCNHM 168 are complete but still in articulation (
Figure 38); the bulla of CCNHM 104 is incomplete (missing the outer lip) but separate from the skull; the bullae of ChM PV 5022 (left) and CCNHM 1077 (both) are complete, and separated from the skull (
Figure 46,
Figure 47 and
Figure 48;
Table 9). The bulla is missing in ChM PV 7677 and the holotype. The tympanic bulla is similar to other xenorophids; it is proportionally quite large for an odontocete of this size (16–17% of bizygomatic width, vs. 8% in
Tursiops truncatus).
The involucrum is large, transversely wide, and dorsoventrally thickest posteriorly (
Figure 46 and
Figure 47). Several transverse creases are present on the anterior two-thirds of the involucrum. In CCNHM 104 and 1077, along the dorsal crest of the involucrum, the ridges separated by these ridges terminate into three–five small finger-like projections, chiefly along the anterior half of the involucrum (
Figure 47). Some creases at the anteroposterior midpoint of the involucrum are deeply incised and divide the involucrum into a deep posterior half and a shallow anterior half, giving the involucrum a step-like dorsal profile in medial view (e.g., ChM PV 5022;
Figure 46). However, other specimens, like CCNHM 104 and 1077, have a far less extremely ‘stepped’ margin (
Figure 46) than in
Albertocetus meffordorum,
Cotylocara, or
Echovenator.
In medial view, the involucrum is nearly bilobate, owing to the aforementioned transverse crease and a shallowly concave ventral margin of the involucrum (
Figure 46); however, this condition is not nearly as extreme as in other xenorophids (
Albertocetus,
Echovenator,
Cotylocara). In CCNHM 1077, the lateral margin is not nearly so excavated, giving the involucrum a less bilobate shape than other specimens.
Recent studies on protocetid earbones have recognized additional structures—tuberosities 1–5 (T1–5) on the involucrum—that help identify isolated bullae [
95]. Subsequent studies of Basilosauridae and early Neoceti have, as of yet, not attempted to recognize or homologize these tuberosities. Owing to the archaic bullar morphology of
Xenorophus, we attempted to evaluate which tuberosities may be present. An anterodorsal prominence just posterior to the transverse crease on the involucrum corresponds to T1; rather than being separate from T5, positioned medial to t1 in protocetids, these two tuberosities seem confluent. A similar condition is present in the mysticete
Coronodon havensteini (CCNHM 108) and basilosaurid whales (e.g., CCNHM 167, cf.
Dorudon, Tupelo Bay Formation), but some basilosaurids possess discrete T1 and T5 separated by a shallow fovea (e.g., CCNHM 4003, Basilosauridae indet.). A deeper fovea, and possibly T1, is present in some Eomysticetidae (e.g., CCNHM 207). The loss of these tuberosities broadly separates Pelagiceti from the protocetids and reflects the reduced contact of the pars cochlearis of the periotic with the tympanic bulla [
95]. However, these observations suggest that some tuberosities persist into Pelagiceti and may prove fruitful for further studies of anatomical changes in the tympanoperiotic across the archaeocete–neocete transition.
A low, somewhat rugose ventral crest is present along the ventromedial edge of the involucrum (
Figure 46); it is more strongly developed in ontogenetically older specimens, like CCNHM 1077 (relative to CCNHM 104). In CCNHM 1077, it is clear and slightly rugose along the posterior two-thirds of the bulla. This crest merges posteriorly with the well-developed horizontal crest on the posterior side of the medial lobe of the bulla. The interprominential notch is shallow and interrupted by the horizontal crest. A well-developed circular elliptical foramen is present dorsally; it is small, perhaps 4–5 mm in length. The inner posterior pedicle is swollen and subspherical; the outer posterior pedicle is much smaller and developed as a delicate (and broken) transverse crest terminating posteriorly into a low tubercle. In dorsal and ventral view, the bulla is “pear”-shaped and very wide (
Figure 47); it widens slightly posteriorly and the greatest width is about 70% of its length. It narrows sharply just anterior to the shallow lateral furrow. The furrow is positioned within the anterior half of the bulla, making the posterior lobe slightly longer than the anterior lobe (
Figure 47). The tympanic cavity is crescent-shaped and transversely narrow; it is divided into anterior and posterior compartments by a low transverse ridge that is positioned far anteriorly, about two-thirds of the distance from the posterior margin (
Figure 47). The outer lip is preserved well in CCNHM 1077 and has a subrectangular outline in medial view. The sigmoid process is well preserved in this specimen and bears a ventral cleft; it nearly completely overlaps the low conical process, expressed as a slight thickening of the outer lip. The ventral surface is gently convex with an anteroposteriorly short and shallow median furrow on the posterior quarter of the bulla; a shallow median pit is present further anteriorly and separated from the furrow by a low ridge. The main ridge is low and evenly convex in cross-section. The involucral ridge (
sensu [
96]) is also low but includes the ventromedial crest.
Upper Dentition: The dental count of Xenorophus sloanii varies; most specimens (CCNHM 104, ChM 7677, USNM 11049) have 13 teeth (3Ix1Cx9PC), but one specimen (CCNHM 168) has 14 upper teeth (3Ix1Cx10PC), giving an upper dental formula of 3Ix1Cx9–10PC. The dental description is based chiefly on CCNHM 168 and 1077, owing to a lesser degree of tooth wear than in some specimens (e.g., CCNHM 104). CCNHM 1077 has an incomplete maxilla, and, for ease of description, is assumed to have the typical dental count and the posteriormost tooth is identified as PC9.
All specimens with well-preserved palates (holotype, CCNHM 104, 168) have asymmetry in tooth positions, generally with the right teeth shifted anteriorly by a few millimeters to nearly a centimeter relative to the teeth on the left (
Figure 49). The posteriormost three–four cheek teeth positions (PC6–9, and PC7–10 in CCNHM 168) and the canines and incisors have the least asymmetry and deviations generally under 3 mm. The anterior cheek teeth (PC1–5) have the most extreme asymmetry, with some teeth shifted as much as 9 mm relative to the position of the opposite side (PC4, CCNHM 168). In CCNHM 104, the left PC4–5 are instead shifted 3–4.5 mm further anterior relative to the right PC4–5; PC6–9 however retain left teeth shifted anterior to those on the left by 0.7–3.6 mm. Anteroposterior dental shifts peak in magnitude around PC4–5, and decrease in magnitude anteriorly and posteriorly.
The upper incisor crowns (I2–3) are conical, distally curved, bear low carinae, and are nearly circular in cross section (
Figure 50,
Figure 51,
Figure 52 and
Figure 53;
Table 4). The labial enamel is smooth; lingually, there are discontinuous striae. The I1 is not preserved in any specimen. The canine is similar to I3 but has discontinuous lingual striae basally, which transition into continuous longitudinal fluting apically.
PC1–2 are similar to the C1, but incipient labial striae are more evident (
Figure 50,
Figure 51 and
Figure 52;
Table 4). PC1–3 are all single-rooted, though the roots of PC2–3 are more inflated. PC2–3 possess crowns that are less conical and, instead, are slightly triangular with a lenticular cross-section rather than round, with smooth and more sharply defined mesial and distal carina. PC4 has a single but bilobate root with labial and lingual sulci; it also bears a crown similar to PC2–3, but with an incipient lingual cingulum.
PC5–6 have triangular crowns with transversely narrower, lenticular cross-sections; they bear a posterolingual bulge and an incipient labial cingulum (
Figure 50,
Figure 51 and
Figure 52;
Table 4). The roots are also bilobate and incompletely separated but are successively more widely divided than in PC4. PC7–8 have strong and dorsally arched lingual cingula and a well-developed nodular labial cingulum, and 3–4 min accessory cusps on the distal carina (
Table 4). Mesial cusps are mostly worn away in CCNHM 168, but, in ChM PV 7677, two–three are present on PC5–9. In PC9 of CCNHM 104, at least one mesial accessory cusp was present. PC9 is absolutely smaller than PC8 (
Table 4), and is single-rooted in the holotype (USNM 11049) and double-rooted in others (CCNHM 104, 1077). Both PC7 and 8 are clearly double-rooted. The PC9 alveolus in CCNHM 168 seems to be larger than PC 10, perhaps suggesting that PC10 is the homologous tooth position of PC 9 in other specimens. The posterior postcanines are more completely preserved in CCNHM 1077, likely corresponding to PC6–9. In this specimen, all are strongly double-rooted with thick cementum (2–3.3 mm thick in upper postcanines; 2–3 mm thick in lower postcanines) and crowns that are triangular and roughly equidimensional. Deep embrasure pits are present within the diastemata between postcanine teeth along the middle of the toothrow. PC6–9 bear one–two mesial denticles and two–four distal denticles. The mesial carina is mostly obliterated by large wear facets on PC6, 8, and 9; the distal carina is unworn, and the apex of the crown is unworn in PC7–9; in PC6, the mesial wear facet extends all the way to the apex. The labial cingulum is irregular and bears a nodular crest; the basal two-thirds of the crown exhibits rugose enamel that becomes smoother apically. The labial surface is similar in being more rugose basally but, overall, is more smooth than the lingual surface. PC9 is double-rooted and the crown is similar to PC8 in CCNHM 104, while the alveolus of PC10 in CCNHM 168 is distinctly smaller than the preceding tooth (PC9); the PC10 alveolus is double-rooted in CCNHM 168.
Mandible: The mandible is long and the horizontal ramus is dorsoventrally shallow for most of its length; anteriorly, the mandible gradually shallows towards the anterior tip (
Figure 53,
Figure 54,
Figure 55,
Figure 56 and
Figure 57;
Table 5). The horizontal ramus is twisted about its longitudinal axis so that the posterior half is facing slightly dorsolaterally rather than laterally. The symphysis is long (220 mm, ~30% of mandibular length); anteriorly, the symphyseal surface is faintly rugose with anteroposteriorly directed striations and transitions posteriorly into a ventrally placed longitudinal furrow (
Figure 55). Within the symphyseal region, the alveoli are set about 10 mm from the medial edge and positioned dorsolaterally (
Figure 56). Laterally positioned shallow embrasure pits are present between I1-PC1 and transition into much deeper pits aligned with the toothrow and between PC2–5, which are positioned along the dorsal edge of the mandible (
Figure 54,
Figure 56 and
Figure 57). These transition into shallow embrasure pits positioned within the toothrow between PC6–7; pits are not developed between PC8–9 in CCNHM 168. However, in CCNHM 168 and 1077, deeply excavated embrasure pits are present between all of the postcanines, and, as preserved in articulation, the upper teeth were aligned with the lower tooth row and the upper postcanine crowns deep into the mandibular embrasure pits (
Figure 58 and
Figure 59).
Most specimens of
Xenorophus sloanii lack mandibles found in articulation, except for CCNHM 1077. Despite being incomplete, the mandible was found in articulation with the skull and informs the manner in which the teeth occlude (
Figure 59). The upper and lower cheek teeth are vertically implanted and are anteroposteriorly aligned, unlike the situation in basilosaurids, where the lower cheek teeth fit lingually to the uppers. In CCNHM 1077, the upper teeth and lower teeth fit in between each other, the crown apices fitting into deep embrasure pits aligned with the toothrow, rather than the uppers being labially offset, as in basilosaurids. The teeth fit so deeply into these pits that, as fossilized, the base of the crown of the upper teeth actually lies a few mm ventral to the base of the enamel crown of the adjacent lower teeth. When occluded, the tooth crowns do not contact their antagonists but instead fit completely within the embrasure pit and, therefore, the crowns likely only contacted gingiva and possibly cementum of the antagonistic teeth.
Six mental foramina (2–3 mm wide) are present on the mandible; they have lanceolate outlines and transition into elongate sulci (15–20 mm long). The posteriormost mental foramina open posteriorly, and the remainder open anteriorly. Mental foramina are positioned below PC2, 4, 5, 6, 8, and 9; anteriorly, they are positioned on the dorsoventral middle of the mandible and become more dorsally elevated posteriorly. Some additional foramina are present near the ventral margin ventral to I1–2.
Posterior to PC5–6, the mandible gradually deepens posteriorly and is expanded ventrally; the lateral surface is broadly convex. The ventral margin has a sinuous outline, and the dorsal margin is concave posteriorly where it leads to the coronoid (
Figure 54 and
Figure 55). The coronoid process of CCNHM 168 is triangular with a low sloping anterior margin and a steep posterior margin. In CCNH 1077, the coronoid process is tongue-shaped rather than triangular (
Figure 54 and
Figure 55). The anterior edge of the coronoid is transversely expanded with a narrow flange for insertion of the temporalis. The masseteric fossa is broadly concave but poorly delimited; it seems to have occupied the dorsal half of the mandible (
Figure 54). Medially, there is a shallow anteroposterior trough on the coronoid, dorsal to the cavernous mandibular foramen. The margins are not preserved, but the mandibular foramen was likely about two-thirds of the dorsoventral depth of the mandible at the level of the coronoid. The ‘pan bone’ is well-developed, and the lateral wall of the mandible here is 2.9–3.5 mm thick.
The mandibular condyle is deeply excavated into a deep medial pit by the mandibular fossa (
Figure 55). The articular surface of the condyle is gently convex and D-shaped in articular view with a straight medial margin. The toothrow is posteriorly slightly elevated above the level of the condyle (e.g., PC9 is entirely dorsal to the dorsal edge of the condyle;
Figure 54).
Lower Dentition: The i1 is missing but i2–3 are similar in morphology to c1 and pc1: conical, slightly posteriorly recurved crowns with smooth (faintly striated) labial enamel and slightly stronger longitudinal striations lingually. Slight carinae are present on pc1 but not incisors or canine; i2–pc1 all lack lingual and labial cingulum. i1–pc1 roots are all anteriorly inclined (
Figure 52,
Figure 54,
Figure 55,
Figure 56 and
Figure 60;
Table 6).
The pc2 is missing but the alveolus accommodated a single oval root, slightly more anteroposterior elongate than pc1. All postcanines posterior to pc3 are double rooted; pc4–9 all bear a dorsally arched lingual margin of the crown (
Figure 52 and
Figure 60;
Table 6). The pc3 has a transversely narrow crown with weakly developed but rugose labial cingulum; it is triangular in lingual view and bears strongly developed mesial and distal carinae with some minute distal denticles. Anterior and mid postcanines (pc2–6) all share incipient, nodular lingual cingulum; in CCNHM 104, the cingulum is somewhat more ridge-like anteriorly in pc2. Lower pc4–5 are triangular like pc3 yet anteroposteriorly broader; they bear incipient labial cingula and strongly developed nodular lingual cingulum. The basal half of the lingual surface of the crown bears apicobasal ridges which are nodular and discontinuous, and become diffuse apically. The cingulum is 1.5–3 mm deep (apicobasally). Four distal accessory cusps are present; the mesial carina is smooth and lacks accessory cusps.
Lower pc6–7 are similar to pc4–5 but have rectangular rather than oval-shaped crowns in apical view (
Figure 52 and
Figure 60;
Table 6). In CCNHM 168 these teeth also have stronger lingual cingula with a nearly continuous crest (anteriorly more strong in CCNHM 104), and stronger labial cingula (
Figure 60). The crowns are anteroposteriorly longer than pc4–5. PC7 has one–two mesial denticles and at least four distal denticles. PC6–7 have larger distal denticles than in pc4–5. PC8 is nearly identical to pc7 but is anteroposteriorly shorter with a more poorly developed labial cingulum; it bears two mesial denticles (
Table 6). PC 9 is smaller yet and molariform with an equilateral crown, and is distally inclined; in occlusal view, the crown is subtriangular. It bears two distal denticles, lacks a labial cingulum, and bears smooth enamel on most of the crown. The mesial carina is convex. In CCNHM 1077, PC 6–9 are preserved (
Figure 60;
Table 6); all are double-rooted with triangular, equidimensional crowns similar to that of their upper counterparts. All possess thick cementum (2–3 mm thick), two–three minute mesial cusps, four distal cusps, and a weak labial cingulum consisting of rugose enamel fading apically into striated enamel. Lingually, the basal two-thirds of the enamel are strongly rugose with two–five nodules present on each apicobasal ridge. The lingual cingulum is strongly developed. Wear facets are present basally on the posterior three teeth, with a large facet removing 3–5 mm of the distal carina on PC8. In CCNHM 168, the posteriormost tooth, pc9, is triangular in occlusal view with a truncated/flattened anterior margin; it is anteroposteriorly shorter and stubbier than other teeth. The lingual cingulum is contiguous with the mesial carina and bears small cusps. A nearly identical (but isolated) tooth, presumed to represent the posteriormost lower postcanine, is preserved in ChM PV 7677. In CCNHM 104 and 1077, the posteriormost lower tooth (pc8) is, instead, nearly identical to the preceding tooth (pc7).
The termination of the toothrow is asymmetrical in CCNHM 168 (
Figure 56). In the right mandible, PC9 is positioned approximately 11 mm further anterior than in the left mandible (measuring from the condyle to the anterior margin of PC9).
Tooth Wear and Dental Erosion: Juvenile and subadult specimens, such as the holotype (USNM 11049), CCNHM 5995, and ChM PV 7677, have no visible tooth wear (
Figure 52; [
5]: Figure 5). In adult specimen CCNHM 168, there is light tooth mesial and distal wear on the upper teeth (
Figure 50). On the anterior teeth (I1–PC4), there is no apical, mesial, or distal wear. PC5 is the anteriormost tooth locus with wear facets; it bears lanceolate mesial facets along the middle third of the mesial carinae, and a long facet on the distal carina on the left PC5, removing three-quarters of the carina. The right PC5 has unworn distal carina. PC6 has some mesial wear basally and a long, narrow distal wear facet removing nearly the entire distal carina. Right PC6 also bears a minute apical wear facet. PC7 is missing nearly the entire mesial carina on both sides, though, on the left PC7, there is a narrow facet, but on the right, there is a large triangular facet that extends down onto the mesial root. In PC8, there is an irregular posterolingually placed wear facet with a tongue extending apically to the cingulum and tongues along the ridges leading to some (but not all) of the accessory cusps. PC8 has similar wear to PC7 but is more extremely worn, with longer tongues developed apical to the cingulum. In PC7–8, the mesial carina may be worn away, but the distal carina retains much of a functional cutting edge as the wear facet is more lingually positioned. In the lower dentition, the i1–pc3 are unworn; the anteriormost wear facet is a small lanceolate facet on the distal carina of pc4. A mesial facet is not developed on left pc5, but the basal three-quarters of the distal carina is worn away by a lanceolate facet. In contrast, right pc5 has two-thirds of the mesial carina worn into a lanceolate wear facet and a small distal facet on the second and third distal accessory cusps. On pc6, there are paired apicobasal wear facets on the ridges leading to the mesial carina and a minute facet on the carina more apically; the distal carina is worn into a lanceolate wear facet along three-quarters of its length along with circular wear facets on the apicalmost two accessory cusps. Large mesial and distal wear facets removing three-quarters of the carinae are developed on pc7–8, but the apical 4–6 mm is intact, along with the principal cusp. A similar mesial oval-shaped mesial wear facet is present on pc9, though this facet is positioned entirely labial to the mesial carina, which is intact. Discontinuous oval-shaped facets are present along the distal edge of the distal accessory cusps in the right pc9; similar but much smaller facets are present in left pc9. Right pc9 also bears a small facet on the distal edge of the principal cusp. Tooth wear in the posterior postcanines (PC6–9, pc6–9) in CCNHM 1077 is less extreme than in CCNHM 168, consisting of only light posterolingual wear of rugose ridges, small mesial wear facets on some teeth (PC6), and large shear facets developed only on a few teeth (distally on pc8, mesially and distally on PC8, and mesially on PC9); apical wear is absent on these teeth (
Figure 59).
In contrast, tooth wear in subadult specimen CCNHM 104 is substantially more extreme than in all other specimens (
Figure 51 and
Figure 60). All preserved anterior teeth have extreme wear of the crowns. I2 preserves the lingual base of the crown only, and a large apico-labial wear facet is present. The crowns of left PC1–2 are completely missing, and these teeth are reduced to lozenge-shaped roots protruding from the alveolar margin with evenly rounded apices. PC3–4 are the least worn teeth in the dentition; they bear large apical wear facets with perhaps only 3–4 mm of the principal cusp missing, small apically positioned mesial wear facets along the carina, and small basal distal wear facets along the carina; the distal wear facet in right PC4 is much larger, occupying virtually the entire distal carina and nearly connecting with the apical wear facet. Right PC5 has a small mesial wear facet along the basal two-thirds of the mesial carina and a large apical wear facet that merges evenly with a continuous distal wear facet that has removed the entire distal carina. In right PC6–7 and left PC6 and PC8, the mesial, apical, and distal cusps are large and continuous, resulting in the loss of the principal and all accessory cusps and carinae. An exception is in left PC8, where a small remnant of carina is present between the base of the principal cusp and apicalmost distal accessory cusp. Left PC9 bears only minute apical wear facets on the two mesial accessory cusps, but a large and continuous wear facet apically and distally. In PC7–8, small facets are present where the enamel on rugose ridges has been removed from wear on the posterolingual surface. PC7–8 have large shear facets that cut basally into the root at least 8–10 mm basal to the crown base; these facets are continuous with the mesial wear facets. A similar, but less basally extensive distal wear facet is present on left PC8.
Evidence of dental erosion is also present in CCNHM 104 (
Figure 51 and
Figure 60). Many teeth (left I2, PC3–4, PC6–9; right PC4–7; left pc2, pc6, right pc6–7) exhibit slight narrowing of the crown cervix (sensu [
97]). This is most pronounced anteriorly, such as left I2 and left PC3–4, where the posterolingual portion of the crown cervix exhibits a transverse trough; in labial and lingual view the distal margin of the tooth is concave just basal to the crown, and the enamel appears slightly undercut in left PC3–4. In left I2, this trough nearly encircles the entire crown base. An isolated incisor tooth, either an upper right or lower left incisor, also has a narrowed crown cervix, albeit not as extreme as in left I2. This isolated tooth (along with left I2, and to a lesser extent, other teeth) bears an irregular base of the enamel crown with a clear ledge-like morphology at the edge of the enamel.
Stylohyal: One rod-like element in CCNHM 168 represents the ?left stylohyal (
Figure 61). It is slightly curved ventrally and bears an oval cross-section with expanded distal and proximal ends; the large end articulates with the basihyal. The distal end has a lenticular pitted facet indicating a cartilaginous joint with the basihyal; the smaller (distal) end is also pitted but less extremely so. Scars are present as sharp ridges along the medial edge and proximally and distally on the lateral edge, identifying this as the left stylohyal. The medial crest on the proximal end is developed into two ridges separated by a crest—small tubercles of bone are developed along this crest.
Cervical Vertebrae: Complete cervical series are preserved in CCNHM 168, CCNHM 1077, and ChM PV 5022; CCNHM 104 preserves an Atlas and three mid-cervicals (C3–C5), and an isolated C3 or C4 is present in ChM PV 7677 (
Figure 62,
Figure 63,
Figure 64,
Figure 65 and
Figure 66;
Table 10,
Table 11,
Table 12 and
Table 13). The atlas is similar to
Albertocetus meffordorum but 135% larger (
Figure 61). The atlas of CCNHM 168 (but not CCNHM 1077) is asymmetrical and slightly wedge-shaped in dorsal view (
Figure 66A), being anteroposteriorly longer on the left (68 mm) than on the right (63 mm). The anterior articular surfaces have an oval outline, and are about 75% as tall as they are wide (
Figure 62). In CCNHM 168, these surfaces are not separated but, in CCNHM 1077, a clearly incised sagittal furrow separates these facets. The ventral part of the body is dorsoventrally thick, almost as thick as the transverse width of the condylar fossa. The hypapophysis is prominent, knob-like, and posteroventrally projecting. A faint intercondylar notch is palpable ventrally on the anterior surface. The neural arch of the atlas is triangular in anterior view and bears a low, knob-like neural spine in CCNHM 104 and 168; it is somewhat more prominent in CCNHM 1077. The neural arch is pierced laterally by a nearly circular 10 mm wide lateral vertebral canal. The neural foramen is circular in anterior view (
Figure 62). The transverse process is developed as a subtriangular, posterolaterally deflected, and anteroposteriorly flattened flange with a single lateral apex in CCNHM 168; a smaller secondary dorsal tubercle is positioned at the dorsal base of the process. In CCNHM 1077, the transverse process is more rectangular with equally sized dorsal and ventral processes. The transverse process is not perforated by a transverse foramen in any specimen. The posterior articular surface of the atlas is trilobate: a large, flat lateral facet and a transversely narrow but dorsoventrally deep median lobe that extends from the odontoid fossa to the posterior side of the hypapophysis; it is dorsoventrally constricted adjacent to the hypapophysis. In lateral view, the atlas is wedge-shaped and widens dorsally. Pathologies on the atlas of CCNHM 168 include pitted, rugose, and unusually spongy bone on the neural spine and hypapophysis and lipping on the lateral and medial edges of the left condylar fossa. In CCNHM 1077, the hypapophysis is longer and more finger-like; a few linear scrape marks attributable to shark scavenging are present on the dorsal edge of the right transverse process of CCNHM 168.
The axis (
Figure 63) has a trilobate atlantal articulation with oval lateral facets and a median anteroventrally facing facet on the ventral half of the low, conical odontoid process; this facet articulates with the posterior face of the hypapophysis (
Figure 63). The odontoid is somewhat more prominent in CCNHM 1077. The neural foramen is subtriangular with a dorsally convex ventral margin; it bears a median sagittal ridge on the centrum, extending from the posterior margin to the odontoid process. Ventrally, a low and indistinct hypapophysis is developed and a shallow fossa is present laterally on each side. The neural spine dorsally transitions into an anteroposteriorly flattened plate; all specimens (CCNHM 168, 1077) possess a neural spine with a bifurcated apex (resembling a heart in outline) like
Albertocetus and
Echovenator. The anterior tip of the neural spine is subtriangular in lateral view and extends anteroventrally, terminating in a flat facet that articulates tightly with a lunate facet on the posterior side of the atlas neural arch; this facet is slightly wider in CCNHM 1077 than in CCNHM 168. In both CCNHM 168 and 1077, the neural spine is rotated 2° (CCNHM 168) and 9.7° (CCNHM 1077) to the right (
Figure 66B). The postzygapophyses are visible in anterior view, as they project dorsolaterally above the lamina. The posterior epiphysis is nearly oval-shaped with a ventral apex for the hypapophysis, and is completely fused in CCNHM 168 yet bears a faint notochordal pit. The transverse processes are short, posterolaterally directed, and rectangular in anterior view, but taper in dorsal view; in CCNHM 168, the right transverse process is slightly larger and longer than the left. In CCNHM 1077, the transverse process extends further posterolaterally. A shallow fossa is present between the centrum and transverse process.
C3 and C4 are similar and possess a nearly circular centrum bearing a ventrally triangular apex formed by a well-developed hypapophysis, larger than that in the axis (
Figure 64). The neural foramen is triangular with a convex ventral margin, giving it a lunate profile. The pedicle is stout and leads to a straight, dorsomedially sloping lamina topped by a low (15 mm high) neural spine. Large lateral vertebral canals are present and encircled by a narrow, splint-like diapophysis and a two-pronged parapophysis with a tongue-shaped dorsal part and shorter ventral part. The diapophysis and parapophysis are fused laterally in C3, completely encircling the canal; they are, however, separated by a 2 mm gap in C4. The entire parapophysis is posteroventrally sloping. In CCNHM 1077, the C3 is similar but bears a smaller ventral parapophysis that projects more anteriorly.
C5 is similar in morphology to C4 but has shorter diapophyses (
Figure 65). C6 is distinguished by possessing elongate and robust parapophyses, that are rectangular in lateral view and distally become transversely swollen, and bears vascular pitting at its ventral apex. The left parapophysis is massive and anteroposteriorly longer than the right. The diapophysis is broken, but the lateral vertebral canal is not closed, as the dorsal parapophysis is developed as a low tubercle and does not appear to be broken. In CCNHM 1077, C5 also lacks a complete lateral bridge around the lateral vertebral canal but possesses longer dorsal and ventral parapophyses than CCNHM 168; these parapophyses also have enlarged tubercles at their apices. C6 lacks a hypapophysis. The centrum is anteroposteriorly thicker than C5. In CCNHM 1077, the C6 has a longer parapophysis that is anteroposteriorly broader distally and splayed more widely apart (60° in CCNHM 168, 90° in CCNHM 1077). In most specimens, the parapophyses are asymmetrical (
Figure 66C–H), with the left parapophysis plunging more closely to vertical (e.g., 39° on left and 47° on right in CCNHM 168, and 40° on left and 49° on right in ChM PV 5022) and the distal end of the left parapophysis (51.9 mm) being 31.6% anteroposteriorly longer than the right (39.2 mm) in CCNHM 168 and approximately 30–24% in CCNHM 1077 (52.1 mm vs. ~40–42 mm, accounting for breakage). In CCNHM 1077, the parapophyses are of asymmetrical size but nearly symmetrical in orientation (47° on left vs. 50° on right).
C7 has a centrum approximately as long as C6 but lacks a parapophysis; it is reduced to a low tubercle on the left side, and is completely absent on the right. The diapophysis (=transverse process) is larger and elevated to the level of the top of the centrum. The transverse process is subhorizontal, slightly ventrally inclined, and dorsoventrally thin. The pedicle is slightly longer than in C6 and the lamina is slightly shorter. The pre- and postzygapophyses are positioned further laterally. The centrum is more oval and dorsoventrally shallow than C6. The neural foramen of C7 is shallowly triangular. The arch bears dorsoventrally thin laminae and a 40 mm tall spine that is rectangular in lateral view. The apex of the spine is rotated 10° clockwise so that the left side faces slightly anterolaterally and the right faces slightly posterolaterally. The apex widens transversely relative to the spine and bears a rugose apical facet, presumably for the nuchal ligament. When placed in articulation, the cervical vertebrae of CCNHM 168 (
Figure 65) indicate that the neutral posture of the neck was dorsally flexed.
Thoracic Vertebrae: T1 is preserved in CCNHM 168 and 1077, and differs from C7 in having knob-like distal ends of the transverse processes, and flat costal facets positioned low and anterolaterally on the centrum (
Figure 67;
Table 10,
Table 11,
Table 12 and
Table 13). T1 in CCNHM 1077 has a spine taller than C5–6, a triangular neural foramen, an oval-shaped centrum with a notochordal pit, shallow prezygapophyseal fossae, a knob-like transverse process with a flat lateral facet, and bears pits on the lateral side of the centrum. T2 and T3 are similar to T1 but possess minute capitular facets dorsolateral on the edge of the centrum; T4 also has more dorsally positioned transverse processes. An isolated anterior thoracic of uncertain position, but likely corresponding to T2–T4, preserves the only complete neural spine for the thoracic series. The arch delineates an oval neural foramen. The spine is tall (11 cm), is subrectangular in lateral view but narrows (in anteroposterior length) dorsally. The spine is slightly curved anteriorly; there is a tear-drop-shaped (posteriorly narrowing) rugose facet at the apex, presumably an attachment for the nuchal ligament.
T4–T6 of CCNHM 1077 are similar to the T2–3 and increase sequentially in centrum length posteriorly (
Figure 67;
Table 12). The anterior face becomes triangular and projects ventrally further than the posterior face. The anterior and posterior faces are at a 5° angle rather than parallel, giving the vertebra a slight wedge shape and widening ventrally. This indicates that the vertebral column was dorsally concave.
T7–T9 of CCNHM 1077 are similar and bear successively large capitular facets along the lateral edges of the centrum (
Figure 67;
Table 12). In T7, the posterior facet is larger than the anterior face, and, in T8, the posterior facet is only slightly larger. In T9, the anterior facet is large and there is no posterior facet; instead, there is a knob-like costal articular facet at the level of the dorsal margin of the centrum. T9 also bears a nearly circular neural foramen.
T10 of CCNHM 1077 and ChM PV 5022 resembles lumbar vertebrae but possesses short, ventrolaterally oriented transverse processes with flat capitular facets for the last rib (
Figure 67;
Table 12). Given that ChM PV 5022 and CCNHM 1077 only possess a single vertebra like this, perhaps
Xenorophus only possessed a single “hanging” rib versus three in
Ankylorhiza. Accordingly, the early toothed mysticete
Coronodon appears to only have a single “hanging” rib [
46].
Lumbar Vertebrae: In CCNHM 1077, six lumbars are preserved, corresponding to L1–4, L9, and L10 (
Figure 68;
Table 12); another nearly complete series is preserved in ChM PV 5022 (
Table 13). The lumbars continuously increase in size posteriorly, continuing this gradual trend from the posterior thoracics. Lumbars are all quite similar in proportion and share a round centrum, small subtriangular neural foramen (decreasing in size posteriorly), ventrolaterally projecting transverse processes, a ventral median ridge, and a high neural spine with rectangular outline in lateral view. A nearly complete series of lumbars (ten) are preserved and follow a similar trend. The anterior lumbars, chiefly L1–3, possess relatively smaller subcircular epiphyses with a truncated dorsal margin; L4 has a larger centrum with a slight dorsal truncation, and L5–L10 have large circular centra. L1–4 have prezygapophyses that are widely projecting, low, and set astride a triangular but equidimensional neural canal; L5–L10 have smaller, more medially positioned, and dorsally higher prezygapophyses adjacent to increasingly deeper and transversely narrower neural canals (
Figure 68). The neural spines of L5 and L8 are curved slightly to the left side, as in all three lumbar vertebrae of CCNHM 168; the L1 of ChM PV 5022 and the T10 of CCNHM 1077 are similarly slightly, but less extremely, deflected to the left. In all lumbars with complete spines in CCNHM 158, 1077, and ChM PV 5022, the neural spines deviate to the left by 5.5–7.5°. The L10 of ChM PV 5022 bears low facets for the first chevron. Three lumbar vertebrae of uncertain position are preserved in CCNHM 104, including an anterior, mid, and posterior (L8/9) vertebra; all are consistent in morphology with ChM PV 5022 and CCNHM 1077 but are smaller in absolute size, similar to CCNHM 168 (
Figure 68). All lumbar vertebrae in CCNHM 1077 have longitudinal ventral keels; the keel is broadly rounded and restricted to the center of the centrum in L1–L3; a sharp and anteroposteriorly longer keel is present in L4 and L9; a somewhat more rounded keel is present in L10. In the anteriormost lumbar vertebra of CCNHM 168 (lumbar A), there is no ventral keel.
Caudal Vertebrae: Six caudal vertebrae are preserved in CCNHM 1077, and three are preserved in ChM PV 5022; this description focuses on CCNHM 1077 (
Figure 69;
Table 12 and
Table 13). Caudal 1 is similar to L10 but bears hemal facets for the chevron posteroventrally, and transverse processes with a sinuous outline in anterior view. Caudal A is an anterior caudal with well-developed anterior and posterior hemal processes, and a narrower neural foramen than Ca1. Caudal B is a mid-caudal and lacks a transverse process, instead possessing anterior and posterior tubercles in the same location but separated by a prominent vertical sinusoidal sulcus for the vertebral canal. The neural arch is absent and consists of two anterior and two posterior tubercles, separated by a median longitudinal fossa (
Figure 69). The hemal process consists of paired elongate anteroposterior ridges, which, on the left side, is separated by the lateral vertebral sulcus and, on the right, is perforated instead by an equidimensional foramen. Ca C and D are posterior caudals and increasingly anteroposteriorly flattened; both possess vertically perforating lateral vertebral canals and an anteroposterior sulcus at the homologous position to the transverse process (
Figure 69). Ca E has bony tubercles extending beyond the centrum articulation (dorsolateral/ventrolateral) and is also rectangular, indicating this is an anterior fluke vertebra. Three caudals, two anterior and one mid caudal, are preserved in ChM PV 5022; these are similar to those of CCNHM 1077. The mid caudal preserves widely flaring prezygapophyses and a dorsoventrally low oval-shaped neural canal. A single posterior caudal is preserved in CCNHM 104, and has a round outline, indicating a position within the peduncle region rather than within the fluke.
Ribs: Several ribs are preserved in CCNHM 168, including left and right R1, and seven nearly complete ribs from other uncertain positions (
Figure 70). R1 is the shortest and the most strongly bowed. Proximally, it bears a large triangular end with a deep notch between the capitulum and the tubercle, and a dorsally prominent tubercle. The entire rib is anteroposteriorly compressed. The distal end is transversely expanded.
Ra likely represents R2 or R3 and was probably longer than R1 but differs from it in having a lower tubercle that is not separated from the capitulum by a notch (
Figure 70). Rb is missing both ends but has a similarly sized and proportioned shaft to Ra. Rc is several positions posterior to these and has a large capitulum and small tubercle; the shaft has an oval cross-section. Re and Rf are similar but possess smaller tubercles than Rc; Rc, Re, and Rf all have a low secondary tubercle positioned about 5–6 cm distal to the primary tubercle (
Figure 70). Rf is about twice as long as R1 and bears a distal facet for the costal cartilage. Rd is similar in proportion to Rc-Rf, but the position is uncertain as it lacks both ends. Rg is long and straighter and is missing the proximal end, but, unlike Rf, the distal end tapers and is transversely flat, indicating it is a posterior rib. Proximal fragments of at least eleven ribs are preserved in CCNHM 1077, including anterior ribs with clear capitula and tubercles and posterior ribs with large capitula and small or absent tubercles. Six mid-thoracic to posterior-thoracic ribs are preserved in ChM PV 5022; all have knob-like capitula. The mid-thoracic ribs have large tubercles positioned quite close to the capitulum and are more laterally bowed than the posterior ribs, which have low tubercles set far distally from the capitulum. One rib, perhaps the last rib, is nearly straight and entirely lacks a tubercle (
Figure 70).