Quantifying Abundance and Mapping Distribution of Loggerhead Turtles in the Mediterranean Sea Using Aerial Surveys: Implications for Conservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area, Survey Design and Data Collection
2.2. Abundance Estimates
2.3. Turtles Usage Areas
2.4. Turtle Potential Biological Removal (PBR)
3. Results
3.1. Sightings and Effort
3.2. Abundance Estimates
3.3. Turtles Usage Areas
3.4. Turtle Potential Biological Removal
4. Discussion
4.1. Loggerhead Turtle Abundance—Results and Limitations
4.2. Sea Turtle Usage Areas
4.3. Potential Biological Removal (PBR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnes, M.D.; Craigie, I.D.; Harrison, L.B.; Geldmann, J.; Collen, B.; Whitmee, S.; Balmford, A.; Burgess, N.D.; Brooks, T.; Hockings, M.; et al. Wildlife Population Trends in Protected Areas Predicted by National Socio-Economic Metrics and Body Size. Nat. Commun. 2016, 7, 12747. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, T.V.; Kovach, A.I. Spatially Explicit Abundance Estimation of a Rare Habitat Specialist: Implications for SECR Study Design. Ecosphere 2018, 9, e02217. [Google Scholar] [CrossRef]
- Burgar, J.M.; Stewart, F.E.C.; Volpe, J.P.; Fisher, J.T.; Burton, A.C. Estimating Density for Species Conservation: Comparing Camera Trap Spatial Count Models to Genetic Spatial Capture-Recapture Models. Glob. Ecol. Conserv. 2018, 15, e00411. [Google Scholar] [CrossRef]
- Wallace, B.P.; DiMatteo, A.D.; Bolten, A.B.; Chaloupka, M.Y.; Hutchinson, B.J.; Abreu-Grobois, F.A.; Mortimer, J.A.; Seminoff, J.A.; Amorocho, D.; Bjorndal, K.A.; et al. Global Conservation Priorities for Marine Turtles. PLoS ONE 2011, 6, e24510. [Google Scholar] [CrossRef]
- Lewison, R.L.; Freeman, S.A.; Crowder, L.B. Quantifying the Effects of Fisheries on Threatened Species: The Impact of Pelagic Longlines on Loggerhead and Leatherback Sea Turtles. Ecol. Lett. 2004, 7, 221–231. [Google Scholar] [CrossRef]
- Hamann, M.; Godfrey, M.; Seminoff, J.; Arthur, K.; Barata, P.; Bjorndal, K.; Bolten, A.; Broderick, A.; Campbell, L.; Carreras, C.; et al. Global Research Priorities for Sea Turtles: Informing Management and Conservation in the 21st Century. Endanger. Species Res. 2010, 11, 245–269. [Google Scholar] [CrossRef]
- Casale, P.; Broderick, A.; Camiñas, J.; Cardona, L.; Carreras, C.; Demetropoulos, A.; Fuller, W.; Godley, B.; Hochscheid, S.; Kaska, Y.; et al. Mediterranean Sea Turtles: Current Knowledge and Priorities for Conservation and Research. Endanger. Species Res. 2018, 36, 229–267. [Google Scholar] [CrossRef]
- Casale, P. Caretta caretta (Mediterranean subpopulation). The IUCN Red List of Threatened Species 2015: E.T83644804A83646294. 2015. Available online: https://www.iucnredlist.org/species/83644804/83646294 (accessed on 23 October 2023).
- Lucchetti, A.; Sala, A. An Overview of Loggerhead Sea Turtle (Caretta caretta) Bycatch and Technical Mitigation Measures in the Mediterranean Sea. Rev. Fish Biol. Fish. 2010, 20, 141–161. [Google Scholar] [CrossRef]
- Casale, P.; Heppell, S. How Much Sea Turtle Bycatch Is Too Much? A Stationary Age Distribution Model for Simulating Population Abundance and Potential Biological Removal in the Mediterranean. Endanger. Species Res. 2016, 29, 239–254. [Google Scholar] [CrossRef]
- Wallace, B.P.; Kot, C.Y.; DiMatteo, A.D.; Lee, T.; Crowder, L.B.; Lewison, R.L. Impacts of Fisheries Bycatch on Marine Turtle Populations Worldwide: Toward Conservation and Research Priorities. Ecosphere 2013, 4, art40. [Google Scholar] [CrossRef]
- Casale, P. Sea Turtle By-Catch in the Mediterranean: Sea Turtle by-Catch in the Mediterranean. Fish Fish. 2011, 12, 299–316. [Google Scholar] [CrossRef]
- Lucchetti, A.; Vasapollo, C.; Virgili, M. An Interview-Based Approach to Assess Sea Turtle Bycatch in Italian Waters. PeerJ 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti, A.; Vasapollo, C.; Virgili, M. Sea Turtles Bycatch in the Adriatic Sea Set Net Fisheries and Possible Hot-Spot Identification. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 1176–1185. [Google Scholar] [CrossRef]
- Clusa, M.; Carreras, C.; Pascual, M.; Gaughran, S.J.; Piovano, S.; Avolio, D.; Ollano, G.; Fernández, G.; Tomás, J.; Raga, J.A.; et al. Potential Bycatch Impact on Distinct Sea Turtle Populations Is Dependent on Fishing Ground Rather than Gear Type in the Mediterranean Sea. Mar. Biol. 2016, 163, 122. [Google Scholar] [CrossRef]
- Houghton, J.D.R. Potential Bycatch Impact on Distinct Sea Turtle Populations Is Dependent on Fishing Ground Rather than Gear Type in the Mediterranean Sea: Editorial Comment on the Feature Article by Clusa et al. (2016). Mar. Biol. 2016, 163, 121. [Google Scholar] [CrossRef]
- Rees, A.; Alfaro-Shigueto, J.; Barata, P.; Bjorndal, K.; Bolten, A.; Bourjea, J.; Broderick, A.; Campbell, L.; Cardona, L.; Carreras, C.; et al. Are We Working towards Global Research Priorities for Management and Conservation of Sea Turtles? Endanger. Species Res. 2016, 31, 337–382. [Google Scholar] [CrossRef]
- Braun McNeill, J.; Goodman Hall, A.; Richards, P. Trends in Fishery-Dependent Captures of Sea Turtles in a Western North Atlantic Foraging Region. Endanger. Species Res. 2018, 36, 315–324. [Google Scholar] [CrossRef]
- Troëng, S.; Chacón, D.; Dick, B. Possible Decline in Leatherback Turtle Dermochelys Coriacea Nesting along the Coast of Caribbean Central America. Oryx 2004, 38, 395–403. [Google Scholar] [CrossRef]
- Kaplan, I.C. A Risk Assessment for Pacific Leatherback Turtles (Dermochelys coriacea). Can. J. Fish. Aquat. Sci. 2005, 62, 1710–1719. [Google Scholar] [CrossRef]
- Broderick, A.C.; Frauenstein, R.; Glen, F.; Hays, G.C.; Jackson, A.L.; Pelembe, T.; Ruxton, G.D.; Godley, B.J. Are Green Turtles Globally Endangered? Glob. Ecol. Biogeogr. 2006, 15, 21–26. [Google Scholar] [CrossRef]
- Pfaller, J.B.; Bjorndal, K.A.; Chaloupka, M.; Williams, K.L.; Frick, M.G.; Bolten, A.B. Accounting for Imperfect Detection Is Critical for Inferring Marine Turtle Nesting Population Trends. PLoS ONE 2013, 8, e62326. [Google Scholar] [CrossRef] [PubMed]
- Broderick, A.C.; Godley, B.J.; Hays, G.C. Trophic Status Drives Interannual Variability in Nesting Numbers of Marine Turtles. Proc. R. Soc. Lond. B Biol. Sci. 2001, 268, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Esteban, N.; Mortimer, J.A.; Hays, G.C. How Numbers of Nesting Sea Turtles Can Be Overestimated by Nearly a Factor of Two. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162581. [Google Scholar] [CrossRef] [PubMed]
- Ceriani, S.A.; Casale, P.; Brost, M.; Leone, E.H.; Witherington, B.E. Conservation Implications of Sea Turtle Nesting Trends: Elusive Recovery of a Globally Important Loggerhead Population. Ecosphere 2019, 10, e02936. [Google Scholar] [CrossRef]
- Warden, M.L.; Haas, H.L.; Richards, P.M.; Rose, K.A.; Hatch, J.M. Monitoring Trends in Sea Turtle Populations: Walk or Fly? Endanger. Species Res. 2017, 34, 323–337. [Google Scholar] [CrossRef]
- Shoop, C.R.; Kenney, R.D. Seasonal Distributions and Abundances of Loggerhead and Leatherback Sea Turtles in Waters of the Northeastern United States. Herpetol. Monogr. 1992, 6, 43–67. [Google Scholar] [CrossRef]
- Epperly, S.P.; Braun, J.; Veishlow, A. Sea Turtles in North Carolina Waters. Conserv. Biol. 1995, 9, 384–394. [Google Scholar] [CrossRef]
- Witt, M.J.; Baert, B.; Broderick, A.C.; Formia, A.; Fretey, J.; Gibudi, A.; Mounguengui, G.A.M.; Moussounda, C.; Ngouessono, S.; Parnell, R.J.; et al. Aerial Surveying of the World’s Largest Leatherback Turtle Rookery: A More Effective Methodology for Large-Scale Monitoring. Biol. Conserv. 2009, 142, 1719–1727. [Google Scholar] [CrossRef]
- Bovery, C.M.; Wyneken, J. Seasonal Variation in Sea Turtle Density and Abundance in the Southeast Florida Current and Surrounding Waters. PLoS ONE 2015, 10, e0145980. [Google Scholar] [CrossRef]
- Eguchi, T.; McClatchie, S.; Wilson, C.; Benson, S.R.; LeRoux, R.A.; Seminoff, J.A. Loggerhead Turtles (Caretta caretta) in the California Current: Abundance, Distribution, and Anomalous Warming of the North Pacific. Front. Mar. Sci. 2018, 5, 452. [Google Scholar] [CrossRef]
- Barco, S.; Burt, M.; DiGiovanni, R.; Swingle, W.; Williard, A. Loggerhead Turtle Caretta caretta Density and Abundance in Chesapeake Bay and the Temperate Ocean Waters of the Southern Portion of the Mid-Atlantic Bight. Endanger. Species Res. 2018, 37, 269–287. [Google Scholar] [CrossRef]
- Panigada, S.; Lauriano, G.; Donovan, G.; Pierantonio, N.; Cañadas, A.; Vázquez, J.A.; Burt, L. Estimating Cetacean Density and Abundance in the Central and Western Mediterranean Sea through Aerial Surveys: Implications for Management. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 141, 41–58. [Google Scholar] [CrossRef]
- Panigada, S.; Lauriano, G.; Burt, L.; Pierantonio, N.; Donovan, G. Monitoring Winter and Summer Abundance of Cetaceans in the Pelagos Sanctuary (Northwestern Mediterranean Sea) Through Aerial Surveys. PLoS ONE 2011, 6, e22878. [Google Scholar] [CrossRef] [PubMed]
- Lauriano, G.; Panigada, S.; Casale, P.; Pierantonio, N.; Donovan, G. Aerial Survey Abundance Estimates of the Loggerhead Sea Turtle Caretta caretta in the Pelagos Sanctuary, Northwestern Mediterranean Sea. Mar. Ecol. Prog. Ser. 2011, 437, 291–302. [Google Scholar] [CrossRef]
- Lauriano, G.; Pierantonio, N.; Donovan, G.; Panigada, S. Abundance and Distribution of Tursiops truncatus in the Western Mediterranean Sea: An Assessment towards the Marine Strategy Framework Directive Requirements. Mar. Environ. Res. 2014, 100, 86–93. [Google Scholar] [CrossRef]
- Lauriano, G.; Pierantonio, N.; Kell, L.; Cañadas, A.; Donovan, G.; Panigada, S. Fishery-Independent Surface Abundance and Density Estimates of Swordfish (Xiphias gladius) from Aerial Surveys in the Central Mediterranean Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 141, 102–114. [Google Scholar] [CrossRef]
- Laran, S.; Pettex, E.; Authier, M.; Blanck, A.; David, L.; Dorémus, G.; Falchetto, H.; Monestiez, P.; Van Canneyt, O.; Ridoux, V. Seasonal Distribution and Abundance of Cetaceans within French Waters—Part I: The North-Western Mediterranean, Including the Pelagos Sanctuary. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 141, 20–30. [Google Scholar] [CrossRef]
- DiMatteo, A.; Cañadas, A.; Roberts, J.; Sparks, L.; Panigada, S.; Boisseau, O.; Moscrop, A.; Fortuna, C.M.; Lauriano, G.; Holcer, D.; et al. Basin-Wide Estimates of Loggerhead Turtle Abundance in the Mediterranean Sea Derived from Line Transect Surveys. Front. Mar. Sci. 2022, 9, 930412. [Google Scholar] [CrossRef]
- Gómez de Segura, A.; Tomás, J.; Pedraza, S.N.; Crespo, E.A.; Raga, J.A. Preliminary Patterns of Distribution and Abundance of Loggerhead Sea Turtles, Caretta caretta, around Columbretes Islands Marine Reserve, Spanish Mediterranean. Mar. Biol. 2003, 143, 817–823. [Google Scholar] [CrossRef]
- Cardona, L.; Revelles, M.; Carreras, C.; San Félix, M.; Gazo, M.; Aguilar, A. Western Mediterranean Immature Loggerhead Turtles: Habitat Use in Spring and Summer Assessed through Satellite Tracking and Aerial Surveys. Mar. Biol. 2005, 147, 583–591. [Google Scholar] [CrossRef]
- Thomas, L.; Buckland, S.T.; Rexstad, E.A.; Laake, J.L.; Strindberg, S.; Hedley, S.L.; Bishop, J.R.B.; Marques, T.A.; Burnham, K.P. Distance Software: Design and Analysis of Distance Sampling Surveys for Estimating Population Size. J. Appl. Ecol. 2010, 47, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Notarbartolo di Sciara, G.; Lauriano, G.; Pierantonio, N.; Cañadas, A.; Donovan, G.; Panigada, S. The Devil We Don’t Know: Investigating Habitat and Abundance of Endangered Giant Devil Rays in the North-Western Mediterranean Sea. PLoS ONE 2015, 10, e0141189. [Google Scholar] [CrossRef]
- Buckland, S.T.; Rexstad, E.A.; Marques, T.A.; Oedekoven, C.S. Distance Sampling: Methods and Applications; Methods in Statistical Ecology; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-19218-5. [Google Scholar]
- Pollock, K.H.; Marsh, H.D.; Lawler, I.R.; Alldredge, M.W. Estimating Animal Abundance in Heterogeneous Environments: An Application to Aerial Surveys for Dugongs. J. Wildl. Manag. 2006, 70, 255–262. [Google Scholar] [CrossRef]
- Fuentes, M.M.P.B.; Bell, I.; Hagihara, R.; Hamann, M.; Hazel, J.; Huth, A.; Seminoff, J.A.; Sobtzick, S.; Marsh, H. Improving In-Water Estimates of Marine Turtle Abundance by Adjusting Aerial Survey Counts for Perception and Availability Biases. J. Exp. Mar. Biol. Ecol. 2015, 471, 77–83. [Google Scholar] [CrossRef]
- Burt, M.L.; Borchers, D.L.; Jenkins, K.J.; Marques, T.A. Using Mark–Recapture Distance Sampling Methods on Line Transect Surveys. Methods Ecol. Evol. 2014, 5, 1180–1191. [Google Scholar] [CrossRef]
- Smolensky, N.L.; Fitzgerald, L.A. Population Variation in Dune-Dwelling Lizards in Response to Patch Size, Patch Quality, and Oil and Gas Development. Southwest. Nat. 2011, 56, 315–324. [Google Scholar] [CrossRef]
- Nykänen, M.; Jessopp, M.; Doyle, T.K.; Harman, L.A.; Cañadas, A.; Breen, P.; Hunt, W.; Mackey, M.; Cadhla, O.Ó.; Reid, D.; et al. Using Tagging Data and Aerial Surveys to Incorporate Availability Bias in the Abundance Estimation of Blue Sharks (Prionace glauca). PLoS ONE 2018, 13, e0203122. [Google Scholar] [CrossRef]
- Hays, G.C.; Webb, P.I.; Hayes, J.P.; Priede, I.G.; French, J. Satellite Tracking of a Loggerhead Turtle (Caretta caretta) in The Mediterranean. J. Mar. Biol. Assoc. U. K. 1991, 71, 743–746. [Google Scholar] [CrossRef]
- Bentivegna, F. Intra-Mediterranean Migrations of Loggerhead Sea Turtles (Caretta caretta) Monitored by Satellite Telemetry. Mar. Biol. 2002, 141, 795–800. [Google Scholar] [CrossRef]
- Godley, B.J.; Broderick, A.C.; Glen, F.; Hays, G.C. Post-Nesting Movements and Submergence Patterns of Loggerhead Marine Turtles in the Mediterranean Assessed by Satellite Tracking. J. Exp. Mar. Biol. Ecol. 2003, 287, 119–134. [Google Scholar] [CrossRef]
- Broderick, A.C.; Coyne, M.S.; Fuller, W.J.; Glen, F.; Godley, B.J. Fidelity and Over-Wintering of Sea Turtles. Proc. R. Soc. B Biol. Sci. 2007, 274, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Hochscheid, S.; Bentivegna, F.; Hamza, A.; Hays, G.C. When Surfacers Do Not Dive: Multiple Significance of Extended Surface Times in Marine Turtles. J. Exp. Biol. 2010, 213, 1328–1337. [Google Scholar] [CrossRef]
- Luschi, P.; Mencacci, R.; Vallini, C.; Ligas, A.; Lambardi, P.; Benvenuti, S. Long-Term Tracking of Adult Loggerhead Turtles (Caretta caretta) in the Mediterranean Sea. J. Herpetol. 2013, 47, 227–231. [Google Scholar] [CrossRef]
- Casale, P.; Mariani, P. The First ‘Lost Year’ of Mediterranean Sea Turtles: Dispersal Patterns Indicate Subregional Management Units for Conservation. Mar. Ecol. Prog. Ser. 2014, 498, 263–274. [Google Scholar] [CrossRef]
- Luschi, P.; Casale, P. Movement Patterns of Marine Turtles in the Mediterranean Sea: A Review. Ital. J. Zool. 2014, 81, 478–495. [Google Scholar] [CrossRef]
- Chimienti, M.; Blasi, M.F.; Hochscheid, S. Movement Patterns of Large Juvenile Loggerhead Turtles in the Mediterranean Sea: Ontogenetic Space Use in a Small Ocean Basin. Ecol. Evol. 2020, 10, 6978–6992. [Google Scholar] [CrossRef]
- Anderson, D.J. The Home Range: A New Nonparametric Estimation Technique. Ecology 1982, 63, 103–112. [Google Scholar] [CrossRef]
- Worton, B.J. Kernel Methods for Estimating the Utilization Distribution in Home-Range Studies. Ecology 1989, 70, 164–168. [Google Scholar] [CrossRef]
- Calenge, C. The Package “Adehabitat” for the R Software: A Tool for the Analysis of Space and Habitat Use by Animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- Wade, P.R. Calculating Limits to the Allowable Human-Caused Mortality of Cetaceans and Pinnipeds. Mar. Mammal Sci. 1998, 14, 1–37. [Google Scholar] [CrossRef]
- Barlow, J.; Swartz, S.L.; Eagle, T.; Wade, P.R. US Marine Mammal Stock Assessments: Guidelines for Preparation, Background, and a Summary of the 1995 Assessments; NOAA Technical Memorandum NMFS-OPR-95-6; U.S. Department of Commerce: Washington, DC, USA, 1995.
- Punt, A.E.; Siple, M.; Francis, T.B.; Hammond, P.S.; Heinemann, D.; Long, K.J.; Moore, J.E.; Sepúlveda, M.; Reeves, R.R.; Sigurðsson, G.M.; et al. Robustness of Potential Biological Removal to Monitoring, Environmental, and Management Uncertainties. ICES J. Mar. Sci. 2020, 77, 2491–2507. [Google Scholar] [CrossRef]
- Marcovaldi, M.Â.; Chaloupka, M. Conservation Status of the Loggerhead Sea Turtle in Brazil: An Encouraging Outlook. Endanger. Species Res. 2007, 3, 133–143. [Google Scholar] [CrossRef]
- Girard, F.; Girard, A.; Monsinjon, J.; Arcangeli, A.; Belda, E.; Cardona, L.; Casale, P.; Catteau, S.; David, L.; Dell’Amico, F.; et al. Toward a Common Approach for Assessing the Conservation Status of Marine Turtle Species within the European Marine Strategy Framework Directive. Front. Mar. Sci. 2022, 9, 790733. [Google Scholar] [CrossRef]
- Clusa, M.; Carreras, C.; Pascual, M.; Gaughran, S.J.; Piovano, S.; Giacoma, C.; Fernández, G.; Levy, Y.; Tomás, J.; Raga, J.A.; et al. Fine-Scale Distribution of Juvenile Atlantic and Mediterranean Loggerhead Turtles (Caretta caretta) in the Mediterranean Sea. Mar. Biol. 2014, 161, 509–519. [Google Scholar] [CrossRef]
- Clusa, M.; Carreras, C.; Pascual, M.; Demetropoulos, A.; Margaritoulis, D.; Rees, A.F.; Hamza, A.A.; Khalil, M.; Aureggi, M.; Levy, Y.; et al. Mitochondrial DNA Reveals Pleistocenic Colonisation of the Mediterranean by Loggerhead Turtles (Caretta caretta). J. Exp. Mar. Biol. Ecol. 2013, 439, 15–24. [Google Scholar] [CrossRef]
- Loisier, A.; Savelli, M.-P.; Arnal, V.; Claro, F.; Gambaiani, D.; Sénégas, J.B.; Cesarini, C.; Sacchi, J.; Miaud, C.; Montgelard, C. Genetic Composition, Origin and Conservation of Loggerhead Sea Turtles (Caretta caretta) Frequenting the French Mediterranean Coasts. Mar. Biol. 2021, 168, 52. [Google Scholar] [CrossRef]
- Bentivegna, F.; Valentino, F.; Falco, P.; Zambianchi, E.; Hochscheid, S. The Relationship between Loggerhead Turtle (Caretta caretta) Movement Patterns and Mediterranean Currents. Mar. Biol. 2007, 151, 1605–1614. [Google Scholar] [CrossRef]
- Zbinden, J.A.; Aebischer, A.; Margaritoulis, D.; Arlettaz, R. Insights into the Management of Sea Turtle Internesting Area through Satellite Telemetry. Biol. Conserv. 2007, 137, 157–162. [Google Scholar] [CrossRef]
- Bolten, A.B.; Martins, H.B.; Bjorndal, K.A.; Cocco, M.; Gerosa, G. Caretta caretta (Loggerhead) Pelagic Movement and Growth. Herpetol. Rev. 1992, 23, 116. [Google Scholar]
- Manzella, S.A.; Fontaine, C.T.; Schroeder, B. Loggerhead Seaturtle Travels from Padre Island, Texas, to the Mouth of the Adriatic Sea. Mar. Turt. Newsl. 1998, 42, 7. [Google Scholar]
- Musick, J.A.; Limpus, C.J. Habitat Utilization and Migration in Juvenile Sea Turtles. In The Biology of Sea Turtles; Musick, J.A., Lutz, P.L., Eds.; CRC Press: Boca Raton, FL, USA, 1996; pp. 137–155. ISBN 978-0-8493-8422-6. [Google Scholar]
- Heppell, S.; Crowder, L.; Crouse, D.; Epperly, S.; Frazer, N. Population Models for Atlantic Loggerheads: Past, Present, and Future. In Loggerhead Sea Turtles; Bolten, A.B., Witherington, B.E., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2003; pp. 255–273. ISBN 978-1-58834-136-5. [Google Scholar]
- Lambert, C.; Authier, M.; Dorémus, G.; Laran, S.; Panigada, S.; Spitz, J.; Van Canneyt, O.; Ridoux, V. Setting the Scene for Mediterranean Litterscape Management: The First Basin-Scale Quantification and Mapping of Floating Marine Debris. Environ. Pollut. 2020, 263, 114430. [Google Scholar] [CrossRef] [PubMed]
- Casale, P.; Mazaris, A.D.; Freggi, D.; Vallini, C.; Argano, R. Growth Rates and Age at Adult Size of Loggerhead Sea Turtles (Caretta caretta) in the Mediterranean Sea, Estimated through Capture-Mark-Recapture Records. Sci. Mar. 2009, 73, 589–595. [Google Scholar] [CrossRef]
- Guarino, F.M.; Di Nocera, F.; Pollaro, F.; Galiero, G.; Iaccarino, D.; Iovino, D.; Mezzasalma, M.; Petraccioli, A.; Odierna, G.; Maio, N. Skeletochronology, Age at Maturity and Cause of Mortality of Loggerhead Sea Turtles Caretta caretta Stranded along the Beaches of Campania (South-Western Italy, Western Mediterranean Sea). Herpetozoa 2020, 33, 39–51. [Google Scholar] [CrossRef]
- Minnett, P.J.; Alvera-Azcárate, A.; Chin, T.M.; Corlett, G.K.; Gentemann, C.L.; Karagali, I.; Li, X.; Marsouin, A.; Marullo, S.; Maturi, E.; et al. Half a Century of Satellite Remote Sensing of Sea-Surface Temperature. Remote Sens. Environ. 2019, 233, 111366. [Google Scholar] [CrossRef]
- Coles, W.C.; Musick, J.A. Satellite Sea Surface Temperature Analysis and Correlation with Sea Turtle Distribution off North Carolina. Copeia 2000, 2000, 551–554. [Google Scholar] [CrossRef]
- Amante, C.; Eakins, B.W. ETOPO1 Global Relief Model Converted to PanMap Layer Format. NOAA Tech. Memorandum NESDIS 2009, 24, 1–19. [Google Scholar] [CrossRef]
- Carreras, C.; Pascual, M.; Tomás, J.; Marco, A.; Hochscheid, S.; Castillo, J.J.; Gozalbes, P.; Parga, M.; Piovano, S.; Cardona, L. Sporadic Nesting Reveals Long Distance Colonisation in the Philopatric Loggerhead Sea Turtle (Caretta caretta). Sci. Rep. 2018, 8, 1435. [Google Scholar] [CrossRef]
- Katsiyiannis, P. Discovery of the First Feeding Area for Adult and Juvenile Green Turtles and Loggerhead Turtles in Greece. Herpetol. Bull. 2019, 32–33. [Google Scholar] [CrossRef]
- Casale, P.; Freggi, D.; Cinà, A.; Rocco, M. Spatio-Temporal Distribution and Migration of Adult Male Loggerhead Sea Turtles (Caretta caretta) in the Mediterranean Sea: Further Evidence of the Importance of Neritic Habitats off North Africa. Mar. Biol. 2013, 160, 703–718. [Google Scholar] [CrossRef]
- Robinson, A.R.; Leslie, W.G.; Theocharis, A.; Lascaratos, A. Mediterranean Sea Circulation. In Encyclopedia of Ocean Sciences; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1689–1705. [Google Scholar]
- Millot, C.; Taupier-Letage, I. Circulation in the Mediterranean Sea. In The Mediterranean Sea; Saliot, A., Ed.; Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2005; pp. 29–66. ISBN 978-3-540-31492-9. [Google Scholar]
- Woodward, J.C. (Ed.) The Physical Geography of the Mediterranean; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Poulain, P.-M.; Menna, M.; Mauri, E. Surface Geostrophic Circulation of the Mediterranean Sea Derived from Drifter and Satellite Altimeter Data. J. Phys. Oceanogr. 2012, 42, 973–990. [Google Scholar] [CrossRef]
- Pascual, A.; Vidal-Vijande, E.; Ruiz, S.; Somot, S.; Papadopoulos, V. Spatiotemporal variability of the surface circulation in the western Mediterranean. In The Mediterranean Sea; American Geophysical Union (AGU): Washington, DC, USA, 2014; pp. 5–23. [Google Scholar]
- Carr, A. Impact of Nondegradable Marine Debris on the Ecology and Survival Outlook of Sea Turtles. Mar. Pollut. Bull. 1987, 18, 352–356. [Google Scholar] [CrossRef]
- Luschi, P.; Hays, G.C.; Papi, F. A Review of Long-Distance Movements by Marine Turtles, and the Possible Role of Ocean Currents. Oikos 2003, 103, 293–302. [Google Scholar] [CrossRef]
- Scott, R.; Marsh, R.; Hays, G.C. Ontogeny of Long Distance Migration. Ecology 2014, 95, 2840–2850. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [PubMed]
- Mannocci, L.; Roberts, J.J.; Halpin, P.N.; Authier, M.; Boisseau, O.; Bradai, M.N.; Cañadas, A.; Chicote, C.; David, L.; Di Méglio, N.; et al. Assessing Cetacean Surveys throughout the Mediterranean Sea: A Gap Analysis in Environmental Space. Sci. Rep. 2018, 8, 3126. [Google Scholar] [CrossRef] [PubMed]
- Parra, G.J.; Bilgmann, K.; Peters, K.J.; Möller, L.M. Abundance and Potential Biological Removal of Common Dolphins Subject to Fishery Impacts in South Australian Waters. Front. Mar. Sci. 2021, 8, 617075. [Google Scholar] [CrossRef]
- Weaver, P.; Johnson, D. Think Big for Marine Conservation. Nature 2012, 483, 399. [Google Scholar] [CrossRef]
- Pilcher, N.J.; Antonopoulou, M.A.; Rodriguez-Zarate, C.J.; Mateos-Molina, D.; Das, H.S.; Bugla, I.; Al Ghais, S.M. Movements of Green Turtles from Foraging Areas of the United Arab Emirates: Regional Habitat Connectivity and Use of Marine Protected Areas. Mar. Biol. 2021, 168, 10. [Google Scholar] [CrossRef]
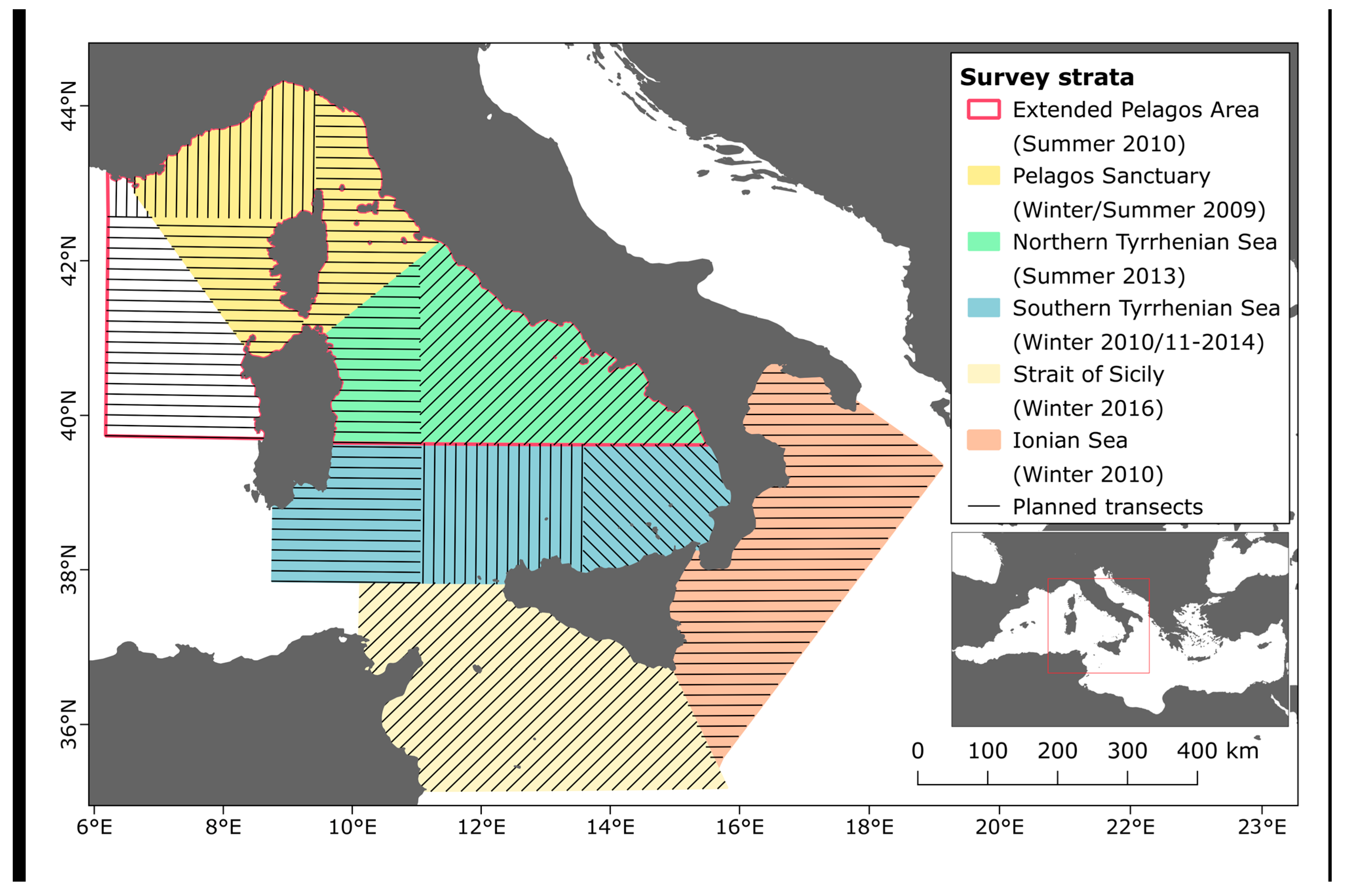




| Area | Year | Season | Strata | Extent (km2) | Transects | Spacing (km) | Length (km) | Coverage (%) |
|---|---|---|---|---|---|---|---|---|
| Pelagos Sanctuary | 2009 | Winter and Summer | 3 | 88,267 | 82 | 10 | 8502 | 15.4 |
| Pelagos Sanctuary * | 2010 | Summer | 3 | 54 | 15 | 5704 | 10.5 | |
| Ionian Sea | 2010 | Winter | 1 | 97,326 | 38 | 15 | 5999 | 9.8 |
| Extended Pelagos Area | 2010 | Summer | 7 | 236,272 | 131 | 15 | 19,753 | 10.5 |
| Northern Tyrrhenian Sea | 2013 | Summer | 2 | 93,216 | 50 | 15 | 6276 | 6.73 |
| Northern Tyrrhenian Sea * | 2010 | Summer | 2 | 50 | 15 | 6107 | 10.5 | |
| Southern Tyrrhenian Sea | 2010–2011 | Winter | 3 | 111,147 | 47 | 15 | 7646 | 6.67 |
| 2014 | 15 | 7646 | 6.67 | |||||
| Strait of Sicily | 2016 | Winter | 1 | 109,709 | 38 | 15 | 8083 | 7.38 |
| Area | Year | Season | Effort (km) | ER | Sightings | D | N (%CV) | 95% CI | G(0) | Nc | Selected Model |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pelagos Sanctuary | 2009 | Winter | 8144.40 | 0.001 | 9 | 0.0026 | 273 (34.33) | 122–461 | 0.43 | 634 | HN/c |
| Pelagos Sanctuary | 2009 | Summer | 8446.90 | 0.018 | 155 | 0.046 | 4083 (14.59) | 3061–5446 | 0.48 | 8506 | HN/c |
| Ionian Sea | 2010 | Winter | 7921.46 | 0.110 | 890 | 0.364 | 35,434 (16.7) | 25,531–49,177 | 0.43 | 82,404 | HN/c |
| Extended Pelagos Area | 2010 | Summer | 19,684.62 | 0.040 | 692 | 0.078 | 33,981 (15.4) | 25,167–45,880 | 0.48 | 70,793 | U/c |
| Pelagos Sanctuary * | 2010 | Summer | 6291.70 | 0.010 | 62 | 0.022 | 1905 (16.2) | 1387–2616 | 0.48 | 3968 | HN/c |
| Central Tyrrhenian Sea * | 2010 | Summer | 9101.63 | 0.060 | 536 | 0.139 | 12,912 (13.3) | 9140–15,308 | 0.48 | 26,900 | HR/c |
| Central Tyrrhenian Sea | 2013 | Summer | 7951.65 | 0.030 | 276 | 0.099 | 9253 (20.4) | 6214–13,780 | 0.48 | 19,277 | HN/c |
| Southern Tyrrhenian Sea | 2010-11 | Winter | 7388.98 | 0.060 | 452 | 0.179 | 17,972 (20.7) | 11,931–29,073 | 0.43 | 41,795 | HN/c |
| Southern Tyrrhenian Sea | 2014 | Winter | 6689.69 | 0.110 | 737 | 0.33 | 33,217 (10.9) | 26,691–41,339 | 0.43 | 74,248 | U/c |
| Sicily Channel | 2016 | Winter | 6325.603 | 0.29 | 1966 | 0.665 | 100,571 (4) | 91,393–110,669 | 0.43 | 233,866 | HN/c |
| Ionian Sea (Winter 2010) | Sicily Channel (Winter 2016) | Southern Tyrrhenian Sea (Winter 2010-11) | Southern Tyrrhenian Sea (Winter 2014) | Northern Tyrrhenian Sea (Summer 2010) * | Northern Tyrrhenian Sea (Summer 2013) | Extended Pelagos Survey Area (Summer 2010) | Pelagos Sanctuary (Summer 2009) | Pelagos Sanctuary (Summer 2010) * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 0.50 | 1.00 | 0.50 | 1.00 | 0.50 | 1.00 | 0.50 | 1.00 | 0.50 | 1.00 | 0.50 | 1.00 | 0.50 | 1.00 | 0.50 | 1.00 | 0.50 | 1.00 |
| N | 566.94 | 1133.89 | 1609.14 | 3218.27 | 287.55 | 575.10 | 531.47 | 1062.94 | 206.59 | 413.18 | 148.05 | 296.10 | 543.70 | 1087.39 | 30.48 | 60.96 | 65.32 | 130.65 |
| L95%CI | 408.50 | 816.99 | 1462.29 | 2924.58 | 190.90 | 381.79 | 427.06 | 854.11 | 146.24 | 292.48 | 99.42 | 198.85 | 402.67 | 805.34 | 22.19 | 44.38 | 48.97 | 97.95 |
| U95%CI | 786.83 | 1573.66 | 1770.7 | 3541.41 | 465.17 | 930.34 | 661.42 | 1322.85 | 244.93 | 489.86 | 220.48 | 443.84 | 734.08 | 1468.16 | 41.85 | 83.17 | 86.97 | 173.95 |
| Nc | 1318.46 | 2636.93 | 3741.86 | 7483.71 | 668.72 | 1337.44 | 1187.63 | 2375.26 | 430.40 | 860.80 | 308.43 | 616.86 | 1132.69 | 2265.38 | 63.48 | 126.97 | 136.09 | 272.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierantonio, N.; Panigada, S.; Lauriano, G. Quantifying Abundance and Mapping Distribution of Loggerhead Turtles in the Mediterranean Sea Using Aerial Surveys: Implications for Conservation. Diversity 2023, 15, 1159. https://doi.org/10.3390/d15121159
Pierantonio N, Panigada S, Lauriano G. Quantifying Abundance and Mapping Distribution of Loggerhead Turtles in the Mediterranean Sea Using Aerial Surveys: Implications for Conservation. Diversity. 2023; 15(12):1159. https://doi.org/10.3390/d15121159
Chicago/Turabian StylePierantonio, Nino, Simone Panigada, and Giancarlo Lauriano. 2023. "Quantifying Abundance and Mapping Distribution of Loggerhead Turtles in the Mediterranean Sea Using Aerial Surveys: Implications for Conservation" Diversity 15, no. 12: 1159. https://doi.org/10.3390/d15121159
APA StylePierantonio, N., Panigada, S., & Lauriano, G. (2023). Quantifying Abundance and Mapping Distribution of Loggerhead Turtles in the Mediterranean Sea Using Aerial Surveys: Implications for Conservation. Diversity, 15(12), 1159. https://doi.org/10.3390/d15121159








