Impact of Complex Oceanographic Features on Seasonal Phytoplankton Community and Biodiversity from 2018 to 2020 in the Vicinity of Dokdo (Island), Offshore Korea
Abstract
:1. Introduction
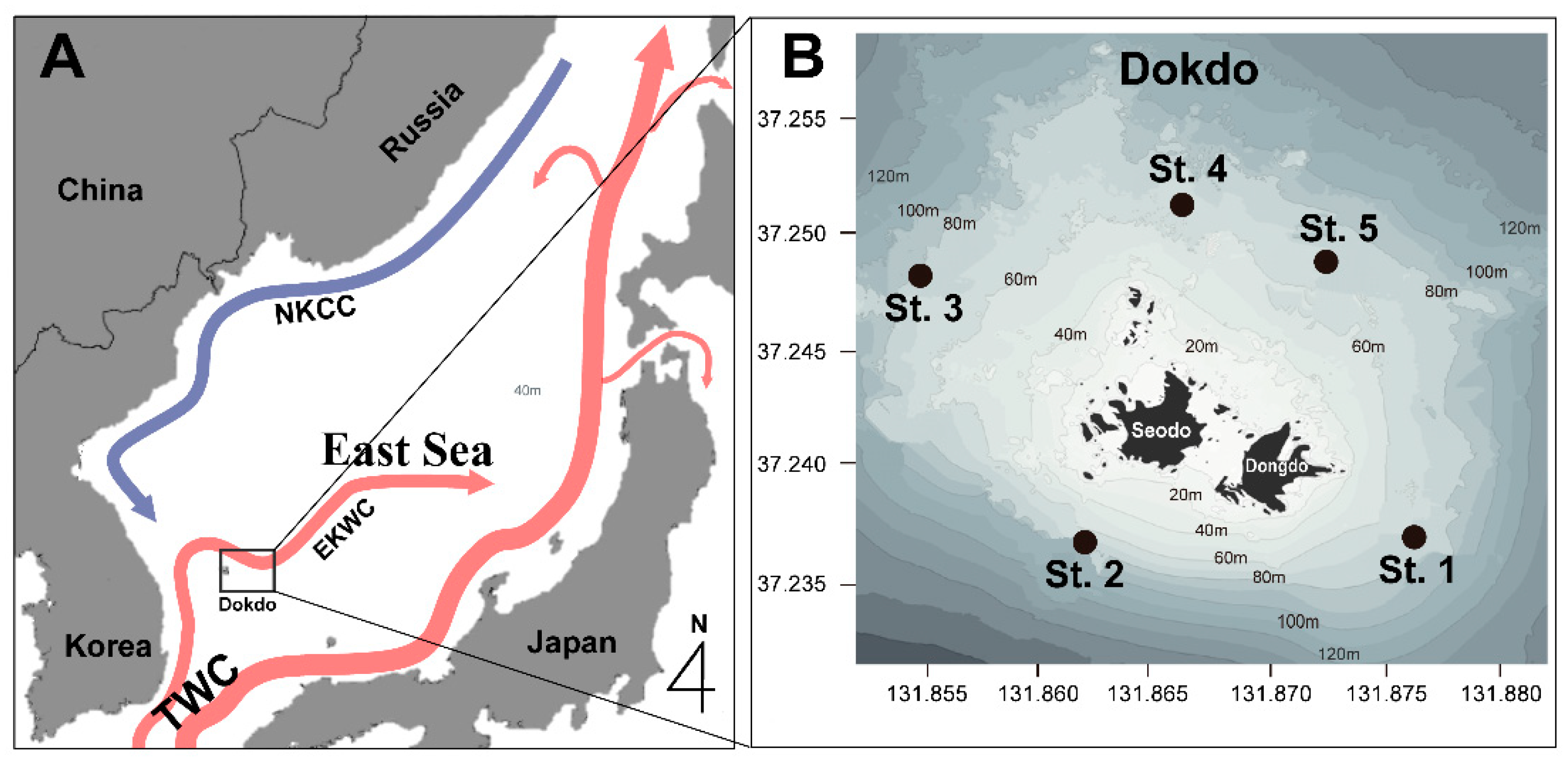
2. Materials and Methods
3. Results
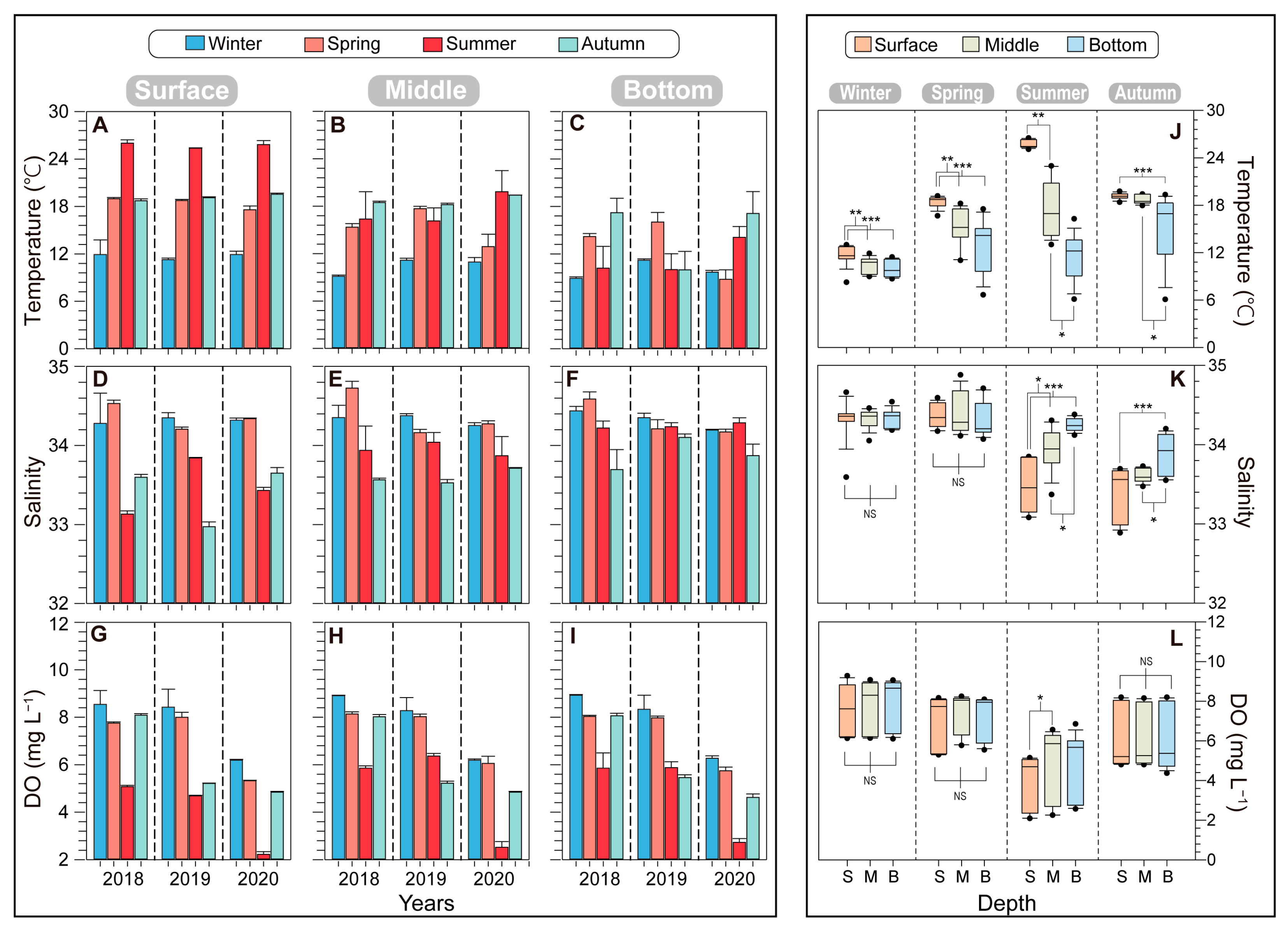
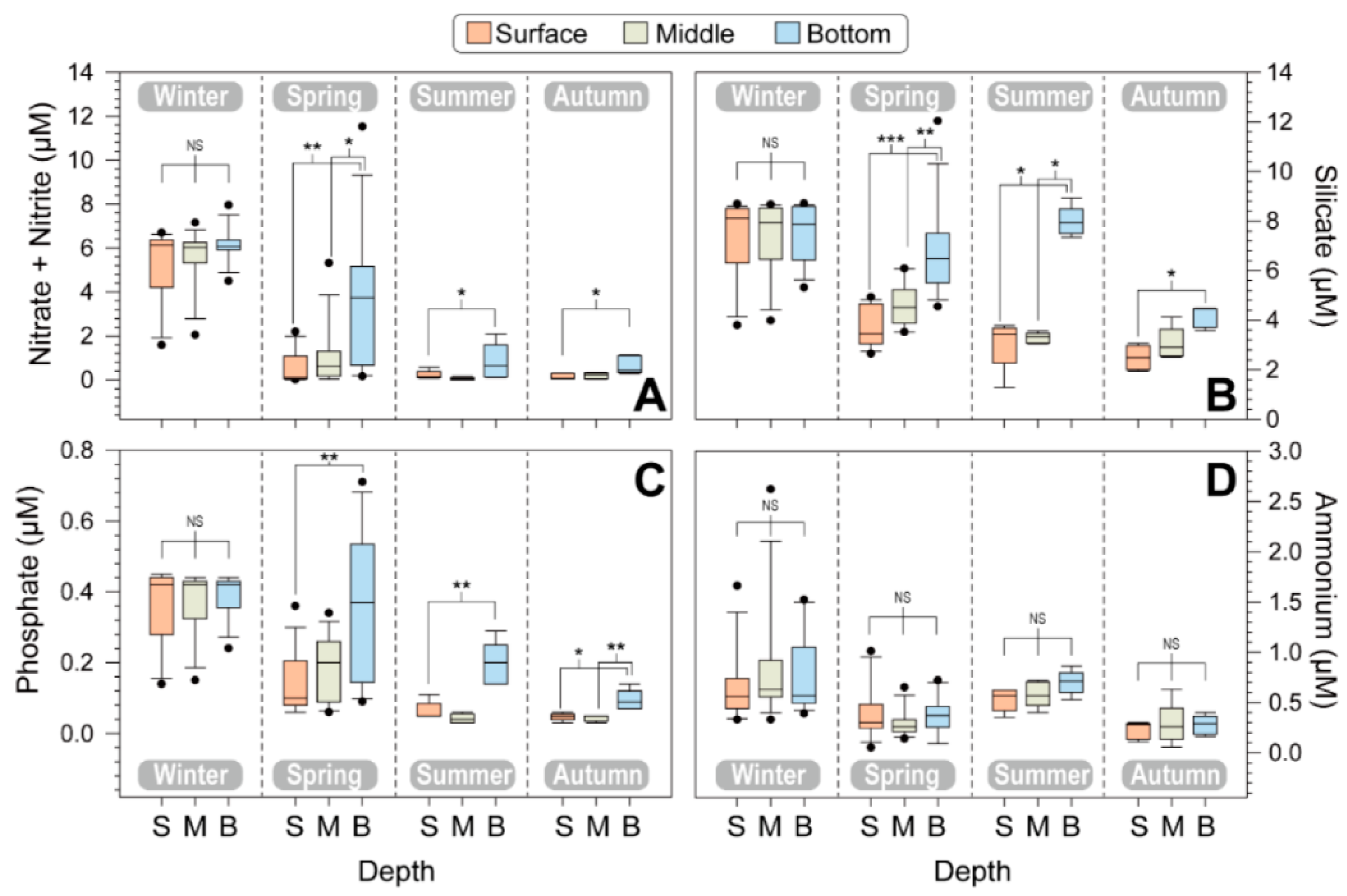
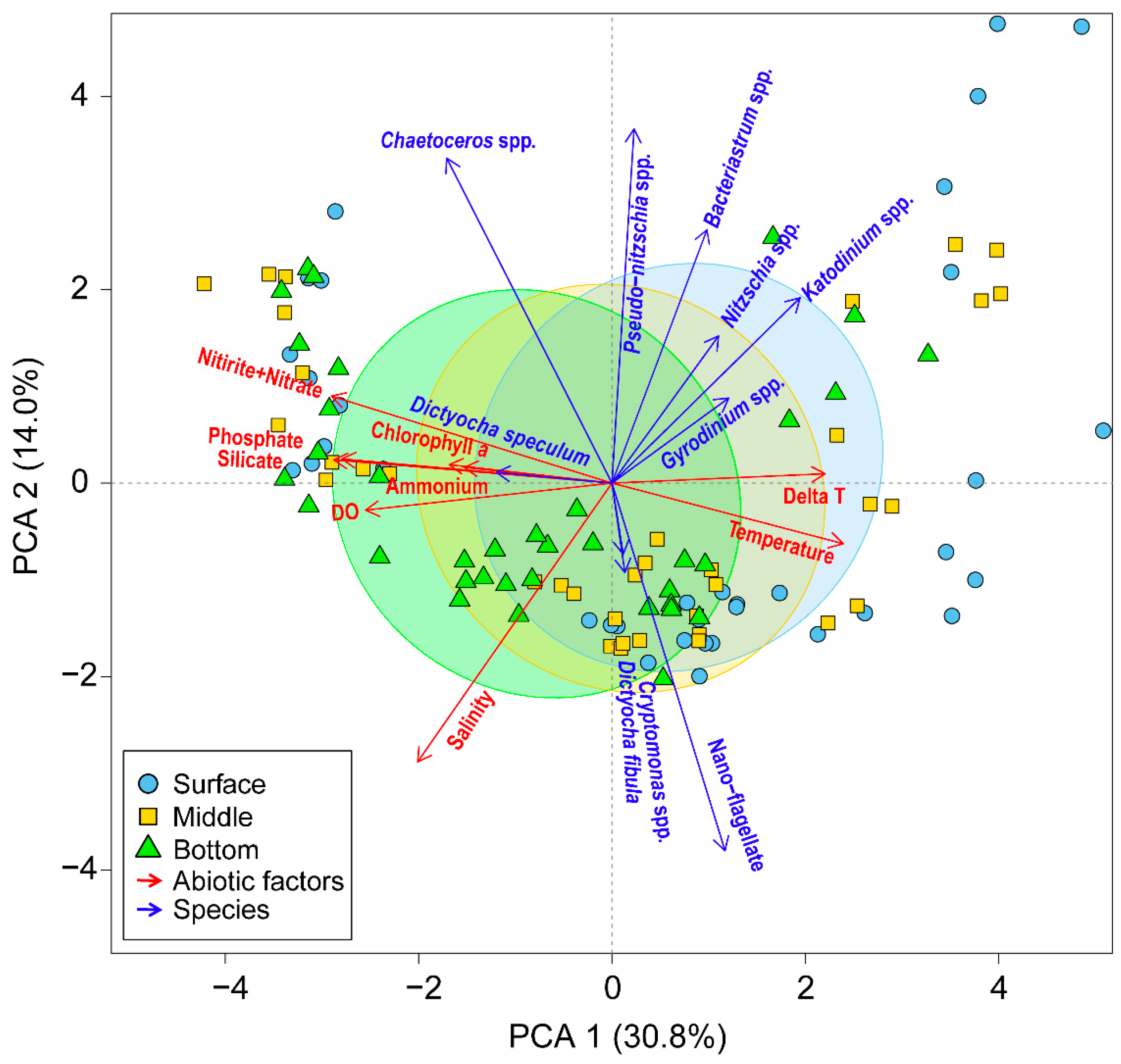
3.1. Abiotic Factors
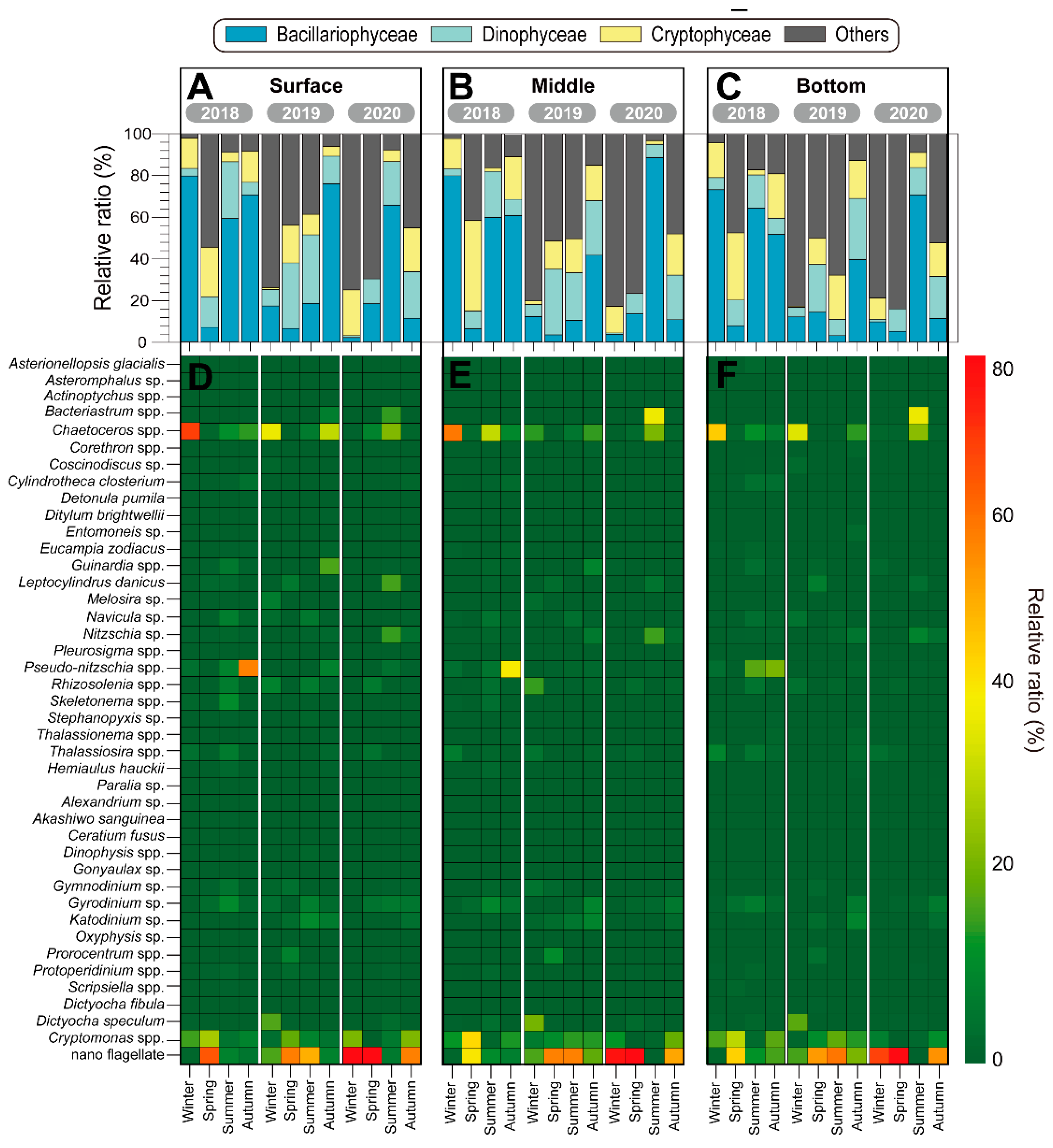
3.2. Biotic Factors in Microcosms
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Townsend, D.W.; Keller, M.D.; Sieracki, M.E.; Ackleson, S.G. Spring phytoplankton blooms in the absence of vertical water column stratification. Nature 1992, 360, 59–62. [Google Scholar] [CrossRef]
- Mozetič, P.; Francé, J.; Kogovšek, T.; Talaber, I.; Malej, A. Plankton trends and community changes in a coastal sea (northern Adriatic): Bottom-up vs. top-down control in relation to environmental drivers. Estuar. Coast. Shelf Sci. 2012, 115, 138–148. [Google Scholar] [CrossRef]
- Thompson, P.A.; Bonham, P.I.; Swadling, K.M. Phytoplankton blooms in the Huon Estuary, Tasmania: Top-down or bottom-up control? J. Plankton Res. 2008, 30, 735–753. [Google Scholar] [CrossRef]
- Kim, T.; Yoon, J.-H. Seasonal variation of upper layer circulation in the northern part of the East/Japan Sea. Cont. Shelf Res. 2010, 30, 1283–1301. [Google Scholar] [CrossRef]
- Takahashi, M. Phytoplankton production during a summer coastal upwelling in the East China Sea. Contin. Shelf Res. 2004, 24, 1321–1338. [Google Scholar]
- Cushing, D.H. A difference in structure between ecosystems in strongly stratified waters and in those that are only weakly stratified. J. Plankton Res. 1989, 11, 1–13. [Google Scholar] [CrossRef]
- Paerl, H.W.; Rudek, J.; Mllin, M.A. Stimulation of phytoplankton production in coastal waters by rainfall inputs: Nutritional and trophic implications. Mar. Biol. 1990, 107, 247–254. [Google Scholar] [CrossRef]
- Carstensen, J.; Klais, R.; Cloern, J.E. Phytoplankton blooms in estuarine and coastal waters: Seasonal patterns and key species. Estuar. Coast. Shelf Sci. 2015, 162, 98–109. [Google Scholar] [CrossRef]
- Yoo, S.; Park, J. Why is the southwest the most productive region of the East Sea/Sea of Japan? J. Mar. Syst. 2009, 78, 301–315. [Google Scholar] [CrossRef]
- Kim, D.-S.; Kim, K.-H.; Shim, J.-H.; Yoo, S.-J. The effect of anticyclonic eddy on nutrients and chlorophyll during spring and summer in the Ulleung Basin, East Sea. Sea 2007, 12, 280–286. [Google Scholar]
- Isoda, Y.; Saitoh, S. The northward intruding eddy along the East coast of Korea. J. Oceanogr. 1993, 49, 443–458. [Google Scholar] [CrossRef]
- Park, K.-A.; Ullman, D.S.; Kim, K.; Chung, J.Y.; Kim, K.-R. Spatial and temporal variability of satellite-observed subpolar front in the East/Japan Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2007, 54, 453–470. [Google Scholar] [CrossRef]
- Lie, H.J.; Cho, C.H. Seasonal circulation patterns of the Yellow and East China Seas derived from satellite-tracked drifter trajectories and hydrographic observations. Prog. Oceanogr. 2016, 146, 121–141. [Google Scholar] [CrossRef]
- Lee, M.; Ro, H.; Kim, Y.B.; Park, C.H.; Baek, S.H. Relationship of spatial phytoplankton variability during spring with eutrophic inshore and oligotrophic offshore waters in the East Sea, including Dokdo, Korea. J. Mar. Sci. Eng. 2021, 9, 1455. [Google Scholar] [CrossRef]
- Lee, M.; Kim, J.H.; Kim, Y.-B.; Park, C.H.; Shin, K.; Baek, S.H. Specific oceanographic characteristics and phytoplankton responses influencing the primary production around the Ulleung Basin area in spring. Acta Oceanol. Sin. 2020, 39, 107–122. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, S.H.; Hwang, J.; Suh, Y.S.; Je Park, H.; Chang, K.I.; Kang, C.K. Summer primary productivity and phytoplankton community composition driven by different hydrographic structures in the East/Japan Sea and the Western Subarctic Pacific. J. Geophys. Res. Oceans 2014, 119, 4505–4519. [Google Scholar] [CrossRef]
- Lee, M.; Kim, Y.B.; Park, C.H.; Baek, S.H. Characterization of seasonal phytoplankton pigments and functional types around offshore island in the East/Japan Sea, based on HPLC pigment analysis. Sustainability 2022, 14, 5306. [Google Scholar] [CrossRef]
- Choi, D.H.; Noh, J.H.; An, S.M.; Choi, Y.R.; Lee, H.; Ra, K.; Kim, D.; Rho, T.K.; Lee, S.H.; Kim, K.T.; et al. Spatial distribution of cold-adapted Synechococcus during spring in seas adjacent to Korea. Algae 2016, 31, 231–241. [Google Scholar] [CrossRef]
- Hyun, M.J.; Won, J.; Choi, D.H.; Lee, H.; Lee, Y.; Lee, C.M.; Noh, J.H. A CHEMTAX Study Based on Picoeukaryotic Phytoplankton Pigments and Next-Generation Sequencing Data from the Ulleungdo–Dokdo Marine System of the East Sea (Japan Sea): Improvement of Long-Unresolved Underdetermined Bias. J. Mar. Sci. Eng. 2022, 10, 1967. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Watts, D.R.; Wimbush, M.; Teague, W.J.; Tracey, K.L.; Book, J.W.; Chang, K.I.; Suk, M.S.; Yoon, J.H. Upper circulation patterns in the Ulleung Basin. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2005, 12, 1617–1638. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, D.J.; Kim, I.N.; Rho, T.K.; Lee, T.S.; Kang, C.K.; Kim, K.R. 2009. Spatial and temporal variability in the pelagic ecosystem of the East Sea (Sea of Japan): A review. J. Mar. Syst. 2009, 78, 288–300. [Google Scholar] [CrossRef]
- Kim, C.H.; Yoon, J.H. A numerical modeling of the upper and the intermediate layer circulation in the East Sea. J. Oceanogr. 1999, 55, 327–345. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A.; Schofield, O.M.; Falkowski, P.G. The role of functional traits and trade-offs in struc-turing phytoplankton communities: Scaling from cellular to ecosystem level. Ecol. Lett. 2007, 10, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. The twelfth tansley lecture. Small is beautiful: The picophytoplankton. Funct. Ecol. 1998, 12, 503–513. [Google Scholar] [CrossRef]
- Deonarine, S.N.; Gobler, C.J.; Lonsdale, D.J.; Caron, D.A. Role of zooplankton in the onset and demise of harmful brown tide blooms (Aureococcus anophagefferens) in US mid-Atlantic estuaries. Aquat. Microb. Ecol. 2006, 44, 181–195. [Google Scholar] [CrossRef]
- Li, W.K. Annual average abundance of heterotrophic bacteria and Synechococcus in surface ocean waters. Limnol. Oceanogr. 1998, 43, 1746–1753. [Google Scholar] [CrossRef]
- Choi, D.H.; Noh, J.H. Phylogenetic diversity of Synechococcus strains isolated from the East China Sea and the East Sea. FEMS Microbiol. Ecol. 2009, 69, 439–448. [Google Scholar] [CrossRef]
- Huang, S.; Wilhelm, S.W.; Harvey, H.R.; Taylor, K.; Jiao, N.; Chen, F. Novel lineages of Prochlorococcus and Synechococcus in the global oceans. ISME J. 2012, 6, 285–297. [Google Scholar] [CrossRef]
- Shim, J.M.; Yun, S.H.; Hwang, J.D.; Jin, H.G.; Lee, Y.H.; Kim, Y.S.; Yun, S.C. Seasonal variability of picoplankton around Ullneung Island. J. Environ. Sci. Int. 2008, 17, 1243–1253. [Google Scholar] [CrossRef]
- Jephson, T.; Carlsson, P. Species-and stratification-dependent diel vertical migration behaviour of three dinoflagellate species in a laboratory study. J. Plankton Res. 2009, 31, 1353–1362. [Google Scholar] [CrossRef]
- de Souza, K.B.; Jephson, T.; Hasper, T.B.; Carlsson, P. Species-specific dinoflagellate vertical distribution in temperature-stratified waters. Mar. Biol. 2014, 161, 1725–1734. [Google Scholar] [CrossRef]
- Sin, E.; Min, W.G.; Kim, Y.-B.; Kim, T.W. Respiration of the sea urchin Mesocentrotus nudus in response to large temperature fluctuations. Mar. Environ. Res. 2019, 144, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Rho, T.-K.; Kim, Y.-B.; Park, J.-I.; Lee, Y.-W.; Im, D.-H.; Kang, D.-J.; Lee, T.-S.; Yoon, S.-T.; Kim, T.-H.; Kwak, J.-H. Plankton community response to physico-chemical forcing in the Ulleung Basin, East Sea during summer 2008. Ocean Polar Res. 2010, 32, 269–289. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.H.; Lee, M.; Lee, C.H.; Park, C.H.; Kim, Y.-B.; Kang, J.H.; Lim, Y.K. Impact of Complex Oceanographic Features on Seasonal Phytoplankton Community and Biodiversity from 2018 to 2020 in the Vicinity of Dokdo (Island), Offshore Korea. Diversity 2023, 15, 1166. https://doi.org/10.3390/d15121166
Baek SH, Lee M, Lee CH, Park CH, Kim Y-B, Kang JH, Lim YK. Impact of Complex Oceanographic Features on Seasonal Phytoplankton Community and Biodiversity from 2018 to 2020 in the Vicinity of Dokdo (Island), Offshore Korea. Diversity. 2023; 15(12):1166. https://doi.org/10.3390/d15121166
Chicago/Turabian StyleBaek, Seung Ho, Minji Lee, Chung Hyeon Lee, Chan Hong Park, Yun-Bae Kim, Jung Hoon Kang, and Young Kyun Lim. 2023. "Impact of Complex Oceanographic Features on Seasonal Phytoplankton Community and Biodiversity from 2018 to 2020 in the Vicinity of Dokdo (Island), Offshore Korea" Diversity 15, no. 12: 1166. https://doi.org/10.3390/d15121166
APA StyleBaek, S. H., Lee, M., Lee, C. H., Park, C. H., Kim, Y.-B., Kang, J. H., & Lim, Y. K. (2023). Impact of Complex Oceanographic Features on Seasonal Phytoplankton Community and Biodiversity from 2018 to 2020 in the Vicinity of Dokdo (Island), Offshore Korea. Diversity, 15(12), 1166. https://doi.org/10.3390/d15121166





