Population Size, Non-Breeding Fraction, and Productivity in a Large Urban Population of Burrowing Parrots (Cyanoliseus patagonus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nesting Sites and Abundance of Breeding Pairs
2.2. Non-Breeding Fraction and Total Population Size
2.3. Proportion of Juveniles and Average Annual Productivity
3. Results
3.1. Nesting Sites and Abundance of Breeding Pairs
3.2. Population Size and Non-Breeding Fraction
3.3. Proportion of Juveniles and Mean Annual Productivity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olah, G.; Butchart, S.H.; Symes, A.; Guzmán, I.M.; Cunningham, R.; Brightsmith, D.J.; Heinsohn, R. Ecological and socio-economic factors affecting extinction risk in parrots. Biodivers. Conserv. 2016, 25, 205–223. [Google Scholar] [CrossRef]
- McClure, C.J.; Rolek, B.W. Relative conservation status of bird orders with special attention to raptors. Front. Ecol. Evol. 2020, 8. [Google Scholar] [CrossRef]
- Snyder, N.; McGowan, P.; Gilardi, J.; Grajal, A. Parrots. Status Survey and Conservation Action Plan 2000–2004; IUCN: Gland, Switzerland; Cambridge, UK, 2000. [Google Scholar]
- Marsden, S.J.; Royle, K. Abundance and abundance change in the world’s parrots. Ibis 2015, 157, 219–229. [Google Scholar] [CrossRef]
- Berkunsky, I.; Quillfeldt, P.; Brightsmith, D.J.; Abbud, M.; Aguilar, J.; Alemán-Zelaya, U.; Aramburú, R.M.; Arias, A.A.; McNab, R.B.; Balsby, T.J. Current threats faced by Neotropical parrot populations. Biol. Conserv. 2017, 214, 278–287. [Google Scholar] [CrossRef]
- Tella, J.L.; Rojas, A.; Carrete, M.; Hiraldo, F. Simple assessments of age and spatial population structure can aid conservation of poorly known species. Biol. Conserv. 2013, 167, 425–434. [Google Scholar] [CrossRef]
- Pacífico, E.C.; Barbosa, E.A.; Filadelfo, T.; Oliveira, K.G.; Silveira, L.F.; Tella, J.L. Breeding to non-breeding population ratio and breeding performance of the globally Endangered Lear’s Macaw Anodorhynchus leari: Conservation and monitoring implications. Bird Conserv. Int. 2014, 24, 466–476. [Google Scholar] [CrossRef]
- Herzog, S.K.; Boorsma, T.; Saldaña-Covarrubias, G.; Calahuma-Arispe, T.; Camacho-Reyes, T.; Dekker, D.; de Vargas, S.E.; García-Cárdenas, M.; García-Solíz, V.H.; Quiroz-Calizaya, J.M.; et al. Breeding and global population sizes of the Critically Endangered Red-fronted Macaw Ara rubrogenys revisited. Bird Conserv. Int. 2023, 33, e14. [Google Scholar] [CrossRef]
- Huck, M.; Fernandez-Duque, E. The floater’s dilemma: Use of space by wild solitary Azara’s owl monkeys, Aotus azarae, in relation to group ranges. Anim. Behav. 2017, 127, 33–41. [Google Scholar] [CrossRef]
- Moreno, J. The Unknown Life of Floaters: The Hidden Face of Sexual Selection. Ardeola 2016, 63, 49–77. [Google Scholar] [CrossRef]
- Robles, H.; Ciudad, C. Floaters may buffer the extinction risk of small populations: An empirical assessment. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170074. [Google Scholar] [CrossRef]
- Penteriani, V.; Ferrer, M.; Delgado, M.M. Floater strategies and dynamics in birds, and their importance in conservation biology: Towards an understanding of nonbreeders in avian populations. Anim. Conserv. 2011, 14, 233–241. [Google Scholar] [CrossRef]
- Tanferna, A.; López-Jiménez, L.; Blas, J.; Hiraldo, F.; Sergio, F. Habitat selection by Black kite breeders and floaters: Implications for conservation management of raptor floaters. Biol. Conserv. 2013, 160, 1–9. [Google Scholar] [CrossRef]
- Masello, J.F.; Quillfeldt, P.; Munimanda, G.K.; Klauke, N.; Segelbacher, G.; Schaefer, H.M.; Failla, M.; Cortés, M.; Moodley, Y. The high Andes, gene flow and a stable hybrid zone shape the genetic structure of a wide-ranging South American parrot. Front. Zool. 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Rojas Martínez, M.E. Estudio de la Interacción entre las Poblaciones de Loro Tricahue Cyanoliseus patagonus Bloxami, y la Actividad Agrícola en las Comunas de Vicuña y Monte Patria, Región de Coquimbo, Chile; Servicio Agrícola y Ganadero, Ministerio de Agricultura, Gobierno de Chile: Santiago de Chile, Chile, 2008. [Google Scholar]
- Bucher, E.H. Distribution y situacion actual del loro barranquero (Cyanoliseus patagonus) en la Argentina. Vida Silv. Neotrop. 1986, 1, 55–61. [Google Scholar]
- Failla, M.; VA, S.; Quillfeldt, P.; Masello, J. Potencial impacto del loro barranquero (Cyanoliseus patagonus): Evaluación de percepción de daño en Patagonia Nordeste, Argentina. Gestión Ambient. 2008, 16, 27–40. [Google Scholar]
- Masello, J.F.; Pagnossin, M.L.; Sommer, C.; Quillfeldt, P. Population size, provisioning frequency, flock size and foraging range at the largest known colony of Psittaciformes: The Burrowing Parrots of the north-eastern Patagonian coastal cliffs. Emu-Austral Ornithol. 2006, 106, 69–79. [Google Scholar] [CrossRef]
- Sanchez, R.; Ballari, S.; Bucher, E.; Masello, J. Foraging by burrowing parrots has little impact on agricultural crops in north-eastern Patagonia, Argentina. Int. J. Pest Manag. 2016, 62, 326–335. [Google Scholar] [CrossRef]
- Tella, J.L.; Canale, A.; Carrete, M.; Petracci, P.; Zalba, S.M. Anthropogenic Nesting Sites Allow Urban Breeding in Burrowing Parrots Cyanoliseus patagonus. Ardeola 2014, 61, 311–321. [Google Scholar] [CrossRef]
- García-Lau, I.; Acosta, M.; Mugica, L.; Rodríguez-Ochoa, A.; González, A. Revisión de los estudios científicos sobre ornitología urbana de La Habana, Cuba. El Hornero 2018, 33, 29–44. [Google Scholar] [CrossRef]
- García-Lau, I.; Vives, A. Selección de cavidades por la Golondrina Azul Cubana (Progne cryptoleuca) en un área urbana. Ornitol. Neotrop. 2016, 27, 189–195. [Google Scholar] [CrossRef]
- Romero-Vidal, P.; Blanco, G.; Hiraldo, F.; Díaz-Luque, J.A.; Luna, Á.; Lera, D.; Zalba, S.; Carrete, M.; Tella, J.L. Nesting innovations in Neotropical parrots associated to anthropogenic environmental changes. Ecol. Evol. 2023, 13, e10462. [Google Scholar] [CrossRef] [PubMed]
- Grilli, P.G.; Soave, G.E.; Arellano, M.L.; Masello, J.F. Relative abundance of the burrowing parrot (Cyanoliseus patagonus) in Buenos Aires province and nearby areas of La Pampa and Río Negro, Argentina. El Hornero 2012, 27, 063–071. [Google Scholar] [CrossRef]
- Lera, D.N.; Cozzani, N.C.; Canale, A.; Tella, J.L.; Zalba, S.M. Variaciones interanuales y cambios estacionales en la abundancia de una población urbana de Loro Barranquero (Cyanoliseus patagonus) en el Sudoeste Bonaerense. El Hornero 2022, 37, 173–180. [Google Scholar] [CrossRef]
- Masello, J.F.; Quillfeldt, P. Chick Growth and Breeding Success of the Burrowing Parrot. Condor 2002, 104, 574–586. [Google Scholar] [CrossRef]
- Ramirez-Herranz, M.; Rios, R.S.; Vargas-Rodriguez, R.; Novoa-Jerez, J.E.; Squeo, F.A. The importance of scale-dependent ravine characteristics on breeding-site selection by the Burrowing Parrot, Cyanoliseus patagonus. PeerJ 2017, 5, e3182. [Google Scholar] [CrossRef] [PubMed]
- Canale, A. El desafío de la conservación de fauna silvestre en áreas urbanas: El loro barranquero (Cyanoliseus patagonus) en Bahía Blanca. Ph.D. Thesis, Universidad Nacional del Sur, Bahía Blanca, Argentina, 2015. [Google Scholar]
- Hunt, W.G. Raptor Floaters at Moffat’s Equilibrium. Oikos 1998, 82, 191–197. [Google Scholar] [CrossRef]
- Katzenberger, J.; Gottschalk, E.; Balkenhol, N.; Waltert, M. Density-dependent age of first reproduction as a key factor for population dynamics: Stable breeding populations mask strong floater declines in a long-lived raptor. Anim. Conserv. 2021, 24, 862–875. [Google Scholar] [CrossRef]
- Lera, D.N.; Cozzani, N.; Tella, J.L.; Zalba, S. Anthropogenic nesting sites and density of Burrowing Parrot (Cyanoliseus patagonus) in northern Argentinian Patagonia. Rev. Chil. Hist. Nat. 2023, 96, 10. [Google Scholar] [CrossRef]
- Brown, C.R.; Brown, M.B. Coloniality in the Cliff Swallow; Chicago Press: London, UK, 1996. [Google Scholar]
- Serrano, D.; Oro, D.; Ursúa, E.; Tella, J.L. Colony size selection determines adult survival and dispersal preferences: Allee effects in a colonial bird. Am. Nat. 2005, 166, 22–31. [Google Scholar] [CrossRef]
- Bonilla, L.M. Monitoreo de la nidificación de la Paraba Frente Roja (Ara rubrogenys) en dos sitios de reproducción en los valles de los Departamentos de Santa Cruz y Cochabamba. Ph.D. Thesis, Universidad Autónoma Gabriel René Moreno, Santa Cruz de La Sierra, Bolivia, 2007. [Google Scholar]
- Sokal, R.R.; Rholf, F.J. Biometry, 2nd ed.; WH Freeman & Co.: San Francisco, CA, USA, 1981. [Google Scholar]
- Carrete, M.; Tella, J.L. Inter-individual variability in fear of humans and relative brain size of the species are related to contemporary urban invasion in birds. PLoS ONE 2011, 6, e18859. [Google Scholar] [CrossRef]
- Dankova, R.; Hula, V. Nesting preference of European Bee-eater (Merops apiaster) in conditions of South Moravia (Czech Republic). MendelNet 2014, 229–233. Available online: https://mnet.mendelu.cz/mendelnet2014/articles/52_dankova_1016.pdf?id=1016&file=52_dankova_1016.pdf (accessed on 5 December 2023).
- Boano, G.; Alberto, T.; Caprio, E. Proper gravel management may counteract population decline of the Collared Sand Martin Riparia riparia. Avocetta 2019, 43, 139–147. [Google Scholar]
- Casagrande, D.G.; Beissinger, S.R. Evaluation of Four Methods for Estimating Parrot Population Size. Condor 1997, 99, 445–457. [Google Scholar] [CrossRef]
- Dénes, F.V.; Tella, J.L.; Beissinger, S.R. Revisiting methods for estimating parrot abundance and population size. Emu-Austral Ornithol. 2018, 118, 67–79. [Google Scholar] [CrossRef]
- Zulian, V.; Müller, E.S.; Cockle, K.L.; Lesterhuis, A.; Tomasi Júnior, R.; Prestes, N.P.; Martinez, J.; Kéry, M.; Ferraz, G. Addressing Multiple Sources of Uncertainty in the Estimation of Global Parrot Abundance from Roost Counts: A Case Study with theVinaceous-Breasted Parrot (Amazona vinacea). Biol. Conserv. 2020, 248, 108672. [Google Scholar] [CrossRef]
- Zulian, V.; Miller, D.A.W.; Ferraz, G. Endemic and Threatened Amazona Parrots of the Atlantic Forest: An Overview of Their Geographic Range and Population Size. Diversity 2021, 13, 416. [Google Scholar] [CrossRef]
- Dupin, M.K.; Dahlin, C.R.; Wright, T.F. Range-Wide Population Assessment of the Endangered Yellow-Naped Amazon (Amazona auropalliata). Diversity 2020, 12, 377. [Google Scholar] [CrossRef]
- Hernández-Brito, D.; Carrete, M.; Tella, J.L. Annual Censuses and Citizen Science Data Show Rapid Population Increases and Range Expansion of Invasive Rose-Ringed and Monk Parakeets in Seville, Spain. Animals 2022, 12, 677. [Google Scholar] [CrossRef]
- Sæther, B.E.; Lande, R.; Engen, S.; Weimerskirch, H.; Lillegård, M.; Altwegg, R.; Becker, P.H.; Bregnballe, T.; Brommer, J.E.; McCleery, R.H.; et al. Generation time and temporal scaling of bird population dynamics. Nature 2005, 436, 99–102. [Google Scholar] [CrossRef]
- Young, A.M.; Hobson, E.A.; Lackey, L.B.; Wright, T.E. Survival on the ark: Life-history trends in captive parrots. Anim. Conserv. 2012, 15, 28–43. [Google Scholar] [CrossRef]
- Negro, J.J. The ghost fraction of populations: A taxon-dependent problem. Anim. Conserv. 2011, 14, 338–339. [Google Scholar] [CrossRef]
- Lenda, M.; Maciusik, B.; Skórka, P. The evolutionary, ecological and behavioural consequences of the presence of floaters in bird populations. North-West. J. Zool. 2012, 8, 394–408. [Google Scholar]
- Penteriani, V.; Otalora, F.; Ferrer, M. Floater mortality within settlement areas can explain the Allee effect in breeding populations. Ecol. Model. 2008, 213, 98–104. [Google Scholar] [CrossRef]
- de la Parra-Martínez, S.M.; Renton, K.; Salinas-Melgoza, A.; Muñoz-Lacy, L.G. Tree-cavity availability and selection by a large-bodied secondary cavity-nester: The Military Macaw. J. Ornithol. 2015, 156, 489–498. [Google Scholar] [CrossRef]
- Renton, K.; Salinas-Melgoza, A.; De Labra-Hernández, M.Á.; de la Parra-Martínez, S.M. Resource requirements of parrots: Nest site selectivity and dietary plasticity of Psittaciformes. J. Ornithol. 2015, 156, 73–90. [Google Scholar] [CrossRef]
- Rivera, L.; Politi, N.; Bucher, E.H.; Pidgeon, A. Effect of forest logging on food availability, suitable nesting habitat, nest density and spatial pattern of a Neotropical parrot. For. Ecol. Manag. 2022, 507, 120005. [Google Scholar] [CrossRef]
- Lewis, T.C.; Vargas, I.G.; Vredenbregt, C.; Jimenez, M.; Hatchwell, B.; Beckerman, A.P.; Childs, D.Z. Nest-site selection and reproductive success of a critically endangered parrot, the Great Green Macaw (Ara ambiguus), in an anthropogenic landscape. Ibis 2023, 457, 1003. [Google Scholar] [CrossRef]
- Ferrelli, F. Assessment of the trends and periodicity of thermal and rainfall events in the southwest of Buenos Aires province (Argentina). Huellas 2020, 24, 11–25. [Google Scholar] [CrossRef]
- Masello, J.F.; Quillfeldt, P. Consequences of La Niña phase of ENSO for the survival and growth of nestling Burrowing Parrots on the Atlantic coast of South America. Emu-Austral Ornithol. 2004, 104, 337–346. [Google Scholar] [CrossRef]
- Masello, J.F.; Balbiano, A. Mortandad de Loros Barranqueros en la Provincia de Río Negro (Parte 4). 2021. [Mensaje en un Blog]. Available online: https://lorosbarranqueros.blogspot.com/2021/02/mortandad-de-loros-barranqueros-en-la.html (accessed on 5 December 2023).

| Colony | Breeding Pairs | Substrate | |||
|---|---|---|---|---|---|
| N° | 2018–2019 | 2019–2020 | 2021–2022 | 2022–2023 | |
| 1 | 41 | 85 | 25 | 72 | Quarry |
| 2 | 130 | 125 | 112 | 72 | Roadside |
| 3 | 8 | 3 | 3 | 3 | Quarry |
| 4 | 5 | No parrots | No parrots | No parrots | Quarry |
| 5 | 84 | 103 | 59 | 73 | Quarry |
| 6 | 5 | 11 | 17 | 9 | Roadside |
| 7 | 16 | 16 | 10 | 6 | Roadside |
| 8 | 10 | 13 | 36 | 39 | Quarry |
| 9 | 12 | 28 | No census | No census | Quarry |
| 10 | 33 | 57 | 18 | 68 | Quarry |
| 11 | 163 | 166 | 240 | 242 | Quarry |
| 12 | 19 | 31 | 13 | No census | Quarry |
| 13 | 69 | 56 | 47 | 38 | Quarry |
| 14 | 75 | 111 | 123 | 129 | Quarry |
| 15 | 645 | 554 | 498 | 367 | Quarry |
| 16 | 29 | 59 | 65 | 54 | Quarry |
| 17 | 19 | 68 | 43 | 41 | Quarry |
| 18 | No parrots | 16 | 17 | 8 | Quarry |
| 19 | No census | 11 | 7 | No census | Quarry |
| 20 | Did not exist | 49 | 59 | 81 | Roadside |
| 21 | Did not exist | 35 | 147 | 131 | Roadside |
| 22 | Unknown | 15 | No census | No census | Roadside |
| 23 | No parrots | No census | 6 | No census | Quarry |
| Season | Pre-Fledgling Population Size | Breeding Pairs | Non-Breeding Fraction (%) | Number of Colonies |
|---|---|---|---|---|
| 2018–2019 | 5802 | 1363 | 53.02 | 18 |
| 2019–2020 | 5110 | 1612 | 36.91 | 22 |
| 2021–2022 | 5845 | 1545 | 47.37 | 21 |
| 2022–2023 | 5095 | 1433 | 43.75 | 18 |
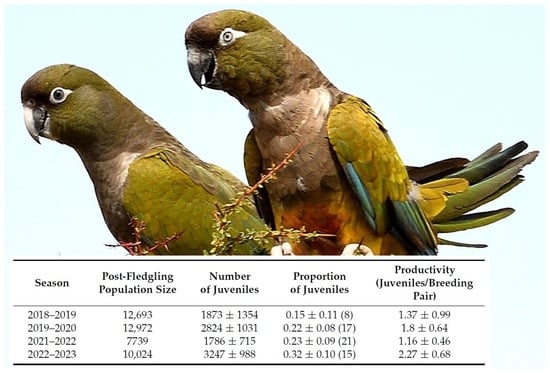
| Season | Post-Fledgling Population Size | Number of Juveniles | Proportion of Juveniles | Productivity (Juveniles/Breeding Pair) |
|---|---|---|---|---|
| 2018–2019 | 12,693 | 1873 ± 1354 | 0.15 ± 0.11 (8) | 1.37 ± 0.99 |
| 2019–2020 | 12,972 | 2824 ± 1031 | 0.22 ± 0.08 (17) | 1.8 ± 0.64 |
| 2021–2022 | 7739 | 1786 ± 715 | 0.23 ± 0.09 (21) | 1.16 ± 0.46 |
| 2022–2023 | 10,024 | 3247 ± 988 | 0.32 ± 0.10 (15) | 2.27 ± 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lera, D.N.; Cozzani, N.; Tella, J.L.; Zalba, S. Population Size, Non-Breeding Fraction, and Productivity in a Large Urban Population of Burrowing Parrots (Cyanoliseus patagonus). Diversity 2023, 15, 1207. https://doi.org/10.3390/d15121207
Lera DN, Cozzani N, Tella JL, Zalba S. Population Size, Non-Breeding Fraction, and Productivity in a Large Urban Population of Burrowing Parrots (Cyanoliseus patagonus). Diversity. 2023; 15(12):1207. https://doi.org/10.3390/d15121207
Chicago/Turabian StyleLera, Daiana N., Natalia Cozzani, José L. Tella, and Sergio Zalba. 2023. "Population Size, Non-Breeding Fraction, and Productivity in a Large Urban Population of Burrowing Parrots (Cyanoliseus patagonus)" Diversity 15, no. 12: 1207. https://doi.org/10.3390/d15121207
APA StyleLera, D. N., Cozzani, N., Tella, J. L., & Zalba, S. (2023). Population Size, Non-Breeding Fraction, and Productivity in a Large Urban Population of Burrowing Parrots (Cyanoliseus patagonus). Diversity, 15(12), 1207. https://doi.org/10.3390/d15121207