Abstract
Water mites are the most diverse freshwater group of Acari and despite growing research interest in the ecology of this group, the environmental influences along longitudinal river gradients on their assemblages are still not fully understood. The objective of this study was to determine how physico-chemical water properties and hydromorphological alterations affect the composition and distribution of water mite assemblages along a longitudinal river gradient. Macroinvertebrate samples were collected from 20 study sites distributed longitudinally along the entire 106 km course of a lowland river (Bednja River) in the Pannonian Lowlands ecoregion of Croatia. At each site, 20 samples were collected with regard to microhabitat composition (+400 samples in total). In parallel with the sampling of macroinvertebrates at each site, the physico-chemical water properties were measured and the degree of hydromorphological alteration was assessed (European Standard EN 15843:2010). Both the number of taxa and water mite abundance were found to increase significantly with increasing distance from the source. However, the assemblages from the upper reaches and those from the lower reaches shared very few species, emphasizing the importance of species-level identification. Water mite species richness and diversity were not reduced with increased levels of variables associated with organic enrichment and eutrophication pressures. Similarly, hydromorphological alteration did not reduce either water mite abundance or species richness and was positively correlated with both. Furthermore, a correspondence analysis on water mite microhabitat preferences revealed that 32% of all species were positively associated with artificial microhabitats (technolithal). These positive associations may be the result of reduced competitive pressure from other larger invertebrates, as well as a possible preference for higher velocity, which usually occurs on smooth technolithal surfaces. A total of 22 different species of water mites were found during this study, 8 of which (or 36% of all species found) were recorded for the first time in Croatia.
1. Introduction
Hydrachnidia (water mites) represent the most diverse and numerous group of mites, with over 7500 recorded species [1], all closely tied to aquatic ecosystems [2]. Unfortunately, this diversity and heterogeneity of species is often accompanied by low population densities and sparse distributions, making it difficult to study the ecology of this group. Lotic ecosystems are sensitive habitats exposed to ever-increasing anthropogenic pressures due to urbanization, industrialization, and agriculture [3,4], which ultimately leads to hydromorphological alteration and eutrophication [5,6,7]. Nowadays, anthropogenic stressors unfortunately play almost as important a role in shaping lowland river communities as natural environmental conditions [8,9]. The taxonomic and functional composition of lotic communities is influenced by hydromorphological alterations, organic and nutrient enrichment, and other stressors, as well as by the relevant natural characteristics, such as altitude, distance from the source, catchment size, geology, etc. [10,11]. Water mite abundance and species distribution patterns are also strongly dependent on physico-chemical water properties [12,13], especially temperature, substrate composition and water velocity [14,15], making them excellent bioindicators of ecological conditions and freshwater quality [16].
Despite the growing research interest in the ecology of this group, the environmental influences along longitudinal river gradients on water mite assemblages are still not fully understood. While it is known that hydromorphological alteration leads to severe changes in macroinvertebrate composition and structure [17,18], it is not entirely clear how, and if at all, this affects meiofaunal structure in general and specifically the composition, abundance and diversity of water mite assemblages.
The first prediction of this study was that the composition of water mite assemblages changes on a longitudinal gradient of the lotic system. To test this, the species richness of water mites was examined in terms of the variables related to the longitudinal gradient: altitude, distance from the source and catchment size. The second prediction was that because of their body size, water mite assemblages are not influenced by hydromorphological alteration, but by microhabitat composition. To test this, substrate preferences with regard to the abundance of specific water mite taxa in specific microhabitats, and the relationship between hydromorphological alteration variables and water mite species richness and abundance were examined. The final prediction was that water mite assemblage composition would change with environmental variables related to organic pollution and eutrophication. In order to test this, water mite species richness and abundance were examined in terms of physico-chemical water properties related to these processes: orthophosphate concentration, total nitrogen and total phosphorous concentration, water conductivity, water temperature, chemical oxygen demand (COD), and oxygen saturation.
2. Materials and Methods
2.1. Study Area
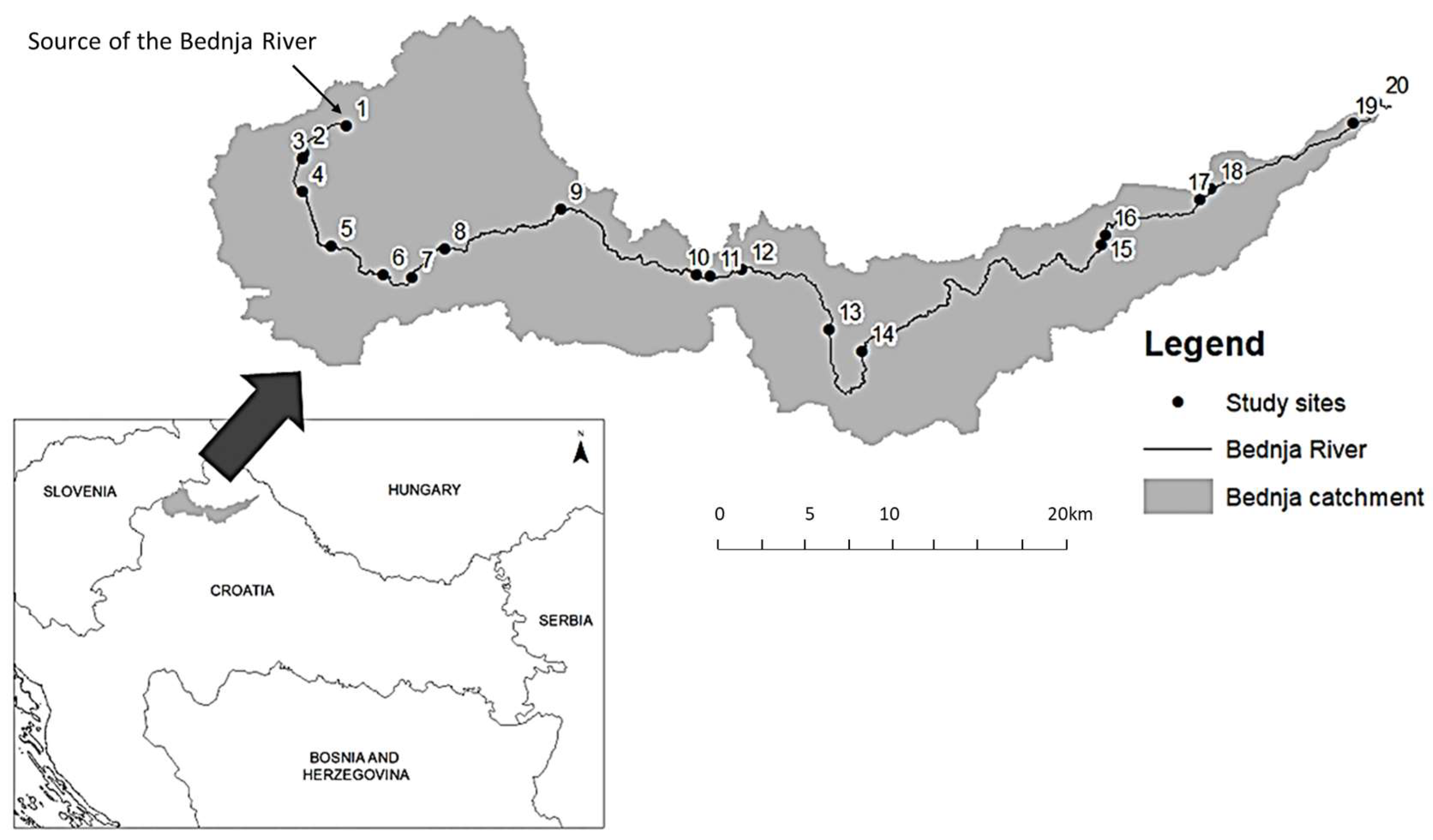
The Bednja River is situated at the north of Croatia and is a right tributary of the Drava River, all of which belong to the Danube River basin. With a total length of 106 km, the typology of the Bednja River changes from its source at the foothills of Ravna Gora Mountain as a small mid-altitude river in the hilly regions to a medium lowland river near the mouth into the Drava River. Throughout its course, the river is exposed to numerous anthropogenic pressures, resulting in a number of degraded habitats. Nevertheless, there are also areas of low anthropogenic influence. While the floodplains are mostly used for intensive and extensive agriculture, the accompanying mountains and hills are mostly naturally occurring forests. Large parts of the river course have been channelized, resulting in a significant loss of natural habitats. Twenty sampling sites were selected along the entire river course, based on the typology of the river and the different types and levels of anthropogenic pressures (Figure 1).
Figure 1.
The location of the study area in northern Croatia—the Bednja River catchment and the distribution of sampling sites (1–20) along the river. (The details of each sampling site are presented in Table S1 in the Supplementary Material).
2.2. Sampling and Laboratory Methods
Samples were collected within one week (30 June, 1, 2, 4, 5 and 7 July) in summer 2015, using the multi-habitat sampling method and microhabitat suggestions according to the AQEM protocol [19]. All benthic samples of macroinvertebrate fauna were collected using a kick-sampler from an area of approximately 0.0625 m2, with the substrate disturbed up to 5 cm. An assessment of microhabitat distribution and composition was conducted at each sampling site. Microhabitats that comprised less than 5% of the total substrate at each sampling site were excluded from sampling (microhabitat composition at each site is presented in Table S3 in the Supplementary Material). A total of 400 benthic invertebrate samples (20 from each site) were collected from a 25 × 25 cm square area using a hand net with a mesh size of 500 μm, as sampling was generalized to all invertebrate fauna and not just water mites. All invertebrate samples were preserved in 70% ethyl alcohol. Water mites were then isolated and identified to the lowest possible taxonomic category (genus or species) using the taxonomic keys by Davids et al. (2006) [20], Di Sabatino et al. (2010) [21] and Gerecke et al. (2016) [22] for adults and Tuzovskij (1990) [23] for deutonymphs. All water mite specimens are deposited at the Department of Biology, Faculty of Science, University of Zagreb, Croatia.
The degree of hydromorphological alteration at each sampling site was assessed using the European Standard EN 15843:2010 “Water quality—Guidance standard on determining of modification of river hydromorphology” (DIN, 2010) [24] which assesses the “departure from naturalness as a result of human pressures on river hydromorphology”. The assessment was performed on a 500 m long reach encompassing the sampled reach and extending upstream to account for drift. Hydromorphological modification scores are grouped into five classes representing different degrees of hydromorphological modification, from near natural (1) to severely modified (5). The hydromorphological scores and measures from each sampling site are presented in Table S2 in the Supplementary Material.
2.3. Environmental Variables
The physico-chemical water properties were measured during sampling at each site. The following parameters were selected as descriptors of water mite distribution on a longitudinal gradient and descriptors of possible eutrophication processes: water temperature, oxygen concentration and saturation (WTW Oxi 330/SET), water pH (WTW pH 300), conductivity (WTW LF 330), chemical oxygen demand (COD; HRN EN ISO 8467:2001 method), biochemical oxygen demand (BOD; HRN EN 1899-1:2004 method), alkalinity (titration with 0.1 M HCl with methyl orange as indicator in titration), nitrate concentration (HRN ISO 7890-3:2001 method), orthophosphate concentration (HRN ISO 6878:2001 method), ammonium ion concentration (HRN ISO 70-3:1998 method), total nitrogen and phosphorus concentration. The physico-chemical water properties of each sampling site are presented in Table S4 in the Supplementary Materials.
2.4. Data Analysis
For all the following tests, statistical significance was determined with a p-value of less than 0.05, with the exception of multiple variable comparison tests where a modified Bonferroni adjustment was used to generate the p value depended on the number of variables used (p = 0.05 × (i + 1)/2i; where i = number of variables used [25]).
Water mite species richness (number of taxa) and local diversity (Shannon diversity index) were calculated using Primer 7.0 software (Primer—E Ltd. 2015) [26]. The correlation between species richness, abundance (number of individuals per m2), and diversity with regard to key parameters that vary along the river longitudinal gradient (distance from the source, altitude, catchment size; i = 3; p = 0.0333) and hydromorphological alteration measures (i = 5; p = 0.03) were determined by Spearman correlation coefficient using Statistica 13.0 [27].
Canonical correspondence analysis (CCA) was used to compare water mite community composition and microhabitat composition data and to determine microhabitat preferences of different water mite taxa. The Monte Carlo permutation test (999 permutations) was used to test the significance of correlations between water mite species and environmental parameters using the CANOCO program [28]. The effects of eutrophication and organic pollution on water mite assemblages were tested with selected physico-chemical variables associated with these processes (orthophosphate concentration, total nitrogen and phosphorus concentration, conductivity, water temperature, COD, oxygen saturation; i = 7; p = 0.0286) using “interactive forward analysis” in CANOCO [28]. The Spearman correlation coefficient was then used to test the relationship between these individual variables and water mite species richness and abundance (i = 7; p = 0.0286). All data related to the abundance of specific species used in these analyses were previously converted to the number of individual water mites per square meter.
3. Results
A total of 451 water mite specimens from 11 genera and 22 species were collected during the study period (Table 1). The most abundant water mite species found in the lotic habitat studied was Hygrobates fluviatilis (complex), whereas nine taxa were represented with a single specimen. A detailed taxa list with calculated abundances for each microhabitat at each site is presented in Table S5 in the Supplementary Materials.

Table 1.
Water mite specimens collected from 20 sites of the Bednja lowland river. No water mites were found in the samples collected on sites 1 and 2.
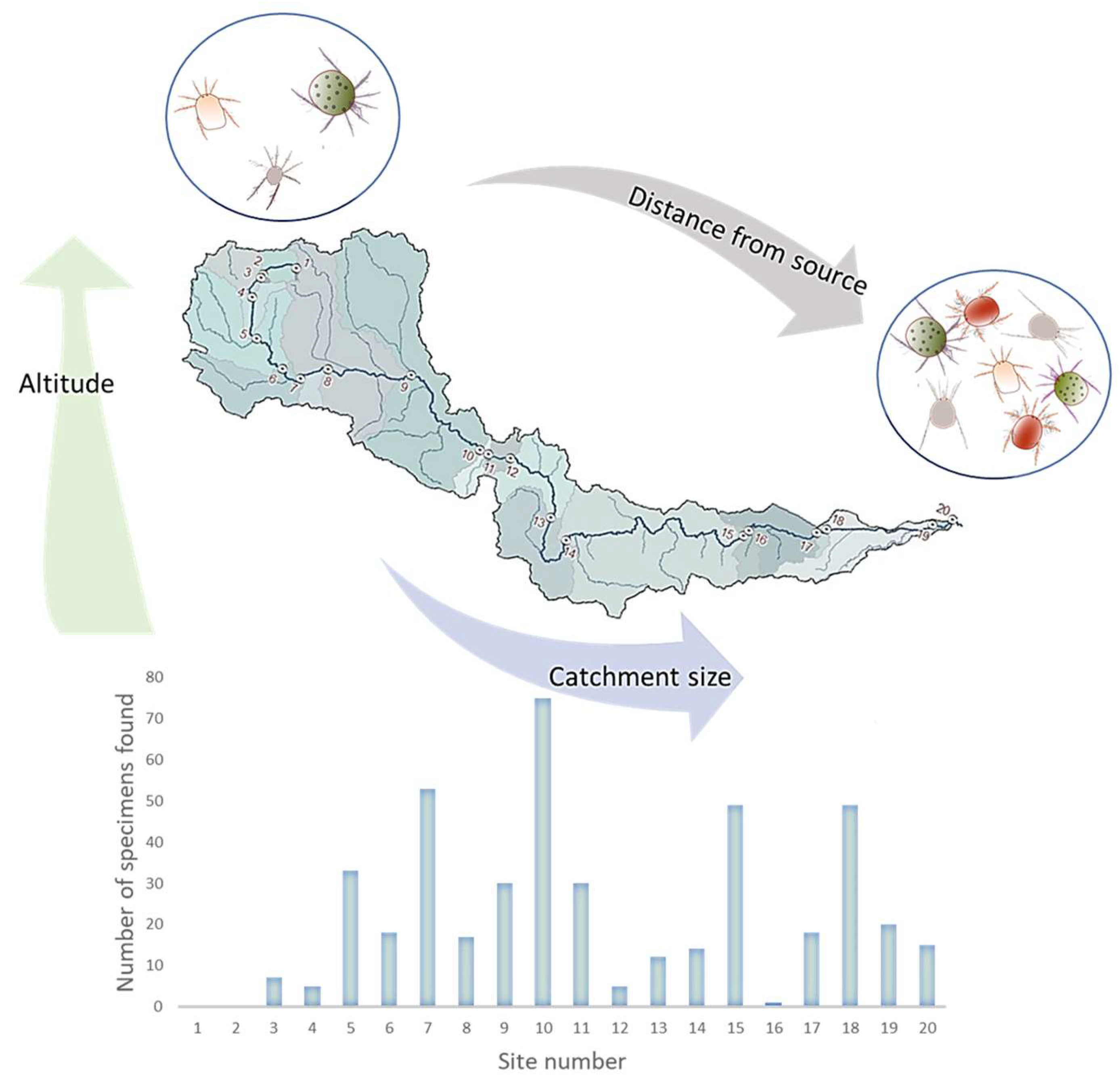
Altitude, catchment size and distance from the source were found to be significantly correlated with water mite abundance, species richness and diversity (Figure 2). Catchment size was found to be positively correlated with both water mite abundance (r = 0.256, p = 0.029) and species richness (r = 0.311; 0.008), as was the distance from the source variable: water mite abundance (r =0.258, p = 0.008) and species richness (r = 0.310, p = 0.008), although the strength of all the correlations above was moderate. Altitude had different effects on water mite assemblages showing a negative correlation with water mite abundance (r = −0.258, p = 0.008) and species richness (r = −0.310, p = 0.008).
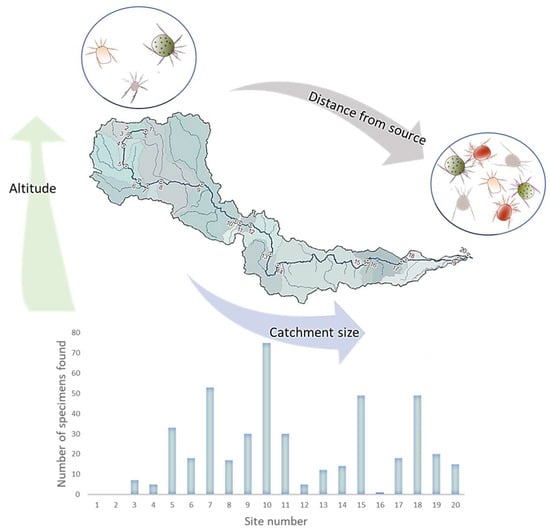
Figure 2.
Graphical presentation of the three main longitudinal characteristics of the study area that significantly correlate with abundance and species richness of water mites. Distance from the source (m) and catchment size (km2) are significantly and positively correlated with abundance and species richness, while altitude (m a.s.l.) is significantly and negatively correlated with abundance and species richness of water mites.
All selected measures of hydromorphological alteration are significantly related to water mite abundance, species richness and local diversity (Table 2).

Table 2.
Values of Spearman correlation coefficient (r) between different hydromorphological (HYMO) features of the sampling sites (riparian vegetation type and structure, degree of channelization, riverbed structure and connection to floodplains, mean value of hydromorphological degradation score) and water mite assemblage characteristics [abundance (N, number of animals/m2), species richness (SR), Shannon diversity index [H′ (loge)]. All the above correlations are statistically significant (adjusted p-value < 0.03).
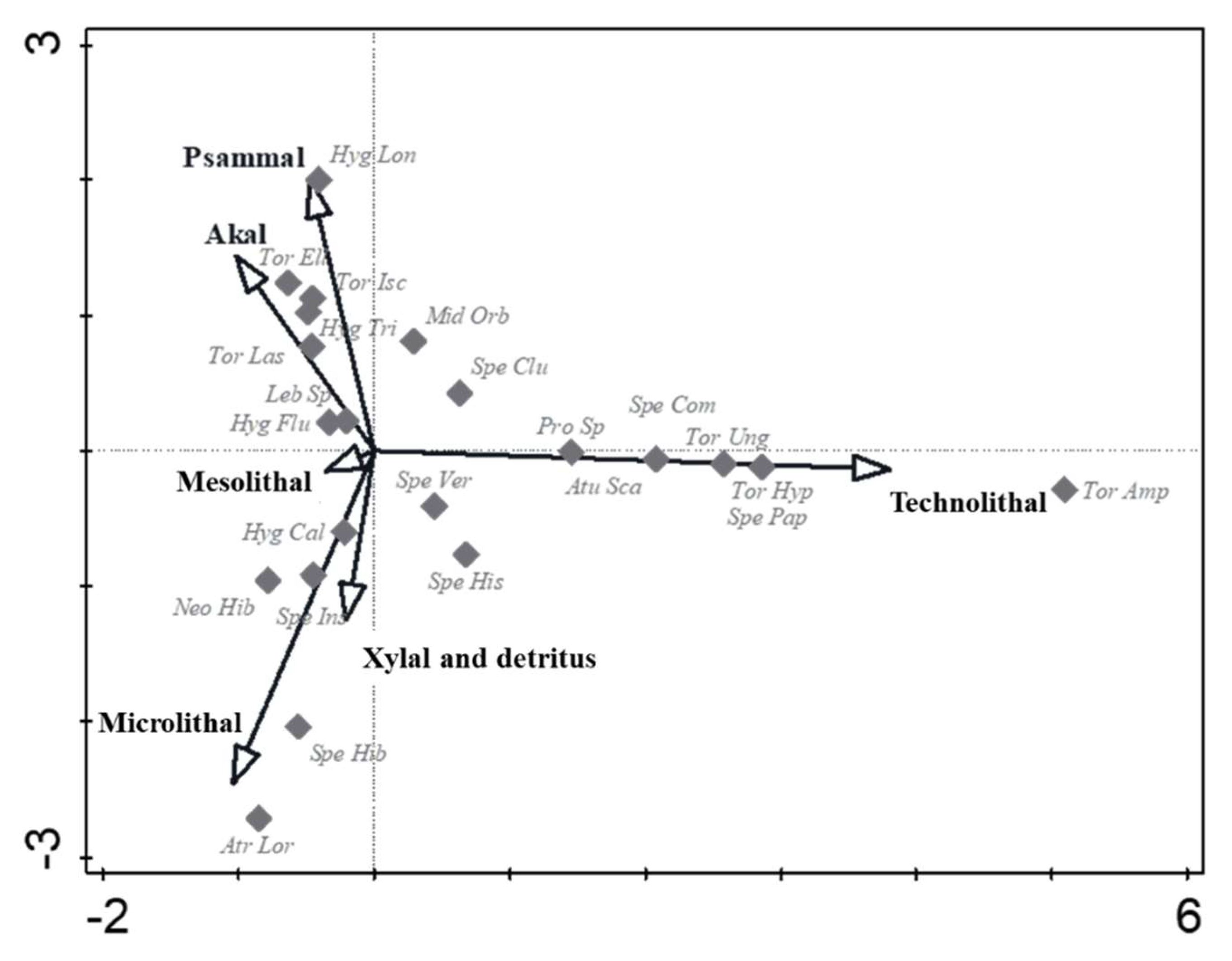
In the CCA analysis (Figure 3), water mite species showing a preference to artificial microhabitats (technolithal) were defined along the Y- axis. Torrenticola amplexa, T. hyporheica, T. ungeri, Sperchon papillosus, S. compactilis, Aturus scaber and Protzia sp. showed a positive association towards technolithal, while Atractides loricatus and S. hibernicus showed a positive association to microlithal microhabitats. Hygrobates longiporus showed a positive association to psammal microhabitats. Other water mite species (Hygrobates calliger, H. fluviatilis, H. trigonicus, Lebertia sp., Mideopsis orbicularis, Neoacarus hibernicus, Sperchon clupeifer, S. hispidus, S. insignis, Sperchonopsis verrucosa, Torrenticola elliptica, T. ischnophallus and T. laskai) were centrally ordinated, with no apparent preference for microhabitats.
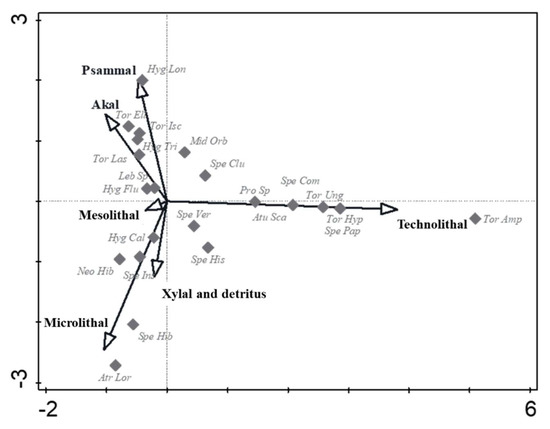
Figure 3.
Canonical correspondence analysis (CCA) ordination of microhabitat structure and water mite variability in a lowland river. Individual water mite species are marked by grey diamonds (♦). Only adult water mite specimens (identified at the species or genus level for Lebertia sp. and Protzia sp.) were considered in this analysis. Available microhabitats are indicated by arrows. The arrow lengths on the ordination show the relative importance of the explanatory variables. The eigenvalues of the first two axes were 0.610 and 0. 543, respectively. A Monte Carlo permutation test indicated that ordination was statistically significant (F = 2.4, p = 0.01). Atractides loricatus = Atr Lor, Aturus scaber = Atu Sca, Hygrobates calliger = Hyg Cal, H. fluviatilis = Hyg Flu, H. longiporus = Hyg Lon, H. trigonicus = Hyg Tri, Lebertia sp. = Leb Sp, Mideopsis orbicularis = Mid Orb, Neoacarus hibernicus = Neo Hib, Protzia sp. = Pro Sp, Sperchon clupeifer = Spe Clu, S. compactilis = Spe Com, S. hibernicus = Spe Hib, S. hispidus = Spe His, S. insignis = Spe Ins, S. papillosus = Spe Pap, Sperchonopsis verrucosa = Spe Ver, Torrenticola amplexa = Tor Amp, T. elliptica = Tor Ell, T. hyporheica = Tor Hyp, T. ischnophallus = Tor Isc, T. laskai = Tor Las, T. ungeri = Tor Ung. Microhabitat named and sampled according to [19].
In the “interactive forward analysis,” seven of the fifteen environmental most prominent water parameters measured in the field (orthophosphate concentration, total nitrogen and phosphorus concentration, conductivity, water temperature, chemical oxygen demand—COD and oxygen saturation) explained less than 10% of the total variation in water mite assemblages (Table 3). The only statistically significant parameter that had an effect on water mite assemblage formation was orthophosphate concentration. The physico-chemical water properties of each site are presented in the Supplementary Materials.

Table 3.
Values of individual physico-chemical parameters in CCA “interactive forward analysis in relation to the variability of the water mite assemblages ((i = 7; p = 0.0286).).
The great taxonomical and functional diversity of water mites along the longitudinal river gradient was best seen when analyzing the effects of individual physico-chemical water properties on specific water mite taxa (Table 4).

Table 4.
Values of Spearman correlation coefficient (r) between environmental parameters and water mite species for which at least one statistically significant correlation was found. Only statistically relevant (i = 12; p = 0.0271) correlations are shown.
4. Discussion
The most abundant water mite species, Hygrobates fluviatilis (complex), is known to inhabit a variety of microhabitats without having a preference for a particular substrate. H. fluviatilis has been described in the past as a widespread species found in various types of freshwaters [22], so the numbers of this species in the analyzed samples are consistent with this literature data. However, molecular studies conducted on this species revealed that it is a complex of several species [29], from which new species are still emerging [30,31,32]. This means that the omnipresence of this species may also be a result of cryptic species and their preferences. Future research efforts should focus on clarifying this issue. Atractides loricatus was found at only one sampling site (site 7) where no substantial nutrient enrichment was present, but a significant degree of hydromorphological alteration was noted, with river embankments and technolithal microhabitats. This finding is unexpected considering that this species mostly inhabits hypocrenic and epirithral zones of mountain freshwater ecosystems [33]. To date, it has been recorded only in the mountainous regions of the Alps, the Dinarides, Sardinia and the Tarta Mountains [34]. Nudomideopsis motasi has only been recorded from four localities in Europe [22], including a find from Markar Cave near Ogulin, Croatia [35]. This species is usually found in hyporheic zones and springs. During this study, a specimen that may belong to this species was collected at a severely hydromorphologically degraded site (Site 17), but due to specimen damage, this finding cannot be confirmed. Future surveys may confirm this finding.
4.1. Longitudinal Gradient
Water mite assemblages from lower river reaches exhibited greater abundance, species richness and diversity than those from upper river reaches. Other research has repeatedly noted the high-water mite abundance and diversity in springs [36,37,38]. In this study, water mite fauna in the upper reaches may have been missed due to the course mesh size (0.05 mm, which is coarser than usually used for efficient collection of water mites) as the sampling method focused on a quantitative description of the invertebrate community in general. In addition, microhabitats that comprised less than 5% of the total substrate at each sampling site were excluded from sampling, likely resulting in the exclusion of important microhabitat from further analysis.
4.2. Hydromorphological Alternation vs. Microhabitat Composition in Shaping Water Mite Assemblages
Surprisingly, all scored variables related to hydromorphological alteration were found to positively affect abundance, species richness and diversity of water mites. The second hypothesis of this study was that water mites, because of their size, are not affected by hydromorphological alterations (i.e., do not have significant relationships with scored variables of hydromorphological alteration) of the watercourse, so this finding can possibly be explained by the lack of competition with larger macroscopic invertebrates due to habitat alteration or that increased water velocity (that is usually associated with canalization) is preferred by some water mite groups.
4.3. Environmental Variables
Physico-chemical water parameters have a relatively small effect on the overall variability of water mite assemblages. One possible explanation is that some variables, such as nutrient input and organic enrichment are partly a natural occurrence [39] that increases along the longitudinal river gradient but may also be the result of anthropogenic influences. The only statistically relevant parameter that influenced the formation of water mite assemblages (in terms of the interactive forward analysis of water mite variability) in the Bednja River was orthophosphate concentration. It is important to note that even the highest value of orthophosphate concentration (0.06 mg PO43−/L) found at site 18 is still relatively unimpacted and can even be considered a benchmark site in monitoring systems [40] with respect to this parameter. Other environmental variables were found to be associated with specific taxa rather than variability of the entire assemblage, but it is important to note that the correlations were mostly moderate, or even weak in some cases.
Water mite species such as Atractides loricatus, Hygrobates fluviatilis (complex) and some species of the genus Lebertia were previously described as rithrobionts [22], which is consistent with the results of this study that positively correlate these species with faster river flows. Mideopsis orbicularis and Sperchon hispidus were found primarily in lower reaches and both species are known to have greater tolerance to organic pollution and higher nutrient levels [22]. These two species are also tied to similar environmental parameters, suggesting a possible natural cohabitation of M. orbicularis and S. hispidus (Table 4). Sperchonopsis verrucosa correlated negatively with higher values of water conductivity, which are primarily present in the upper river sections in our study. This result is consistent with the work of Zawal et al. (2017) [15].
A total of 22 water mite species were found during this study, of which eight are new records for the water mite fauna of Croatia, making the total number of species recorded in Croatia to 107 [41]. Three newfound species are usually associated with the hyporheic zone: Neoacarus hibernicus, Torrenticola hyporheica and Torrenticola ungeri [22,42]. Hygrobates trigonicus is a rithrobiont species, spread throughout the entire western Palearctic [22], so this result is not surprising. Sperchon compactilis and Sperchon papillosus are both rheobionts commonly found in cohabitation throughout Europe, Turkey and Iran [21] which was also the case in this study. Torrenticola laskai has been detected at numerous sites in southern and central Europe [21] and was recorded in Croatia for the first time in this study. Torrenticola ischnophallus is a rare and poorly known species that has only been found at a few sites in Europe [43]. In this study, six specimens of Torrenticola ischnophallus were found, which is a valuable discovery that provides better insight into the ecology of this species.
5. Conclusions
A recent ascent in research papers recognizing water mites as potential bioindicators has highlighted a huge gap in basic ecological data on this unique animal group. The present study aimed to address a basic question of water mite ecology using a huge data set of 400 samples from lentic habitats. During this study, which was conducted in a single lotic ecosystem, a staggering 36% of all species were found for the first time in Croatia. This is often the case in ecological surveys that include water mites, with new species often being described in this research we discussed several poorly researched and interesting ecological features of water mite assemblages, including changes of species abundance and composition along a lowland watershed, an association of certain species with the “technolithal” substrate, as well as the association of certain species with other microhabitats. As this is the first research on water mites at the studied lotic ecosystem, our results represent an important basis for future research, especially given the expected impacts of climate change.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15020139/s1, Table S1: Geographic location and main characteristics of each sampling site; Table S2: Selected environmental characteristics as indicators of hydromorphological habitat degradation at the Bednja River sampling sites (score 5—severely modified habitat, score 1—near-natural habitat).; Table S3: Representation of the respective substrate (microhabitat) at the sampling sites along the course of the Bednja River; Table S4: Physico- chemical water properties at the selected sampling sites of the Bednja River. (COD = chemical oxygen demand, BOD= biochemical oxygen demand, NH4+ = ammonium ion concentration, NO2− = nitrite concentration, NO3− = nitrate concentration, ORG. N = organic nitrogen, Ʃ N = total nitrogen, PO43− = ortophosphate concentration, ƩP = total phosphorous); Table S5: Water mite taxa abundance on all microhabitats (calculated number of individuals/m2) at 20 sampling sites along the Bednja River in 2015.
Author Contributions
Conceptualization, I.P.; methodology, I.P., I.V.M. and Z.M.; formal analysis, I.P.; investigation, I.V.M.; resources, I.V.M.; data curation, I.P.; writing—original draft preparation, T.Ž.V. and I.P.; writing—review and editing, I.V.M. and Z.M.; supervision, Z.M.; project administration, I.V.M.; funding acquisition, I.V.M.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data shared in Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Smit, H. Water mites of the world, with keys to the families, subfamilies, genera and subgenera (Acari: Hydrachnidia). In Monografieën van de Nederlandse Entomologische Vereniging; Nederlandse Entomologische Vereniging (NEV): Leiden, The Netherlands, 2020; Volume 12, pp. 1–774. [Google Scholar]
- Krantz, G.W.; Walter, D.E. A Manual of Acarology, 3rd ed.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 1–807. [Google Scholar]
- Tockner, K.; Pusch, M.; Borchardt, D.; Lorang, M.S. Multiple Stressors in Coupled River-Floodplain Ecosystems. Freshw. Biol. 2010, 55, 135–151. [Google Scholar] [CrossRef]
- Villeneuve, B.; Piffady, J.; Valette, L.; Souchon, Y.; Usseglio-Polatera, P. Direct and Indirect Effects of Multiple Stressors on Stream Invertebrates across Watershed, Reach and Site Scales: A Structural Equation Modelling Better Informing on Hydromorphological Impacts. Sci. Total Environ. 2018, 612, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Allan, J.D. Landscapes and Riverscapes: The Influence of Land Use on Stream Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Novotny, V.; Bedoya, D.; Virani, H.; Manolakos, E. Linking Indices of Biotic Integrity to Environmental and Land Use Variables: Multimetric Clustering and Predictive Models. Water Sci. Technol. 2009, 59, 1–8. [Google Scholar] [CrossRef]
- Paul, M.J.; Meyer, J.L. Streams in the Urban Landscape. Annu. Rev. Ecol. Syst. 2001, 32, 333–365. [Google Scholar] [CrossRef]
- Maoduš, I.V.; Pozojević, I.; Vilenica, M.; Mihaljević, Z. Longitudinal Dynamics of Odonata Assemblages in an Anthropogenically Impacted Lotic System. Int. J. Lim. 2022, 58, 7. [Google Scholar] [CrossRef]
- Vilenica, M.; Mihaljević, Z. Odonata Assemblages in Anthropogenically Impacted Habitats in the Drava River—A Long-Term Study. Water 2022, 14, 3119. [Google Scholar] [CrossRef]
- Šumanović, M.; Pozojević, I.; Mihaljević, Z.; Vučković, N.; Dorić, V.; Miliša, M. Invertebrate Functional Responses to Hydromorphological Degradation in Mediterranean Croatian Rivers. Fundam. Appl. Limnol. 2021, 194, 259–270. [Google Scholar] [CrossRef]
- Vilenica, M.; Kerovec, M.; Pozojević, I.; Mihaljević, Z. Mayfly Response to Different Stress Types in Small and Mid-Sized Lowland Rivers. ZooKeys 2020, 980, 57–77. [Google Scholar] [CrossRef]
- Kowalik, W.; Biesiadka, E. Occurence of water mites (Hydracarina) in the river Wieprz polluted with domestic-industry sewage. Acta Hydrobiol. 1981, 23, 331–348. [Google Scholar]
- Smit, H.; van der Hammen, H. Water Mites as Indicators of Natural Aquatic Ecosystems of the Coastal Dunes of the Netherlands and Northwestern France. Hydrobiologia 1992, 231, 51–64. [Google Scholar] [CrossRef]
- Pozojević, I.; Ternjej, I.; Mihaljević, Z.; Gottstein, S.; Vučković, N.; Dorić, V.; Rumišek, M. Prey Abundance Supporting Unusual Water Mite (Acari: Hydrachnidia) Community in a Sublacustrine Spring and Tributary River. Acta Biol. 2018, 25, 69–75. [Google Scholar] [CrossRef]
- Zawal, A.; Stryjecki, R.; Stępień, E.; Buczyńska, E.; Buczyński, P.; Czachorowski, S.; Pakulnicka, J.; Śmietana, P. The Influence of Environmental Factors on Water Mite Assemblages (Acari, Hydrachnidia) in a Small Lowland River: An Analysis at Different Levels of Organization of the Environment. Limnology 2017, 18, 333–343. [Google Scholar] [CrossRef]
- Miccoli, F.P.; Lombardo, P.; Cicolani, B. Indicator Value of Lotic Water Mites (Acari: Hydrachnidia) and Their Use in Macroinvertebrate-Based Indices for Water Quality Assessment Purposes. Knowl. Managt. Aquatic Ecosyst. 2013, 411, 8. [Google Scholar] [CrossRef]
- Knehtl, M.; Podgornik, S.; Urbanič, G. Scale-Depended Effects of Hydromorphology and Riparian Land-Use on Benthic Invertebrates and Fish: Implications for Large River Management. Hydrobiologia 2021, 848, 3447–3467. [Google Scholar] [CrossRef]
- Urbanič, G.; Mihaljević, Z.; Petkovska, V.; Pavlin Urbanič, M. Back to Ecology: Reference Conditions as a Basis for Assessment, Restoration and Sustainable Management of Large Rivers. Water 2021, 13, 2596. [Google Scholar] [CrossRef]
- AQEM Consortium: Manual for the Application of the AQEM Method A Comprehensive Method to Assess European Streams Using Benthic Macroinvertebrates, Developed for the Purpose of the Water Framework Directive; Version 1.0. 2002. Available online: https://scholar.google./scholar?q=AQEM+Consortium.+2002.+Manual+for+the+application+of+the+AQEM+system%3A+a+comprehensive+method+to+assess+European+streams+using+benthic+macroinvertebrates%2C+developed+for+the+purpose+of+the+Water+Framework+Directive.+Version+1.0%2C+February+2002%2C+202+%D1%80 (accessed on 16 January 2023).
- Bartsch, I.; Davids, K.; Deichsel, R.; Di Sabatino, A.; Gabrys, G.; Gledhill, T.; Jäger, P.; Makol, J.; Smit, H.; van der Hammen, H.; et al. Süßwasserfauna von Mitteleuropa, Vol. 7/2-1 Chelicerata: Araneae/Acari I; Gerecke, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Gerecke, R.; Gledhill, T.; Smit, H. Süßwasserfauna von Mitteleuropa, Vol. 7/2-2 Chelicerata: Acari II; Gerecke, R., Ed.; Spektrum: Heidelberg, Germany, 2010. [Google Scholar]
- Gerecke, R.; Gledhill, T.; Pešić, V.; Smit, H. Süßwasserfauna von Mitteleuropa, Bd. 7/2-3 Chelicerata; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Tuzovskij, P.V. Key to Deutonymphs of Water Mites [Opredelitel’ Deutonymphs Vodyanykh Kleshchey]; Akademia Nauka UdSSR: Moscow, Russia, 1990; pp. 1–238. [Google Scholar]
- UNE EN 15843; Water Quality—Guidance Standard on Determining the Degree of Modification of River Hydromorphology. CEN: Brussels, Belgium, 2010. Available online: https://www.en-standard.eu/une-en-15843-2010-water-quality-guidance-standard-on-determining-the-degree-of-modification-of-river-hydromorphology/ (accessed on 11 November 2022).
- Rom, D.M. An improved Hochberg procedure for multiple tests of significance. Br. J. Math. Stat. Psychol. 2013, 66, 189–196. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v7 User Manual/Tutorial. PRIMER-EPlymouth—References—Scientific Research Publishing. 2015. Available online: https://www.scirp.org/(S(oyulxb452alnt1aej1nfow45))/reference/ReferencesPapers.aspx?ReferenceID=1894960 (accessed on 11 November 2022).
- TIBCO Statistica® Document Management System 13.3.0. Available online: https://docs.tibco.com/products/tibco-statistica-document-management-system-13-3-0 (accessed on 11 November 2022).
- ScienceOpen. Canoco Reference Manual and User’s Guide: Software for Ordination (Version 5.0). Available online: https://www.scienceopen.com/document?vid=8b35db20-453f-48e0-89e5-d4f23f0cd6df (accessed on 11 November 2022).
- Pešić, V.; Asadi, M.; Cimpean, M.; Dabert, M.; Esen, Y.; Gerecke, R.; Martin, P.; Savić, A.; Smit, H.; Stur, E. Six species in one: Evidence of cryptic speciation in the Hygrobates fluviatilis complex (Acariformes, Hydrachnidia, Hygrobatidae). Syst. Appl. Acarol. 2017, 22, 1327–1377. [Google Scholar] [CrossRef]
- Pešić, V.; Saboori, A.; Zawal, A.; Dabert, M. Hidden but not enough: DNA barcodes reveal two new species in Hygrobates fluviatilis complex from Iran (Acariformes, Hydrachnidia, Hygrobatidae). Syst. Appl. Acarol. 2019, 24, 2439–2459. [Google Scholar] [CrossRef]
- Pešić, V.; Jovanović, M.; Manović, A.; Zawal, A.; Bańkowska, A.; Lyubomirova, L.; Karaouzas, I.; Dabert, M. Molecular evidence for two new species of the Hygrobates fluviatilis-complex from the Balkan Peninsula (Acariformes, Hydrachnidia, Hygrobatidae). Syst. Appl. Acarol. 2020, 25, 1702–1719. [Google Scholar] [CrossRef]
- Tuzovskij, P.V. A New Water Mite Species of the Hygrobates Fluviatilis-Complex from Russia (Acari, Hydrachnidia, Hygrobatidae). Zootaxa 2021, 4974. [Google Scholar] [CrossRef] [PubMed]
- Gerecke, R.; Hörweg, C. Water Mites of the Genus Atractides (Acari: Hydrachnidia: Hygrobatidae) from the Gesäuse National Park (Austria, Styria). Lauterbornia 2013, 76, 69–76. [Google Scholar]
- Gerecke, R. The Water Mites of the Genus Atractides Koch, 1837 (Acari, Hydrachnidia: Hygrobatidae) in Corsica and Sardinia. Zoosystema 2014, 36, 735–759. [Google Scholar] [CrossRef]
- Smit, H.; Gerecke, R.; Di Sabatino, A. A catalogue of water-mites of the superfamily Arrenuroidea (Acari: Hydrachnidia) from the Mediterranean countries. Arch. Hydrobiol. Suppl. 2000, 121, 201–267. [Google Scholar]
- Gerecke, R.; Martin, P.; Gledhill, T. Water Mites (Acari: Parasitengona: Hydrachnidia) as Inhabitants of Groundwater-Influenced Habitats—Considerations Following an Update of Limnofauna Europaea. Limnologica 2018, 69, 81–93. [Google Scholar] [CrossRef]
- Pešić, V.; Savić, A.; Jabłońska, A.; Michoński, G.; Grabowski, M.; Bańkowska, A.; Zawal, A. Environmental factors affecting water mite assemblages along eucrenon-hypocrenon gradients in Mediterranean karstic springs. Exp. Appl. Acarol. 2019, 77, 471–486. [Google Scholar] [CrossRef]
- Pozojević, I.; Pešić, V.; Goldschmidt, T.; Gottstein, S. Crenal Habitats: Sources of Water Mite (Acari: Hydrachnidia) Diversity. Diversity 2020, 12, 316. [Google Scholar] [CrossRef]
- Carpenter, S.R. Submersed vegetation: An internal factor in lake ecosystem succession. Am. Nat. 1981, 118, 372–383. [Google Scholar] [CrossRef]
- Opatrilova, L. (Ed.) WFD Intercalibration Phase 2: Milestone 5 Report—River/EC GIG/Benthic Invertebrates; European Commission Directorate General, JRC, Institute of Environment and Sustainability: Brussels, Belgium, 2011. [Google Scholar]
- Pozojević, I.; Vučković, N.; Dorić, V.; Šumanović, M.; Ternjej, I. Contribution to the knowledgeof the water mite (Acari: Hydrachnidia) fauna of Croatia—New data and records from a permanent pool in the Dinaric karst region. Persian J. Acarol. 2021, 10, 1–7. [Google Scholar]
- Di Sabatino, A.; Cicolani, B. On the Presence of the Family Torrenticolidae Piersig (Acari, Hydrachnidia) in Interstitial Waters of Sicily (South Italy): Description of a New Species. Ann. Limnol.-Int. J. Lim. 1993, 29, 31–39. [Google Scholar] [CrossRef]
- Esen, Y.; Erman, O. Kahramanmaraş ili Torrenticola Piersig, 1896 ve Monatractides K. Viets, 1926 (Acari: Hydrachnidia: Torrenticolidae) türleri ve Türkiye faunası için iki yeni kayıt. Firat Univ. J. Sci. 2014, 26, 39–44. Available online: https://www.bingol.edu.tr/documents/Mara%C5%9F_Torrenticolidae_Esen&Erman_2014.pdf (accessed on 16 January 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).