Fire Impact on the Formation and Development of the Boreal Pine Wooded Mires
Abstract
1. Introduction
2. Materials and Methods
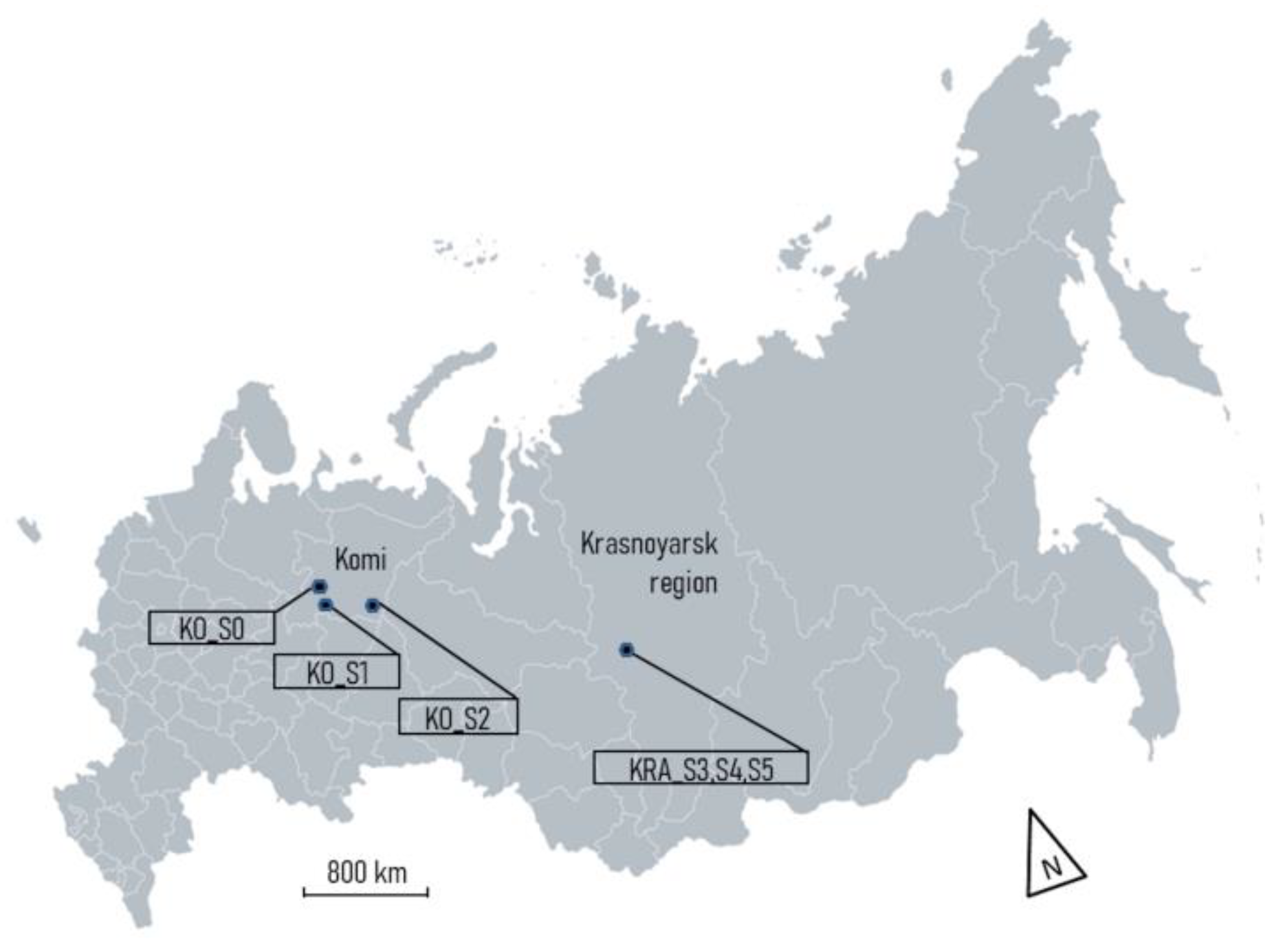
2.1. Site Description
2.2. Vegetation Study and Peat Sampling
2.3. Plant Macrofossil Analysis
2.4. Chronology
3. Results
3.1. Age Models and Chronologies
3.2. Macrofossil Analysis
4. Discussion
4.1. Post-Glacial Pine Wooded Bogs’ Initiation and Development
4.2. The Bogs’ Development and Fire Activity
4.3. The Impact of Pyrogenic Factor on the Bog Plant Communities during the Recent (Oligotrophic) Stage of Their Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, Z.; Loisel, J.; Brosseau, D.P.; Beilman, D.W.; Hunt, S.J. Global peatland dynamics since the last glacial maximum. Geophys. Res. Lett. 2010, 37, L13402. [Google Scholar] [CrossRef]
- Joosten, H. Peatlands, Climate Change Mitigation and Biodiversity Conservation; Nordic Council of Minister: Copenhagen, Denmark, 2015. [Google Scholar]
- Cubizolle, H.; Fassion, F.; Argant, J.; Latour-Argant, C.; Galet, P.; Oberlin, C. Mire initiation, climatic change and agricultural expansion over the course of the Late-Holocene in the Massif Central mountain range (France): Causal links and implications for mire conservation. Quat. Int. 2012, 251, 77–96. [Google Scholar] [CrossRef]
- Lavoie, M.; Pellerin, S.; Larocque, M. Examining the role of allogenous and autogenous factors in the longterm dynamics of a temperate headwater peatland (southern Quebec, Canada). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 386, 336–348. [Google Scholar] [CrossRef]
- Kur’ina, I.V.; Veretennikova, E.E. Impact of climate change of the Holocene on the development of the ridge-hollow swamp complex of Western Siberia. Proc. RAS Geogr. 2015, 2, 74–87. [Google Scholar] [CrossRef]
- Dyakonov, K.N.; Novenko, E.Y.; Mazei, N.G.; Kusilman, M.V. The age of peatlands and peatland formation stages in Polesie landscapes of the East European plain. Dokl. Earth Sci. 2020, 492, 464–470. [Google Scholar] [CrossRef]
- Wein, R.W.; MacLean, D.A. The Role of Fire in Northern Circumpolar Ecosystems; Chichester Wiley: Hoboken, NJ, USA, 1983; pp. 1–18. [Google Scholar]
- Bond, W.J.; Keeley, J.E. Fire as a global ‘herbivore’: The ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 2005, 20, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Bowman, D.M.J.S.; Balch, J.K.; Artaxo, P.; Bond, W.J.; Carlson, J.M.; Cochrane, M.A.; D’Antonio, C.M.; DeFries, R.S.; Doyle, J.C.; Harrison, S.P.; et al. Fire in the Earth system. Science 2009, 324, 481–484. [Google Scholar] [CrossRef]
- Driscoll, D.A.; Lindenmayer, D.B.; Bennett, A.F.; Bode, M.; Bradstock, R.A.; Cary, G.J.; Clarke, M.F.; Dexter, N.; Fensham, R.; Friend, G.; et al. Fire management for biodiversity conservation: Key research questions and our capacity to answer them. Biol. Conserv. 2010, 143, 1928–1939. [Google Scholar] [CrossRef]
- Kettridge, N.; Thompson, D.K.; Waddington, J.M. Impact of wildfire on the thermal behavior of northern peatlands: Observations and model simulations. J. Geophys. Res. 2012, 117, G02014. [Google Scholar] [CrossRef]
- Thompson, D.K. Wildfire Impacts on Peatland Ecohydrology. Ph.D. Thesis, School of Geography and Earth Sciences, McMaster University, Hamilton, ON, Canada, 2012. [Google Scholar]
- Pressler, Y.; Moore, J.C.; Cotrufo, M.F. Belowground community responses to fire: Meta-analysis reveals contrasting responses of soil microorganisms and mesofauna. Oikos 2019, 128, 309–327. [Google Scholar] [CrossRef]
- Doerr, S.H.; Santín, C. Global trends in wildfire and its impacts: Perceptions versus realities in a changing world. Phil. Trans. R. Soc. B 2016, 371, 20150345. [Google Scholar] [CrossRef] [PubMed]
- Kharuk, V.I.; Ponomarev, E.I.; Ivanova, G.A.; Dvinskaya, M.L.; Coogan, S.C.; Flannigan, M.D. Wildfires in the Siberian taiga. Ambio 2021, 50, 1953–1974. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.C.; Kobziar, L.N. Smoldering Combustion and Ground Fires: Ecological Effects and Multi-Scale Significance. Fire Ecol. 2013, 9, 124–132. [Google Scholar] [CrossRef]
- Parish, F.; Sirin, A.; Charman, D.; Joosten, H.; Minayeva, T.; Silvius, M.; Stringer, L. (Eds.) Assessment on Peatlands, Biodiversity and Climate Change: Main Report; Global Environment Centre: Osaka, Japan, 2008; 179p. [Google Scholar]
- Minayeva, T.; Sirin, A.A.; Stracher, G.B. The peat fires of Russia. In Coal and Peat Fires: A Global Perspective; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 2, pp. 376–394. [Google Scholar]
- Bixby, R.J.; Cooper, S.D.; Gresswell, R.E.; Brown, L.E.; Dahm, C.N.; Dwire, K.A. Fire effects on aquatic ecosystems: An assessment of the current state of the science. Freshw. Sci. 2015, 34, 1340–1350. [Google Scholar] [CrossRef]
- Joosten, H.; Sirin, A.; Couwenberg, J.; Laine, J.; Smith, P. The role of peatlands in climate regulation. In Peatland Restoration and Ecosystem Services: Science, Policy and Practice; Bonn, A., Allott, T., Evans, M., Joosten, H., Stoneman, R., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 63–76. [Google Scholar]
- Martin, D.A. At the nexus of fire, water and society. Philos. Trans. R. Soc. B 2016, 371, 20150172. [Google Scholar] [CrossRef]
- Harper, A.R.; Doerr, S.H.; Santin, C.; Froyd, C.A.; Sinnadurai, P. Prescribed fire and its impacts on ecosystem services in the UK. Sci. Total Environ. 2018, 624, 691–703. [Google Scholar] [CrossRef]
- Kiely, L.; Spracklen, D.V.; Wiedinmyer, C.; Conibear, L.A.; Reddington, C.L.; Arnold, S.R.; Knote, C.; Khan, M.F.; Latif, M.T.; Syaufina, L.; et al. Air quality and health impacts of vegetation and peat fires in Equatorial Asia during 2004–2015. Environ. Res. Lett. 2020, 15, 094054. [Google Scholar] [CrossRef]
- Flanagan, N.E.; Wang, H.; Winton, S.; Richardson, C.J. Low-severity fire as a mechanism of organic matter protection in global peatlands: Thermal alteration slows decomposition. Glob. Change Biol. 2020, 36, 3930–3946. [Google Scholar] [CrossRef] [PubMed]
- Joosten, H.; Clarke, D. Wise Use of Mires and Peatlands; International Mire Conservation Group and International Peat Society: Saarijärvi, Finland, 2002; 304p. [Google Scholar]
- Minaeva, T.Y.; Sirin, A.A. Peat fires—causes and ways of prevention. Sci. Ind. 2002, 9, 3–8. [Google Scholar]
- Sirin, A.; Minaeva, T.; Vozbrannaya, A.; Bartalev, S. How to avoid peat fires? Sci. Russ. 2011, 2, 13–21. [Google Scholar]
- Turetsky, M.R.; Benscoter, B.; Page, S.; Rein, G.; Van Der Werf, G.R.; Watts, A. Global vulnerability of peatlands to fire and carbon loss. Nat. Geosci. 2015, 8, 11–14. [Google Scholar] [CrossRef]
- Mętrak, M.; Malawska, M.; Kamiński, J.; Wiłkomirski, B. Chemical changes of peat soils and plant succesion on the deeply burnt mires. Pol. J. Environ. Stud. 2006, 15, 57–66. [Google Scholar]
- Sulwiński, M.; Mętrak, M.; Suska-Malawska, M. Long-term fire effects of the drained open fen on organic soils. Arch. Environ. Prot. 2017, 43, 11–19. [Google Scholar] [CrossRef]
- Van Beest, C.; Petrone, R.; Nwaishi, F.; Waddington, J.M.; Macrae, M. Increased peatland nutrient availability following the Fort Mcmurray Horse River wildfire. Diversity 2019, 11, 142. [Google Scholar] [CrossRef]
- Gorham, E. Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecol. Appl. 1991, 1, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Poulter, B.; Christensen, N.L.; Halpin, P.N. Carbon emissions from a temperate peat fire and its relevance to interannual variability of trace atmospheric greenhouse gases. J. Geophys. Res. 2006, 111, D06301. [Google Scholar] [CrossRef]
- Sirin, A.A.; Makarov, D.A.; Maslov, A.A.; Gul’be, Y.I.; Gummert, I. Depth of peat burning and carbon loss during an underground forest fire. Contemp. Probl. Ecol. 2020, 13, 769–779. [Google Scholar] [CrossRef]
- Kucherov, I.B.; Kutenkov, S.A. Dwarf shrub sphagnum-feathermoss and sphagnum pine forests of northern and middle taiga of Europaean Russia. Trans. Karelian Res. Cent. Russ. Acad. Sci. 2012, 1, 16–32. [Google Scholar]
- Yurkovskaya, T.K. Geography and Cartography of the Vegetation of the Mires of European Russia and Adjacent Territories. Proc. Komarov Bot. Institute 1992, 4, 1–256. [Google Scholar]
- Yurkovskaya, T.K. Regularities of distribution of mires in Russia. Bot. J. 2006, 91, 1777–1786. [Google Scholar]
- Kuznetsov, O.L. Vegetation dynamics of raised bogs. News Samara Sci. Cent. Russ. Acad. Sci. 2012, 14, 1288–1291. [Google Scholar]
- Kutenkov, S.A.; Kuznetsov, O.L. Diversity and dynamics of forested mires and paludified forests on the European North of Russia. In Diversity and Dynamics of Forest Ecosystems in Russia; Publishing house KMK: Moscow, Russia, 2013; Volume 2, pp. 152–204. [Google Scholar]
- Gromtsev, A.N. Fire regime in spontaneous forests of the North-Western taiga landscapes. Ecology 1993, 3, 22–26. [Google Scholar]
- Feurdean, A.; Diaconu, A.-C.; Pfeiffer, M.; Gałka, M.; Hutchinson, S.M.; Butiseaca, G.; Gorina, N.; Tonkov, S.; Niamir, A.; Tantau, I.; et al. Holocene wildfire regimes in forested peatlands in western Siberia: Interaction between peatland moisture conditions and the composition of plant functional types. Clim. Past 2021, 18, 1255–1274. [Google Scholar] [CrossRef]
- Taskaev, A.I. (Ed.) Atlas of the Komi Republic on Climate and Hydrology; DiK, Drofa: Moscow, Russia, 1997; p. 116. [Google Scholar]
- Schulze, E.D.; Vygodskaya, N.N.; Tchebakova, N.M.; Czimczik, C.I.; Kozlov, D.N.; Lloyd, J.; Mollicone, D.; Parfenova, E.; Sidorov, K.N.; Varlagin, A.V.; et al. The Eurosiberian Transect: An introduction to the experimental region. Chem. Phys. Meteorol. 2002, 54, 421–428. [Google Scholar] [CrossRef]
- Jiroušek, M.; Peterka, T.; Chytrý, M.; Jiménez-Alfaro, B.; Kuznetsov, O.L.; Pérez-Haase, A.; Aunina, L.; Biurrun, I.; Dítě, D.; Goncharova, N.; et al. Classification of European bog vegetation of the Oxycocco-Sphagnetea class. Appl. Veget. Sci. 2022, 25, e12646. [Google Scholar] [CrossRef]
- Dymov, A.A.; Gorbach, N.M.; Goncharova, N.N.; Karpenko, L.V.; Gabov, D.N.; Kutyavin, I.N.; Startsev, V.V.; Mazur, A.S.; Grodnitskaya, I.D. Holocene and recent fires influence on soil organic matter, microbiological and physico-chemical properties of peats in the European North-East of Russia. Catena 2022, 217, 106449. [Google Scholar] [CrossRef]
- Gorodnitskaya, I.D.; Karpenko, L.V.; Pashkeeva, O.E.; Goncharova, N.N.; Startsev, V.V.; Baturina, O.A.; Dymov, A.A. Impact of forest fires on the microbiological properties of oligotrophic peat soils and gleyed peat podzols of bogs in the Northern part of the Sym-Dubches Interfluve, Krasnoyarsk Region. Eurasian Soil Sci. 2022, 55, 460–473. [Google Scholar] [CrossRef]
- Ipatov, V.S.; Mirin, D.M. Description of Phythocoenosis. Metodical Recommendations; St. Petersburg State University Press: St. Petersburg, Russia, 2008; 71p. [Google Scholar]
- WFO (2022): World Flora Online. Available online: http://www.worldfloraonline.org (accessed on 22 November 2022).
- Dombrovskaya, A.V.; Koronieva, M.M.; Tyuremnov, S.N. Atlas of Plant Macroremains Occurring in Peat; State Energy Publishing: Moscow, Russia, 1959; pp. 1–89. [Google Scholar]
- Katz, N.Y.; Katz, S.V.; Skobeeva, E.I. Atlas of Plant Remains in Peat; Nedra: Moscow, Russia, 1977; 376p. [Google Scholar]
- Kutenkov, S.A. Korpi software for plotting stratigraphic diagrams of peat composition. Trans. Karelian Res. Cent. Russ. Acad. Sci. 2013, 6, 171–176. [Google Scholar]
- Chichagova, O.A. Radiocarbon Dating of Soil Humus; Nauka: Moscow, Russia, 1985; 157p. [Google Scholar]
- Blaauw, M. Methods and code for ’classical’ age-modelling of radiocarbon sequences. Quat. Geochron. 2010, 5, 512–518. [Google Scholar] [CrossRef]
- Reimer, P.J.; Bard, E.; Bayliss, A.; Beck, J.W.; Blackwell, P.G.; Ramsey, C.B.; Buck, C.E.; Cheng, H.; Edwards, R.L.; Friedrich, M. IntCal13 and Marine13 Radiocarbon Age Calibration Curves 0-50,000 years cal BP. Radiocarbon 2013, 55, 1869–1887. [Google Scholar] [CrossRef]
- Walker, M.; Head, M.J.; Lowe, J.; Berkelhammer, M.; BjÖrck, S.; Cheng, H.; Cwynar, L.C.; Fisher, D.; Gkinis, V.; Long, A.; et al. Subdividing the Holocene Series/Epoch: Formalization of stages/ages and subseries/subepochs, and designation of GSSPs and auxiliary stratotypes. J. Quat. Sci. 2019, 34, 173–186. [Google Scholar] [CrossRef]
- Khotinskiy, N.A. Controversial problems of reconstruction and correlation of Holocene paleoclimates. In Paleoclimates of Later Glaciation and Holocene; Nauka: Moscow, Russia, 1989; pp. 12–17. [Google Scholar]
- Golubeva, Y. Climate and vegetation of the post-glacial period on the territory of the Komi Republic. Lithosphere 2008, 2, 124–132. [Google Scholar]
- Andreicheva, L.N.; Bratushak, Y.V.; Marchenko-Vagapova, T.I. Development of the Natural Environment and Climate in the Pleistocene and Holocene in the North of the European Russia; Geoprint: Syktyvkar, Russia, 2006; 23p. [Google Scholar]
- Khotinskiy, N.A. The Holocene of the Northern Eurasia; Nauka: Moscow, Russia, 1977; 200p. [Google Scholar]
- Madany, M.N.; Swetnam, T.W.; West, N.E. Comparison of two approaches for determining fire dates from tree scars. For. Sci. 1982, 28, 856–861. [Google Scholar] [CrossRef]
- Fritts, H.C. Dendroclimatology and dendroecology. Quat. Res. 1971, 1, 419–449. [Google Scholar] [CrossRef]
- Grissino-Mayer, H.A. Manual and tutorial for the proper use of an increment borer. Tree-Ring Res. 2003, 59, 63–79. [Google Scholar]
- Rinn, F.T. Reference Manual. Computer Program for Tree-Ring Analysis and Presentation; Frank Rinn: Helenberg, Germany, 1996; Version 3.5; 264p. [Google Scholar]
- Ali, M.I.; Feng, F.; Liu, X.; Min, W.K.; Shabir, M. On some new operations in soft set theory. Comput. Math. Appl. 2009, 57, 1547–1553. [Google Scholar] [CrossRef]
- Van Bellen, S.; Garneau, M.; Ali, A.A.; Bergeron, Y. Did fires drive Holocene carbon sequestration in boreal ombrotrophic peatlands of eastern Canada? Quat. Res. 2012, 78, 50–59. [Google Scholar] [CrossRef]
- Ouarmim, S.; Asselin, H.; Hely, C.; Bergeron, Y.; Ali, A.A. Long-term dynamics of fire refuges in boreal mixedwood forests. J. Quat. Sci. 2014, 29, 123–129. [Google Scholar] [CrossRef]
- Dymov, A.A.; Grodnitskaya, I.D.; Yakovleva, E.V.; Dubrovskiy, Y.A.; Kutyavin, I.N.; Startsev, V.V.; Milanovsky, E.Y.; Prokushkin, A.S. Albic Podzols of Boreal Pine Forests of Russia: Soil Organic Matter, Physicochemical and Microbiological Properties across Pyrogenic History. Forests 2022, 13, 1831. [Google Scholar] [CrossRef]
- Liss, O.L.; Abramova, L.I.; Avetov, N.A.; Berezina, N.A.; Inisheva, L.I.; Kurnishkova, T.V.; Sluka, Z.A.; Tolpysheva, T.Y.; Shvedchikova, N.K. The Mire Systems of Western Siberia and Their Environmental Significance; Kuvaev, V.B., Ed.; Grif Publications: Tula, Russia, 2001; 584p. [Google Scholar]
- Kremenetski, K.V.; Velichko, A.A.; Borisova, O.K.; MacDonald, G.M.; Smith, L.C.; Frey, K.E.; Orlova, L.A. Peatlands of the Western Siberian lowlands: Current knowledge on zonation, carbon content and Late Quaternary history. Quat. Sci. Rev. 2003, 23, 703–723. [Google Scholar] [CrossRef]
- Smith, L.C.; Macdonald, G.M.; Velichko, A.A.; Beilman, D.W.; Borisova, O.K.; Frey, K.E.; Kremenetski, K.V.; Sheng, Y. Siberian peatlands a net carbon sink and global methane source since the early Holocene. Science 2004, 303, 353–356. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, G.M.; Beilman, D.W.; Kremenetski, K.V.; Sheng, Y.; Smith, L.C.; Velichko, A.A. Rapid Early Development of Circumarctic Peatlands and Atmospheric CH4 and CO2 Variations. Science 2006, 314, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Korhola, A.; Ruppel, M.; Seppä, H.; Väliranta, M.; Virtanen, T.; Weckström, J. The Importance of northern Peatland Expansion to the Late-Holocene Rise of Atmospheric Methane. Quat. Sci. Rev. 2010, 29, 611–617. [Google Scholar] [CrossRef]
- Ruppel, M.; Väliranta, M.; Virtanen, T.; Korhola, A. Postglacial spatiotemporal peatland initiation and lateral expansion dynamics in North America and northern Europe. Holocene 2013, 23, 1596–1606. [Google Scholar] [CrossRef]
- Kuryina, I.V.; Veretennikova, E.E.; Golovatskaya, E.A.; Blyakharchuk, T.A.; Smirnov, S.V. Dynamics of the level of watering of swamps in the southern taiga subzone of Western Siberia in the middle and late Holocene. Bull. Tomsk State Univ. Biol. 2018, 42, 218–241. [Google Scholar]
- Zemtsov, V.A.; Inisheva, L.I. Mires of Western Siberia—Their Role in the Biosphere, 2nd ed.; TGU: Tomsk, Russia, 2000. [Google Scholar]
- Elina, G.A.; Lukashov, A.D.; Yurkovskaya, T.K. Late Glacial and Holocene Palaeovegetation and Palaeogeography of Eastern Fennoscandia; Finnish Environment Institute: Helsinki, Finland, 2010; Volume 4, 300p. [Google Scholar]
- Borisova, O.K. Landscape and climate change in Holocene. News RAS Geogr. 2014, 2, 5–20. [Google Scholar]
- Andreicheva, L.N.; Marchenko-Vagapova, T.I.; Buravskaya, M.N.; Golubeva, Y.V. The Natural Environment of the Neopleistocene and Holocene in the European Northeast of Russia; GEOS: Moscow, Russia, 2015; p. 224. [Google Scholar]
- Dendievel, A.-M.; Jouffroy-Bapicot, I.; Argant, J.; Scholtès, A.; Tourman, A.; Beaulieu, J.-L.; Cubizolle, H. From natural to cultural mires during the last 15 ka years: An integrated approach comparing 14C14C ages on basal peat layers with geomorphological, palaeoecological and archaeological data (Eastern Massif Central, France). Quat. Sci. Rev. 2020, 233, 106219. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Mazei, N.G.; Kupriyanov, D.A.; Kusilman, M.V.; Olchev, A.V. Peatland initiation in Central European Russia during the Holocene: Effect of climate conditions and fires. Holocene 2021, 31, 545–555. [Google Scholar] [CrossRef]
- Kuznetsov, O.L. Bog ecosystems of the Karelian part of the green belt of Fennoscandia. Tr. Kar. Sci. Cent. RAS 2014, 6, 77–88. [Google Scholar]
- Pyavchenko, N.I. On the age of peatlands and changes in vegetation in the south of Western Siberia in the Holocene. Bull. Quat. Study Comm. 1983, 52, 164–170. [Google Scholar]
- Pyavchenko, N.I. Peat Bogs, Their Natural and Economic Importance; Nauka: Moscow, Russia, 1985; 152p. [Google Scholar]
- Jones, M.C.; Yu, Z. Rapid deglacial and early Holocene expansion of peatlands in Alaska. Proc. Natl. Acad. Sci. USA 2010, 107, 7347–7352. [Google Scholar] [CrossRef] [PubMed]
- Antipin, V.K.; Elina, G.A.; Tokarev, P.N.; Brazovskaya, T.I. Mire ecosystems of ≪Vodlozersky≫ National Nature Park: Past, present and future. Bot. J. 1996, 81, 21–37. [Google Scholar]
- Elina, G.A.; Arslanov, K.A.; Klimanov, V.A.; Usova, L.I. Vegetation and climatochronology of Holocene in Lovozero plain of Kola Peninsula (according to spore-pollen diagrams of pulsa mire). Bot. J. 1995, 80, 1–16. [Google Scholar]
- Elina, G.A.; Arslanov, K.A.; Klimanov, V.A. Stages of development of Holocene vegetation in southern and eastern Karelia. Bot. J. 1996, 81, 1–17. [Google Scholar]
- Efremova, T.T.; Efremov, S.P.; Kosykh, N.P.; Mironicheva-Tokareva, N.P.; Titlyanova, A.A. Biological productivity and soils of southern Vasyugany bogs. Sib. Ecol. J. 1994, 1, 253–269. [Google Scholar]
- Pitkänen, A.; Huttunen, P.; Jungner, H.; Meriläinen, J.; Tolonen, K. Holocene fire history of middle boreal pine forest sites in eastern Finland. Ann. Bot. Fennici. 2003, 40, 15–33. [Google Scholar]
- Franzén, L.G.; Malmgren, B.A. Microscopic charcoal and tar (CHAT) particles in peat: A 6500-year record of palaeo-fires in southern Sweden. Mires Peat 2012, 10, 1–25. [Google Scholar]
- Kozlovskaya, L.S.; Medvedeva, V.M.; Pyavchenko, N.I. Dynamics of Organic Matter during Peat Formation; Nauka: Leningrad, Russia, 1978; 173p. [Google Scholar]
- Glebov, F.Z. The Relationships between Forest and Mire in the Taiga Zone; Science Siberian Department: Novosibirsk, Russia, 1988; 184p. [Google Scholar]
- Vasiliev, S.V. Forests and Wetlands of West Siberia; NTL: Tomsk, Russia, 2007; 276p, ISBN 978-5-89503-334-0. [Google Scholar]
- Akhmetieva, N.P.; Belova, S.E.; Jamalov, R.G.; Kulichevskaya, I.S.; Lapina, E.E.; Mikhailova, A.V. Natural restoration of mires after fires. Water Res. 2014, 41, 343–354. [Google Scholar]
- Badmazhapova, I.A.; Gyninova, A.B.; Gonchikov, B.N. The chemical property change of the drained peat soils under the fire factor influence. Bull. KrasGAU 2014, 5, 50–55. [Google Scholar]
- Vasilevich, V.I. Swampy birch forests of the North-West of European Russia. Bot. J. 1997, 82, 19–29. [Google Scholar]
- Grishutkin, O.G. Influence of the fires of 2010 on the swamp ecosystems of the Mordovian State Nature Reserve. Proc. Smidovich Mordovian State Nat. Reserve 2012, 10, 261–265. [Google Scholar]
- Napreenko-Dorokhova, T.V.; Napreenko, M.G. Development of the natural complex of Tselau (according to the structure of the peat deposit). Bull. Balt. Fed. Univ. I Kant. Nat. Med. Sci. 2015, 1, 50–64. [Google Scholar]
- Pyavchenko, N.I. Forest Swamp Science; Publishing House of the Academy of Sciences of the USSR: Moscow, Russia, 1963; 192p. [Google Scholar]
- Marchenko-Vagapova, T.I.; Golubeva, Y.V. Warming or cooling? The question is still open! Bull. Inst. Geol. Komi SC UB RAS 2010, 189, 45–46. [Google Scholar]
- Furyaev, V.V.; Furyaev, E.A. Pyroecological properties of Scotch pine in Central Siberia. Conifers Boreal Zone 2008, 25, 103–109. [Google Scholar]
- Peteet, D.; Andreev, A.; Bardeen, W.; Mistretta, F. Long-term Arctic peatland dynamics, vegetation and climate history of the Pur-Taz region, western Siberia. Boreas 1998, 27, 115–126. [Google Scholar] [CrossRef]
- Gorbach, N.M.; Kutyavin, I.N.; Startsev, V.V.; Dymov, A.A. Dynamics of fires in the northeast of the European part of Russia in the Holocene. Theor. Appl. Ecol. 2021, 3, 104–110. [Google Scholar] [CrossRef]
- Karpenko, L.V.; Prokushkin, A.S. Genesis and history of the post-glacial evolution of forest bog in the valley of the Dubches river. Sib. For. J. 2018, 5, 33–44. [Google Scholar]
- Kupriyanov, D.A.; Novenko, E.Y. Reconstruction of the dynamics of forest fires in Central Meshchera in the Holocene (according to paleoanthracological analysis data). Sib. Ecol. J. 2019, 26, 253–263. [Google Scholar]
- Marlon, J.R.; Bartlein, P.J.; Walsh, M.K.; Harrison, S.P.; Brown, K.J.; Edwards, M.E.; Higuera, P.E.; Power, M.J.; Anderson, R.S.; Briles, C.; et al. Wildfire responses to abrupt climate change in North America. Proc. Natl. Acad. Sci. USA 2009, 106, 2519–2524. [Google Scholar] [CrossRef]
- Barhoumi, C.; Peyron, O.; Joannin, S.; Subetto, D.; Kryshen, A.; Drobyshev, I.; Girardin, M.P.; Brossier, B.; Paradis, L.; Pastor, T.; et al. Gradually increasing forest fire activity during the Holocene in the northern Ural region (Komi Republic, Russia). Holocene 2019, 29, 1906–1920. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; pp. 3–32. [Google Scholar] [CrossRef]
- Karpenko, L.V.; Prokushkin, A.S. Reconstruction of fires in virgin forests at Sym-Dubches interfluve in Holocene. Sib. For. J. 2019, 5, 61–69. [Google Scholar]
- Ryzhkova, N.I.; Kutyavin, I.N.; Pinto, G.; Kryshen, A.M.; Aleinikov, A.A.; Vozmitel, F.K.; Drobyshev, I.V. 2020 Dynamics of fire activity in the pine forests of the Pechora-Ilychsky reserve according to the data of dendrochronological studies. Proc. Pechora-Ilychsky Reserve 2020, 19, 101–106. [Google Scholar]









| Site | Laboratory Number | Depth, cm | 14C yr BP (±σ) | Calibrated Age (yr BP, before 1950 AD) |
|---|---|---|---|---|
| 0 | 14C2106 | 25–35 | 990 ± 90 | 894 |
| 14C2111 | 55–65 | 2730 ± 115 | 2870 | |
| 14C2139 | 89–90 | 7350 ± 300 | 8190 | |
| I | 14C1976 | 50–70 | 4433 ± 150 | 5075 |
| 14C1919 | 104–105 | 9030 ± 200 | 10,133 | |
| II | 14C1903 | 30–40 | 455 ± 80 | 442 |
| 14C1884 | 70–80 | 1875 ± 55 | 1853 | |
| 14C1882 | 100–110 | 3840 ± 55 | 4212 | |
| 14C1883 | 129–130 | 5080 ± 65 | 5860 | |
| 14C1895 | 160–170 | 6228 ± 120 | 7078 | |
| 14C1969 | 210–220 | 6730 ± 120 | 7624 | |
| III | 14C1926 | 35–40 | 504 ± 65 | 537 |
| 14C1930 | 100–120 | 1363 ± 65 | 1214 | |
| 14C1923 | 140–160 | 2040 ± 80 | 1999 | |
| 14C1925 | 180–200 | 2405 ± 95 | 2396 | |
| IV | 14C1918 | 40–55 | 214 ± 70 | 203 |
| 14C1923 | 55–65 | 1810 ± 65 | 1710 | |
| V | 14C1925 | 60–70 | 1430 ± 120 | 1337 |
| 14C1920 | 107–108 | 2915 ± 115 | 3082 | |
| 14C1924 | 120–130 | 4125 ± 95 | 4623 | |
| 14C1890 | 160–170 | 4690 ± 130 | 5418 | |
| 14C1897 | 239–240 | 7535 ± 120 | 8319 |
| Site | Dates of Fires | Accounting Year | The Last Fire, Years |
|---|---|---|---|
| Komi Republic (European North) | |||
| 0 | 1919 | 2020 | 100 ± 5 |
| I | 1879 | 2020 | 140 ± 5 |
| II | 1906 | 2020 | 113 ± 5 |
| Krasnoyarsk Region (Western Siberia) | |||
| III | - | - | - |
| IV | 1938, 1959 | 2019 | ±5 |
| V | 1940, 1986 | 2019 | 79 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goncharova, N.; Dubrovskiy, Y.A.; Miglovets, M.; Kutyavin, I.N.; Dymov, A. Fire Impact on the Formation and Development of the Boreal Pine Wooded Mires. Diversity 2023, 15, 159. https://doi.org/10.3390/d15020159
Goncharova N, Dubrovskiy YA, Miglovets M, Kutyavin IN, Dymov A. Fire Impact on the Formation and Development of the Boreal Pine Wooded Mires. Diversity. 2023; 15(2):159. https://doi.org/10.3390/d15020159
Chicago/Turabian StyleGoncharova, Nadezhda, Yuri A. Dubrovskiy, Mikhail Miglovets, Ivan N. Kutyavin, and Alexey Dymov. 2023. "Fire Impact on the Formation and Development of the Boreal Pine Wooded Mires" Diversity 15, no. 2: 159. https://doi.org/10.3390/d15020159
APA StyleGoncharova, N., Dubrovskiy, Y. A., Miglovets, M., Kutyavin, I. N., & Dymov, A. (2023). Fire Impact on the Formation and Development of the Boreal Pine Wooded Mires. Diversity, 15(2), 159. https://doi.org/10.3390/d15020159







