North–South Differentiation of Black Flies in the Western Cordillera of North America: A New Species of Prosimulium (Diptera: Simuliidae) †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection Sites
2.2. Chromosomes
2.3. Morphology
2.4. Type Depositories
3. Results
3.1. Chromosomal Description
3.2. Morphological Description
3.3. Diagnosis
3.4. Type Material
3.5. Additional Specimens Examined
3.6. Distribution
3.7. Bionomics
3.8. Etymology
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schweikert, R.A.; Bogan, N.L.; Girty, G.H.; Hanson, R.E.; Merguerian, C. Timing and structural expression of the Nevadan Orogeny, Sierra Nevada, California. Geol. Soc. Am. Bull. 1984, 95, 967–979. [Google Scholar] [CrossRef]
- McMillan, M.E.; Heller, P.L.; Wing, S.L. History and causes of post-Laramide relief in the Rocky Mountain orogenic plateau. Geol. Soc. Am. Bull. 2006, 118, 393–405. [Google Scholar] [CrossRef]
- Clark, P.U.; Dyke, A.S.; Shakun, J.D.; Carlson, A.E.; Clark, J.; Wohlfarth, B.; Mitrovica, J.X.; Hostetler, S.W.; McCabe, A.M. The Last Glacial Maximum. Science 2009, 325, 710–714. [Google Scholar] [CrossRef]
- Hewitt, G. The genetic legacy of the Quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef]
- Brunsfeld, S.J.; Sullivan, J.; Soltis, D.E.; Soltis, P.S. A comparative phylogeography of northwestern North America: A synthesis. In Integrating Ecology and Evolution in a Spatial Context; Silvertown, J., Antonovics, J., Eds.; Blackwell Science: Oxford, UK, 2001; pp. 319–339. [Google Scholar]
- Ehlers, J.; Gibbard, P. Quaternary glaciation. In Encyclopedia of Snow, Ice and Glaciers; Singh, V.P., Singh, P., Haritashya, U.K., Eds.; Springer: New York, NY, USA, 2011; pp. 873–882. [Google Scholar] [CrossRef]
- Adler, P.H.; Cheke, R.A.; Post, R.J. Evolution, epidemiology, and population genetics of black flies (Diptera: Simuliidae). Infect. Genet. Evol. 2010, 10, 846–865. [Google Scholar] [CrossRef] [PubMed]
- Adler, P.H. World blackflies (Diptera: Simuliidae): A comprehensive revision of the taxonomic and geographical inventory [2022]. 2022, p. 145. Available online: http://biomia.sites.clemson.edu/pdfs/blackflyinventory.pdf (accessed on 11 November 2022).
- Weinmann, C.J.; Anderson, J.R.; Longhurst, W.M.; Connolly, G. Filarial worms of Columbian black-tailed deer in California. 1. Observations in the vertebrate host. J. Wildl. Dis. 1973, 9, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Key, H.; Wenk, P. The transmission of Onchocerca tarsicola (Filarioidea: Onchocercidae) by Odagmia ornata and Prosimulium nigripes (Diptera: Simuliidae). J. Helminthol. 1981, 55, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Adler, P.H.; Currie, D.C.; Wood, D.M. The Black Flies (Simuliidae) of North America; Cornell University Press: Ithaca, NY, USA, 2004. [Google Scholar]
- Rivera, J.; Currie, D.C. Identification of Nearctic black flies using DNA barcodes (Diptera: Simuliidae). Mol. Ecol. Resour. 2009, s1, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Adler, P.H.; Kúdelová, T.; Kúdela, M.; Seitz, G.; Ignjatović-Ćupina, A. Cryptic biodiversity and the origins of pest status revealed in the macrogenome of Simulium colombaschense (Diptera: Simuliidae), history’s most destructive black fly. PLoS ONE 2016, 11, e0147673. [Google Scholar] [CrossRef]
- Basrur, P.K. The salivary gland chromosomes of seven segregates of Prosimulium (Diptera: Simuliidae) with a transformed centromere. Can. J. Zool. 1959, 37, 527–570. [Google Scholar] [CrossRef]
- Basrur, P.K. The salivary gland chromosomes of seven species of Prosimulium (Diptera: Simuliidae) from Alaska and British Columbia. Can. J. Zool. 1962, 40, 1019–1033. [Google Scholar] [CrossRef]
- Chance, M.M. The functional morphology of the mouthparts of blackfly larvae (Diptera: Simuliidae). Quaest. Entomol. 1970, 6, 254–284. [Google Scholar]
- Peterson, B.V.; Kondratieff, B.C. The black flies (Diptera: Simuliidae) of Colorado: An annotated list with keys, illustrations and descriptions of three new species. Mem. Am. Entomol. Soc. 1995, 42, 1–121. [Google Scholar]
- Sommerman, K.M. Prosimulium esselbaughi n. sp., the Alaskan P. hirtipes 2 (Diptera: Simuliidae). Proc. Entomol. Soc. Wash. 1964, 66, 141–145. [Google Scholar]
- Peterson, B.V. The Prosimulium of Canada and Alaska. Mem. Entomol. Soc. Can. 1970, 69, 1–216. [Google Scholar]
- Mason, P.G.; Shemanchuk, J.A. Black flies. Agric. Can. Publ. 1990, 1499/E, 1–19. [Google Scholar]
- Hull, J.M.; Keane, J.J.; Savage, W.K.; Godwin, S.A.; Shafer, J.A.; Jepsen, E.P.; Gerhardt, R.; Stermer, C.; Ernest, H.B. Range-wide genetic differentiation among North American great gray owls (Strix nebulosa) reveals a distinct lineage restricted to the Sierra Nevada, California. Mol. Phylogen. Evol. 2010, 56, 212–221. [Google Scholar] [CrossRef]
- Bayard de Volo, S.; Reynolds, R.T.; Sonsthagen, S.A.; Talbot, S.L.; Antolin, M.F. Phylogeography, postglacial gene flow, and population history of North American Northern Goshawks (Accipiter gentilis). Auk 2013, 130, 342–354. [Google Scholar] [CrossRef]
- Krejsa, D.M.; Talbot, S.L.; Sage, G.K.; Sonsthagen, S.A.; Jung, T.S.; Magoun, A.J.; Cook, J.A. Dynamic landscapes in northwestern North America structured populations of wolverines (Gulo gulo). J. Mammal. 2021, 102, 891–908. [Google Scholar] [CrossRef]
- Nadeau, S.; Godbout, J.; Lamothe, M.; Gros-Louis, M.-C.; Isabel, N.; Ritland, K. Contrasting patterns of genetic diversity across the ranges of Pinus monticola and P. strobus: A comparison between eastern and western North American postglacial colonization histories. Am. J. Bot. 2015, 102, 1342–1355. [Google Scholar] [CrossRef]
- Dulfer, H.E.; Margold, M.; Darvill, C.M.; Stroeven, A.P. Reconstructing the advance and retreat dynamics of the central sector of the last Cordilleran Ice Sheet. Quatern. Sci. Rev. 2022, 284, 107465. [Google Scholar] [CrossRef]
- Colella, J.P.; Lan, T.; Talbot, S.L.; Lindqvist, C.; Cook, J.A. Whole-genome resequencing reveals persistence of forest-associated mammals in Late Pleistocene refugia along North America’s North Pacific Coast. J. Biogeogr. 2021, 48, 1153–1169. [Google Scholar] [CrossRef]
- Rothfels, K.H. Cytotaxonomy of black flies (Simuliidae). Annu. Rev. Entomol. 1979, 24, 507–539. [Google Scholar] [CrossRef]
- Madahar, D.P. Cytogenetic study in triploidy in Prosimulium ursinum (Simuliidae: Diptera). Genetics 1973, 74 (Suppl. S2), 170–171. [Google Scholar]
- Finn, D.; Adler, P.H. Population genetic structure of a rare high-elevation black fly, Metacnephia coloradensis, occupying Colorado lake outlet streams. Freshwat. Biol. 2006, 51, 2240–2251. [Google Scholar] [CrossRef]
- Mee, J.A.; Moore, J.-S. The ecological and evolutionary implications of microrefugia. J. Biogeogr. 2014, 41, 837–841. [Google Scholar] [CrossRef]
- Latch, E.K.; Heffelfinger, J.R.; Fike, J.A.; Rhodes, O.E., Jr. Species-wide phylogeography of North American mule deer (Odocoileus hemionus): Cryptic glacial refugia and postglacial recolonization. Mol. Ecol. 2009, 18, 1730–1745. [Google Scholar] [CrossRef] [PubMed]
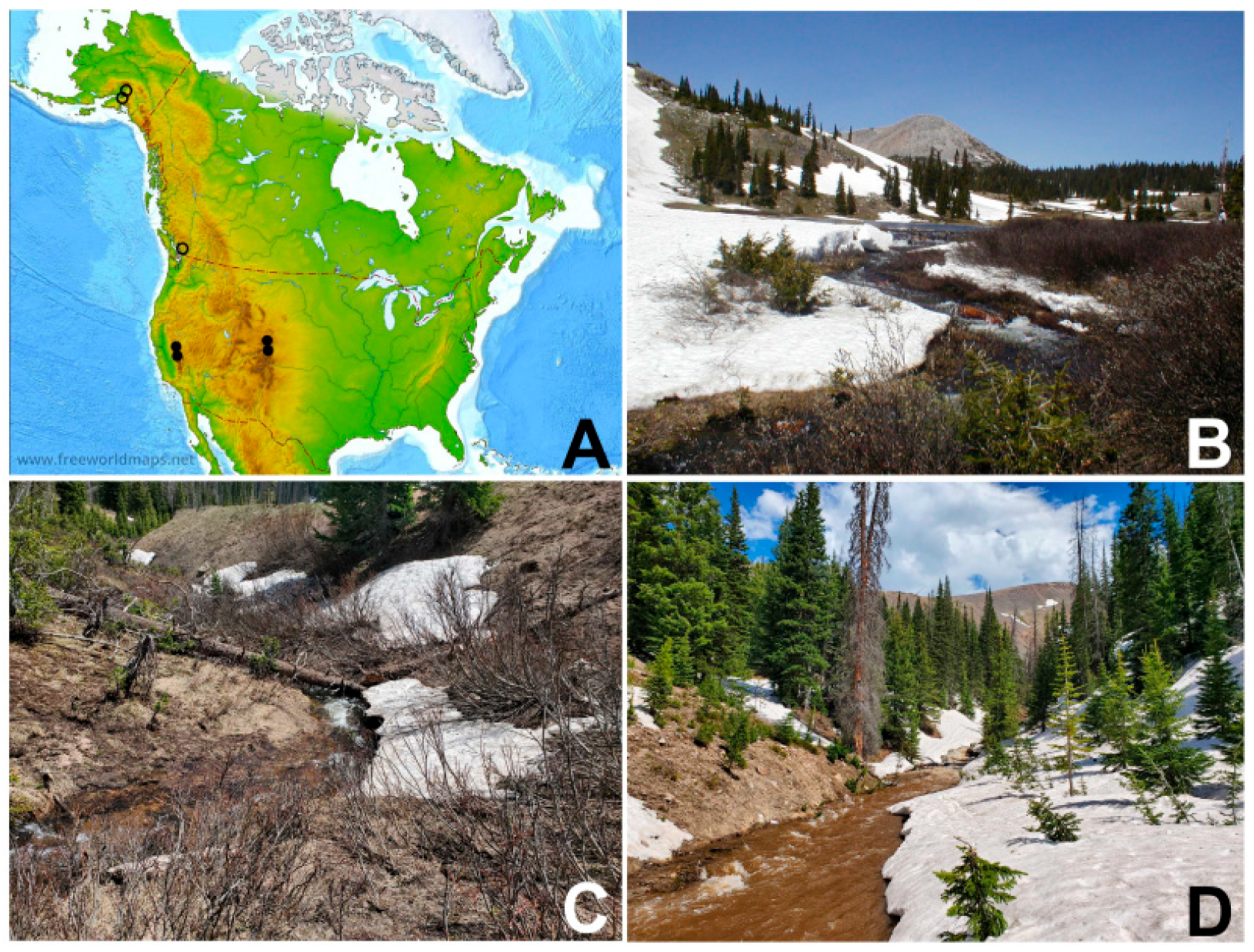







| Site | Location (Stream Width) | Latitude Longitude | Elevation (m above Sea Level) | Date | Life Stage |
|---|---|---|---|---|---|
| 1a | WY, Albany County, Snowy Range Pass, Libby Creek (2.0–2.5 m) | 41°21′07″ N 106°17′01″ W | 3233 | 11 June 2007 | 13 larvae (4 chromosome preparations) |
| 1b | 12 June 2008 | 2 larvae (2 chromosome preparations) | |||
| 1c | 7 July 2008 | 7 pupae, 2 males and 5 females with pupal exuviae | |||
| 1d | 17 July 2008 | 4 pupae, 2 females with pupal exuviae | |||
| 2 | CO, Jackson County, near Cameron Pass, snowmelt trib. Michigan River (0.5–1.0 m) | 40°30′31″ N 105°53′05″ W | 3089 | 11 June 2022 | 6 larvae (6 chromosome preparations) |
| 3 | CO, Jackson County, trib. Michigan River (0.5–1.0 m) | 40°30′56″ N 105°53′11″ W | 3139 | 20 June 2022 | 2 larvae (2 chromosome preparations) |
| 4 | CO, Jackson County, Cameron Pass, Michigan Ditch (2.0–2.5 m) | 40°31′13″ N 105°53′32″ W | 3135 | 20 June 2022 | 2 larvae (2 chromosome preparations) |
| Site | |||||
|---|---|---|---|---|---|
| 1a 1 | 1b 1 | 2 | 3 | 4 | |
| Female:Male | 3:3 | 0:2 | 4:2 | 2:0 | 1:1 |
| IS-37 | – 2 | – | 0.08 | – | – |
| IIS-17 | – | – | – | 0.25 | 0.25 |
| IIL-15 | 0.42 | – | – | – | |
| IIL-16 | – | – | – | – | 0.25 |
| IIIS hb80 | – | – | – | 0.25 | – |
| IIIS hb81 | 1.00 | 1.00 | 0.08 | – | |
| IIIL-23 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| IIIL-3 4 | * | * | * | * | * |
| IIIL-36 | – | – | 0.17 | – | 0.25 |
| IIIL-37 | – | – | 0.17 | 0.50 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adler, P.H.; Reeves, W.K. North–South Differentiation of Black Flies in the Western Cordillera of North America: A New Species of Prosimulium (Diptera: Simuliidae). Diversity 2023, 15, 212. https://doi.org/10.3390/d15020212
Adler PH, Reeves WK. North–South Differentiation of Black Flies in the Western Cordillera of North America: A New Species of Prosimulium (Diptera: Simuliidae). Diversity. 2023; 15(2):212. https://doi.org/10.3390/d15020212
Chicago/Turabian StyleAdler, Peter H., and Will K. Reeves. 2023. "North–South Differentiation of Black Flies in the Western Cordillera of North America: A New Species of Prosimulium (Diptera: Simuliidae)" Diversity 15, no. 2: 212. https://doi.org/10.3390/d15020212
APA StyleAdler, P. H., & Reeves, W. K. (2023). North–South Differentiation of Black Flies in the Western Cordillera of North America: A New Species of Prosimulium (Diptera: Simuliidae). Diversity, 15(2), 212. https://doi.org/10.3390/d15020212







