The Effects of Vegetation and the Environment on Testate Amoeba Assemblages in Sphagnum Peatlands in the Northern Caucasus Mountains
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Region
2.2. Study Sites
2.3. Field Sampling and Measurements
2.4. Testate Amoeba Analysis
2.5. Data Analyses
3. Results
3.1. Variation in Environmental Characteristics
3.2. General Characteristics of the Testate Amoeba, Vascular Plant and Bryophyte Assemblages
3.3. Multiple Factor Analysis of the Environment, Vegetation and Testate Amoeba Data
3.4. Effects of Abiotic Variables on Vegetation and Testate Amoeba Assembalges
3.5. Variance Paritioning of the Environment and Vegetation Effects on the Species Composition of Testate Amoebae
4. Discussion
4.1. Sphagnum-Dwelling Testate Amoebae in the Northern Caucasus Mountains
4.2. The Relationships among the Environment, Vegetation and Testate Amoebae
4.3. The Effect of Environmental Variables on the Species Structure of Testate Amoeba Assemblages
4.4. The Ecological Preferences of Testate Ameoba Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wardle, D.A. Communities and Ecosystems: Linking the Aboveground and Belowground Components (MPB-34); Princeton University Press: Princeton, NJ, USA, 2002; ISBN 0-691-07487-9. [Google Scholar]
- Hooper, D.U.; Bignell, D.E.; Brown, V.K.; Brussard, L.; Mark Dangerfield, J.; Wall, D.H.; Wardle, D.A.; Coleman, D.C.; Giller, K.E.; Lavelle, P.; et al. Interactions between Aboveground and Belowground Biodiversity in Terrestrial Ecosystems: Patterns, Mechanisms, and Feedbacks. BioScience 2000, 50, 1049. [Google Scholar] [CrossRef]
- Bezemer, T.M.; Lawson, C.S.; Hedlund, K.; Edwards, A.R.; Brook, A.J.; Igual, J.M.; Mortimer, S.R.; Van Der Putten, W.H. Plant Species and Functional Group Effects on Abiotic and Microbial Soil Properties and Plant-Soil Feedback Responses in Two Grasslands. J. Ecol. 2006, 94, 893–904. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Hester, A.J.; Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Hewison, R.L.; Potts, J.M. Is Vegetation Composition or Soil Chemistry the Best Predictor of the Soil Microbial Community? Plant Soil. 2010, 333, 417–430. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Bardgett, R.D.; van Straalen, N.M. The Unseen Majority: Soil Microbes as Drivers of Plant Diversity and Productivity in Terrestrial Ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Millard, P.; Singh, B.K. Does Grassland Vegetation Drive Soil Microbial Diversity? Nutr. Cycl. Agroecosyst. 2010, 88, 147–158. [Google Scholar] [CrossRef]
- Reynolds, H.L.; Packer, A.; Bever, J.D.; Clay, K. Grassroots Ecology: Plant-Microbe-Soil Interactions as Drivers of Plant Community Structure and Dynamics. Ecology 2003, 84, 2281–2291. [Google Scholar] [CrossRef]
- Cheng, D.-L.; Niklas, K.J. Above- and Below-Ground Biomass Relationships Across 1534 Forested Communities. Ann. Bot. 2007, 99, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.S.; Geisen, S.; Morriën, E.; Snoek, B.L.; van der Putten, W.H. Network Analyses Can Advance Above-Belowground Ecology. Trends Plant Sci. 2018, 23, 759–768. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, G.; Hai, X.; Li, J.; Shangguan, Z.; Peng, C.; Deng, L. Long-Term Forest Succession Improves Plant Diversity and Soil Quality but Not Significantly Increase Soil Microbial Diversity: Evidence from the Loess Plateau. Ecol. Eng. 2020, 142, 105631. [Google Scholar] [CrossRef]
- Artz, R.R.E.; Chapman, S.J.; Siegenthaler, A.; Mitchell, E.A.D.; Buttler, A.; Bortoluzzi, E.; Gilbert, D.; Yli-Petays, M.; Vasander, H.; Francez, A.-J. Functional Microbial Diversity in Regenerating Cutover Peatlands Responds to Vegetation Succession. J. Appl. Ecol. 2008, 45, 1799–1809. [Google Scholar] [CrossRef]
- Jassey, V.E.; Chiapusio, G.; Binet, P.; Buttler, A.; Laggoun-Défarge, F.; Delarue, F.; Bernard, N.; Mitchell, E.A.; Toussaint, M.-L.; Francez, A.-J.; et al. Above- and Belowground Linkages in Sphagnum Peatland: Climate Warming Affects Plant-Microbial Interactions. Glob. Chang. Biol. 2013, 19, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Rydin, H.; Jeglum, J.K.; Bennett, K.D. The Biology of Peatlands, 2nd ed.; Oxford University Press: Oxford, UK, 2013; ISBN 978-0-19-960299-5. [Google Scholar]
- Yu, Z.C. Northern Peatland Carbon Stocks and Dynamics: A Review. Biogeosciences 2012, 9, 4071–4085. [Google Scholar] [CrossRef]
- Belyea, L.R.; Malmer, N. Carbon Sequestration in Peatland: Patterns and Mechanisms of Response to Climate Change. Glob. Chang. Biol. 2004, 10, 1043–1052. [Google Scholar] [CrossRef]
- Gilbert, D.; Mitchell, E.A.D. Chapter 13 Microbial Diversity in Sphagnum Peatlands. In Peatlands; Martini, I.P., Cortizas, A.M., Chesworth, W., Eds.; Developments in Earth Surface Processes; Elsevier: Amsterdam, The Netherlands, 2006; Volume 9, pp. 287–318. [Google Scholar]
- Mazei, Y.; Tsyganov, A. Freshwater Testate Amoebae, 1st ed.; KMK Scientific Press Ltd.: Moscow, Russia, 2006; ISBN 5-873317-336-2. [Google Scholar]
- Gilbert, D.; Amblard, C.; Bourdier, G.; Francez, A.-J. The Microbial Loop at the Surface of a Peatland: Structure, Function, and Impact of Nutrient Input. Microb. Ecol. 1998, 35, 83–93. [Google Scholar] [CrossRef]
- Gilbert, D.; Amblard, C.; Bourdier, G.; André-Jean, F.; Mitchell, E.A.D. Le régime alimentaire des Thécamoebiens (Protista, Sarcodina). L’Année Biol. 2000, 39, 57–68. [Google Scholar] [CrossRef]
- Potapov, A.M.; Rozanova, O.L.; Semenina, E.E.; Leonov, V.D.; Belyakova, O.I.; Bogatyreva, V.Y.; Degtyarev, M.I.; Esaulov, A.S.; Korotkevich, A.Y.; Kudrin, A.A.; et al. Size Compartmentalization of Energy Channeling in Terrestrial Belowground Food Webs. Ecology 2021, 102, e03421. [Google Scholar] [CrossRef]
- Mitchell, E.A.D.; Buttler, A.; Grosvernier, P.; Rydin, H.; Albinsson, C.; Greenup, A.L.; Heijmans, M.M.P.D.; Hoosbeek, M.R.; Saarinen, T. Relationships among Testate Amoebae (Protozoa), Vegetation and Water Chemistry in Five Sphagnum-Dominated Peatlands in Europe: Testate Amoeba, Vegetation and Water Chemistry in Bogs. New Phytol. 2000, 145, 95–106. [Google Scholar] [CrossRef]
- Opravilová, V.; Hájek, M. Others The Variation of Testacean Assemblages (Rhizopoda) along the Complete Baserichness Gradient in Fens: A Case Study from the Western Carpathians. Acta Protozool. 2006, 45, 191. [Google Scholar]
- Lamentowicz, M.; Lamentowicz, Ł.; van der Knaap, W.O.; Gąbka, M.; Mitchell, E.A.D. Contrasting Species—Environment Relationships in Communities of Testate Amoebae, Bryophytes and Vascular Plants Along the Fen–Bog Gradient. Microb. Ecol. 2010, 59, 499–510. [Google Scholar] [CrossRef]
- Carlson, M.L.; Flagstad, L.A.; Gillet, F.; Mitchell, E.A.D. Community Development along a Proglacial Chronosequence: Are above-Ground and below-Ground Community Structure Controlled More by Biotic than Abiotic Factors?: Above- and below-Ground Communities in Succession. J. Ecol. 2010, 98, 1084–1095. [Google Scholar] [CrossRef]
- Koenig, I.; Feldmeyer-Christe, E.; Mitchell, E.A.D. Comparative Ecology of Vascular Plant, Bryophyte and Testate Amoeba Communities in Four Sphagnum Peatlands along an Altitudinal Gradient in Switzerland. Ecol. Indic. 2015, 54, 48–59. [Google Scholar] [CrossRef]
- Katz, N.Y. Swamps of the Earth; Nauka: Moscow, Russia, 1971. [Google Scholar]
- Tarnogradsky, D. Microflora and microfauna of the peat bogs of the Caucasus. 2. Tarskoe peaty moor. Proc. North-Cauc. Hydrobiol. Stn. North-Ossetia Agrar. Inst. 1947, 5, 19–34. [Google Scholar]
- Tarnogradsky, D. Microflora and microfauna of the peat bogs of the Caucasus. 6. Devdorakskoe Sphagnum peatland. Proc. North-Cauc. Hydrobiol. Stn. North-Ossetia Agrar. Inst. 1957, 6, 3–56. [Google Scholar]
- Tarnogradsky, D. Microflora and microfauna of the peat bogs of the Caucasus. 8. Sedge-Sphagnum lakes in the upper course of Balkarskiy Cherek River. Proc. North-Cauc. Hydrobiol. Stn. North-Ossetia Agrar. Inst. 1959, 6, 3–91. [Google Scholar]
- Tarnogradsky, D. Microflora and Microfauna of the Peat Bogs of the Caucasus. 5. Sphagnetum of the Makhar Gorge (Karachaevo-Cherkesskaya A. Republic). Proc. North-Cauc. Hydrobiol. Stn. North-Ossetia Agrar. Inst. 1961, 7, 3–33. [Google Scholar]
- Malysheva, E.A.; Mazei, N.G.; Shapovalov, M.I.; Saprykin, M.A.; Mazei, Y.A. Freshwater Testate Amoebae in the Belaya River Basin (Northwestern Caucasus). Inland Water Biol. 2017, 10, 92–96. [Google Scholar] [CrossRef]
- Mischenko, A.L. General characteristics of the Norther Caucasus region and its wetlands. In Wetlands in Russia. Wetlands in the North Caucasus; Mischenko, A.L., Ed.; Wetlands International: Moscow, Russia, 2006; Volume 6, p. 316. ISBN 978-90-5882-028-0. [Google Scholar]
- Komarov, Y.V. Republic of North Ossetia-Alania. In Wetlands in Russia. Wetlands in the North Caucasus; Mischenko, A.L., Ed.; Wetlands International: Moscow, Russia, 2006; Volume 6, p. 316. ISBN 978-90-5882-028-0. [Google Scholar]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth. BioScience 2001, 51, 933. [Google Scholar] [CrossRef]
- Botch, M.S.; Mazig, V.V. Mire Ecosystems in the USSR; Nauka: Leningrad, Russia, 1979. [Google Scholar]
- Katz, N.Y. Mires and Peatlands; UCHPEDGIZ: Moscow, Russia, 1941. [Google Scholar]
- Kvavadze, E.V.; Efremov, Y.V. Palynological Studies of Lake and Lake-Swamp Sediments of the Holocene in the High Mountains of Arkhyz (Western Caucasus). Acta Palaeobot. 1996, 36, 107–120. [Google Scholar]
- Connor, S.E.; Kvavadze, E.V. Modelling Late Quaternary Changes in Plant Distribution, Vegetation and Climate Using Pollen Data from Georgia, Caucasus. J. Biogeogr. 2009, 36, 529–545. [Google Scholar] [CrossRef]
- Knyasev, A.; Savinetsky, A.; Gey, N. Holocene History of Vegetation in North Ossetia. In Historical Ecology of Wild and Domestic Ungulates: History of Pasture Ecosystems; Nauka: Moscow, Russia, 1992; pp. 84–108. [Google Scholar]
- Prokin, A.A.; Sazhnev, A.S.; Philippov, D.A. Water Beetles (Insecta: Coleoptera) of Some Peatlands in the North Caucasus. Nat. Conserv. Res. 2019, 4, 57–66. [Google Scholar] [CrossRef]
- Doroshina, G.Y.; Nikolajev, I.A. Sphagnum mosses (Sphagnaceae, Bryophyta) of mires Chefandzar and Masota in North Ossetia (Caucasus). Nov. Sist. Nizshikh Rastenii 2018, 52, 455–462. [Google Scholar] [CrossRef]
- Busch, E.A. On the mires limnogeneus origin of the Balkaria and Digoria. Proc. Bot. Mus. USSR 1932, 25, 7–16. [Google Scholar]
- Maevsky, P.F. Flora of the Middle Zone of the European Part of Russia, 11th ed.; Scientific Press Ltd. KMK: Moscow, Russia, 2014; ISBN 978-5-87317-958-9. [Google Scholar]
- Ignatov, M.S.; Ignatova, E.A. Moss Flora of the Middle European Russia. Vol. 1: Sphagnaceae—Hedwigiaceae.; KMK Scientific Press Ltd.: Moscow, Russia, 2003; Volume 1, ISBN 5-87317-104-1. [Google Scholar]
- Ignatov, M.S.; Ignatova, E.A. Moss Flora of the Middle European Russia. Vol. 2: Fontinalaceae—Amblystegiaceae; KMK Scientific Press Ltd.: Moscow, Russia, 2004; Volume 2, ISBN 5-87317-149-1. [Google Scholar]
- Mazei, Y.A.; Chernyshov, V.A. Testate Amoebae Communities in the Southern Tundra and Forest-Tundra of Western Siberia. Biol. Bull. 2011, 38, 789–796. [Google Scholar] [CrossRef]
- Todorov, M.; Bankov, N. An Atlas of Sphagnum-Dwelling Testate Amoebae in Bulgaria, 1st ed.; Pensoft Publishers: Sofia, Bulgaria, 2019; ISBN 978-954-642-972-8. [Google Scholar]
- Lahr, D.J.G.; Kosakyan, A.; Lara, E.; Mitchell, E.A.D.; Morais, L.; Porfirio-Sousa, A.L.; Ribeiro, G.M.; Tice, A.K.; Pánek, T.; Kang, S.; et al. Phylogenomics and Morphological Reconstruction of Arcellinida Testate Amoebae Highlight Diversity of Microbial Eukaryotes in the Neoproterozoic. Curr. Biol. 2019, 29, 991–1001.e3. [Google Scholar] [CrossRef]
- Macumber, A.L.; Blandenier, Q.; Todorov, M.; Duckert, C.; Lara, E.; Lahr, D.J.G.; Mitchell, E.A.D.; Roe, H.M. Phylogenetic Divergence within the Arcellinida (Amoebozoa) Is Congruent with Test Size and Metabolism Type. Eur. J. Protistol. 2020, 72, 125645. [Google Scholar] [CrossRef] [PubMed]
- Marcisz, K.; Jassey, V.E.J.; Kosakyan, A.; Krashevska, V.; Lahr, D.J.G.; Lara, E.; Lamentowicz, Ł.; Lamentowicz, M.; Macumber, A.; Mazei, Y.; et al. Testate Amoeba Functional Traits and Their Use in Paleoecology. Front. Ecol. Evol. 2020, 8, 340. [Google Scholar] [CrossRef]
- Mitchell, E.A.D.; Lamentowicz, M.; Payne, R.J.; Mazei, Y. Effect of Taxonomic Resolution on Ecological and Palaeoecological Inference—A Test Using Testate Amoeba Water Table Depth Transfer Functions. Quat. Sci. Rev. 2014, 91, 62–69. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. Available online: https://CRAN.R-project.org/package=vegan (accessed on 30 October 2022).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: A Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R.; Use R! Springer: New York, NY, USA, 2011; ISBN 978-1-4419-7975-9. [Google Scholar]
- Josse, J.; Pagès, J.; Husson, F. Testing the Significance of the RV Coefficient. Comput. Stat. Data Anal. 2008, 53, 82–91. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically Meaningful Transformations for Ordination of Species Data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Legendre, P.; Anderson, M.J. Distance-Based Redundancy Analysis: Testing Multispecies Responses in Multifactorial Ecological Experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Peres-Neto, P.R.; Legendre, P.; Dray, S.; Borcard, D. Variation Partitioning of Species Data Matrices: Estimation and Comparison of Fractions. Ecology 2006, 87, 2614–2625. [Google Scholar] [CrossRef]
- Bobrov, A.A.; Charman, D.J.; Warner, B.G. Ecology of Testate Amoebae (Protozoa: Rhizopoda) on Peatlands in Western Russia with Special Attention to Niche Separation in Closely Related Taxa. Protist 1999, 150, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Marcisz, K.; Lamentowicz, Ł.; Słowińska, S.; Słowiński, M.; Muszak, W.; Lamentowicz, M. Seasonal Changes in Sphagnum Peatland Testate Amoeba Communities along a Hydrological Gradient. Eur. J. Protistol. 2014, 50, 445–455. [Google Scholar] [CrossRef]
- Trappeniers, K.; Kerckvoorde, A.V.; Chardez, D.; Nijs, I.; Beyens, L. Testate Amoebae Assemblages from Soils in the Zackenberg Area, Northeast Greenland. Arct. Antarct. Alp. Res. 2002, 34, 94–101. [Google Scholar] [CrossRef]
- Mazei, Y.A.; Tsyganov, A.N.; Chernyshov, V.A.; Ivanovsky, A.A.; Payne, R.J. First Records of Testate Amoebae from the Novaya Zemlya Archipelago (Russian Arctic). Polar Biol. 2018, 41, 1133–1142. [Google Scholar] [CrossRef]
- Mazei, Y.A.; Lebedeva, N.V.; Taskaeva, A.A.; Ivanovsky, A.A.; Chernyshov, V.A.; Tsyganov, A.N.; Payne, R.J. What Role Does Human Activity Play in Microbial Biogeography?: The Revealing Case of Testate Amoebae in the Soils of Pyramiden, Svalbard. Pedobiologia 2018, 67, 10–15. [Google Scholar] [CrossRef]
- Payne, R.J.; Bobrov, A.A.; Tsyganov, A.N.; Babeshko, K.V.; Sloan, T.J.; Kay, M.; Kupriyanov, D.A.; Surkov, N.V.; Novenko, E.Y.; Andreev, A.A.; et al. First Records of Contemporary Testate Amoeba Assemblages from the Kamchatka Peninsula, Russia and Potential for Palaeoenvironmental Reconstruction. Boreas 2021, 50, 998–1010. [Google Scholar] [CrossRef]
- Beyens, L.; Chardez, D.; De Baere, D.; Verbruggen, C. The Aquatic Testate Amoebae Fauna of the Strømness Bay Area, South Georgia. Antarct. Sci. 1995, 7, 3–8. [Google Scholar] [CrossRef]
- Heal, O.W. The Abundance and Micro-Distribution of Testate Amoebae (Rhizopoda: Testacea) in Sphagnum. Oikos 1962, 13, 35. [Google Scholar] [CrossRef]
- Amesbury, M.J.; Swindles, G.T.; Bobrov, A.; Charman, D.J.; Holden, J.; Lamentowicz, M.; Mallon, G.; Mazei, Y.; Mitchell, E.A.D.; Payne, R.J.; et al. Development of a New Pan-European Testate Amoeba Transfer Function for Reconstructing Peatland Palaeohydrology. Quat. Sci. Rev. 2016, 152, 132–151. [Google Scholar] [CrossRef]
- Swindles, G.T.; Reczuga, M.; Lamentowicz, M.; Raby, C.L.; Turner, T.E.; Charman, D.J.; Gallego-Sala, A.; Valderrama, E.; Williams, C.; Draper, F.; et al. Ecology of Testate Amoebae in an Amazonian Peatland and Development of a Transfer Function for Palaeohydrological Reconstruction. Microb. Ecol. 2014, 68, 284–298. [Google Scholar] [CrossRef]
- Tsyganov, A.N.; Bobrov, A.A.; Shimano, S.D.; Mitchell, E.A.D.; Hagiwara, Y.; Wall, A.A.J.; Mazei, N.G.; Chernyshov, V.A.; Wanner, M.; Zhong, Y.; et al. Distribution of Soil Testate Amoeba Assemblages along an Elevation Gradient on Mount Fuji (Japan). Eur. J. Protistol. 2022, 84, 125894. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, H.; Mazei, Y.; Kurina, I.; Swindles, G.T.; Bobrov, A.; Tsyganov, A.N.; Gu, Y.; Huang, X.; Xue, J.; et al. Developing a Continental-Scale Testate Amoeba Hydrological Transfer Function for Asian Peatlands. Quat. Sci. Rev. 2021, 258, 106868. [Google Scholar] [CrossRef]
- Tahvanainen, T. Water Chemistry of Mires in Relation to the Poor-Rich Vegetation Gradient and Contrasting Geochemical Zones of the North-Eastern Fennoscandian Shield. Folia Geobot. 2004, 39, 353–369. [Google Scholar] [CrossRef]
- Booth, R.K. Ecology of Testate Amoebae (Protozoa) in Two Lake Superior Coastal Wetlands: Implications for Paleoecology and Environmental Monitoring. Wetlands 2001, 21, 564–576. [Google Scholar] [CrossRef]
- Lamentowicz, M.; Mitchell, E.A.D. The Ecology of Testate Amoebae (Protists) in Sphagnum in North-Western Poland in Relation to Peatland Ecology. Microb. Ecol. 2005, 50, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Sim, T.G.; Swindles, G.T.; Morris, P.J.; Baird, A.J.; Charman, D.J.; Amesbury, M.J.; Beilman, D.; Channon, A.; Gallego-Sala, A.V. Ecology of Peatland Testate Amoebae in Svalbard and the Development of Transfer Functions for Reconstructing Past Water-Table Depth and PH. Ecol. Indic. 2021, 131, 108122. [Google Scholar] [CrossRef]
- Tsyganov, A.N.; Babeshko, K.V.; Novenko, E.Y.; Malysheva, E.A.; Payne, R.J.; Mazei, Y.A. Quantitative Reconstruction of Peatland Hydrological Regime with Fossil Testate Amoebae Communities. Russ. J. Ecol. 2017, 48, 191–198. [Google Scholar] [CrossRef]
- Tolonen, K.; Warner, B.G.; Vasander, H. Ecology of Testaceans (Protozoa: Rhizopoda) in Mires in Southern Finland: I. Autecology. Arch. Für Protistenkd. 1992, 142, 119–138. [Google Scholar] [CrossRef]
- Heal, O. The Distribution of Testate Amoebae (Rhizopoda: Testacea) in Some Fens and Bogs in Northern England. Zool. J. Linn. Soc. 1961, 44, 369–382. [Google Scholar] [CrossRef]


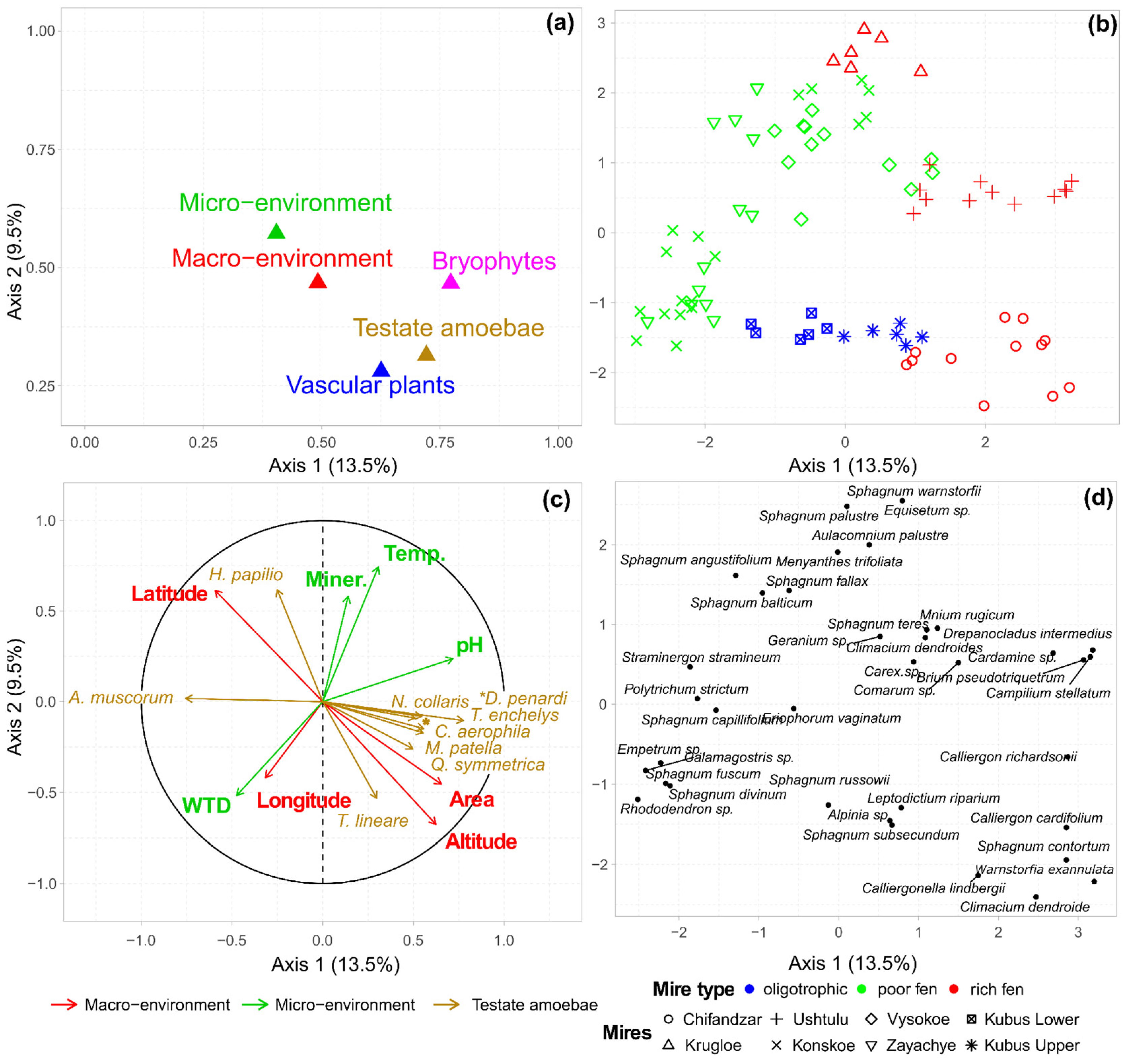
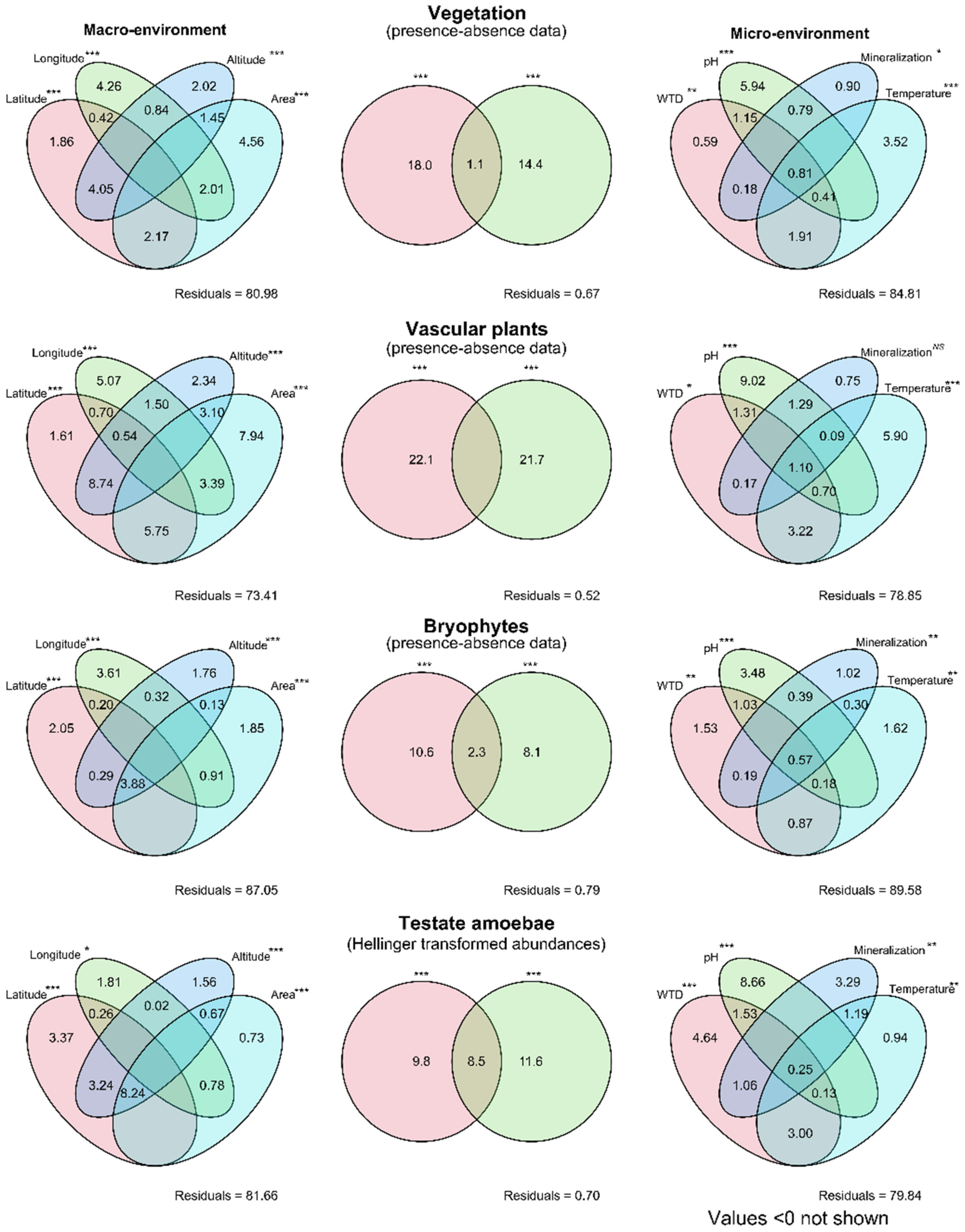

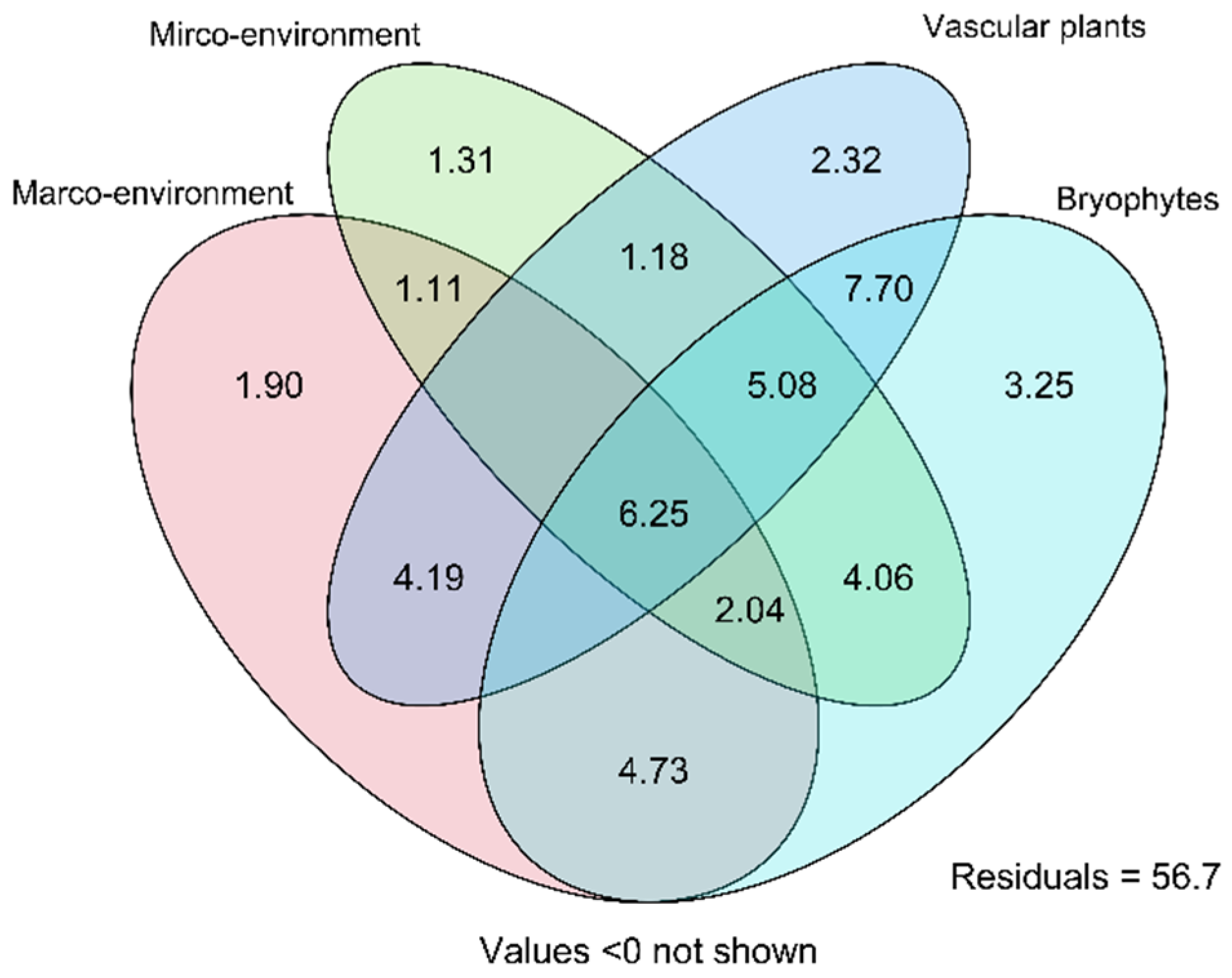
| No | Location (Sample Size) | Latitude, °N, Longitude, °E | Altitude, m a.s.l. | Area, km2 | Trophic State | Dominating Plants |
|---|---|---|---|---|---|---|
| North Ossetia-Alania | ||||||
| 1 | Chifandzar (12) | 42.919, 43.514 | 2289 | 0.556 | eutrophic | Calamagrostis-Sphagnum |
| Carex-Sphagnum | ||||||
| 2 | Lower Kubus (6) | 42.893, 43.576 | 2077 | 0.0049 | oligotrophic | Eriophorum-Sphagnum |
| 3 | Upper Kubus (6) | 42.893, 43.577 | 2080 | 0.002 | oligotrophic | Eriophorum-Sphagnum |
| Kabardino-Balkaria | ||||||
| 4 | Konskoe (18) | 43.101, 43.49 | 1776 | 0.0002 | (meso-) oligotrophic | Calamagrostis-Rhododendron-Sphagnum |
| Empetrum-Calamagrostis-Sphagnum | ||||||
| Carex-Sphagnum | ||||||
| 5 | Zayachye (12) | 43.098, 43.478 | 1810 | 0.0001 | (meso-) oligotrophic | Empetrum-Sphagnum |
| Eriophorum-Sphagnum | ||||||
| 6 | Vysokoe (12) | 43.097, 43.479 | 1836 | 0.0015 | (meso-) oligotrophic | Carex-Sphagnum |
| Eriophorum-Sphagnum | ||||||
| 7 | Krugloe (6) | 43.106, 43.476 | 1648 | 0.0001 | eutrophic | Carex-Sphagnum |
| 8 | Ushtulu (12) | 42.975, 43.335 | 1995 | 0.173 | eutrophic | Carex-Sphagnum |
| Comarum-Sphagnum | ||||||
| Environmental Characteristics | Water Table Depth, cm | Acidity, pH | Mineralization, ppm |
|---|---|---|---|
| Acidity, pH | −0.57 *** | ||
| Mineralization, ppm | −0.24 | 0.29 | |
| Temperature, °C | −0.61 *** | 0.31 * | 0.52 *** |
| Macro-Environment | Micro-Environment | Vegetation | Testate Amoebae | ||
| Macro-environment | 1.00 | <0.001 | <0.001 | <0.001 | |
| Micro-environment | 0.15 | 1.00 | <0.001 | <0.001 | |
| Vegetation | 0.33 | 0.33 | 1.00 | <0.001 | |
| Testate amoebae | 0.38 | 0.28 | 0.37 | 1.00 | |
| MFA | 0.61 | 0.59 | 0.84 | 0.70 | |
| Macro-environment | Micro-environment | Vascular plants | Bryophytes | Testate amoebae | |
| Macro-environment | 1.00 | <0.001 | <0.001 | <0.001 | <0.001 |
| Micro-environment | 0.15 | 1.00 | <0.001 | <0.001 | <0.001 |
| Vascular plants | 0.25 | 0.30 | 1.00 | <0.001 | <0.001 |
| Bryophytes | 0.30 | 0.26 | 0.41 | 1.00 | <0.001 |
| Testate amoebae | 0.38 | 0.28 | 0.27 | 0.35 | 1.00 |
| MFA | 0.56 | 0.54 | 0.71 | 0.80 | 0.64 |
| Data Sets | R2, % | Adjusted R2, % | Df | Pseudo-F | p-Value |
|---|---|---|---|---|---|
| Macro-environment | 22.3 | 18.3 | 4, 79 | 5.66 | 0.001 |
| Micro-environment | 24 | 20.2 | 4, 79 | 6.24 | 0.001 |
| Vascular plants | 34.8 | 24.8 | 11, 72 | 3.49 | 0.001 |
| Mosses | 53.4 | 32.1 | 26, 57 | 2.51 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsyganov, A.N.; Chertoprud, E.S.; Mazei, N.G.; Esaulov, A.S.; Sadchikov, I.P.; Mazei, Y.A. The Effects of Vegetation and the Environment on Testate Amoeba Assemblages in Sphagnum Peatlands in the Northern Caucasus Mountains. Diversity 2023, 15, 258. https://doi.org/10.3390/d15020258
Tsyganov AN, Chertoprud ES, Mazei NG, Esaulov AS, Sadchikov IP, Mazei YA. The Effects of Vegetation and the Environment on Testate Amoeba Assemblages in Sphagnum Peatlands in the Northern Caucasus Mountains. Diversity. 2023; 15(2):258. https://doi.org/10.3390/d15020258
Chicago/Turabian StyleTsyganov, Andrey N., Elena S. Chertoprud, Natalia G. Mazei, Anton S. Esaulov, Ivan P. Sadchikov, and Yuri A. Mazei. 2023. "The Effects of Vegetation and the Environment on Testate Amoeba Assemblages in Sphagnum Peatlands in the Northern Caucasus Mountains" Diversity 15, no. 2: 258. https://doi.org/10.3390/d15020258
APA StyleTsyganov, A. N., Chertoprud, E. S., Mazei, N. G., Esaulov, A. S., Sadchikov, I. P., & Mazei, Y. A. (2023). The Effects of Vegetation and the Environment on Testate Amoeba Assemblages in Sphagnum Peatlands in the Northern Caucasus Mountains. Diversity, 15(2), 258. https://doi.org/10.3390/d15020258










