The Effect of Salinity on the Egg Production Rate of the Sac-Spawning Calanoid Copepod, Pseudodiaptomus hessei, in a Temporarily Open/Closed Southern African Estuary
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Chlorophyll-a Analysis
2.3. Egg Production Rates
2.4. Statistical Analysis
3. Results
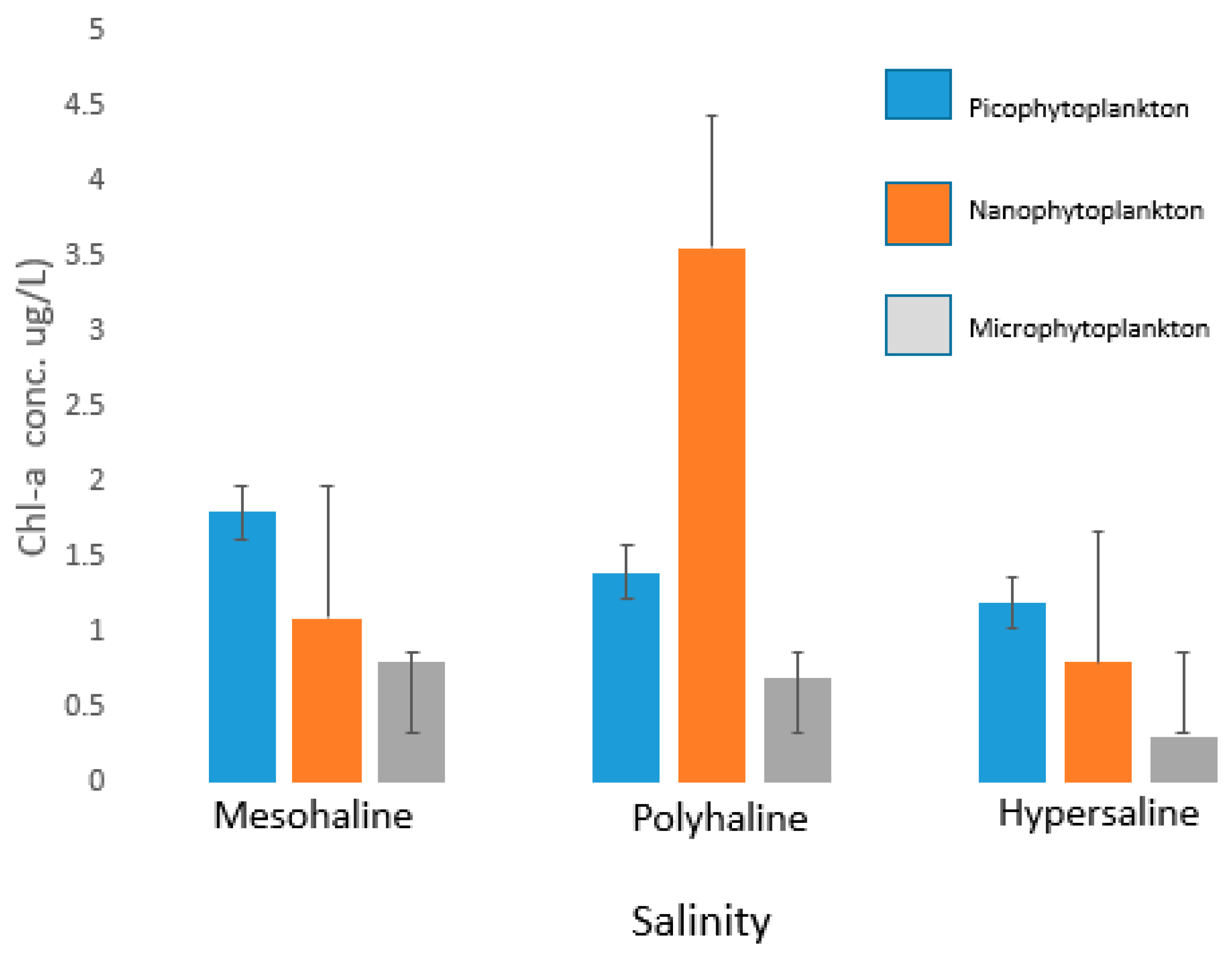
3.1. Chlorophyll-a Concentrations
3.2. Egg Production Rates
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitfield, A.K.; Adams, J.B.; Bate, G.C.; Bezuidenhout, K.; Bornman, T.G.; Cowley, P.D.; Froneman, P.W.; Gama, P.T.; James, N.C.; Mackenzie, B.; et al. A multidisciplinary study of a small, temporarily open/closedSouth African estuary, with particular emphasis on the influence of mouth state on the ecology of the system. Afr. J. Mar. Sci 2008, 30, 453–473. [Google Scholar] [CrossRef]
- Perissinotto, R.; Stretch, D.D.; Whitfield, A.K.; Adams, J.B.; Forbes, A.T. Demetriades NTEcosystem functioning of temporarily open/closed estuaries in South Africa. In Estuaries: Types, Movement Patterns and Climatical Impacts; Crane, J.R., Solomon, A.E., Eds.; Nova Science Publishers: New York, NY, USA, 2010; pp. 1–69. [Google Scholar]
- Froneman, P.W. Seasonal changes in zooplankton biomass and grazing in a temperate estuary, South Africa. Estuar. Coast. Shelf Sci. 2001, 52, 543–553. [Google Scholar] [CrossRef]
- Froneman, P.W. Feeding studies on selected zooplankton in a temperate estuary, South Africa. Estuar. Coast. Shelf Sci. 2000, 51, 543–552. [Google Scholar] [CrossRef]
- Kemp, J.O.G.; Froneman, P.W. Recruitment of ichthyoplankton and macrozooplankton during overtopping events into a temporarily open/closed southern African estuary. Estuar. Coast. Shelf Sci. 2004, 61, 529–537. [Google Scholar] [CrossRef]
- Froneman, P.W.; Vorwerk, P. Feeding ecology of juvenile Gilchristella aestuaria and Atherina breviceps (Pisces) in a temperate estuary, South Africa. Afr. J. Aqua. Sci. 2003, 28, 35–41. [Google Scholar] [CrossRef]
- Whitfield, A.K. Biology and Ecology of Fishes in Southern African Estuaries. Inst. Ichthyol. 1998, 2, 223. [Google Scholar]
- Strydom, N.A. Patterns in Larval Fish Diversity, Abundance, and Distribution in Temperate South African Estuaries. Estuaries Coasts 2015, 38, 268–284. [Google Scholar] [CrossRef]
- Hart, R. Horizontal distribution of copepod Pseudodiaptomus hessei in sub-tropical Lake Sibaya. Freshw. Biol. 1978, 8, 415–421. [Google Scholar] [CrossRef]
- Jerling, H.L.; Wooldridge, T.H. Population dynamics and estimates of production for the calanoid copepod Pseudodiaptomus hessei in a warm temperate estuary. Estuar. Coast. Shelf Sci. 1991, 33, 121–135. [Google Scholar] [CrossRef]
- Froneman, P.W. Food web dynamics in a temperate temporarily open/closed estuary (South Africa). Estuar Coast. Shelf Sci. 2004, 59, 87–95. [Google Scholar] [CrossRef]
- Jerling, H.L.; Wooldridge, T.H.S. Plankton distribution and abundance in the Sundays River estuary, South Africa, with comments on potential feeding interactions. Afr. J. Mar. Sci. 1995, 15, 169–184. [Google Scholar] [CrossRef] [Green Version]
- Wooldridge, T.H.; Callahan, R. The effects of a single freshwater release into the Kromme Estuary. 3: Estuarine zooplankton response. Water SA 2000, 26, 311–318. [Google Scholar]
- Noyon, M.; Froneman, P.W. Variability in egg production rates of the calanoid copepod, Pseudodiaptomus hessei, in a South African estuary in relation to environmental factors. Estuar. Coast. Shelf Sci. 2013, 135, 306–316. [Google Scholar] [CrossRef]
- Dube, K.; Nhamo, G.; Chikodzi, D. Flooding trends and their impacts on coastal communities of Western Cape Province, South Africa. GeoJournal 2021, 87, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Mahlalela, P.T.; Blamey, R.C.; Hart, N.C.G.; Reason, C.J.C. Drought in the Eastern Cape region of South Africa and trends in rainfall characteristics. Clim. Dyn. 2020, 55, 2743–2759. [Google Scholar] [CrossRef]
- Wasserman, R.J.; Noyon, M.; Avery, T.S.; Froneman, P.W. Trophic level stability-inducing effects of predaceous early juvenile fish in an estuarine mesocosm study. PLoS ONE 2013, 8, e61019. [Google Scholar] [CrossRef]
- Pagano, M.; Kouassi, E.; Saint-Jean, L.; Arfi, R.; Bouvy, M. Feeding of Acartia clausi and Pseudodiaptomus hessei (Copepoda: Calanoida) on natural particles in a tropical lagoon (Ebrié, Côte d’Ivoire). Estuar. Coast. Shelf Sci. 2003, 56, 433–445. [Google Scholar] [CrossRef]
- Venables, W.N.; David, M.; Smith, D.M. The R development core team. In An Introduction to R; Version 1.0; The R Development Core Team: Vienna, Austria, 2003. [Google Scholar]
- Isla, J.A.; Perissinotto, R. Effects of temperature, salinity and sex on the basal metabolic rate of the estuarine copepod Pseudodiaptomus hessei. J. Plankton Res. 2004, 26, 579–583. [Google Scholar] [CrossRef]
- Miller, D.D.; Marcus, N.H. The effects of salinity and temperature on the density and sinking velocity of eggs of the calanoid copepod Acartia tonsa Dana. J. Exp. Mar. Biol. Ecol. 1994, 179, 235–252. [Google Scholar] [CrossRef]
- Peck, M.A.; Holste, L. Effects of salinity, photoperiod and adult stocking density on egg production and egg hatching success in Acartia tonsa (Calanoida: Copepoda): Optimizing intensive cultures. Aquaculture 2006, 255, 341–350. [Google Scholar] [CrossRef]
- Richoux, N.R.; Froneman, P.W. Assessment of spatial variation in carbon utilization by benthic and pelagic invertebrates in a temperate South African estuary using stable isotope signatures. Estuar. Coast. Shelf Sci. 2007, 71, 545–558. [Google Scholar] [CrossRef]
- Beyrend-Dur, D.; Kumar, R.; Rao, T.R.; Souissi, S.; Cheng, S.H.; Hwang, J.S. Demographic parameters of adults of Pseudodiaptomus annandalei (Copepoda: Calanoida): Temperature-salinity and generation effects. J. Exp. Mar. Biol. Ecol. 2011, 404, 1–14. [Google Scholar] [CrossRef]
- Devreker, D.; Pierson, J.J.; Souissi, S.; Kimmel, D.G.; Roman, M.R. An experimental approach to estimate egg production and development rate of the calanoid copepod Eurytemora affinis in Chesapeake Bay, USA. J. Exp. Mar. Biol. Ecol. 2012, 416, 72–83. [Google Scholar] [CrossRef]
- Rhyne, A.L.; Ohs, C.L.; Stenn, E. Effects of temperature on reproduction and survival of the calanoid copepod Pseudodiaptomus pelagicus. Aquaculture 2009, 292, 53–59. [Google Scholar] [CrossRef]
- Halsband-Lenk, C.; Pierson, J.J.; Leising, A.W. Reproduction of Pseudocalanus newmani (Copepoda: Calanoida) is deleteriously affected by diatom blooms–a field study. Prog. Oceanogr. 2005, 67, 332–348. [Google Scholar] [CrossRef]
- Berasategui, A.A.; Hoffmeyer, M.S.; Dutto, M.S.; Biancalana, F. 2012. Seasonal variation in the egg morphology of the copepod Eurytemora americana and its relationship with reproductive strategy in a temperate estuary in Argentina. ICES J. Mar. Sci. 2012, 69, 380–388. [Google Scholar] [CrossRef]
- Dur, G.; Souissi, S.; Schmitt, F.G.; Beyrend-Dur, D.; Hwang, H.S. Mating and mate choice in Pseudodiaptomus annandalei (Copepoda: Calanoida). J. Exp. Mar. Biol. Ecol. 2011, 402, 1–11. [Google Scholar] [CrossRef]
- Kiørboe, T.; Sabatini, M. Reproductive and life cycle strategies in egg-carrying cyclopoid and free-spawning calanoid copepods. J. Plankton Res. 1994, 16, 1353–1366. [Google Scholar] [CrossRef]
- Auel, H. Egg size and reproductive adaptations among Arctic deep-sea copepods (Calanoida, Paraeuchaeta). Helgol. Mar. Res. 2004, 58, 1472004153. [Google Scholar] [CrossRef] [Green Version]
- Wooldridge, T. Estuarine Zooplankton Community Structure and Dynamics. In Estuaries of South Africa; Allanson, B.R., Baird, D., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 141–166. [Google Scholar]

| Salinity | Temperature | Salinity |
|---|---|---|
| Mesohaline | 21.6 (±0.1) | 5 (±0.2) |
| Polyhaline | 20.5 (±0.3) | 24 (±0.5) |
| Hypersaline | 22.5 (±0.6) | 38 (±0.5) |
| Variable | Mesohaline | Polyhaline | Hypersaline |
|---|---|---|---|
| Egg production rate (F d−1) (n = 20) | 6.8–12.0 (8.81 ± 2.2) | 20.3–28.1 (23.5 ± 2.2) | 5.9–11.3 (8.3 ± 2.5) |
| Clutch size (n = 20) | 13–25 (18.0 ± 3.1) | 26–36 (30.1 ± 2.9) | 12–25 (18.4 ± 6.2) |
| Egg size (µm) | 75–86 (80.4 ± 4.1) | 68–91 (83.2 ± 9.3) | 57–85 (78.2 ± 7.2) |
| Hatching success (%) | 65–78 (72.8 ± 4.5) | 81–94 (88.8 ± 4.7) | 48–84 (63.0 ± 13.2) |
| % spawning females | 52 | 78 | 46 |
| Prosome length (mm) | 0.91–1.08 (1.12 ± 0.81) | 1.05–1.33 (0.91 ± 0.79) | 0.76–1.01 (0.88 ± 0.89) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Froneman, P.W. The Effect of Salinity on the Egg Production Rate of the Sac-Spawning Calanoid Copepod, Pseudodiaptomus hessei, in a Temporarily Open/Closed Southern African Estuary. Diversity 2023, 15, 263. https://doi.org/10.3390/d15020263
Froneman PW. The Effect of Salinity on the Egg Production Rate of the Sac-Spawning Calanoid Copepod, Pseudodiaptomus hessei, in a Temporarily Open/Closed Southern African Estuary. Diversity. 2023; 15(2):263. https://doi.org/10.3390/d15020263
Chicago/Turabian StyleFroneman, Pierre William. 2023. "The Effect of Salinity on the Egg Production Rate of the Sac-Spawning Calanoid Copepod, Pseudodiaptomus hessei, in a Temporarily Open/Closed Southern African Estuary" Diversity 15, no. 2: 263. https://doi.org/10.3390/d15020263






