Diversity and Endemism of Southern African Gekkonids Linked with the Escarpment Has Implications for Conservation Priorities
Abstract
:1. Introduction
1.1. Gekkonid Diversity
1.2. Conservation
1.3. Study, Research Question, and Aims
2. Materials and Methods
2.1. Map Production
2.2. Phylogenetic Tree Construction
2.3. Diversity and Endemism Estimates
3. Results
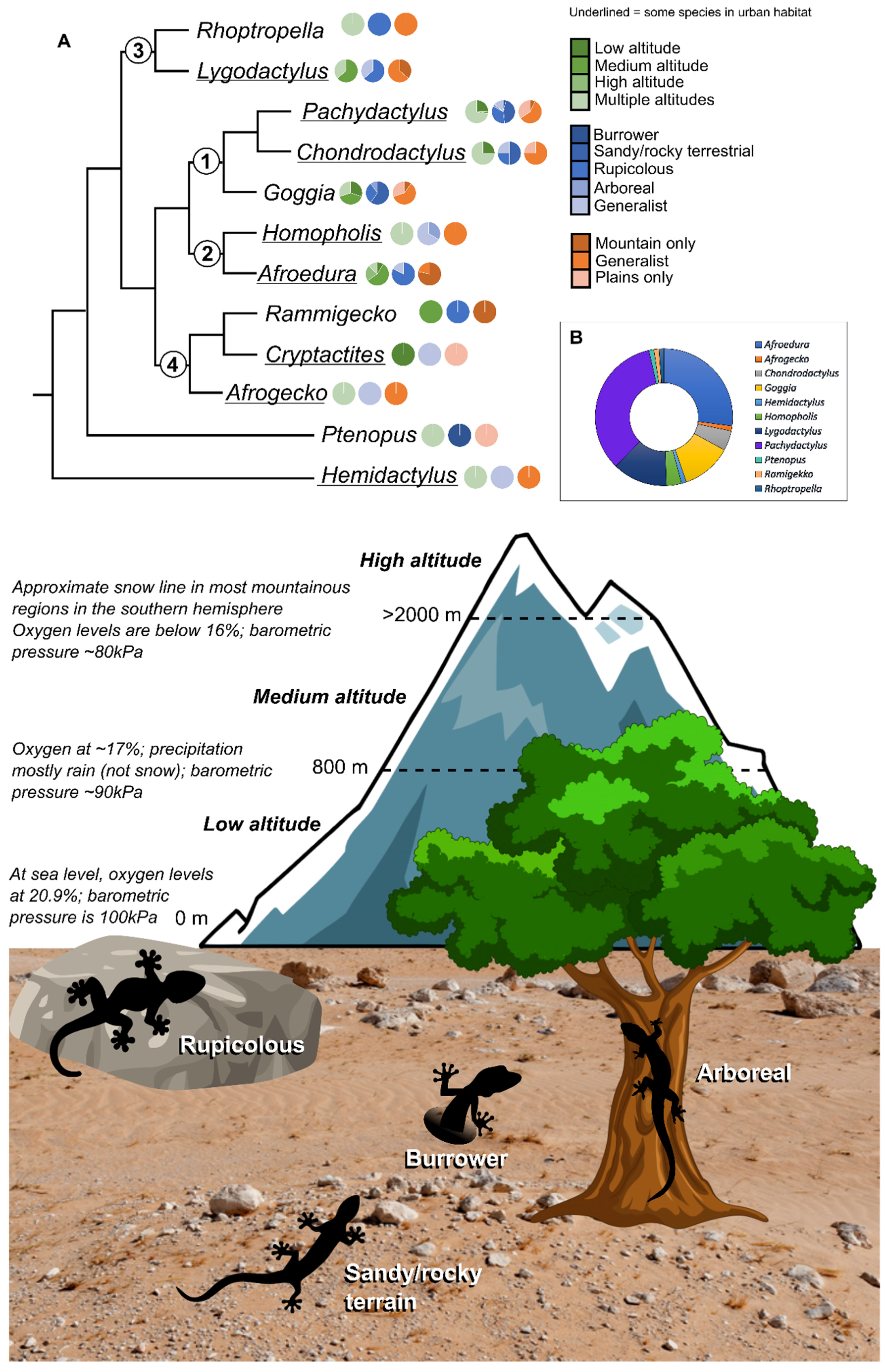
3.1. Generic Distribution
3.2. Phylogenetic Relationships between Southern African Gekkonids
3.3. Gekkonid Diversity and Endemism
4. Discussion
4.1. Diversity, Species Richness, and Endemism
4.2. Conservation Considerations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoorn, C.; Wesselingh, F.P.; ter Steege, H.; Bermudez, M.A.; Mora, A.; Sevink, J.; Sanmartín, I.; Sanchez-Meseguer, A.; Anderson, C.L.; Figueiredo, J.P.; et al. Amazonia through time: Andean uplift, climate change, landscape evolution and biodiversity. Science 2010, 330, 927–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, B.T.; McCormack, J.E.; Cuervo, A.M.; Hickerson, M.J.; Aleixo, A.; Cadena, C.D.; Pérez-Emán, J.; Burney, C.W.; Xie, X.; Harvey, M.G.; et al. The drivers of tropical speciation. Nature 2014, 515, 406–409. [Google Scholar] [CrossRef]
- Hurlbert, A.H.; Jetz, W. Species richness, hotspots, and the scale dependence of range maps in ecology and conservation. Proc. Natl. Acad. Sci. USA 2007, 104, 13384–13389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiens, J.J.; Donoghue, M.J. Historical biogeography, ecology and species richness. Trends Ecol. Evol. 2004, 19, 639–644. [Google Scholar] [CrossRef]
- Waide, R.B.; Willig, M.R.; Steiner, C.F.; Mittelbach, G.; Gough, L.; Dodson, S.I.; Juday, G.P.; Parmenter, R. The relationship between productivity and species richness. Annu. Rev. Ecol. Evol. Syst. 1999, 30, 257–300. [Google Scholar] [CrossRef] [Green Version]
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Uetz, P.; Freed, P.; Aguilar, R.; Hošek, J. (Eds.) The Reptile Database. 2022. Available online: https://www.reptile-database.org (accessed on 5 December 2022).
- Lewin, A.; Feldman, A.; Bauer, A.M.; Belmaker, J.; Broadley, D.G.; Chirio, L.; Itescu, Y.; LeBreton, M.; Maza, E.; Meirte, D.; et al. Patterns of species richness, endemism and environmental gradients of African reptiles. J. Biogeogr. 2016, 43, 2380–2390. [Google Scholar] [CrossRef]
- Kissling, W.D.; Blach-Overgaard, A.; Zwaan, R.E.; Wagner, P. Historical colonization and dispersal limitation supplement climate and topography in shaping species richness of African lizards (Reptilia: Agaminae). Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kafash, A.; Ashrafi, S.; Yousefi, M.; Rastegar-Pouyani, E.; Rajabizadeh, M.; Ahmadzadeh, F.; Grünig, M.; Pellissier, L. Reptile species richness associated to ecological and historical variables in Iran. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Doan, T.M. A south-to-north biogeographic hypothesis for Andean speciation: Evidence from the lizard genus Proctoporus (Reptilia, Gymnophthalmidae). J. Biogeog. 2003, 30, 361–374. [Google Scholar] [CrossRef]
- Agarwal, I.; Bauer, A.M.; Jackman, T.R.; Karanth, K.P. Insights into Himalayan biogeography from geckos: A molecular phylogeny of Cyrtodactylus (Squamata: Gekkonidae). Mol. Phyl. Evol. 2014, 80, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Tolley, K.A.; Alexander, G.J.; Branch, W.R.; Bowles, P.; Maritz, B. Conservation status and threats for African reptiles. Biol. Conserv. 2016, 204, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Bates, M.F.; Branch, W.R.; Bauer, A.M.; Burger, M.; Marias, J.; Alexander, G.J.; De Villiers, M.S. Suricata 1: Atlas and Red List of the Reptiles of South Africa, Lesotho, and Swaziland; South African Biodiversity Institute: Pretoria, South Africa, 2014; Volume 46, pp. 331–397. [Google Scholar]
- Jacobsen, N.H.; Kuhn, A.L.; Jackman, T.R.; Bauer, A.M. A phylogenetic analysis of the southern African gecko genus Afroedura Loveridge (Squamata: Gekkonidae), with the description of nine new species from Limpopo and Mpumalanga provinces of South Africa. Zootaxa 2014, 3846, 451–501. [Google Scholar] [CrossRef] [Green Version]
- Travers, S.L.; Jackman, T.R.; Bauer, A.M. A molecular phylogeny of Afromontane dwarf geckos (Lygodactylus) reveals a single radiation and increased species diversity in a South African montane center of endemism. Mol. Phyl. Evol. 2014, 80, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Tolley, K.A.; Bowie, R.C.K.; Measey, G.J.; Price, B.W.; Forest, F. The shifting landscape of genes since the Pliocene: Terrestrial phylogeography in the greater cape floristic region. In Fynbos: Ecology, Evolution and Conservation of a Megadiverse Region; Allsopp, N., Colville, J.F., Verboom, G.A., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 143–163. [Google Scholar]
- Tolley, K.A.; Tilbury, C.R.; Burger, M. Convergence and vicariance: Speciation of chameleons in the Cape Fold Mountains, South Africa, and the description of three new species of Bradypodion Fitzinger, 1843. Afr. J. Herpetol. 2022, 71, 14–38. [Google Scholar] [CrossRef]
- Partridge, T.C.; Maud, R.R. Geomorphic evolution of southern Africa since the Mesozoic. S. Afr. J. Geol. 1987, 90, 179–208. [Google Scholar]
- McCarthy, T.; Rubridge, B. The Story of Earth and Life; Struik Publishers: Cape Town, South Africa, 2005. [Google Scholar]
- Conradie, W.; Schmitz, A.; Lobón-Rovira, J.; Becker, F.S.; Pinto, P.V.; Hauptfleisch, M.L. Rock island melody remastered: Two new species in the Afroedura bogerti Loveridge, 1944 group from Angola and Namibia. Zoosyst. Evol. 2022, 98, 435–453. [Google Scholar] [CrossRef]
- Branch, B. Field Guide to Snakes and Other Reptiles of Southern Africa; Struik Publishers: Cape Town, South Africa, 1998. [Google Scholar]
- Heinicke, M.P.; Daza, J.D.; Greenbaum, E.; Jackman, T.R.; Bauer, A.M. Phylogeny, taxonomy and biogeography of a circum-Indian Ocean clade of leaf-toed geckos (Reptilia: Gekkota), with a description of two new genera. Syst. Biodiv. 2014, 12, 23–42. [Google Scholar] [CrossRef]
- Heinz, M.D.; Brennan, I.G.; Jackman, T.R.; Bauer, A.M. Phylogeny of the genus Chondrodactylus (Squamata: Gekkonidae) with the establishment of a stable taxonomy. Bull. Mus. Comp. 2021, 163, 151–210. [Google Scholar] [CrossRef]
- Lamb, T.; Bauer, A.M. Footprints in the sand: Independent reduction of subdigital lamellae in the Namib-Kalahari burrowing geckos. Proc. Royal Soc. B 2006, 273, 855–864. [Google Scholar] [CrossRef] [Green Version]
- Gamble, T.; Greenbaum, E.; Jackman, T.R.; Russell, A.P.; Bauer, A.M. Repeated origin and loss of adhesive toepads in geckos. PLoS ONE 2012, 7, e39429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolau, G.K.; Petford, M.; Edwards, S.; Busschau, T.; Lynch, K.; Kemp, L.; Conradie, W. New insights into the geographical distribution, ecology and conservation status of South Africa’s endemic Coastal Leaf-toed Gecko, Cryptactites peringueyi (Boulenger, 1910). Herp. Notes 2021, 14, 439–450. [Google Scholar]
- Heinicke, M.P.; Jackman, T.R.; Bauer, A.M. The measure of success: Geographic isolation promotes diversification in Pachydactylus geckos. BMC Evol. Biol. 2017, 17, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebelo, A.D.; Bates, M.F.; Burger, M.; Branch, W.R.; Conradie, W. Range expansion of the Common Dwarf Gecko, Lygodactylus capensis: South Africa’s most successful reptile invader. Herp. Notes 2019, 12, 643–650. [Google Scholar]
- Bauer, A.M.; Lamb, T. Phylogenetic relationships of southern African geckos in the Pachydactylus group (Squamata: Gekkonidae). Afr. J. Herp. 2005, 54, 105–129. [Google Scholar] [CrossRef]
- Branch, W.R.; Bauer, A.M.; Good, D.A. A review of the Namaqua gecko, Pachydactylus namaquensis (Reptilia: Gekkonidae) from southern Africa, with the description of two new species. Afr. Zool. 1996, 31, 53–69. [Google Scholar] [CrossRef] [Green Version]
- Bauer, A.M.; Lamb, T.; Branch, W.R. A revision of the Pachydactylus serval and P. weberi groups (Reptilia: Gekkota: Gekkonidae) of Southern Africa, and with the description of eight new species. Proc. Calif. Acad. Sci. 2006, 57, 595–709. [Google Scholar]
- Hibbitts, T.J.; Whiting, M.J.; Stuart-Fox, D.M. Shouting the odds: Vocalization signals status in a lizard. Behav. Ecol. Sociobiol. 2007, 61, 1169–1176. [Google Scholar] [CrossRef]
- Branch, W.R.; Bauer, A.M. Notes on two poorly-known Phyllodactylus (Squamata: Gekkonidae) from South Africa. Herpetol. Nat. Hist. 1996, 4, 127–134. [Google Scholar]
- Haacke, W.D. Description of a new species of Phyllodactylus Gray (Reptilia: Gekkonidae) from the Cape Fold Mountains, South Africa. Ann. Transvaal Mus. 1996, 36, 229–237. [Google Scholar]
- Bauer, A.M.; Good, D.A.; Branch, B. The taxonomy of the southern african leaf-toed geckos (Squamata: Gekkonidae), with a review of Old World “Phyllodactylus” and the description of five new genera. Proc. Calif. Acad. Sci. 1997, 49, 447–497. [Google Scholar]
- Pyron, R.A.; Burbrink, F.T.; Wiens, J.J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 2013, 13, 1–54. [Google Scholar] [CrossRef] [Green Version]
- Makhubo, B.G.; Tolley, K.A.; Bates, M.F. Molecular phylogeny of the Afroedura nivaria (Reptilia: Gekkonidae) species complex in South Africa provides insight on cryptic speciation. Mol. Phylogenet. Evol. 2015, 82, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Busschau, T.; Conradie, W.; Daniels, S.R. Evidence for cryptic diversification in a rupicolous forest-dwelling gecko (Gekkonidae: Afroedura pondolia) from a biodiversity hotspot. Mol. Phylogenet. Evol. 2019, 139, 106549. [Google Scholar] [CrossRef] [PubMed]
- Branch, W.R.; Schmitz, A.; Lobón-Rovira, J.; Baptista, N.L.; António, T.; Conradie, W. Rock island melody: A revision of the Afroedura bogerti Loveridge, 1944 group, with descriptions of four new endemic species from Angola. Zoosyst. Evol. 2021, 97, 55. [Google Scholar] [CrossRef]
- Lobon-Rovira, J.; Conradie, W.; Pinto, P.V.; Keates, C.; Edwards, S.; Plessis, A.D.; Branch, W.R. Systematic revision of Afrogecko ansorgii (Boulenger, 1907) (Sauria: Gekkonidae) from western Angola. Zootaxa 2022, 5124, 401–430. [Google Scholar] [CrossRef]
- Cox, N.; Young, B.E.; Bowles, P.; Fernandez, M.; Marin, J.; Rapacciuolo, G.; Böhm, M.; Brooks, T.M.; Hedges, S.B.; Hilton-Taylor, C.; et al. A global reptile assessment highlights shared conservation needs of tetrapods. Nature 2022, 605, 285–290. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Dinerstein, E.; Vynne, C.; Sala, E.; Joshi, A.R.; Fernando, S.; Lovejoy, T.E.; Mayorga, J.; Olson, D.; Asner, G.P.; Baillie, J.E.M.; et al. A global deal for nature: Guiding principles, milestones, and targets. Sci. Adv. 2019, 5, eaaw2869. [Google Scholar] [CrossRef] [Green Version]
- Burgess, N.; Küper, W.; Mutke, J.; Brown, J.; Westaway, S.; Turpie, S.; Meshack, C.; Taplin, J.; McClean, C.; Lovett, J.C. Major gaps in the distribution of protected areas for threatened and narrow range Afrotropical plants. Biodivers. Conserv. 2005, 14, 1877–1894. [Google Scholar] [CrossRef]
- Kearney, S.G.; Adams, V.M.; Fuller, R.A.; Possingham, H.P.; Watson, J.E. Estimating the benefit of well-managed protected areas for threatened species conservation. Oryx 2020, 54, 276–284. [Google Scholar] [CrossRef]
- Carruthers, J. National Park Science: A Century of Research in South Africa; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Sinclair, S.P.; Milner-Gulland, E.J.; Smith, R.J.; McIntosh, E.J.; Possingham, H.P.; Vercammen, A.; Knight, A.T. The use, and usefulness, of spatial conservation prioritizations. Conserv. Lett. 2018, 11, e12459. [Google Scholar] [CrossRef]
- Botts, E.A.; Pence, G.; Holness, S.; Sink, K.; Skowno, A.; Driver, A.; Harris, L.R.; Desmet, P.; Escott, B.; Lötter, M.; et al. Practical actions for applied systematic conservation planning. Conserv. Biol. 2019, 33, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Tickner, D.; Opperman, J.J.; Abell, R.; Acreman, M.; Arthington, A.H.; Bunn, S.E.; Cooke, S.J.; Dalton, J.; Darwall, W.; Edwards, G.; et al. Bending the curve of global freshwater biodiversity loss: An emergency recovery plan. BioScience 2020, 70, 330–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stats, S.A. The Nature of South Africa’s Protected Area Estate. 2021. Available online: https://www.statssa.gov.za/?p=14732 (accessed on 14 January 2023).
- Department of Environmental Affairs. National Protected Areas Expansion Strategy for South Africa 2016; Department of Environmental Affairs: Pretoria, South Africa, 2016. [Google Scholar]
- Tolley, K.A.; Weeber, J.; Maritz, B.; Verburgt, L.; Bates, M.F.; Conradie, W.; Hofmeyr, M.D.; Turner, A.A.; da Silva, J.M.; Alexander, G.J. No safe haven: Protection levels show imperilled South African reptiles not sufficiently safe-guarded despite low average extinction risk. Biol. Conserv. 2019, 233, 61–72. [Google Scholar] [CrossRef]
- Tolley, K.A.; Weeber, J.; Bates, M.F.; Bauer, A.M. Afroedura multiporis. The IUCN Red List of Threatened Species 2022: E.T115648679A197428768. Available online: https://www.iucnredlist.org/species/115648679/197428768 (accessed on 17 December 2022).
- Tolley, K.A.; Alexander, G.J.; Conradie, W.; Pietersen, D.; Weeber, J. Homopholis mulleri. The IUCN Red List of Threatened Species 2022: E.T10235A197398514. Available online: https://www.iucnredlist.org/species/10235/197398514 (accessed on 17 December 2022).
- Tolley, K.A.; Weeber, J.; Pietersen, D.; Conradie, W.; Alexander, G.J. Lygodactylus methueni. The IUCN Red List of Threatened Species 2022: E.T12439A197400102. Available online: https://www.iucnredlist.org/species/12439/197400102 (accessed on 17 December 2022).
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2022. Available online: http://qgis.osgeo.org (accessed on 30 November 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Hollister, J.; Shah, T.; Robitaille, A.; Beck, M.; Johnson, M. elevatr: Access Elevation Data from Various APIs. R Package Version 0.4.2. 2021. Available online: https://github.com/jhollist/elevatr/ (accessed on 30 November 2022).
- Sievert, C. Interactive Web-Based Data Visualization with R, Plotly, and Shiny. Chapman and Hall/CRC 2020. Available online: https://plotly-r.com (accessed on 5 December 2022).
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Xia, X. DAMBE6: New tools for microbial genomics, phylogenetics, and molecular evolution. J. Hered. 2017, 108, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [Green Version]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Rosauer, D.; Laffan, S.W.; Crisp, M.D.; Donnellan, S.D.; Cook, L.G. Phylogenetic endemism: A new approach for identifying geographical concentrations of evolutionary history. Mol. Ecol. 2009, 18, 4061–4072. [Google Scholar] [CrossRef] [PubMed]
- Mishler, B.D.; Knerr, N.; González-Orozco, C.E.; Thornhill, A.H.; Laffan, S.W.; Miller, J.T. Phylogenetic measures of biodiversity and neo-and paleo-endemism in Australian Acacia. Nat. Commun. 2014, 5, 4473. [Google Scholar] [CrossRef] [PubMed]
- Albassatneh, M.C.; Escudero, M.; Monnet, A.; Arroyo, J.; Bacchetta, G.; Bagnoli, F.; Dimopoulos, P.; Hampe, A.; Leriche, A.; Médail, F.; et al. Spatial patterns of genus-level phylogenetic endemism in the tree flora of Mediterranean Europe. Divers. Distrib. 2021, 27, 913–928. [Google Scholar] [CrossRef]
- Laffan, S.W.; Lubarsky, E.; Rosauer, D.F. Biodiverse, a tool for the spatial analysis of biological and related diversity. Ecography 2010, 33, 643–647, (Version 4.0). [Google Scholar] [CrossRef] [Green Version]
- Crisp, M.D.; Laffan, S.; Linder, H.P.; Monro, A. Endemism in the Australian flora. J. Biogeogr. 2001, 28, 183–198. [Google Scholar] [CrossRef]
- Lamoreux, J.F.; Morrison, J.C.; Ricketts, T.H.; Olson, D.M.; Dinerstein, E.; McKnight, M.W.; Shugart, H.H. Global tests of biodiversity concordance and the importance of endemism. Nature 2006, 440, 212–214. [Google Scholar] [CrossRef]
- Forest, F.; Grenyer, R.; Rouget, M.; Davies, T.J.; Cowling, R.M.; Faith, D.P.; Balmford, A.; Manning, J.C.; Procheş, Ş.; Van Der Bank, M.; et al. Preserving the evolutionary potential of floras in biodiversity hotspots. Nature 2007, 445, 757–760. [Google Scholar] [CrossRef]
- Molina-Venegas, R.; Rodríguez, M.Á.; Pardo-de-Santayana, M.; Ronquillo, C.; Mabberley, D.J. Maximum levels of global phylogenetic diversity efficiently capture plant services for humankind. Nat. Ecol. Evol. 2021, 5, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Gumbs, R.; Chaudhary, A.; Daru, B.H.; Faith, D.P.; Forest, F.; Gray, C.L.; Kowalska, A.; Lee, W.S.; Pellens, R.; Pipins, S. The Post-2020 Global Biodiversity Framework must safeguard the Tree of Life. BioRxiv 2021, arXiv:2021.03.03.433783. [Google Scholar] [CrossRef]
- Magurran, A.E.; McGill, B.J. (Eds.) Biological Diversity: Frontiers in Measurement and Assessment; OUP: Oxford, UK, 2010. [Google Scholar]
- Linder, H.P. On areas of endemism, with an example from the African Restionaceae. Syst. Biol. 2001, 50, 892–912. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, G.; Farris, E.; Pontecorvo, C. A new method to set conservation priorities in biodiversity hotspots. Plant Biosyst. 2012, 146, 638–648. [Google Scholar] [CrossRef]
- Margules, C.R.; Pressey, R.L. Systematic conservation planning. Nature 2000, 405, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Murali, G.; Gumbs, R.; Meiri, S.; Roll, U. Global determinants and conservation of evolutionary and geographic rarity in land vertebrates. Sci. Adv. 2021, 7, eabe5582. [Google Scholar] [CrossRef]
- Rosauer, D.F.; Jetz, W. Phylogenetic endemism in terrestrial mammals. Glob. Ecol. Biogeogr. 2015, 24, 168–179. [Google Scholar] [CrossRef]
- Rahbek, C.; Borregaard, M.K.; Colwell, R.K.; Dalsgaard, B.; Holt, B.G.; Morueta-Holme, N.; Nogues-Bravo, D.; Whittaker, R.J.; Fjeldsåa, J. Humboldt’s enigma: What causes global patterns of mountain biodiversity? Science 2019, 365, 1108–1113. [Google Scholar] [CrossRef]
- Böhning-Gaese, K.; Caprano, T.; Ewijk, K.V.; Veith, M. Range size: Disentangling current traits and phylogenetic and biogeographic factors. Am. Nat. 2006, 167, 555–567. [Google Scholar] [CrossRef]
- Aragón, P.; Lobo, J.M.; Olalla-Tárraga, M.Á.; Rodríguez, M.Á. The contribution of contemporary climate to ectothermic and endothermic vertebrate distributions in a glacial refuge. Glob. Ecol. Biogeogr. 2010, 19, 40–49. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolau, G.K.; Edwards, S. Diversity and Endemism of Southern African Gekkonids Linked with the Escarpment Has Implications for Conservation Priorities. Diversity 2023, 15, 306. https://doi.org/10.3390/d15020306
Nicolau GK, Edwards S. Diversity and Endemism of Southern African Gekkonids Linked with the Escarpment Has Implications for Conservation Priorities. Diversity. 2023; 15(2):306. https://doi.org/10.3390/d15020306
Chicago/Turabian StyleNicolau, Gary K., and Shelley Edwards. 2023. "Diversity and Endemism of Southern African Gekkonids Linked with the Escarpment Has Implications for Conservation Priorities" Diversity 15, no. 2: 306. https://doi.org/10.3390/d15020306
APA StyleNicolau, G. K., & Edwards, S. (2023). Diversity and Endemism of Southern African Gekkonids Linked with the Escarpment Has Implications for Conservation Priorities. Diversity, 15(2), 306. https://doi.org/10.3390/d15020306





