First Record of Beauveria varroae, Cordyceps blackwelliae, and Purpureocillium lavendulum from Greece and Their Pathogenicity against Thaumetopoea pityocampa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Baits
2.2. Sampling
2.3. Isolation
2.4. Morphological Observation
2.5. Genomic DNA Extraction and Polymerase Chain Reaction (PCR)
2.6. Sequencing and Phylogenetic Analysis
2.7. Virulence Estimation against T. pityocampa Larvae
2.8. Statistical Analysis
3. Results
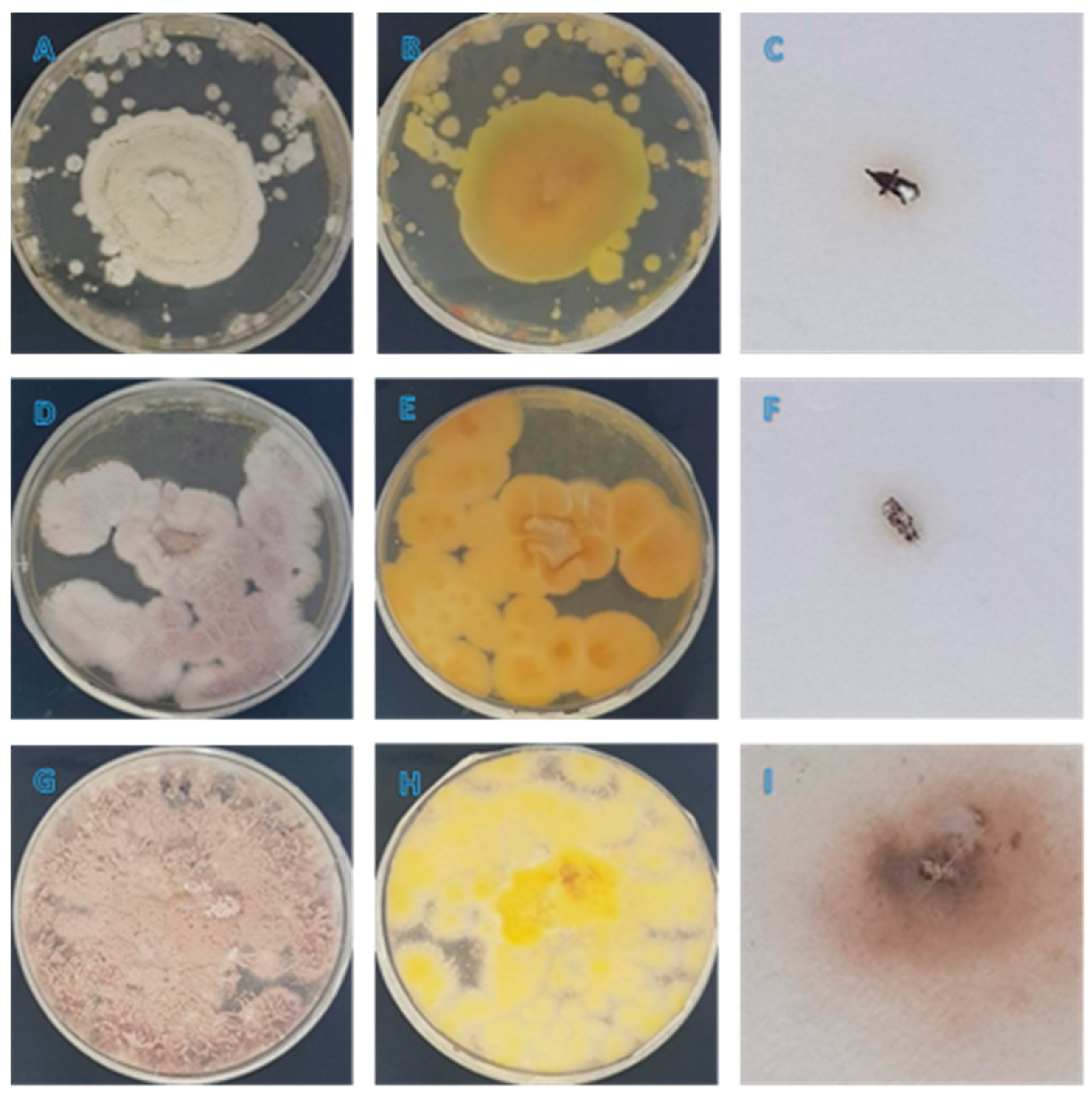
3.1. Morphological Observation
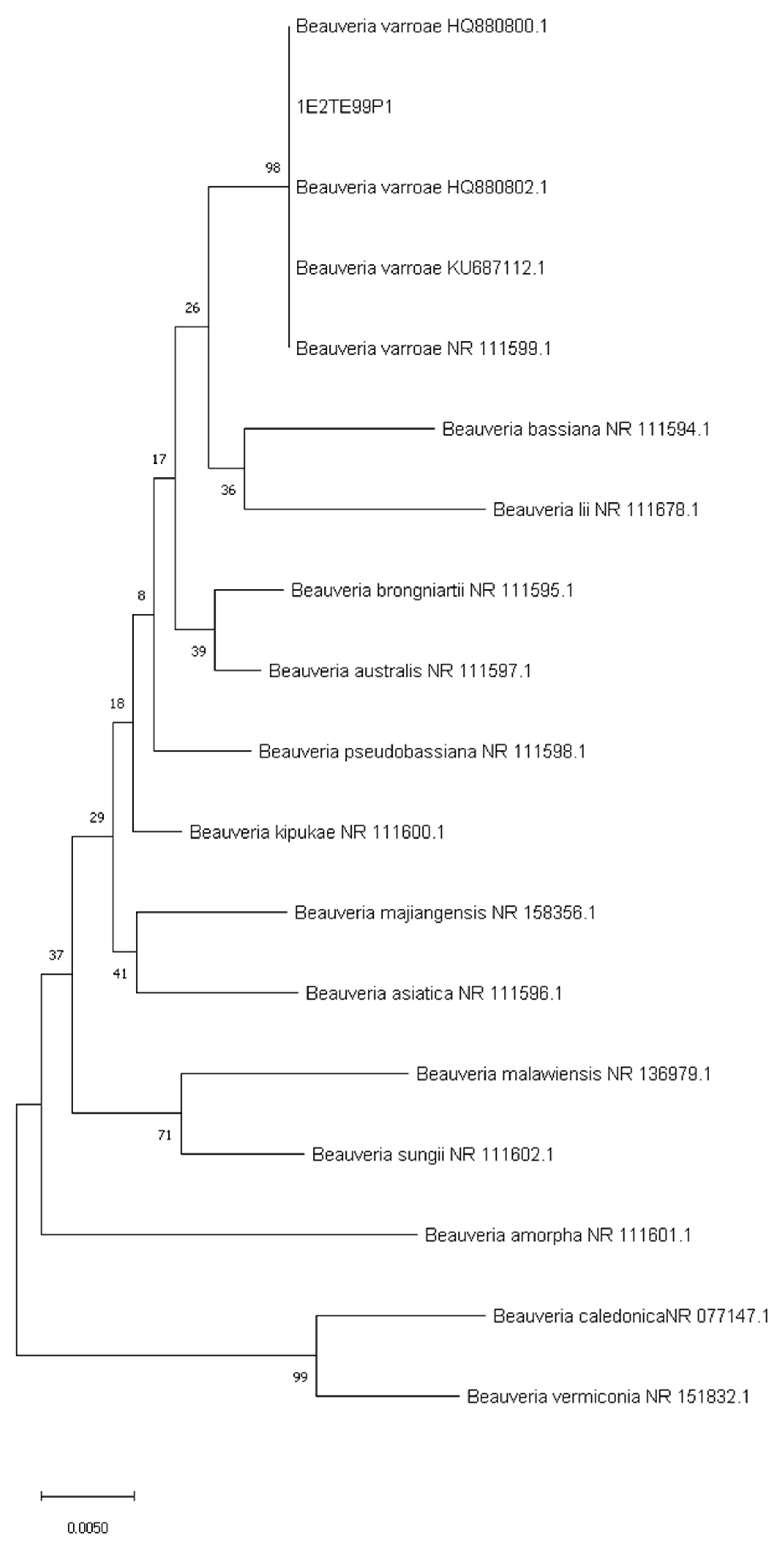
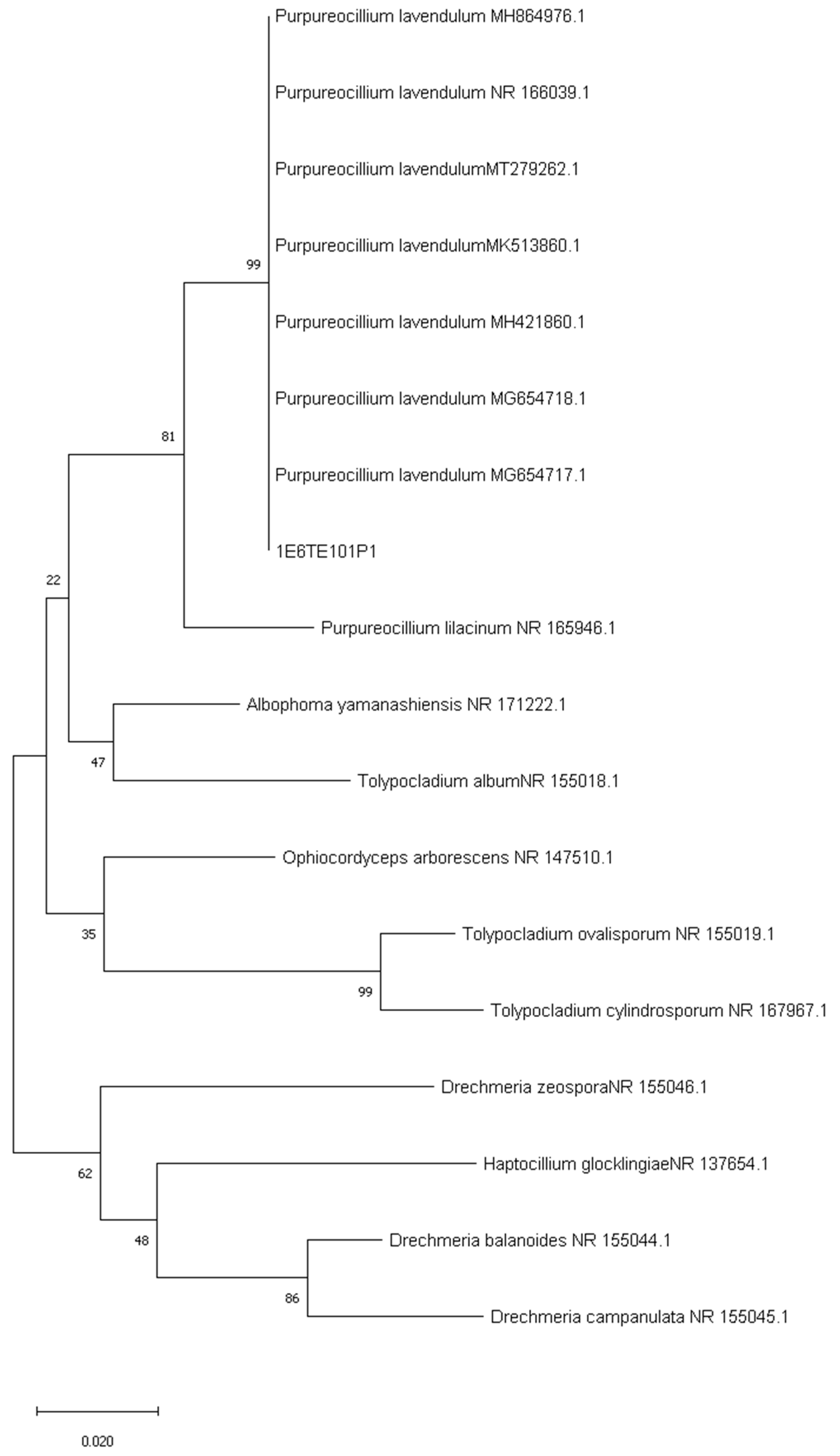
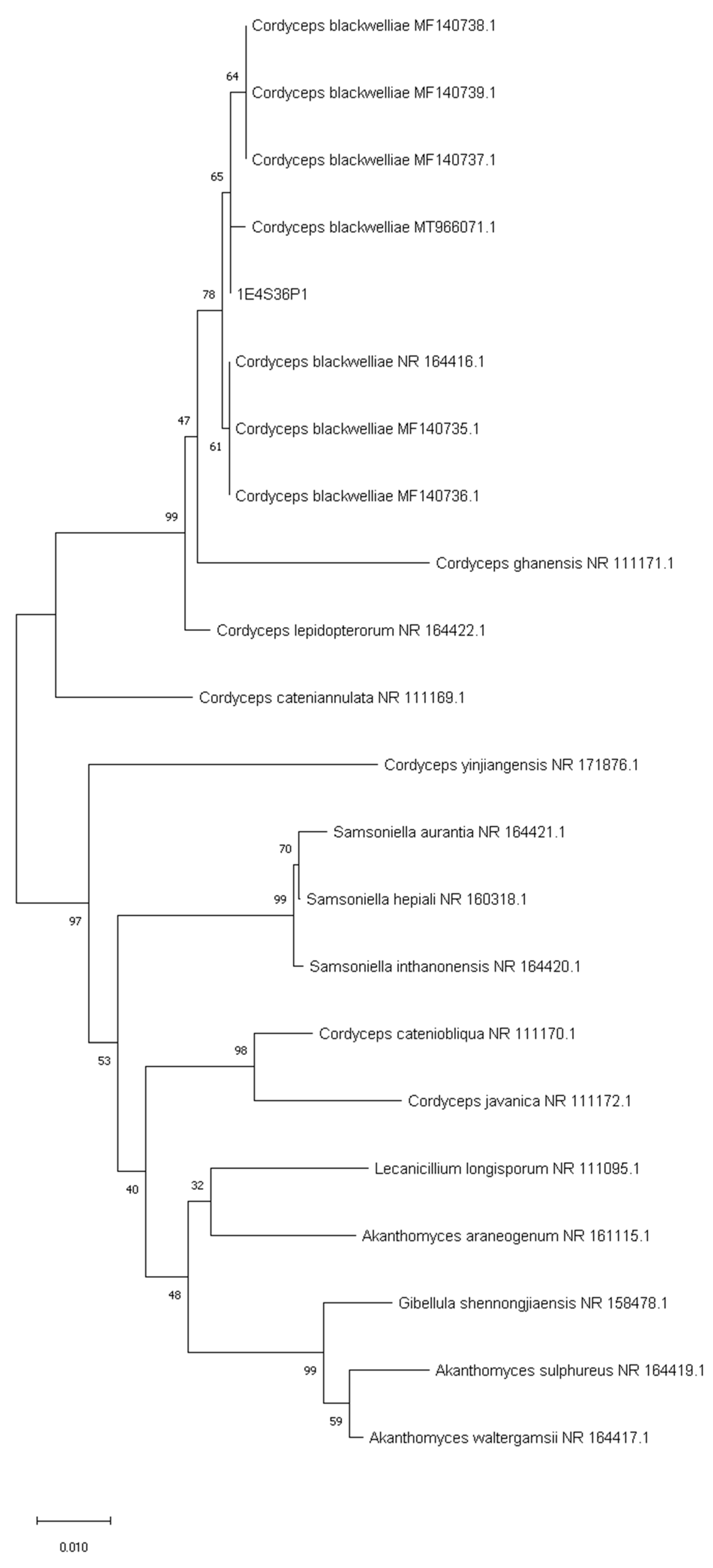
3.2. Sequencing of ITS and Phylogenetic Analysis
3.3. Insecticidal Action of EPF on T. pityocampa Larvae
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qu, S.; Wang, S. Interaction of entomopathogenic fungi with the host immune system. Dev. Comp. Immunol. 2018, 83, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.O.; Noda, H.; Blackwell, M. Insect symbiosis: Derivation of yeast-like endosymbionts within an entomopathogenic filamentous lineage. Mol. Biol. Evol. 2001, 18, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Mueller, U.G.; Gerardo, N.M.; Aanen, D.K.; Six, D.L.; Schultz, T.R. The evolution of agriculture in insects. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 563–595. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Eliopoulos, P.A. Endophytic entomopathogenic fungi: A valuable biological control tool against plant pests. Appl. Sci. 2020, 10, 360. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.A.; Pell, J.K. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.E.; Goettel, M.S.; Blackwell, M.; Chandler, D.; Jackson, M.A.; Keller, S.; Koike, M.; Maniania, N.K.; Monzón, A.; Ownley, B.H.; et al. Fungal entomopathogens: New insights on their ecology. Fungal Ecol. 2009, 2, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Mantzoukas, S.; Lagogiannis, I.; Mpousia, D.; Ntoukas, A.; Karmakolia, K.; Eliopoulos, P.A.; Poulas, K. Beauveria bassiana endophytic strain as plant growth promoter: The case of the grape vine Vitis vinifera. J. Fungi 2021, 7, 142. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Kjer, J.; Proksch, P. Fungal endophytes from higher plants: A prolific source of phytochemicals and other bioactive natural products. Fungal Divers. 2010, 41, 1–16. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Pettas, I.; Lagogiannis, I. Stored product pests as models for trapping entomopathogenic fungi from olive tree orchards in Western Greece. J. Stored Prod. Res. 2020, 87, 101584. [Google Scholar] [CrossRef]
- Zimmermann, G. The Galleria bait method for detection of entomopathogenic fungi in soil. J. Appl. Entomol. 1986, 102, 213–215. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Lagogiannis, I.; Ntoukas, A.; Eliopoulos, P.A.; Kouretas, D.; Karpouzas, D.G.; Poulas, K. Trapping entomopathogenic fungi from vine terroir soil samples with insect baits for controlling serious pests. Appl. Sci. 2020, 10, 3539. [Google Scholar] [CrossRef]
- Lovett, B.; St. Leger, R.J. The insect pathogens. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Meyling, N.V.; Eilenberg, J. Occurrence and distribution of soil borne entomopathogenic fungi within a single organic agroecosystem. Agric. Ecosyst. Environ. 2006, 113, 336–341. [Google Scholar] [CrossRef]
- Tkaczuk, C.; Mietkiewski, R. Occurrence of entomopathogenic fungi in different kinds of soil. Rocz. Nauk. Rol. Ser. E 1996, 25, 41–46. [Google Scholar]
- Quesada-Moraga, E.; Navas-Cortés, J.A.; Maranhao, E.A.A.; Ortiz-Urquiza, A.; Santiago-Álvarez, C. Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol. Res. 2007, 111, 947–966. [Google Scholar] [CrossRef]
- Krysa, A.; Ropek, D.; Kuzniar, T. The occurrence of entomopathogenic fungi depending on season in selected organic farm. J. Res. Appl. Agric. Eng. 2012, 57, 226–230. [Google Scholar]
- Oliveira, I.; Pereira, J.A.; Quesada-Moraga, E.; Lino-Neto, T.; Bento, A.; Baptista, P. Effect of soil tillage on natural occurrence of fungal entomopathogens associated to Prays oleae Bern (Lepidoptera, Plutellidae). Sci. Hortic. 2013, 159, 190–196. [Google Scholar] [CrossRef]
- Jabbour, R.; Barbercheck, M.E. Soil management effects on entomopathogenic fungi during the transition to organic agriculture in a feed grain rotation. Biol. Control 2009, 51, 435–443. [Google Scholar] [CrossRef]
- Roberts, D.W.; Hajek, A.E. Entomopathogenic fungi as bioinsecticides. In Frontiers in Industrial Mycology; Leatham, G.F., Ed.; Chapman & Hall: New York, NY, USA, 1992; pp. 144–159. [Google Scholar]
- Goettel, M.S.; Eilenberg, J.; Glare, T.R. Entomopathogenic fungi and their role in regulation of insect populations. In Comprehensive Molecular Insect Science; Gilbert, L.I., Iatrou, K., Gill, S., Eds.; Elsevier: Oxford, UK, 2005; Volume 6, pp. 361–406. [Google Scholar] [CrossRef]
- Rehner, S.A.; Minnis, A.M.; Sung, G.H.; Luangsa-ard, J.J.; Devotto, L.; Humber, R.A. Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia 2011, 103, 1055–1073. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, D.X.; Duan, D.E.; Wang, Y.B.; Yu, H. Morphology, molecular characterization, and virulence of Beauveria pseudobassiana isolated from different hosts. J. Invertebr. Pathol. 2020, 172, 107333. [Google Scholar] [CrossRef]
- Zhang, S.L.; He, L.M.; Chen, X.; Huang, B. Beauveria lii sp. nov. isolated from Henosepilachna vigintioctopunctata. Mycotaxon 2012, 121, 199–206. [Google Scholar] [CrossRef]
- Imoulan, A.; Hussain, M.; Kirk, P.M.; El Meziane, A.; Yao, Y.J. Entomopathogenic fungus Beauveria: Host specificity, ecology and significance of morpho-molecular characterization in accurate taxonomic classification. J. Asia Pac. Entomol. 2017, 20, 1204–1212. [Google Scholar] [CrossRef]
- Ghikas, D.V.; Kouvelis, V.N.; Typas, M.A. Phylogenetic and biogeographical implications inferred by mitochondrial intergenic region analyses and ITS1- 5.8S-ITS2 of the entomopathogenic fungi B. bassiana and B. brongniartii. BMC Microbiol. 2010, 10, 174–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Huang, C.; He, L.; Zhang, S.; Li, Z. Molecular tracing of white muscardine in the silkworm, Bombyx mori (Linn.) II. Silkworm white muscardine is not caused by artificial release or natural epizootic of Beauveria bassiana in China. J. Invert. Path. 2015, 125, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Coates, B.S.; Hellmich, R.L.; Lewis, L.C. Beauveria bassiana haplotype determination based on nuclear rDNA internal transcribed spacer PCR-RFLP. Mycol. Res. 2002, 106, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Hassan, F.R.; Abdullah, S.K.; Assaf, L.H. First record of Βeauveria varroae from Iraq. Nov. Hedwigia 2019, 108, 427–433. [Google Scholar] [CrossRef]
- Perdomo, H.; Cano, J.; García, D.; Gené, J.; Hernández, M.; Guarro, J. Polyphasic analysis of Purpureocillium lilacinum isolates from different origins and proposal of the new species Purpureocillium lavendulum. Mycologia 2013, 105, 151–161. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Y.R.; Zhang, C.C.; Fan, H.F.; Guo, Z.Y.; Yang, H.Y.; Chen, M.; Han, J.J.; Xu, J.; Zhang, K.Q.; et al. An efficient gene disruption system for the nematophagous fungus Purpureocillium lavendulum. Fungal Biol. 2019, 123, 274–282. [Google Scholar] [CrossRef]
- Liang, L.M.; Zhang, Y.; Xu, J.; Zhang, K.Q.; Cao, Y.R. Characterization of the complete mitochondrial genome of the nematophagous fungus Purpureocillium lavendulum. Mitochondrial DNA Part B Resour. 2021, 6, 33–35. [Google Scholar] [CrossRef]
- Luangsa-Ard, J.; Houbraken, J.; van Doorn, T.; Hong, S.B.; Borman, A.M.; Hywel-Jones, N.L.; Samson, R.A. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol. Lett. 2011, 321, 141–149. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The genus Cordyceps: An extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2018, 129, 293–316. [Google Scholar] [CrossRef]
- Panda, A.K.; Swain, K.C. Traditional uses and medicinal potential of Cordyceps sinensis of Sikkim. J. Ayurveda Integr. Med. 2011, 2, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Holliday, J.C.; Cleaver, M.P. Medicinal value of the caterpillar fungi species of the genus Cordyceps (Fr.) Link (Ascomycetes). A review. Int. J. Med. Mushrooms 2008, 10, 219–234. [Google Scholar] [CrossRef]
- Zhou, X.; Gong, Z.; Su, Y.; Lin, J.; Tang, K. Cordyceps fungi: Natural products, pharmacological functions and developmental products. J. Pharm. Pharmacol. 2009, 61, 279–291. [Google Scholar] [CrossRef]
- Jacquet, J.-S.; Orazio, C.; Jactel, H. Defoliation by processionary moth significantly reduces tree growth, a quantitative review. Ann. Forest Sci. 2012, 69, 857–866. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Mahillo, A.I.; Gonzalez-Muñoz, M.; Vega, J.M.; López, J.A.; Yart, A.; Kerdelhué, C.; Camafeita, E.; Ortiz, J.C.G.; Vogel, H.; Toffolo, E.P.; et al. Setae from the pine processionary moth (Thaumetopoea pityocampa) contain several relevant allergens. Contact Derm. 2012, 67, 367–374. [Google Scholar] [CrossRef]
- Chang, J.C.; Wu, S.S.; Liu, Y.C.; Yang, Y.H.; Tsai, Y.F.; Li, Y.H.; Tseng, C.T.; Tang, L.C.; Nai, Y.S. Construction and selection of an entomopathogenic fungal library from soil samples for controlling Spodoptera litura. Front. Sustain. Food Syst. 2021, 5, 596316. [Google Scholar] [CrossRef]
- Rogers, S.O.; Bendich, A.J. Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant. Mol. Biol. 1985, 5, 69–76. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; Version 27.0; IBM Corp: Armonk, NY, USA, 2020. [Google Scholar]
- Mongkolsamrit, S.; Noisripoom, W.; Thanakitpipattana, D.; Wutikhun, T.; Spatafora, J.W.; Luangsa-Ard, J. Disentangling cryptic species with isaria-like morphs in Cordycipitaceae. Mycologia 2018, 110, 230–257. [Google Scholar] [CrossRef] [PubMed]
- Rehner, S.A.; Posada, F.; Buckley, E.P.; Infante, F.; Castillo, A.; Vega, F.E. Phylogenetic origins of African and Neotropical Beauveria bassiana s.l. pathogens of the coffee berry borer, Hypothenemus hampei. J. Invertebr. Pathol. 2006, 93, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Glare, T.R.; Reay, S.D.; Nelson, T.L.; Moore, R. Beauveria caledonica is a naturally occurring pathogen of forest beetles. Mycol. Res. 2008, 112, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Aquino De Muro, M.; Elliott, S.; Moore, D.; Parker, B.L.; Skinner, M.; Reid, W.; El Bouhssini, M. Molecular characterisation of Beauveria bassiana isolates obtained from overwintering sites of Sunn Pests (Eurygaster and Aelia species). Mycol. Res. 2005, 109, 294–306. [Google Scholar] [CrossRef]
- Meyling, N.V.; Hajek, A.E. Principles from community and metapopulation ecology: Application to fungal entomopathogens. BioControl 2010, 55, 39–54. [Google Scholar] [CrossRef]
- Sharma, L.; Oliveira, I.; Torres, L.; Marques, G. Entomopathogenic fungi in Portuguese vineyards soils: Suggesting a ‘Galleria-Tenebrio-bait method’ as bait-insects Galleria and Tenebrio significantly underestimate the respective recoveries of Metarhizium robertsii and Beauveria bassiana. MycoKeys 2018, 38, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Er, M.K.; Tunaz, H.; Ücük, C.; Bariş, C.; Işikber, A.A. Occurrence of entomopathogenic fungi on insect pests of stored wheat and maize in Central and South Anatolia in Turkey. Turk. Entomoloji Derg. 2016, 40, 249–263. [Google Scholar] [CrossRef] [Green Version]
- Darsouei, R.; Karimi, J.; Ghadamyari, M.; Hosseini, M. Natural Enemies of the Sugar Beet Army Worm, Spodoptera exigua (Lepidoptera: Noctuidae) in Northeast Iran. Entomolog. News 2018, 127, 446–464. [Google Scholar] [CrossRef]
- Samson, R.A. Paecilomyces and some allied hyphomycetes. Stud. Mycol. 1974, 6, 1–119. [Google Scholar]
- Sung, G.H.; Hywel-Jones, N.L.; Sung, J.M.; Luangsa-ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef] [Green Version]
- Gallou, A.; Serna-Domínguez, M.G.; Berlanga-Padilla, A.M.; Ayala-Zermeño, M.A.; Mellín-Rosas, M.A.; Montesinos-Matías, R.; Arredondo-Bernal, H.C. Species clarification of Isaria isolates used as biocontrol agents against Diaphorina citri (Hemiptera: Liviidae) in Mexico. Fungal Biol. 2016, 120, 414–423. [Google Scholar] [CrossRef]
- Er, M.K.; Tunaz, H.; Gökçe, A. Pathogenicity of entomopathogenic fungi to Thaumetopoea pityocampa (Schiff.) (Lepidoptera: Thaumatopoeidae) larvae in laboratory conditions. J. Pest Sci. 2007, 80, 235–239. [Google Scholar] [CrossRef]
- Paparatti, B.; Fabozzi, R. A new pathogen of the pine processionary caterpillar (Thaumetopoea pityocampa Den. et Schif.), Lepidoptera: Thaumetopoeidae. Inf. Fitopatol. 1988, 38, 45–48. [Google Scholar]
- Sönmez, E.; Demir, İ.; Bull, J.C.; Butt, T.M.; Demirbağ, Z. Pine processionary moth (Thaumetopoea pityocampa, Lepidoptera: Thaumetopoeidae) larvae are highly susceptible to the entomopathogenic fungi Metarhizium brunneum and Beauveria bassiana. Biocontrol Sci. Tech. 2017, 27, 1168–1179. [Google Scholar] [CrossRef]
- Akinci, H.A.; Ozman-Sullivan, S.K.; Diler, H.; Celik, N.; Sullivan, G.T.; Karaca, G. Entomopathogenic fungi isolated from Thaumetopoea pityocampa and their efficacies against its larvae. Fresenius Environ. Bull. 2017, 26, 5251–5257. [Google Scholar]
- Ozdemir, I.O.; Kushiyev, R.; Erper, I.; Tuncer, C. Efficacy of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae against Thaumetopoea pityocampa Shiff.(Lepidoptera: Thaumatopoeidae). Arch. Phytopathol. Plant Prot. 2019, 52, 470–480. [Google Scholar] [CrossRef]
- Sevim, A.; Demir, I.; Demirbağ, Z. Molecular characterization and virulence of Beauveria spp. from the pine processionary moth, Thaumetopoea pityocampa (Lepidoptera: Thaumetopoeidae). Mycopathologia 2010, 170, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Sevim, A.; Demir, I.; Sönmez, E.; Kocacevik, S.; Demirbağ, Z. Evaluation of entomopathogenic fungi against the sycamore lace bug, Corythucha ciliata (Say) (Hemiptera: Tingidae). Turk. J. Agric. Forest. 2013, 37, 595–603. [Google Scholar] [CrossRef]

| Treatment | Concentration | Exposure Time | ||
|---|---|---|---|---|
| 48 HAT | 96 HAT | 144 HAT | ||
| B. varroae | 103 | 20.0 ± 0.0 d | 47.0 ± 1.8 d | 60.0 ± 5.8 c |
| 104 | 36.3 ± 3.1 c | 53.0 ± 3.8 c | 63.0 ± 5.8 c | |
| 105 | 43.3 ± 1.8 b | 60.0 ± 5.3 b | 67.0 ± 5.8 c | |
| 106 | 46.4± 5.8 b | 63.0 ± 6.2 b | 80.0 ± 1.3 b | |
| 107 | 50.0 ± 2.2 a | 73.0 ± 1.5 a | 89.0 ± 1.5 a | |
| 108 | 53.0 ± 1.3 a | 83.0 ± 5.3 a | 91.0 ± 5.8 a | |
| Control | H2O + Tergitol NP-9 | 0.0 ± 0.0 e | 3.7 ± 4.3 e | 4.7 ± 1.3 d |
| Treatment | Concentration | Exposure Time | ||
|---|---|---|---|---|
| 48 HAT | 96 HAT | 144 HAT | ||
| P. lavendulum | 103 | 20.0 ± 2.0 e | 37.0 ± 3.8 c | 43.0 ± 6.1 c |
| 104 | 36.0 ± 1.0 d | 43.0 ± 4.8 c | 53.0 ± 2.8 b | |
| 105 | 40.0 ± 0.0 c | 43.0 ± 1.3 c | 57.0 ± 2.8 b | |
| 106 | 43.4 ± 2.8 b | 60.0 ± 1.0 b | 60.0 ± 2.1 b | |
| 107 | 50.0 ± 3.1 a | 63.0 ± 3.5 b | 73.0 ± 1.5 a | |
| 108 | 53.0 ± 5.3 a | 73.0 ± 5.3 a | 77.0 ± 2.1 a | |
| Control | H2O + Tergitol NP-9 | 0.0 ± 0.0 f | 3.7± 4.3 d | 4.7 ± 1.3 d |
| Treatment | Concentration | Exposure Time | ||
|---|---|---|---|---|
| 48 HAT | 96 HAT | 144 HAT | ||
| Cordyceps Blackwelliae | 103 | 12.1 ± 1.9 e | 13.3 ± 5.8 e | 20.0 ± 0.0 d |
| 104 | 20.0 ± 0.0 d | 33.0 ± 3.8 d | 43.0 ± 3.8 c | |
| 105 | 36.0 ± 2.1 c | 43.0 ± 1.3 c | 47.0 ± 4.8 c | |
| 106 | 43.4 ± 5.8 b | 50.0 ± 0.0 b | 60.0 ± 0.0 b | |
| 107 | 50.0 ± 3.2 b | 53.0 ± 6.2 b | 67.0 ± 2.3 a | |
| 108 | 63.0 ± 1.3 a | 63.0 ± 3.3 a | 71.0 ± 1.8 a | |
| Control | H2O + Tergitol NP-9 | 0.0 ± 0.0 f | 3.7 ± 4.3 f | 4.7 ± 1.3 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagogiannis, I.; Mantzoukas, S.; Eliopoulos, P.A.; Poulas, K. First Record of Beauveria varroae, Cordyceps blackwelliae, and Purpureocillium lavendulum from Greece and Their Pathogenicity against Thaumetopoea pityocampa. Diversity 2023, 15, 312. https://doi.org/10.3390/d15030312
Lagogiannis I, Mantzoukas S, Eliopoulos PA, Poulas K. First Record of Beauveria varroae, Cordyceps blackwelliae, and Purpureocillium lavendulum from Greece and Their Pathogenicity against Thaumetopoea pityocampa. Diversity. 2023; 15(3):312. https://doi.org/10.3390/d15030312
Chicago/Turabian StyleLagogiannis, Ioannis, Spiridon Mantzoukas, Panagiotis A. Eliopoulos, and Konstantinos Poulas. 2023. "First Record of Beauveria varroae, Cordyceps blackwelliae, and Purpureocillium lavendulum from Greece and Their Pathogenicity against Thaumetopoea pityocampa" Diversity 15, no. 3: 312. https://doi.org/10.3390/d15030312
APA StyleLagogiannis, I., Mantzoukas, S., Eliopoulos, P. A., & Poulas, K. (2023). First Record of Beauveria varroae, Cordyceps blackwelliae, and Purpureocillium lavendulum from Greece and Their Pathogenicity against Thaumetopoea pityocampa. Diversity, 15(3), 312. https://doi.org/10.3390/d15030312









