Decreasing Trends of Chinstrap Penguin Breeding Colonies in a Region of Major and Ongoing Rapid Environmental Changes Suggest Population Level Vulnerability
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strycker, N.; Wethington, M.; Borowicz, A.; Forrest, S.; Witharana, C.; Hart, T.; Lynch, H.J. A global population assessment of the Chinstrap penguin (Pygoscelis antarctica). Sci. Rep. 2020, 10, 19474. [Google Scholar] [CrossRef]
- Lynch, H.J.; Naveen, R.; Trathan, P.N.; Fagan, W.F. Spatially integrated assessment reveals widespread changes in penguin populations on the Antarctic Peninsula. Ecology 2012, 93, 1367–1377. [Google Scholar] [CrossRef]
- McMahon, K.W.; Michelson, C.I.; Hart, T.; McCarthy, M.D.; Patterson, W.P.; Polito, M.J. Divergent trophic responses of sympatric penguin species to historic anthropogenic exploitation and recent climate change. Proc. Natl. Acad. Sci. USA 2019, 116, 25721–25727. [Google Scholar] [CrossRef] [PubMed]
- Nicol, S.; Constable, A.J.; Pauly, T.; Hkh, O. Estimates of circumpolar abundance of antarctic krill based on recent acoustic density measurements. CCAMLR Sci. 2000, 7, 87–99. [Google Scholar]
- Atkinson, A.; Siegel, V.; Pakhomov, E.A.; Rothery, P.; Loeb, V.; Ross, R.M.; Quetin, L.B.; Schmidt, K.; Fretwell, P.; Murphy, E.J.; et al. Oceanic circumpolar habitats of Antarctic krill. Mar. Ecol. Prog. Ser. 2008, 362, 1–23. [Google Scholar] [CrossRef]
- Rombolá, E.F.; Marschoff, E.; Coria, N. Inter-annual variability in Chinstrap penguin diet at South Shetland and South Orkneys Islands. Polar Biol. 2010, 33, 799–806. [Google Scholar] [CrossRef]
- Panasiuk, A.; Wawrzynek-Borejko, J.; Musiał, A.; Korczak-Abshire, M. Pygoscelis penguin diets on King George Island, South Shetland Islands, with a special focus on the krill Euphausia superba. Antarct. Sci. 2020, 32, 21–28. [Google Scholar] [CrossRef]
- Dimitrijević, D.; Paiva, V.H.; Ramos, J.A.; Seco, J.; Ceia, F.R.; Chipev, N.; Valente, T.; Barbosa, A. Isotopic niches of sympatric Gentoo and Chinstrap Penguins: Evidence of competition for Antarctic krill? Polar Biol. 2018, 41, 1655–1669. [Google Scholar] [CrossRef]
- Emmerson, L.; Southwell, C.; Clarke, J.; Tierney, M.; Kerry, K. Adélie penguin response parameters signal reduced prey accessibility: Implications for predator–prey response curves. Mar. Biol. 2015, 162, 1187–1200. [Google Scholar] [CrossRef]
- Youngflesh, C.; Jenouvrier, S.; Li, Y.; Ji, R.; Ainley, D.G.; Ballard, G.; Barbraud, C.; Delord, K.; Dugger, K.M.; Emmerson, L.M.; et al. Circumpolar analysis of the Adélie Penguin reveals the importance of environmental variability in phenological mismatch. Ecology 2017, 98, 940–951. [Google Scholar] [CrossRef]
- Nicol, S.; Worby, A.; Leaper, R. Changes in the Antarctic sea ice ecosystem: Potential effects on krill and baleen whales. Mar. Freshw. Res. 2008, 59, 361. [Google Scholar] [CrossRef]
- Atkinson, A.; Hill, S.L.; Pakhomov, E.A.; Siegel, V.; Reiss, C.S.; Loeb, V.J.; Steinberg, D.K.; Schmidt, K.; Tarling, G.A.; Gerrish, L.; et al. Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nat. Clim. Change 2019, 9, 142–147. [Google Scholar] [CrossRef]
- Atkinson, A.; Hill, S.L.; Reiss, C.S.; Pakhomov, E.A.; Beaugrand, G.; Tarling, G.A.; Yang, G.; Steinberg, D.K.; Schmidt, K.; Edwards, M.; et al. Stepping stones towards Antarctica: Switch to southern spawning grounds explains an abrupt range shift in krill. Glob. Chang. Biol. 2022, 28, 1359–1375. [Google Scholar] [CrossRef]
- WG-ASAM e-group. Results from the WG-ASAM intersessional e-group on Krill biomass estimates from acoustic surveys. In Proceedings of the Working Group on Ecosystem Monitoring and Management-WG-EMM-2021, Hobart, Australia, 5–9 July 2021. [Google Scholar]
- Trathan, P.N.; Warwick-Evans, V.; Young, E.; Friedlaender, A.; Kim, J.; Kokubun, N. The ecosystem approach to management of the Antarctic krill fishery—The ‘devils are in the detail’ at small spatial and temporal scales. J. Mar. Syst. 2022, 225, 103598. [Google Scholar] [CrossRef]
- Santa Cruz, F.; Krüger, L.; Cárdenas, C.A. Spatial and temporal catch concentrations for Antarctic krill: Implications for fishing performance and precautionary management in the Southern Ocean. Ocean Coast. Manag. 2022, 223, 106146. [Google Scholar] [CrossRef]
- Trivelpiece, W.Z.; Hinke, J.T.; Miller, A.K.; Reiss, C.S.; Trivelpiece, S.G.; Watters, G.M. Variability in krill biomass links harvesting and climate warming to penguin population changes in Antarctica. Proc. Natl. Acad. Sci. USA 2011, 108, 7625–7628. [Google Scholar] [CrossRef]
- Salmerón, N. Warming Precludes Lower Biomass of Antarctic Krill in the South Shetland Islands: Consequences for Foraging and Breeding Success of a Chinstrap Penguin Population. Master’s Thesis, University of Ghant, Ghent, Belgium, 2022. [Google Scholar]
- Hinke, J.T.; Salwicka, K.; Trivelpiece, S.G.; Watters, G.M.; Trivelpiece, W.Z. Divergent responses of Pygoscelis penguins reveal a common environmental driver. Oecologia 2007, 153, 845–855. [Google Scholar] [CrossRef]
- Barbosa, A.; Benzal, J.; De León, A.; Moreno, J. Population decline of chinstrap penguins (Pygoscelis antarctica) on Deception Island, South Shetlands, Antarctica. Polar Biol. 2012, 35, 1453–1457. [Google Scholar] [CrossRef]
- Herman, R.; Borowicz, A.; Lynch, M.; Trathan, P.; Hart, T.; Lynch, H. Update on the global abundance and distribution of breeding Gentoo Penguins (Pygoscelis papua). Polar Biol. 2020, 43, 1947–1956. [Google Scholar] [CrossRef]
- Petry, M.V.; Valls, F.C.L.; Petersen, E.S.; Finger, J.V.G.; Krüger, L. Population trends of seabirds at Stinker Point, Elephant Island, Maritime Antarctica. Antarct. Sci. 2018, 30, 220–226. [Google Scholar] [CrossRef]
- Petry, M.V.; Valls, F.C.L.; Petersen, E.D.S.; Krüger, L.; Piuco, R.D.C.; dos Santos, C.R. Breeding sites and population of seabirds on Admiralty Bay, King George Island, Antarctica. Polar Biol. 2016, 39, 1343–1349. [Google Scholar] [CrossRef]
- Krüger, L.; Huerta, M.F.; Santa Cruz, F.; Cárdenas, C.A. Antarctic krill fishery effects over penguin populations under adverse climate conditions: Implications for the management of fishing practices. Ambio 2021, 50, 560–571. [Google Scholar] [CrossRef]
- Watters, G.M.; Hinke, J.T.; Reiss, C.S. Long-term observations from Antarctica demonstrate that mismatched scales of fisheries management and predator-prey interaction lead to erroneous conclusions about precaution. Sci. Rep. 2020, 10, 2314. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, N.; Deagle, B.; Love, K.; Polanowski, A.; Fielding, S.; Wood, A.G.; Hill, S.; Grant, S.; Belchier, M.; Fleming, A.; et al. Changes in prey fields increase the potential for spatial overlap between gentoo penguins and a krill fishery within a marine protected area. Divers. Distrib. 2021, 27, 552–563. [Google Scholar] [CrossRef]
- BirdLife International. Pygoscelis Antarcticus. The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2020; p. 8235. [Google Scholar]
- Humphries, G.R.W.; Naveen, R.; Schwaller, M.; Che-Castaldo, C.; McDowall, P.; Schrimpf, M.; Lynch, H. Mapping Application for Penguin Populations and Projected Dynamics (MAPPPD): Data and tools for dynamic management and decision support. Polar Rec. 2017, 53, 160–166. [Google Scholar] [CrossRef]
- Bozkurt, D.; Bromwich, D.H.; Carrasco, J.; Hines, K.M.; Maureira, J.C.; Rondanelli, R. Recent Near-surface Temperature Trends in the Antarctic Peninsula from Observed, Reanalysis and Regional Climate Model Data. Adv. Atmos. Sci. 2020, 37, 477–493. [Google Scholar] [CrossRef]
- Carrasco, J.F.; Bozkurt, D.; Cordero, R.R. A review of the observed air temperature in the Antarctic Peninsula. Did the warming trend come back after the early 21st hiatus? Polar Sci. 2021, 28, 100653. [Google Scholar] [CrossRef]
- Eayrs, C.; Li, X.; Raphael, M.N.; Holland, D.M. Rapid decline in Antarctic sea ice in recent years hints at future change. Nat. Geosci. 2021, 14, 460–464. [Google Scholar] [CrossRef]
- Parkinson, C.L. A 40-y record reveals gradual Antarctic sea ice increases followed by decreases at rates far exceeding the rates seen in the Arctic. Proc. Natl. Acad. Sci. USA 2019, 116, 14414–14423. [Google Scholar] [CrossRef]
- Meyer, B.; Atkinson, A.; Bernard, K.S.; Brierley, A.S.; Driscoll, R.; Hill, S.L.; Marschoff, E.; Maschette, D.; Perry, F.A.; Reiss, C.S.; et al. Successful ecosystem-based management of Antarctic krill should address uncertainties in krill recruitment, behaviour and ecological adaptation. Commun. Earth Environ. 2020, 1, 28. [Google Scholar] [CrossRef]
- Lynch, H.; Che-Castaldo, C.; Humphries, G.; Naveen, R. MAPPPD (Mapping Application for Penguin Populations and Projected Dynamics). 2022. Available online: https://www.penguinmap.com/ (accessed on 23 August 2022).
- Rizzo, M.; Szekely, G. E-Statistics: Multivariate Inference vie the Energy of Data; The Comprehensive R Archive Network: Vienna, Austria, 2022. [Google Scholar]
- Rizzo, M.L.; Székely, G.J. Energy distance. Wiley Interdiscip. Rev. Comput. Stat. 2016, 8, 27–38. [Google Scholar] [CrossRef]
- Hadfield, J. MCMC Generalised Linear Mixed Models; The Comprehensive R Archive Network: Vienna, Austria, 2022. [Google Scholar]
- Hadfield, J.D. MCMC Methods for Multi-Response Generalized Linear Mixed Models: The MCMCglmm R Package. J. Stat. Softw. 2010, 33, 1–21. [Google Scholar] [CrossRef]
- Houslay, T.M.; Wilson, A.J. Avoiding the misuse of BLUP in behavioural ecology. Behav. Ecol. 2017, 28, 948–952. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Casanovas, P.; Naveen, R.; Forrest, S.; Poncet, J.; Lynch, H.J. A comprehensive coastal seabird survey maps out the front lines of ecological change on the western Antarctic Peninsula. Polar Biol. 2015, 38, 927–940. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List Categories and Criteria: Version 3.1, 2nd ed.; IUCN International Union for Conservation of Nature: Gland, Switzerland; Cambridge, MA, USA, 2012; ISBN 9782831714356. [Google Scholar]
- Green, D.B.; Bestley, S.; Corney, S.P.; Trebilco, R.; Lehodey, P.; Hindell, M.A. Modelling Antarctic Krill Circumpolar Spawning Habitat Quality to Identify Regions with Potential to Support High Larval Production. Geophys. Res. Lett. 2021, 48, e2020GL091206. [Google Scholar] [CrossRef]
- David, C.L.; Schaafsma, F.L.; van Franeker, J.A.; Pakhomov, E.A.; Hunt, B.P.V.; Lange, B.A.; Castellani, G.; Brandt, A.; Flores, H. Sea-Ice Habitat Minimizes Grazing Impact and Predation Risk for Larval Antarctic Krill. Polar Biol. 2021, 44, 1175–1193. [Google Scholar] [CrossRef]
- Veytia, D.; Corney, S.; Meiners, K.M.; Kawaguchi, S.; Murphy, E.J.; Bestley, S. Circumpolar Projections of Antarctic Krill Growth Potential. Nat. Clim. Change 2020, 10, 568–575. [Google Scholar] [CrossRef]
- Sylvester, Z.T.; Long, M.C.; Brooks, C.M. Detecting Climate Signals in Southern Ocean Krill Growth Habitat. Front. Mar. Sci. 2021, 8, 669508. [Google Scholar] [CrossRef]
- Testa, G.; Neira, S.; Giesecke, R.; Piñones, A. Projecting Environmental and Krill Fishery Impacts on the Antarctic Peninsula Food Web in 2100. Prog. Oceanogr. 2022, 206, 102862. [Google Scholar] [CrossRef]
- Godø, O.R.; Trathan, P. Voluntary Actions by the Antarctic Krill Fishing Industry Help Reduce Potential Negative Impacts on Land-Based Marine Predators during Breeding, Highlighting the Need for CCAMLR Action. ICES J. Mar. Sci. 2022, 79, 1457–1466. [Google Scholar] [CrossRef]
- Roberts, C.M.; O’Leary, B.C.; McCauley, D.J.; Cury, P.M.; Duarte, C.M.; Lubchenco, J.; Pauly, D.; Sáenz-Arroyo, A.; Sumaila, U.R.; Wilson, R.W.; et al. Marine Reserves Can Mitigate and Promote Adaptation to Climate Change. Proc. Natl. Acad. Sci. USA 2017, 114, 6167–6175. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.L.; Tittensor, D.P.; Worm, B.; Lotze, H.K. Incorporating Climate Change Adaptation into Marine Protected Area Planning. Glob. Change Biol. 2020, 26, 3251–3267. [Google Scholar] [CrossRef] [PubMed]
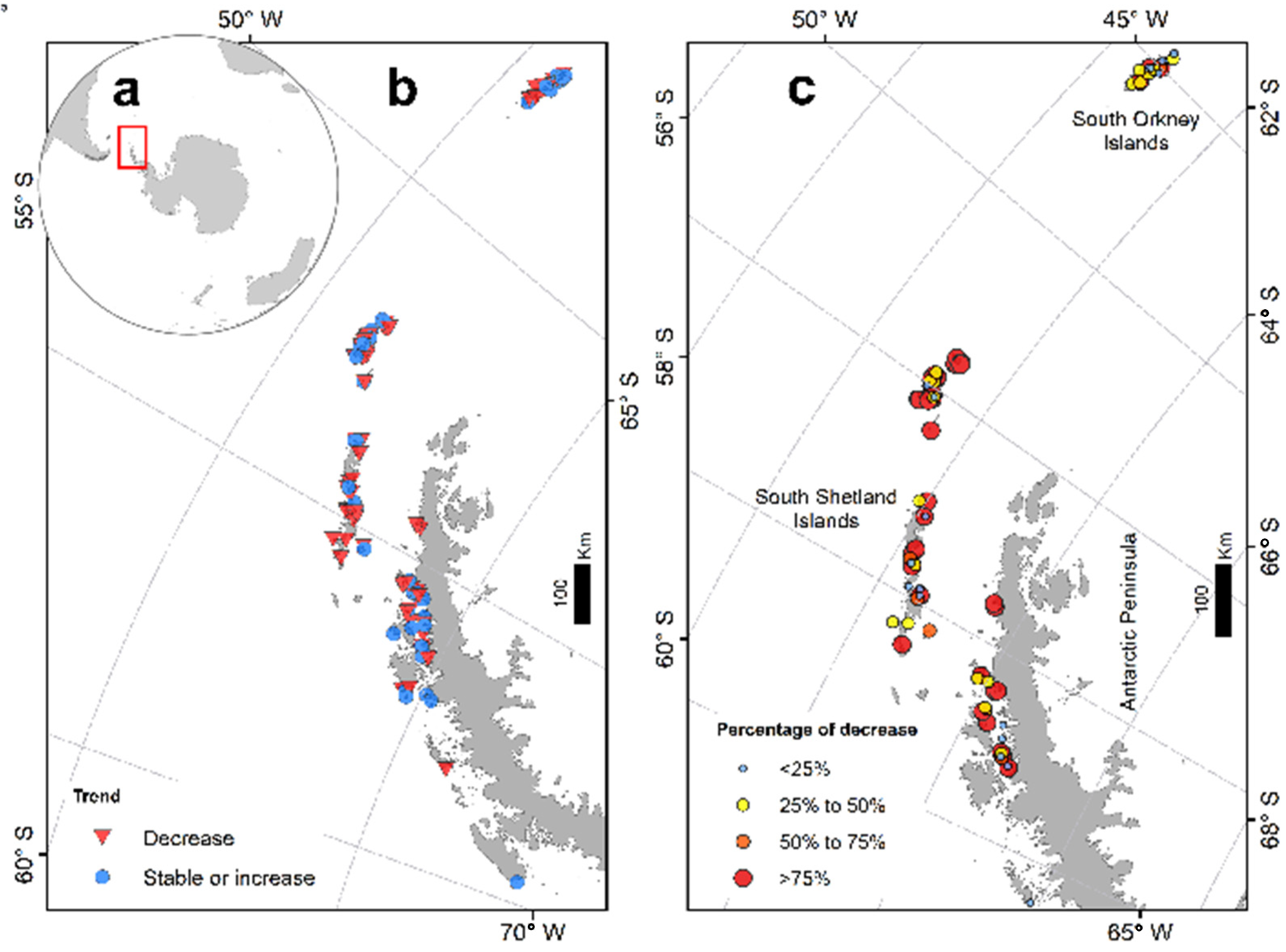
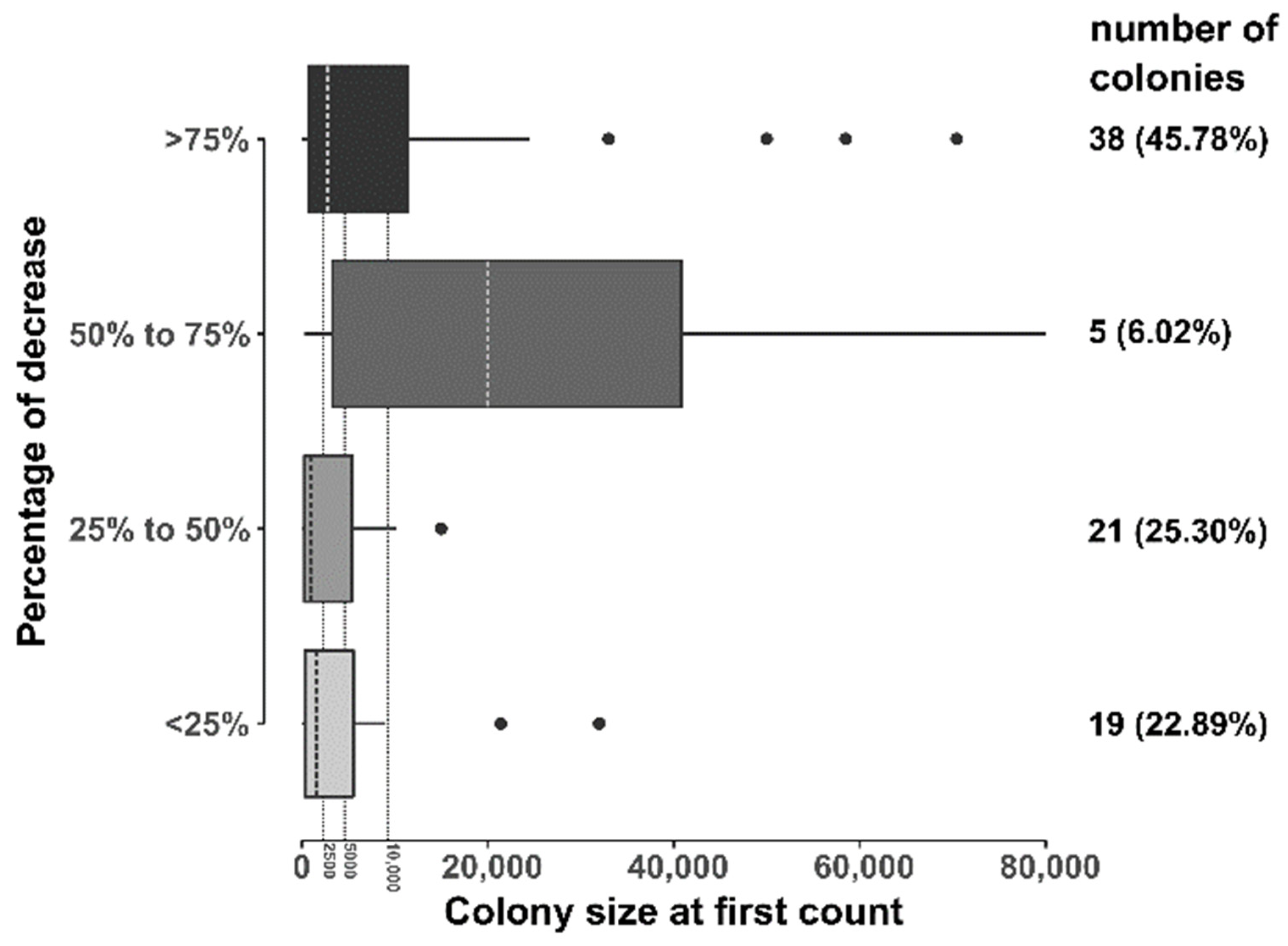

| Area | Abundance from Counts | Model Estimations | 95% CI |
|---|---|---|---|
| Antarctic Peninsula and South Shetlands—48.1 | 32.11% | 31.93% | 30.49–33.13% |
| South Orkneys—48.2 | 28.06% | 28.20% | 23.30–31.53% |
| Total | 60.18% | 60.12% | 53.78–64.67% |
| IUCN Red List Criteria | ||
|---|---|---|
| A taxon is Vulnerable when the best available evidence indicates that it meets any of the following criteria, and it is therefore considered to be facing a high risk of extinction in the wild: | ||
| A. Reduction in population size based on any of the following: | Apply? | Justification |
| No | Despite the levels of decrease found in [1] and this study, the rapid change of the Antarctic Peninsula is an ongoing process that is clearly not reversible in the short term, and the effects on penguins are not completely understood, despite existing evidence [17,24,25]. |
| Yes | Declines over 30% happened for the majority of the global population ([1]; [this study]). Future declines are very likely, as probable causes of penguin decreases [17] have not ceased, nor are they reversible in the short term. Chinstrap penguin generation length is 9.4 years [27]; therefore, these levels of decline might have occurred in 3 generations based on results from this study. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, L. Decreasing Trends of Chinstrap Penguin Breeding Colonies in a Region of Major and Ongoing Rapid Environmental Changes Suggest Population Level Vulnerability. Diversity 2023, 15, 327. https://doi.org/10.3390/d15030327
Krüger L. Decreasing Trends of Chinstrap Penguin Breeding Colonies in a Region of Major and Ongoing Rapid Environmental Changes Suggest Population Level Vulnerability. Diversity. 2023; 15(3):327. https://doi.org/10.3390/d15030327
Chicago/Turabian StyleKrüger, Lucas. 2023. "Decreasing Trends of Chinstrap Penguin Breeding Colonies in a Region of Major and Ongoing Rapid Environmental Changes Suggest Population Level Vulnerability" Diversity 15, no. 3: 327. https://doi.org/10.3390/d15030327
APA StyleKrüger, L. (2023). Decreasing Trends of Chinstrap Penguin Breeding Colonies in a Region of Major and Ongoing Rapid Environmental Changes Suggest Population Level Vulnerability. Diversity, 15(3), 327. https://doi.org/10.3390/d15030327







