Abstract
Maternal hormones such as estrogens deposited into the yolk of turtle eggs follow circulating levels in adult females, and they may alter the sexual fate of developing embryos in species with temperature-dependent sex determination (TSD). In temperate regions, this deposition occurs during the spring when estrogens increase in adult females as ambient temperatures rise, drop after the first clutch, and peak again (albeit less) in the fall. Global warming alters turtle nesting phenology (inducing earlier nesting), but whether it affects circulating hormones remains unknown, hindering our understanding of all potential challenges posed by climate change and the adaptive potential (or lack thereof) of turtle populations. Here, we addressed this question in painted turtles (Chrysemys picta) by quantifying estradiol, estrone, and testosterone via mass spectrometry in the blood of wild adult females exposed to 26 °C and 21 °C in captivity between mid-August and mid-October (15 females per treatment). Results from ANOVA and pairwise comparisons revealed no differences between treatments in circulating hormones measured at days 0, 2, 7, 14, 28, and 56 of the experiment. Further research is warranted (during the spring, using additional temperatures) before concluding that females are truly buffered against the indirect risk of climate change via maternal hormone allocation.
1. Introduction
The maternal allocation of hormones by mothers influences sexual development of turtles that possess temperature-dependent sex determination (TSD) [1]. This mechanism is prevalent and ancestral in this vertebrate group, from which genotypic sex determination with sex chromosomes evolved multiple times [2]. Most TSD turtles generally produce male offspring at low incubation temperatures and females at high incubation temperatures (TSDIa or MF pattern) [3], such that populations would risk feminization under current climate change predictions by exposing embryos to warmer conditions in their nest that produce more females. Additionally, higher yolk estrogen concentrations can also feminize embryos when incubated around the pivotal temperature (the value that produces a 1:1 population sex ratio), as seen in painted and red-eared slider, as well as other turtles, but not all [4,5,6]. The feminizing capability of estrogens is demonstrated by the sex reversal of eggs of multiple TSD turtles incubated at male-producing temperatures when exogenous estradiol is applied topically to the eggs [7,8,9] and even in GSD turtle species with XX/XY sex chromosomes [10]. Importantly, yolk estrogen concentration varies seasonally coinciding with the circulating levels in females during vitellogenesis [4,11], which is captured in the hormonal variation detected among egg layers [12] and between clutch follicles [4]. Some have suggested that such seasonal variation might yield more female-biased clutches later in the reproductive season [1].
Indeed, plasma estrogens in painted turtles are undetectable during the winter brumation period, but they increase dramatically after their emergence from brumation, coinciding with warming spring temperatures and a lengthening photoperiod. Estrogen levels continue rising until the oviposition of the first clutch (which is determined by environmental temperatures) and drop to low levels for the remainder of the summer [13,14]. Then, a second and lesser estrogen peak occurs during the fall, likely coinciding with ovarian growth [13]. Thus, any factor that alters an adult female’s circulating hormone profile during yolk deposition could impact the sex ratios of hatchlings and ultimately affect population dynamics and the rate of loss of genetic variation.
One such factor might be ambient temperature via its effect on female behavior. Namely, reproductive females adjust their body temperature behaviorally, warming up above the water temperature by basking more frequently during the spring, which influences their gonadal cycle and, thus, their first nesting date and ability to lay a second clutch [15]. Indeed, warmer spring temperatures induce earlier onset of nesting in painted and snapping turtles [16,17,18,19], revealing that temperature (perhaps more than photoperiod) is an important trigger of the reproductive cycle in females, during which estrogen rises early in the spring and later in the summer. Earlier nesting would also expose eggs to lower environmental temperatures, which might produce more males earlier in the nesting season and more females thereafter, all else being equal [19]. However, all else is not equal. Indeed, climate change is elevating the ambient temperatures that adult female turtles experience, and yet, how elevated temperatures affect female hormonal levels beyond their effects on basking and nesting phenology remains unknown. This gap hinders our understanding of the potential for females to respond to climate change.
Some observations lead to the hypothesis that global warming could induce higher estrogen levels in adult females. For instance, aromatase, the enzyme responsible for estrogen synthesis in turtles by converting androgens into estrogen, catalyzes more estrogen production in the gonads of embryos incubated at warmer temperatures in all TSD turtle species studied so far, including Dermochelys coriacea, Emys orbicularis, Malaclemys terrapin, Chelydra serpentina, Trachemys scripta, and Chrysemys picta [20,21,22]. The aromatase gene also exhibits higher transcription in the gonads of embryos incubated at higher temperatures [23,24,25,26,27,28]. Both the enhanced gene transcription and catalytic activity results in greater estrogen levels in embryos developing at warmer conditions until the peak temperature within the optimal range is reached, past which production begins decreasing [21]. Aromatase is also essential for adult female steroidogenesis and maintenance of ovarian differentiation in turtles and other vertebrates [29,30,31,32]. Similar to embryos, aromatase is more metabolically active in the brain of adult painted turtles at 27 °C compared to 17 °C and 37 °C [33], similar to Anolis carolinensis lizards where aromatase was more metabolically active in the brain at 27 °C compared to 37 °C [34]. Combined, these observations suggest an optimal temperature for aromatase activity within the optimal range of temperatures for a given species and the denaturation of this enzyme at extreme values past this range (e.g., 37 °C). Thus, only very extreme global warming may reduce estrogen levels in females, given current predictions that temperatures would increase ~2.5 °C by 2100 under the RCP 3.4 scenario (more likely case) [35] and by ~5–6 °C under the RCP 8.5 scenario (worst case) [19].
Therefore, we hypothesize that warmer ambient temperatures, as predicted under more likely climate change scenarios [36,37], may increase circulating estrogen levels in adult females, perhaps induced by higher aromatase activity at warmer conditions in adults as it does in embryos. If true, higher maternal circulating estrogens during vitellogenesis could result in higher estrogen deposition in the eggs, potentially feminizing clutches incubated around the pivotal temperature [1,6]. Such an effect would add to the accelerating feminizing effect that is expected from rising average temperatures and exacerbated thermal fluctuations expected under climate change [38,39,40]. Alternatively, if temperature does not impact estrogen production, females would be shielded from this indirect effect of climate change, and some other environmental and/or physiological process/es must drive the elevating estrogen during the breeding season. Here, we investigate the effect of constant temperature on circulating estrogen levels in adult female painted turtles as a first step to illuminate the potential for global warming to affect TSD sex ratios indirectly, via maternal effects.
2. Materials and Methods
The objective of this study was to test the hypothesis that temperature affects the levels of circulating estrogens in adult female painted turtles. To determine this, we quantified the levels of unbound estrogen in the blood plasma of females exposed to colder (21 °C) and warmer (26 °C) ambient water temperatures. We predicted that circulating estrogens in adult females will be higher when exposed to constant elevated ambient temperatures over a two-month period. Alternatively, adult females may be buffered from thermal effects on their hormone production, resulting in similar levels of estrogens in both treatments. Because the testosterone (T) to estrogen ratio (T:E2) in the yolk has been studied for its potential to affect sex determination rather than estrogens alone [5], we also measured T levels and examined T:E2 ratios among treatments over time.
2.1. Animal Collection
Thirty adult female painted turtles were captured during the summer of 2021 under Iowa DNR permit (between 15 May and 7 August) and kept at the Iowa State University (ISU) Horticulture Research Station in an outdoor artificial pond until 30 females were captured that were larger than the smallest gravid female we have captured from this population. Females were then retrieved and transported to our indoor animal facility at ISU, where they were randomly assigned to a warmer (~26 °C) or colder (~21 °C) treatment in equal numbers (n = 15 per treatment) in separate temperature-controlled rooms and kept in a 12:12 light:dark cycle in a 1.2 × 1.2 × 0.3 m water pool. All adult females were non-gravid by the start of the experiment, having laid eggs while they were held in the outdoor artificial pond (5 out of 30 females) or before they were trapped. No basking platform was provided during the experiment to minimize variation in body temperature among females due to behavioral differences. Blood was extracted from the subcarapacial sinus at 6 different time points (days 0, 2, 7, 14, 28, and 56 of the experiment, between 11 August and 7 October) and centrifuged at 4 °C for 5 min to separate the plasma from the red blood cells. Samples were immediately frozen on dry ice and stored at −80 °C. Turtles were released back into the lake at the Horticulture Research Station after all blood sampling concluded. All animal procedures were approved by ISU IACUC. Because different amounts of lymph could be drawn when collecting blood samples from the subcarapacial sinus [41,42], which could dilute the plasma, we weighed the vials containing the plasma and red blood cell (RBC) fractions, and we calculated a dilution factor as the ratio of plasma to RBC weights. We multiplied the raw values of hormones by this dilution factor before statistical tests, and compared the results using these relative values to those using raw values.
The samples were submitted to the Iowa State Metabolomics facility where hormone levels were quantified using Mass Spectrometry in an Agilent 6470 Triple Quadrupole LC/MS machine. Levels of estrogens (estrone and 17-beta estradiol) and testosterone in the plasma were assessed following [43], after extraction following [44], with a few modifications to optimize the protocol for turtle plasma, as described below.
2.2. Sample Preparation
Non-labeled standards from Sigma for estrone (E1) (cat. 46573) and 17-beta estradiol (E2) (cat. PHR1353) were used and diluted 10-fold (E1 in methanol and E2 in acetonitrile) to a final concentration of E1 and E2 of 516.6 pg/µL and 519.4 pg/µL, respectively.
Then, 200 µL of plasma from each sample was spiked with 10 µL of each of the E1 and E2 diluted internal standards, and 0.4 mL of ice-cold 100% methanol was added. Samples were then vortexed for 30 s, incubated on ice for 1 h, vortexed for 30 s, and sonicated in a cold-water bath for 10 min (or longer if needed to dissolve all of the pellets). Samples were vortexed a third time for 30 s and centrifuged at 13,000× g to precipitate out any proteins. The supernatant was removed and placed into a clean Eppendorf tube rinsed with methanol. This process was repeated with another 200 µL of plasma and 300 µL of methanol instead of 400 µL, and the resulting supernatant products were combined. Then, 1 mL of ethyl acetate was added to each sample, vortexed well, and 300–400 µL of water was added to achieve phase separation. Samples were vortexed for 10 min at full speed and refrigerated overnight. Next day, samples were centrifuged for 10 min at 4000× g, and the upper organic layer (about 400–500 µL) was transferred into a GCMS vial and speed-vac for 4–5 h.
For the derivatization for LC-MS, sample extracts were reconstituted in 100 µL of NaHCO3 (0.1 M, pH 9.85–10.5), and 100 µL of Dansyl Chloride was added followed by vortexing. Samples were incubated on a shaking heat block for 10 min at 60 °C and vortexed twice while shaking on the heat block. Samples were allowed to cool and placed into the inserts for LC-MS.
2.3. QQQ-MS Analysis
The column Agilent Eclipse plus C18 RRHD 1.8 µm 2.1 × 100 mm was used. Gradients were set to a flow rate of 0.400 mL/min at 40 °C. Solvent A consisted of 0.1% formic acid and Solvent B contained Acetonitrile with 0.1% formic acid. The column pressure was approximately 515 bar with a 50/50 mix of solvent A and B. The gradient (time in minutes; % of Solvent A) was: (initial; 50), (5; 50), (13; 10), (15; 1), (16; 1), (16.01; 50), and (20; 50), and triggered by a 10 µL injection.
2.4. Statistical Analysis
Raw hormone values (pg/mg) were Box-Cox transformed to improve normality of their distribution, and these transformed values were used in all downstream analyses. An ANOVA was conducted to test for the effect of temperature (T°), time (t), and their interaction on hormone levels (T° × t), followed by a separate ANOVA of differences in hormone level by temperature at each timepoint. ANOVA tests were conducted for each hormone separately and for the T:E2 ratio, using (a) the raw hormonal values, (b) multiplying raw values by their dilution factor to obtain relative hormone levels, (c) subtracting the baseline value at day 0 from the raw values to obtain delta hormone levels, and (d) subtracting the baseline relative value at day 0 to obtain relative delta hormone levels. Then, all analyses were re-run after outliers, i.e., extreme values with z-score > 3, were removed from the dataset. Significance was assessed at an alpha of 0.05 for the ANOVA, with Bonferroni correction for multiple comparisons in the separate ANOVA tests. Statistical tests were conducted in R version 4.0.3 [45].
3. Results
Over the course of our two-month experiment, circulating levels remained low for Estradiol (E2) and Estrone (E1), and lower yet for testosterone (T) compared to either E1 or E2 (Figure 1, Table 1).
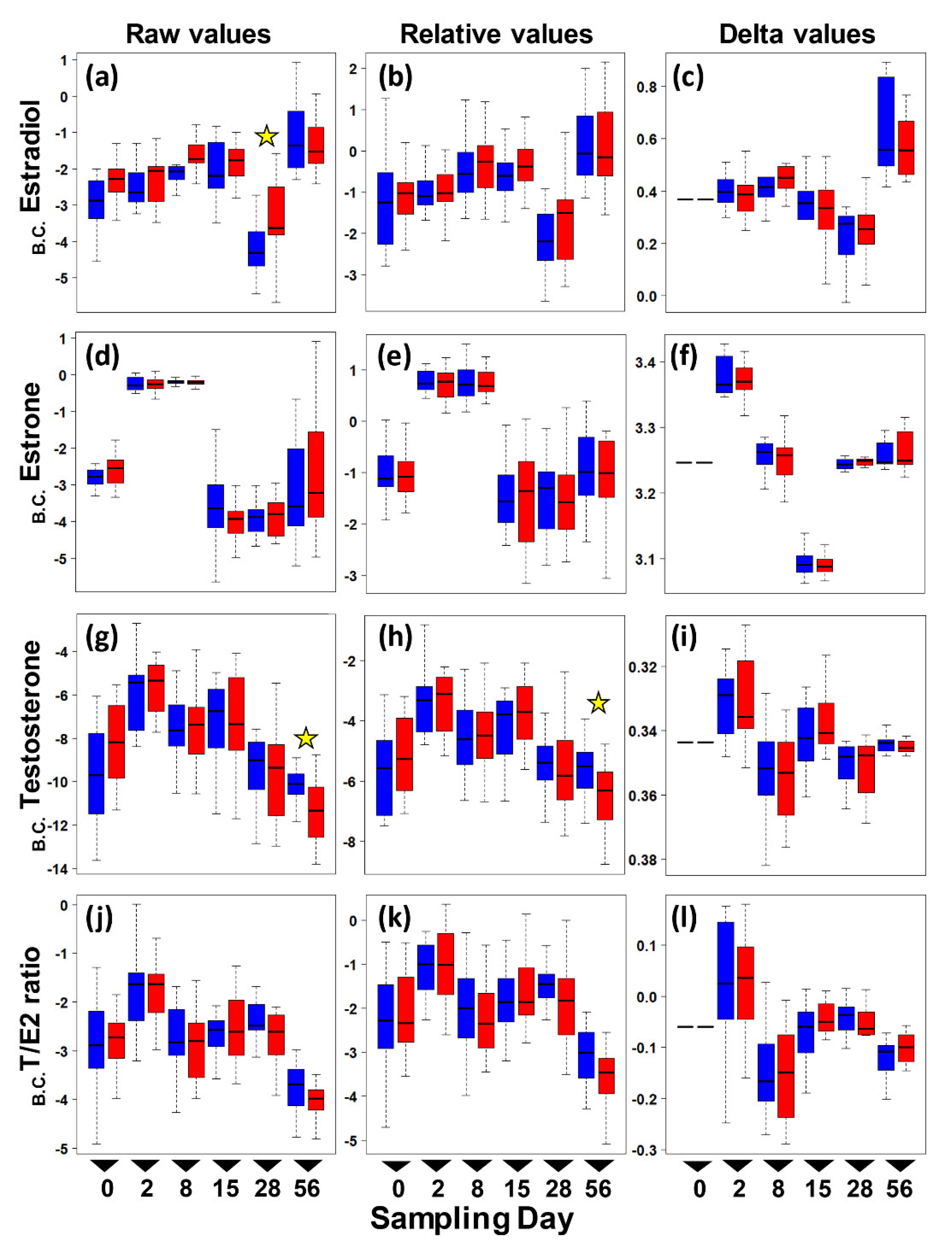
Figure 1.
Boxplots of circulating sex hormone levels in adult female painted turtles (Chrysemys picta) after 0, 2, 7, 14, 28, and 56 days of exposure to colder (21 °C—blue) or warmer (26 °C—red) water temperatures indoors. Each boxplot denotes the interquartile range (IQR) with median values (black solid horizontal line), 25th to 75th percentile (box), and 1.5 IQR (dotted line whiskers) for Box–Cox transformed (B.C.) pg/mg values (no outliers were removed from these plots). Left panels present raw E2 (a), E1 (d), T (g), and T:E2 Ratio (j) levels. Middle panels present relative E2 (b), E1 (e), T (h), and T:E2 Ratio (k) levels. Right panels present changes from day 0 in E2 (c), E1 (f), T (i), and T:E2 Ratio (l) levels (i.e., delta values). Stars denote significant thermal effect on hormone level detected by separate ANOVAs conducted at each sampling day.

Table 1.
Average and range (in pg/mg) of raw values (including outliers) of the sex hormones quantified in Chrysemys picta reproductive females per temperature and sampling day. Temp = temperature treatment. N = sample size. Min = minimum value. Max = Maximum value.
ANOVA results revealed no significant interaction between temperature and day in raw circulating hormone levels (p > 0.32 in all cases. Table S1). Thus, a reduced ANOVA was run that included only the main effects (temperature and day), and results indicated that day, and not temperature, had a significant effect on the level of estrone, testosterone, and the T:E2 ratio (Table 2). In contrast, temperature had a significant effect on raw estradiol levels, as did day.

Table 2.
Results from the ANOVA tests using Box–Cox-transformed corrected (relative) hormone values (i.e., raw values multiplied by dilution factor), after outliers were removed (see text for details).
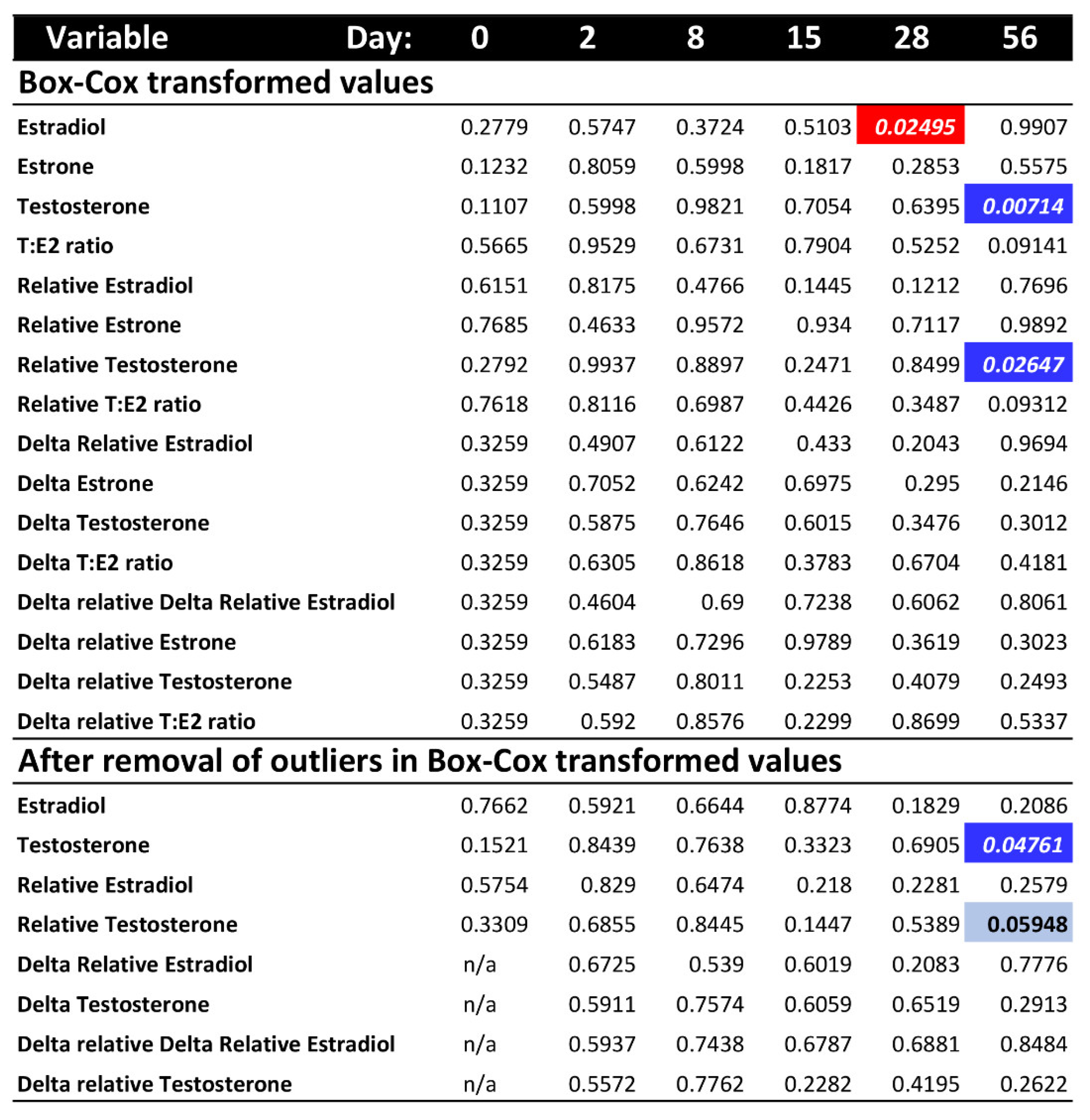
When correcting the raw hormone values by their dilution factor (i.e., using relative values), the effect of day remained significant for all hormones, whereas the significant difference in estradiol between temperatures disappeared (Table 2). These results were robust to using the delta values (raw delta and relative delta values) to examine the change in hormone levels from the initial baseline at day 0 for estradiol and the T:E2 ratio (Table S2) or for delta relative values (Table S3), all of which were only affected by day, except for delta-estrone and delta-testosterone, which remained unaltered by any factor.
To further explore whether differences in hormone levels might exist on any one particular sampling day that could have been masked by the overall ANOVA test, we conducted a post hoc separate ANOVA at each sampling time. Results revealed a significant effect of temperature on estradiol at day 28, and on raw testosterone and relative testosterone at day 56, whereas all other hormone values were not significantly different between the colder and warmer treatments at any given sampling period (Figure 2). However, these differences for estradiol and relative testosterone disappeared after removing outliers, leaving only one significant difference by temperature (raw testosterone) out of all post hoc comparisons, which is not different than expected by chance (p-values surpass the Bonferroni-corrected alpha).
Figure 2.
p-values of the post hoc ANOVA tests per sampling day for each hormone variable, after Box-Cox transformation and outlier removal (no outliers were detected after Box-Cox transformation for estrone or T:E2 ratio). Colored cells indicate significant p-values < 0.05 (bright colors) or marginally significant (light blue). Red indicates higher hormone level at warmer temperature. Blue indicates higher hormone level at colder temperature.
4. Discussion
Many environmental and maternal factors influence sex determination in TSD vertebrates, and improving our understanding of the physiological and hormonal mechanisms underlying sexual development is important to illuminate this process, particularly in the context of climate change. In a warming world, turtles must adapt to increasing global temperatures or they will face population declines or, potentially, extinction. TSD species are among the most vulnerable taxa due to skewing of sex ratios that reduces population viability. While extensive research has concentrated on the direct effect that climate change has on sex ratios by exposing developing embryos of TSD taxa to warmer conditions, ours is the first study, to our knowledge, to investigate the potential indirect impact that global warming might have via endocrine maternal effects, by exploring the influence of environmental temperature on sex hormone levels in reproductive female painted turtles, Chrysemys picta.
Results suggest that turtles are resilient to warming conditions in their estrogen, estrone, and testosterone levels during the two-month length of our study. In general, levels were low for Estradiol (E2) and Estrone (E1) and even lower for testosterone (T) compared to values detected in the spring in another population of this species, but they were similar to values reported for the fall [13]. Indeed, while all three hormones differed in concentration by sampling date, consistent with the seasonal changes reported previously for this taxon [13] and other taxa [46,47,48], our data revealed only a modest tendency for estradiol to increase and for testosterone to decrease by temperature. However, these differences disappeared after accounting for the relative dilution of the blood samples or after removing outliers. Once these corrections were made, only testosterone showed a significant thermal effect, but this observation is indistinguishable from a false positive expected by chance given the number of post hoc comparisons (i.e., the p-values are greater than the Bonferroni-corrected alpha). Although we found no strong evidence for an indirect effect of warming temperature on maternal circulating hormones that could potentially alter hatchling sex ratios, the direct threat of feminization of developing embryos of TSDIa (MF) taxa remains critical due to elevated average and fluctuations in ambient temperatures [38,39,49], particularly as these risks appear not to be offset by maternal behavioral responses that alter nesting phenology adaptively [19,50,51].
Intriguingly, our results also indicate that an environmental cue (s) other than temperature is responsible for raising estrogen levels in adult female painted turtles. It is likely that the lengthening photoperiod alone, which coincides with elevated spring estrogen levels [13], plays an important role. If so, such an effect must be lagging, because our study was conducted indoors and females were exposed to a constant 12:12 light:dark cycle, yet circulating hormones changed over time (as evidenced by the significant day effect in the ANOVA tests—Table 2 and Figure 1).
Further research is warranted to test alternative explanations for why we did not observe a more substantial increase in estrogens at warmer temperatures. One possibility is that our experimental design may not have captured the true environmental effect of temperature in an ecologically relevant manner. For example, our study took place from August to October (as logistical issues precluded collecting females earlier that year), a period of time when other studies detected a second (albeit much attenuated) peak in circulating estradiol in other painted turtle populations, which was attributed to ovarian growth [13]. Yet, other studies reported undetectable sex hormones at an equivalent time [52], perhaps because females had laid their clutches for that season and could have entered a state of quiescence [52]. We note that all the females included in our study were non-gravid by the time they entered the experiment. Our data match the pattern of estradiol reported in [13], with very low levels in August and September, and elevated values (accompanied by greater variance) in October (Figure 1). Thus, females may not have been physiologically capable of greater hormonal production at this time in the reproductive season for our temperature treatments to have had an effect, whereas perhaps an effect would be detected if the experiment were conducted in the spring months. Another possibility is that our chosen temperatures were not different enough, or the higher temperature treatment warm enough, to elicit significantly different levels of hormone production.
5. Conclusions
Results from our study revealed no effect (or undistinguishable from random) of ambient temperature on the level of estrogens, testosterone, or their ratio, in reproductive painted turtles. However, further research in the spring, using a wider range of temperatures is warranted before concluding whether females are truly buffered against the indirect risk posed by climate change to further feminize sex ratios beyond the direct effect that global warming has on developing embryos. The observed tendencies for estradiol to increase at the warmer temperature on day 28 and for testosterone to decrease at day 56 suggest that under proper conditions, a difference may be detected in a future study. Thus, our work underscores the urgency of conducting additional studies to better understand the complex relationships between environmental factors and turtle physiology. For instance, if research conducted in the spring does find a thermal effect on hormone levels of reproductive females, then further work would be warranted to test for the interaction between such effects and endocrine disrupting contaminants in the habitats of TSD taxa, which not only affect embryos but also the mothers during vitellogenesis [53,54]. Such information would be relevant for conservation as it will allow managers to develop management efforts for populations where intervention to directly alter sex ratios may be needed to ensure their survival.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15030428/s1, Supplementary Materials include Tables S1–S4.
Author Contributions
Conceptualization, N.E.T. and N.V.; Data curation, N.E.T. and N.V.; formal analysis, N.E.T. and N.V.; funding acquisition, N.E.T. and N.V.; investigation, N.E.T. and N.V.; methodology, N.E.T. and N.V.; project administration, N.V.; resources, N.E.T. and N.V.; supervision, N.V.; visualization, N.E.T. and N.V.; writing—original draft, N.E.T.; writing—review and editing, N.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant-in-aid-of-research from The Justin Congdon and Nancy Dickson Research Fund (CDRF) Turtle Ecology Fund at the Chelonian Research Foundation (CRF) to N.V. and N.T., and from National Science Foundation grants IOS 2129793 and IOS 2127995 to N.V. The APC was waived by Diversity.
Institutional Review Board Statement
All animal procedures were approved by the Iowa State University Institutional Animal Care and Use Committee (protocol IACUC-21-060 approved 20 April 2021).
Data Availability Statement
All data are provided in Table S4 in the Supplementary Materials.
Acknowledgments
We thank M. Meyer for her assistance in the field and with animal care, N. Howell and C. Arnold for advice, equipment, and access to the ISU Horticulture Research Station, and D. Adams for statistical advice. We acknowledge the W.M. Keck Metabolomics Research Laboratory (Office of Biotechnology, Iowa State University, Ames IA) for providing analytical instrumentation and we thank Ann M Perera for her assistance and support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bowden, R.M.; Ewert, M.A.; Nelson, C.E. Environmental sex determination in a reptile varies seasonally and with yolk hormones. Proc. Biol. Sci. 2000, 267, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Bista, B.; Valenzuela, N. Turtle insights into the evolution of the reptilian karyotype and the genomic architecture of sex determination. Genes 2020, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Ewert, M.A.; Etchberger, C.R.; Nelson, C.E. Turtle sex-determining modes and TSD patterns, and some TSD pattern correlates. In Temperature-Dependent Sex Determination in Vertebrates; Valenzuela, N., Lance, V.A., Eds.; Smithsonian Book: Washington, DC, USA, 2004; pp. 21–32. [Google Scholar]
- Bowden, R.M.; Ewert, M.A.; Freedberg, S.; Nelson, C.E. Maternally derived yolk hormones vary in follicles of the painted turtle, Chrysemys picta. J. Exp. Zool. 2002, 293, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Radder, R.S. Maternally derived egg yolk steroid hormones and sex determination: Review of a paradox in reptiles. J. Biosci. 2007, 32, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Bowden, R.M.; Paitz, R.T. Temperature fluctuations and maternal estrogens as critical factors for understanding temperature-dependent sex determination in nature. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 177–184. [Google Scholar] [CrossRef]
- Raynaud, A.; Pieau, C. Embryonic development of the genital system. In Biology of the Reptilia; Gans, C., Billet, F., Eds.; John Wiley & Sons: New York, NY, USA, 1985; pp. 151–300. [Google Scholar]
- Wibbels, T.; Crews, D. Steroid-induced sex determination at incubation temperatures producing mixed sex ratios in a turtle with TSD. Gen. Comp. Endocrinol. 1995, 100, 53–60. [Google Scholar] [CrossRef]
- Freedberg, S.; Nelson, C.E.; Ewert, M.A. Estradiol-17 beta induces lasting sex reversal at male-producing temperatures in kinosternid turtles. J. Herpetol. 2006, 40, 95–98. [Google Scholar] [CrossRef]
- Freedberg, S.; Bowden, R.M.; Ewert, M.A.; Sengelaub, D.R.; Nelson, C.E. Long-term sex reversal by oestradiol in amniotes with heteromorphic sex chromosomes. Biol. Lett. 2006, 2, 378–381. [Google Scholar] [CrossRef]
- Janzen, F.J.; Wilson, M.E.; Tucker, J.K.; Ford, S.P. Experimental manipulation of steroid concentrations in circulation and in egg yolks of turtles. J. Exp. Zool. 2002, 293, 58–66. [Google Scholar] [CrossRef]
- Bowden, R.M.; Ewert, M.A.; Lipar, J.L.; Nelson, C.E. Concentrations of steroid hormones in layers and biopsies of chelonian egg yolks. Gen. Comp. Endocrinol. 2001, 121, 95–103. [Google Scholar] [CrossRef]
- Callard, I.P.; Lance, V.; Salhanick, A.R.; Barad, D. The annual ovarian cycle of Chrysemys picta: Correlated changes in plasma steroids and parameters of vitellogenesis. Gen. Comp. Endocrinol. 1978, 35, 245–257. [Google Scholar] [CrossRef]
- Crawford, K.M. The winter environment of painted turtles, Chrysemys picta—temperature, dissolved-oxygen, and potential cues for emergence. Can. J. Zool.—Rev. Can. De Zool. 1991, 69, 2493–2498. [Google Scholar] [CrossRef]
- Topping, N.E.; Valenzuela, N. Turtle nest-site choice, anthropogenic challenges, and evolutionary potential for adaptation. Front. Ecol. Evol. 2021, 9, 808621. [Google Scholar] [CrossRef]
- Obbard, M.E.; Brooks, R.J. Prediction of the onset of the annual nesting season of the common snapping turtle, Chelydra serpentina. Herpetologica 1987, 43, 324–328. [Google Scholar]
- Rowe, J.W.; Coval, K.A.; Campbell, K.C. Reproductive characteristics of female midland painted turtles (Chrysemys picta marginata) from a population on Beaver Island, Michigan. Copeia 2003, 2003, 326–336. [Google Scholar] [CrossRef]
- Grayson, K.L.; Dorcas, M.E. Seasonal temperature variation in the painted turtle (Chrysemys picta). Herpetologica 2004, 60, 325–336. [Google Scholar] [CrossRef]
- Schwantes, A.M.; Swenson, J.J.; González-Roglich, M.; Johnson, D.M.; Domec, J.C.; Jackson, R.B. Measuring canopy loss and climatic thresholds from an extreme drought along a fivefold precipitation gradient across Texas. Glob. Chang. Biol. 2017, 23, 5120–5135. [Google Scholar] [CrossRef] [PubMed]
- Desvages, G.; Pieau, C. Aromatase-activity in gonads of turtle embryos as a function of the incubation-temperature of eggs. J. Steroid Biochem. Mol. Biol. 1992, 41, 851–853. [Google Scholar] [CrossRef]
- Desvages, G.; Girondot, M.; Pieau, C. Sensitive Stages for the Effects of Temperature on gonadal aromatase-activity in embryos of the marine turtle Dermochelys coriacea. Gen. Comp. Endocrinol. 1993, 92, 54–61. [Google Scholar] [CrossRef]
- Jeyasuria, P.; Roosenburg, W.M.; Place, A.R. Role of P-450 Aromatase in sex determination of the diamondback terrapin, Malaclemys terrapin. J. Exp. Zool. 1994, 270, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Jeyasuria, P.; Place, A.R. Temperature-dependent aromatase expression in developing diamondback terrapin (Malaclemys terrapin) embryos. J. Steroid Biochem. Mol. Biol. 1997, 61, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Jeyasuria, P.; Place, A.R. Embryonic brain-gonadal axis in temperature-dependent sex determination of reptiles: A role for P450 aromatase (CYP19). J. Exp. Zool. 1998, 281, 428–449. [Google Scholar] [CrossRef]
- Rhen, T.; Metzger, K.; Schroeder, A.; Woodward, R. Expression of putative sex-determining genes during the thermosensitive period of gonad development in the snapping turtle, Chelydra Serpentina. Sex. Dev. 2007, 1, 255–270. [Google Scholar] [CrossRef]
- Ramsey, M.; Crews, D. Adrenal-kidney-gonad complex measurements may not predict gonad-specific changes in gene expression patterns during temperature-dependent sex determination in the red-eared slider turtle (Trachemys scripta elegans). J. Exp. Zool. A Ecol. Genet. Physiol. 2007, 307, 463–470. [Google Scholar] [CrossRef]
- Valenzuela, N.; Neuwald, J.L.; Literman, R. Transcriptional evolution underlying vertebrate sexual development. Dev. Dyn. 2013, 242, 307–319. [Google Scholar] [CrossRef]
- Lance, V.A. Is regulation of aromatase expression in reptiles the key to understanding temperature-dependent sex determination? J. Exp. Zool. A Ecol. Genet. Physiol. 2009, 311, 314–322. [Google Scholar] [CrossRef]
- Dorizzi, M.; Richard-Mercier, N.; Pieau, C. The ovary retains male potential after the thermosensitive period for sex determination in the turtle Emys orbicularis. Differentiation 1996, 60, 193–201. [Google Scholar] [CrossRef]
- Britt, K.L.; Findlay, J.K. Estrogen actions in the ovary revisited. J. Endocrinol. 2002, 175, 269–276. [Google Scholar] [CrossRef]
- Meinhardt, U.; Mullis, P.E. The aromatase cytochrome P-450 and its clinical impact. Horm. Res. 2002, 57, 145–152. [Google Scholar] [CrossRef]
- Veitia, R.A. Le facteur de transcription FOXL2: Un acteur clé de la différenciation de l’ovaire, de son maintien et de la fertilité. Bull. De L’académie Natl. De Méd. 2016, 200, 1115–1127. [Google Scholar] [CrossRef]
- Callard, G.V.; Petro, Z.; Ryan, K.J. Identification of aromatase in the reptilian brain. Endocrinology 1977, 100, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Wade, J. Androgen metabolism in the brain of the green anole lizard (Anolis carolinensis). Gen. Comp. Endocrinol. 1997, 106, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Pielke, R., Jr.; Burgess, M.G.; Ritchie, J. Most plausible 2005-2040 emissions scenarios project less than 2.5 degrees C of warming by 2100. SocArXiv 2021. [Google Scholar] [CrossRef]
- Fuentes, M.; Limpus, C.J.; Hamann, M.; Dawson, J. Potential impacts of projected sea-level rise on sea turtle rookeries. Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, 132–139. [Google Scholar] [CrossRef]
- Stanford, C.B.; Iverson, J.B.; Rhodin, A.G.; van Dijk, P.P.; Mittermeier, R.A.; Kuchling, G.; Berry, K.H.; Bertolero, A.; Bjorndal, K.A.; Blanck, T.E.G.; et al. Turtles and tortoises are in trouble. Curr. Biol. 2020, 30, R721–R735. [Google Scholar] [CrossRef]
- Neuwald, J.L.; Valenzuela, N. The lesser known challenge of climate change: Thermal variance and sex-reversal in vertebrates with temperature-dependent sex determination. PLoS ONE 2011, 6, e18117. [Google Scholar] [CrossRef]
- Valenzuela, N.; Literman, R.; Neuwald, J.L.; Mizoguchi, B.; Iverson, J.B.; Riley, J.L.; Litzgus, J.D. Extreme thermal fluctuations from climate change unexpectedly accelerate demographic collapse of vertebrates with temperature-dependent sex determination. Sci. Rep. 2019, 9, 4254. [Google Scholar] [CrossRef]
- Bowden, R.M.; Paitz, R.T. Is thermal responsiveness affected by maternal estrogens in species with temperature-dependent sex determination? Sex. Dev. 2021, 15, 69–79. [Google Scholar] [CrossRef]
- Hernandez-Divers, S.M.; Hernandez-Divers, S.J.; Wyneken, J. Angiographic, anatomic and clinical technique descriptions of a subcarapacial venipuncture site for chelonians. J. Herpetol. Med. Surg. 2002, 12, 32–37. [Google Scholar] [CrossRef]
- Mans, C. Venipuncture techniques in chelonian species. Lab Anim. 2008, 37, 303–304. [Google Scholar] [CrossRef]
- Boggs, A.S.; Bowden, J.A.; Galligan, T.M.; Guillette, L.J.; Kucklick, J.R. Development of a multi-class steroid hormone screening method using Liquid Chromatography/Tandem Mass Spectrometry (LC-MS/MS). Anal. Bioanal. Chem. 2016, 408, 4179–4190. [Google Scholar] [CrossRef]
- Bussy, U.; Chung-Davidson, Y.-W.; Buchinger, T.J.; Li, K.; Li, W. High-sensitivity determination of estrogens in fish plasma using chemical derivatization upstream UHPLC–MSMS. Steroids 2017, 123, 13–19. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing, version 4.0.3; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 25 February 2023).
- McPherson, R.; Boots, L.; MacGregor, R., III; Marion, K. Plasma steroids associated with seasonal reproductive changes in a multiclutched freshwater turtle, Sternotherus odoratus. Gen. Comp. Endocrinol. 1982, 48, 440–451. [Google Scholar] [CrossRef]
- Rostal, D.C.; Owens, D.W.; Grumbles, J.S.; MacKenzie, D.S.; Amoss, M.S., Jr. Seasonal reproductive cycle of the Kemp’s ridley sea turtle (Lepidochelys kempi). Gen. Comp. Endocrinol. 1998, 109, 232–243. [Google Scholar] [CrossRef]
- Allman, P.; Bowden, R.M.; Donini, J.; Serra, I. Year-round plasma steroid hormone profiles and the reproductive ecology of gopher tortoises (Gopherus polyphemus) at the southernmost edge of their range. Gen. Comp. Endocrinol. 2019, 282, 113213. [Google Scholar] [CrossRef]
- Hulin, V.; Delmas, V.; Girondot, M.; Godfrey, M.H.; Guillon, J.-M. Temperature-dependent sex determination and global change: Are some species at greater risk? Oecologia 2009, 160, 493–506. [Google Scholar] [CrossRef]
- Morjan, C.L. How rapidly can maternal behavior affecting primary sex ratio evolve in a reptile with environmental sex determination? Am. Nat. 2003, 162, 205–219. [Google Scholar] [CrossRef]
- Laloë, J.-O.; Hays, G.C. Can a present-day thermal niche be preserved in a warming climate by a shift in phenology? A case study with sea turtles. R. Soc. Open Sci. 2023, 10, 221002. [Google Scholar] [CrossRef]
- Blanvillain, G.; Owens, D.W.; Kuchling, G. Hormones and reproductive cycles in turtles. In Hormones and Reproduction of Vertebrates; Norris, D.O., Lopez, K.H., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 277–303. [Google Scholar]
- Marquez, E.C.; Traylor-Knowles, N.; Novillo-Villajos, A.; Callard, I.P. Cloning of estrogen receptor alpha and aromatase cDNAs and gene expression in turtles (Chrysemys picta and Pseudemys scripta) exposed to different environments. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 154, 213–225. [Google Scholar] [CrossRef]
- Mizoguchi, B.A.; Valenzuela, N. Ecotoxicological perspectives of sex determination. Sex. Dev. 2016, 10, 45–57. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).