Reproductive Biology of the Golden Cuttlefish Sepia esculenta (Cephalopoda, Sepiida)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection, Initial Treatment, Fixation, and Storage
2.2. Morphological Analysis
2.3. Histological Analysis
2.4. Statistical Analysis
3. Results
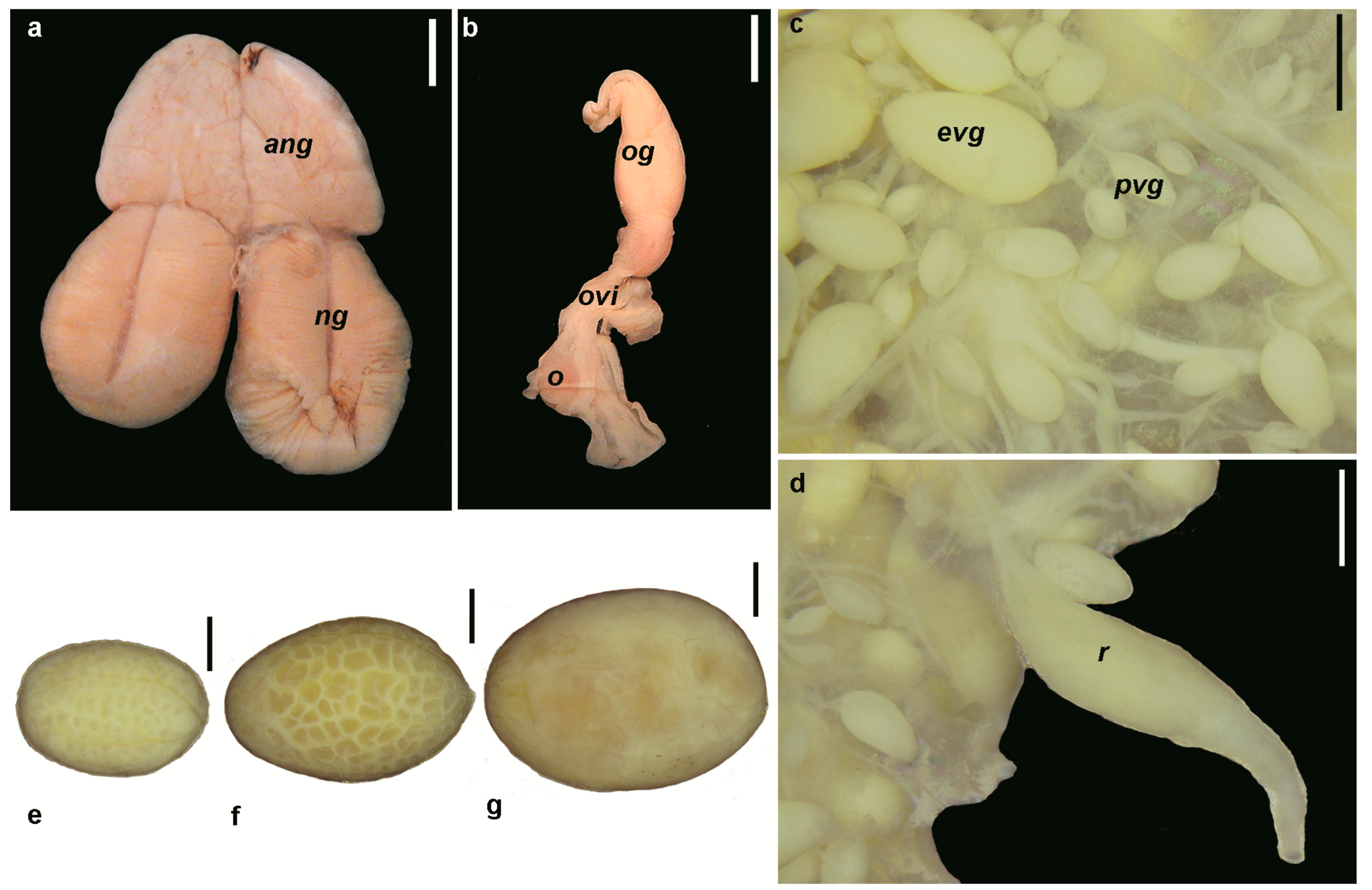
3.1. Female Reproductive System
3.1.1. Morphology of the Reproductive System
3.1.2. Potential Fecundity
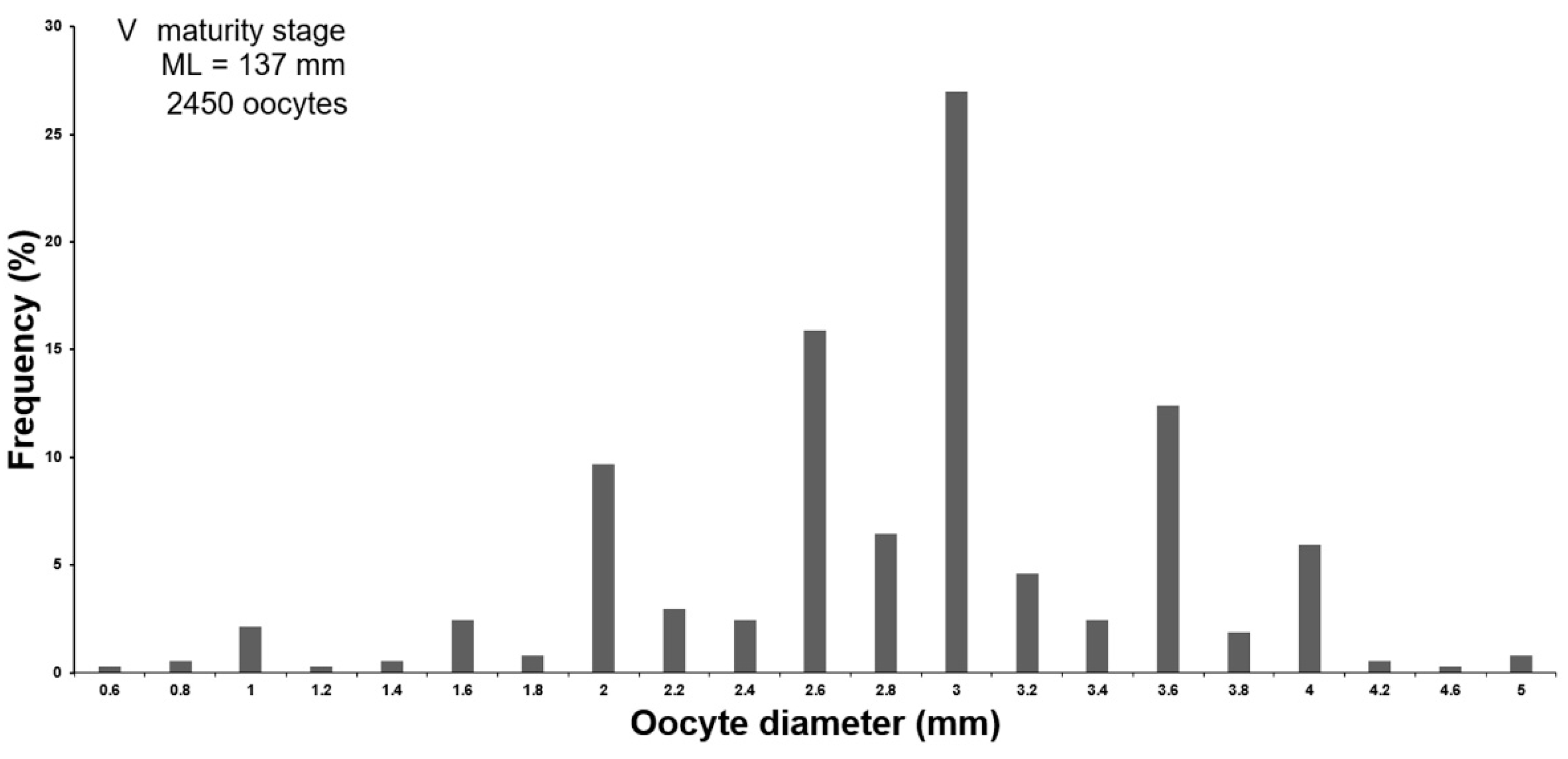
3.1.3. Oogenesis
Period of Oogonia Production
- Phase 1. Pre-meiotic oocytes
Protoplasmic Growth Period
- Phase 2. Primary growth
- Phase 3. Simple follicle
Interstitial Period
- Phase 4. Early yolkless
- Phase 5. Late yolkless
Trophoplasmic Growth Period
- Phase 6. Early vitellogenesis
- Phase 7. Middle vitellogenesis
- Phase 8. Late vitellogenesis
- Phase 9. Ripe oocyte
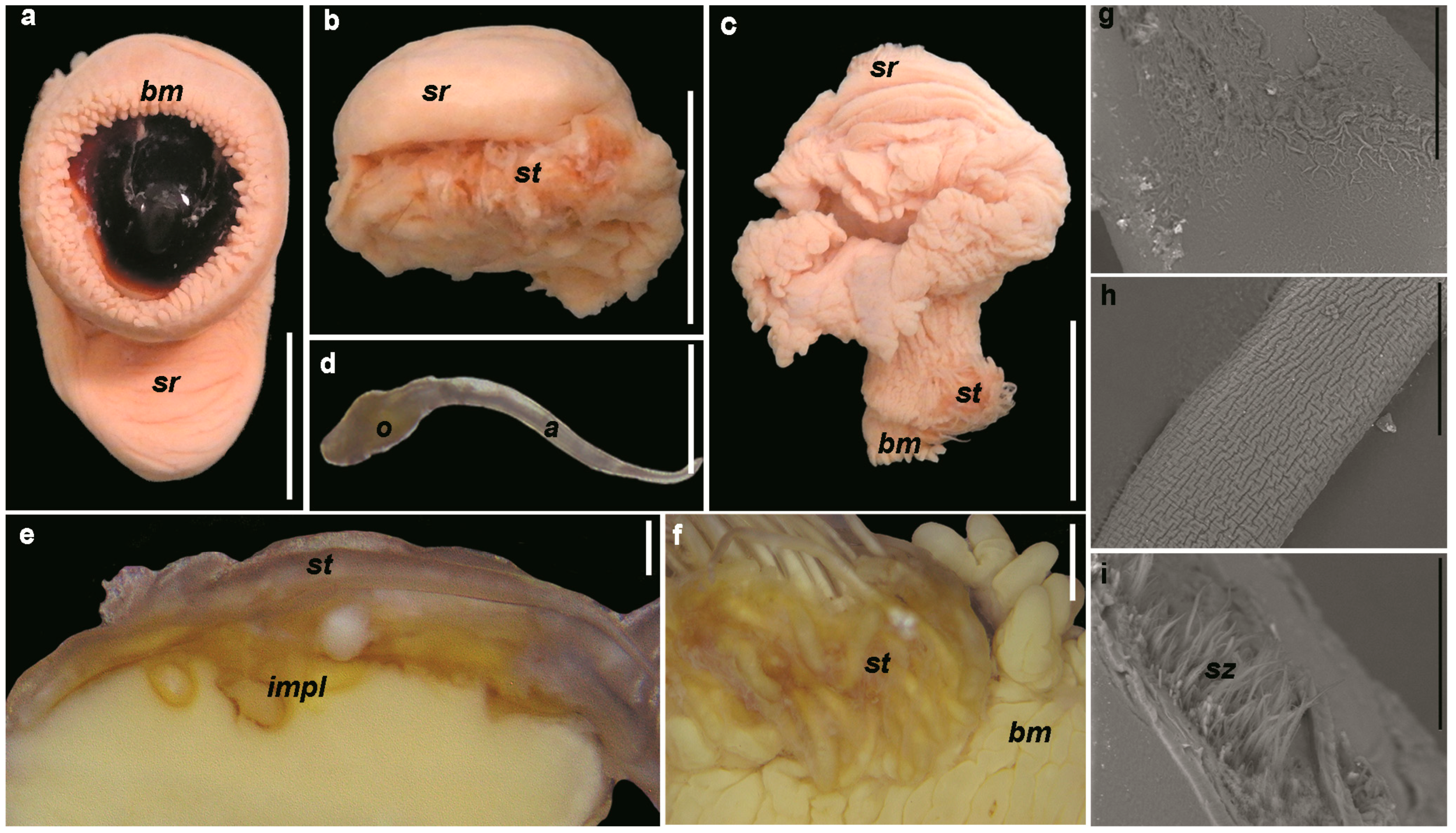
3.2. Male Reproductive System
3.2.1. Morphology of the Reproductive System
3.2.2. Number of Spermatophores
3.2.3. Spermatophores
3.2.4. Spermatangia
4. Discussion
4.1. Female Reproductive System
4.2. Male Reproductive System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reid, A.; Jereb, P.; Roper, C.F.E. Family Sepiidae. In Cephalopods of the World. An Annotated and Illustrated Catalogue of Species Known to Date; Jereb, P., Roper, C.F.E., Eds.; Chambered nautiluses and sepioids (Nautilidae, Sepiidae, Sepiolidae, Sepiadariidae, Idiosepiidae and Spirulidae); FAO Species Catalogue for Fishery Purposes. No. 4; FAO: Rome, Italy, 2005; Volume 1, pp. 57–152. [Google Scholar]
- Neige, P. The geography of body size in cuttlefishes (Cephalopoda, Sepiidae). Swiss J. Palaeontol. 2021, 140, 17. [Google Scholar] [CrossRef]
- Khromov, D.N.; Lu, C.C.; Guerra, A.; Dong, Z.H.; Boletzky, S.V. A synopsis of sepiidae outside Australian waters (Cephalopoda: Sepioidea). In Systematics and Biogeography of Cephalopods; Voss, N.A., Vecchione, M., Toll, R.B., Sweeney, M.J., Eds.; Smithson. Contrib. Zool.; Smithsonian Institution Press: Washington, DC, USA, 1998; Volume I, pp. 77–139. [Google Scholar]
- Rosa, R.; Pissarra, V.; Borges, F.O.; Xavier, J.; Gleadall, I.G.; Golikov, A.; Bello, G.; Morais, L.; Lishchenko, F.; Roura, Á.; et al. Global patterns of species richness in coastal Cephalopods. Front. Mar. Sci. 2019, 6, 469. [Google Scholar] [CrossRef] [Green Version]
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; FAO: Rome, Italy, 2020; 244p. [Google Scholar]
- Syda Rao, G.S. Aspects of biology and exploitation of Sepia aculeata Orbigny from Mangalore Area, Karnataka. Indian J. Fish. 1997, 44, 247–254. [Google Scholar]
- Gabr, H.R.; Hanlon, R.T.; Hanafy, M.H.; El-Etreby, S.G. Maturation, fecundity and seasonality of reproduction of two commercially valuable cuttlefish, Sepia pharaonis and S. dollfusi, in the Suez Canal. Fish. Res. 1998, 36, 99–115. [Google Scholar] [CrossRef]
- Onsoy, B.; Salman, A. Reproductive biology of the common Cuttlefish Sepia Officinalis L. (Sepiida: Cephalopoda) in the Aegean Sea. Turk. J. Vet. Anim. Sci. 2005, 29, 613–619. [Google Scholar]
- Güven, O.; Özbaş, M. Reproductıon of common Cuttlefısh (Sepıa offıcınalıs, l.,1758) ın Antalya Bay. Rapp. Comm. Int. Mer. Médit. 2007, 38, 494. [Google Scholar]
- Neves, A.; Cabral, H.; Sequeira, V.; Figueiredo, I.; Moura, T.; Gordo, L.S. Distribution patterns and reproduction of the cuttlefish, Sepia officinalis in the Sado Estuary (Portugal). J. Mar. Biol. Assoc. UK 2009, 89, 579–584. [Google Scholar] [CrossRef]
- Akyol, O.; Tellibayraktar, B.; Ceyhan, T. Preliminary results on the cuttlefish, Sepia officinalis, reproduction in Izmir Bay (Aegean Sea). J. Fish. Sci. 2011, 5, 122–130. [Google Scholar] [CrossRef]
- Sundaram, S.; Khan, M.Z. Biology of the spineless cuttlefish Sepiella inermis (Orbigny, 1848) from Mumbai waters. Indian J. Fish. 2011, 58, 7–13. [Google Scholar]
- Dursun, D.; Eronat, E.G.T.; Akalin, M.; Salman, A. Reproductive biology of pink cuttlefish Sepia orbignyana in the Aegean Sea (Eastern Mediterranean). Turk. J. Zool. 2013, 37, 576–581. [Google Scholar] [CrossRef]
- Sundaram, S. Fishery and biology of Sepia pharaonis Ehrenberg, 1831 off Mumbai, northwest coast of India. J. Mar. Biol. Assoc. India 2014, 56, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Riad, R.; Atta, M.; Halim, Y.; Elebiary, N. Reproductive biology of Sepia pharaonis Ehrenberg, 1831(Cephalopoda: Sepioidea) from the Suez Gulf (Red Sea), Egypt. Egypt J. Aquat. Biol. Fish. 2015, 19, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Salman, A. Reproductive biology of the elegant cuttlefish (Sepia elegans) in the Eastern Mediterranean. Turk. J. Fish. Aquat. Sci. 2015, 15, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Gras, M.; Safi, G.; Lebredonchel, H.; Quinquis, J.; Foucher, É.; Koueta, N.; Robin, J.P. Stock structure of the English Channel common cuttlefish Sepia officinalis (Linnaeus, 1758) during the reproduction period. J. Mar. Biol. Assoc. UK 2016, 96, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Krstulović Šifner, S.; Damjanović, T.; Isajlović, I. Distribution, Length-weight relationships and reproductive characteristics of Sepia orbignyana, Férussac, 1826 in the northern and central Adriatic Sea. Cah. Biol. Mar. 2018, 59, 43–51. [Google Scholar]
- Amin, A.M.; Elhalfawy, A.M.M.; Khouraiba, H.M.M.; Ali, M.A.M. Some biological aspect and gonads histology of Sepia savignyi (Blainville, 1827), in the Gulf of Suez, Egypt. Egypt J. Aquatic. Biol. Fish. 2019, 23, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Saddikioui, L.; Abi-Ayad, S.M.E.A. Maturation, fecundity and spawning season of the cuttlefish Sepia officinalis in Oran Bay (Western Algeria Coasts). In Proceedings of the 2st International Conference on Food, Agriculture and Animal Sciences (ICOFAAS 2019), Antalya, Turkey, 8–11 November 2019; Dadasoglu, F., Tozly, F., Cig, F., Yildirim, E., Eds.; p. 142. [Google Scholar]
- Aly, S.M.; El-Dakar, A.Y.; El-Aiatt, A.A.O.; Al-Beak, A.M. Feeding habits and reproduction period of Sepia officinalis (Linnaeus, 1758) captured from East Mediterranean Sea. Egypt J. Aquat. Biol. Fish. 2020, 24, 171–182. [Google Scholar] [CrossRef]
- Daghooghi, B.; Salarpouri, A.; Darvishi, M.; Momeni, M. Reproduction biology of Pharaoh cuttlefish Sepia pharaonis (Ehrenberg, 1831) from Hormozgan province, Persian Gulf. J. Anim. Environ. 2021; in press. [Google Scholar]
- Mai, M.D.; Nguyen, T.T.X. Reproductive aspects and tolerance to temperature and salinity of egg of pharaon cuttlefish Sepia pharaonis Ehrenberg, 1831 from Vietnam waters. Phuket. Mar. Biol. Cent. Res. Bull. 2021, 78, 155–161. [Google Scholar]
- Kavitha, M.; Sasikumar, G.; Iyadurai, J.; Lakshmanan, R.; Felix, J. Insight on the reproductive biology of small striped cuttlefish, Sepia prabahari in Gulf of Mannar, Indian Ocean and recommendation for a minimum legal size. Fish. Res. 2022, 248, 106227. [Google Scholar] [CrossRef]
- Ezzedine-Najai, S. Fecundity of cuttlefish, Sepia officinalis L. (Mollusca: Cephalopoda) from the Gulf of Tunis. Vie Milieu 1985, 35, 283–284. [Google Scholar]
- Laptikhovsky, V.V.; Salman, A.; Onsoy, B.; Katagan, T. Fecundity of the common cuttlefish, Sepia officinalis L. (Cephalopoda, Sepiidae): A new look at an old Problem. Sci. Mar. 2003, 67, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Beasley, A.L.; Hall, K.C.; Latella, C.I.; Harrison, P.L.; Morris, S.G.; Scott, A.; Beasley, A.L.; Hall, K.C.; Latella, C.I.; Harrison, P.L.; et al. Reproductive characteristics of three small-bodied cuttlefish in subtropical waters. Mar. Freshwater. Res. 2018, 69, 403–417. [Google Scholar] [CrossRef]
- Lin, D.; Xuan, S.; Chen, Z.; Chen, X. The Ovarian development, fecundity and hypothesis on spawning pattern of common cuttlefish Sepia officinalis off Mauritania. Fish. Res. 2019, 210, 193–197. [Google Scholar] [CrossRef]
- Yin, Y.N.; Liu, C.L.; Peng, H.U.; Zhang, J.Y.; Liu, S.F.; Zhuang, Z.M.; Xue, T.M. Histology of oogenesis and ovarian development in cultured Sepia esculenta. J. Fish. Sci. China 2018, 25, 503–511. [Google Scholar] [CrossRef]
- Boletzky, S.V. Fecundity variation in relation to intermittent or chronic spawning in the cuttlefish, Sepia officinalis L. (Mollusca, Cephalopoda). Bull. Mar. Sci. 1987, 40, 382–388. [Google Scholar]
- Hoyle, W.E. XX.—Diagnoses of New Species of Cephalopoda Collected during the Cruise of H.M.S. ‘Challenger.’—Part II. The Decapoda. Ann. Mag. Nat. Hist. 1885, 16, 181–203. [Google Scholar] [CrossRef] [Green Version]
- Natsukari, Y.; Tashiro, M. Neritic squid resources and cuttlefish resources in Japan. Mar. Beh. Physiol. 1991, 18, 149–226. [Google Scholar] [CrossRef]
- Okutani, T. Cuttlefishes and Squids of the World [New Edition]; Tokyo University Press: Kanagawa, Japan, 2015. [Google Scholar]
- Yin, Y.E. Studies on Structure of Reproductive System and Oogenesis in Sepia esculenta Hoyle. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2018. [Google Scholar]
- Yasuda, J. Some ecological notes on the cuttlefish, Sepia esculenta Hoyle. Bull. Jpn. Soc. Sci. Fish. 1951, 16, 350–356. [Google Scholar] [CrossRef]
- Tomiyama, A. Ecological studies on the useful sea animals of Yamaguchi Pref. Inland Sea, 17: On the Cuttlefish, Sepia esculenta Hoyle. J. Yamaguchi Prefect. Naikai Fish. Exp. Stn. 1957, 9, 29–39. [Google Scholar]
- Arima, S.; Hiramatsu, T.; Norimatsu, T.; Segawa, K.; Tako, N. Research on seeding production techniques and cultivation technology of the cuttlefishes–IV. Buzen Fish. Exp. Stn. Fukuoka Prefect. Res. Rep. 1963, 1964, 1–56. [Google Scholar]
- Zhang, X.; Wang, L.; Zhang, Y.; Luo, G.; Gao, H. Strategy optimization of stock enhancement of golden cuttlefish, (Sepia esculenta) based on structural characteristics of reproductive and recruitment populations. J. Fish. China 2019, 43, 1890–1899. [Google Scholar]
- Wang, Z.; Wang, L.; Li, W.; Zhang, X. Histological Structure of the Seminal Receptacle, Sperm Storage and Utilization in the Golden Cuttlefish (Sepia Esculenta). J. Fish. China 2020, 44, 419–428. [Google Scholar]
- Fujita, T.; Hirayama, I.; Matsuoka, T.; Kawamura, G. Spawning behavior and selection of spawning substrate by cuttlefish Sepia esculenta. Nippon. Suisan Gakkaishi 1997, 63, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Wada, T.; Takegaki, T.; Mori, T.; Natsukari, Y. Sperm displacement behavior of the cuttlefish Sepia esculenta (Cephalopoda: Sepiidae). J. Ethol. 2005, 23, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhan, W.; Zhang, X. Effects of different substrate and temperature on hunger tolerance in Sepia esculenta juveniles. J. Fish. Sci. China 2018, 25, 1071–1081. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Zhan, W.; Song, N.; Gao, T. Morphological characteristics and genetic differentiation of a breeding Population of Sepia esculenta in Qingdao. J. Fish. Sci. China 2019, 26, 342. [Google Scholar] [CrossRef]
- Arkhipkin, A.I. Reproductive system structure, development and function in Cephalopods with a new general scale for maturity stages. J. Northw. Atl. Fish. Sci. 1992, 12, 63–74. [Google Scholar] [CrossRef]
- Nigmatullin, C.M.; Sabirov, R.M. Ontogenetic trends in spermatophore formation in coleoid Cephalopods. In Proceedings of the Contribution to Current Cephalopod Research: Morphology, Systematics, Evolution, Ecology and Boistratygraphy. Proc. Conf., Moscow, Russia, 2–4 April 2015; pp. 24–26. [Google Scholar]
- Golikov, A.V. Distribution and reproductive biology of ten-armed Cephalopods (Sepiolida, Teuthida) in the Barents Sea and adjacent areas. Ph.D. Thesis, Moscow State University, Moscow, Russia, 2015. [Google Scholar]
- Golikov, A.V.; Blicher, M.E.; Jørgensen, L.L.; Walkusz, W.; Zakharov, D.V.; Zimina, O.L.; Sabirov, R.M. Reproductive biology and ecology of the boreoatlantic armhook squid Gonatus fabricii (Cephalopoda: Gonatidae). J. Moll. Stud. 2019, 85, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Romeis, B. Mikroscopische Technik; Urban und Schwarzenberg: Munchen, German, 1989; 697p. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Prentice Hall: Upper Saddle River, NJ, USA, 2010; 944p. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Paleontol. Electron. 2001, 4, 9. [Google Scholar]
- Lu, C.C.; Reid, A.L. Two new cuttlefishes (Cephalopoda: Sepiidae) from the North West Shelf, and a redescription of Sepia sulcata Hoyle, 1885. Rec. West. Aust. Mus. 1997, 18, 277–310. [Google Scholar]
- Reid, A.L. A complete description of Sepia mira (Cotton 1932) (Cephalopoda: Sepiidae) from Eastern Australia. Proc. Linn. Soc. N. S. W. 1998, 119, 155–171. [Google Scholar]
- Reid, A.L. Australian cuttlefishes (Cephalopoda, Sepiidae): The ‘Doratosepion’ species complex, with descriptions of three new species. Invertebr. Taxon. 2000, 14, 1–76. [Google Scholar] [CrossRef]
- Reid, A.L. A new cuttlefish, Sepia grahami, sp. nov. (Cephalopoda: Sepiidae) from Eastern Australia. Proc. Linn. Soc. N. S. W. 2001, 123, 159–172. [Google Scholar]
- Reid, A.L. Sepia hedleyi Berry, 1918 (Cephalopoda: Sepiidae): A complete description and clarification of the status of S. dannevigi Berry, 1918 and S. rex (Iredale, 1926). Rec. South Aust. Mus. 2001, 34, 79–97. [Google Scholar]
- Reid, A.L.; Lu, C.C. New Sepiella Gray, 1849 (Cephalopoda: Sepiidae) from Northern Australia, with a Redescription of Sepiella weberi Adam, 1939. Beagle Rec. Mus. Art Galleries North. Territ. 1998, 14, 71–102. [Google Scholar] [CrossRef]
- Reid, A.L.; Lu, C.C. A new cuttlefish, Sepia filibrachia n. sp., from the South China Sea with a redescription of Sepia mestus Gray, 1849 (Cephalopoda: Sepiidae) from eastern Australia. Zootaxa 2005, 911, 1–22. [Google Scholar] [CrossRef]
- Nigmatullin, C.M. Ovary development, potential and actual fecundity and oocyte resorption in coleoid cephalopods: A review. Berliner. Paläobiol. Abh. 2002, 1, 82–84. [Google Scholar]
- Melo, Y.C.; Sauer, W.H.H. Ovarian atresia in cephalopods. S. Afr. J. Mar. Sci. 1998, 20, 143–151. [Google Scholar] [CrossRef]
- Rocha, F.; Guerra, Á.; González, Á.F. A review of reproductive strategies in Cephalopods. Biol. Rev. 2001, 76, 291–304. [Google Scholar] [CrossRef]
- Iredale, T. The cuttlefish “Bones” of the Sydney beaches (Phylum Mollusca-Class Cephalopoda). Aust. Zool. 1926, 4, 186–196. [Google Scholar]
- Gray, J. Catalogue of the Mollusca in the Collection of the British Museum I: Cephalopoda Nntepedia; Order of the trustees: London, UK, 1849; 164p. [Google Scholar]
- Blainville, H.M.D.; de Sèche, S.M. Dictionnaire Des Sciences Naturelles; Cuvier, F., Ed.; F. G. Levrault: Strasbourg, France; Le Normant: Paris, France, 1827; pp. 257–293. [Google Scholar] [CrossRef] [Green Version]
- d’Orbigny, A.D. Tableau méthodique de la classe des Céphalopodes. Ann. Sci. Nat. 1826, 7, 96–314. [Google Scholar]
- Laptikhovsky, V.V.; Arkhipkin, A.I. Oogenesis and gonad development in the cold water loliginid squid Loligo gahi (Cephalopoda: Myopsida) on the Falkland shelf. J. Molluscan. Stud. 2001, 67, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Hoving, H.J.T.; Laptikhovsky, V.V.; Lipinski, M.R.; Jürgens, E. Fecundity, oogenesis, and ovulation pattern of dsouthern African Lycoteuthis lorigera (Steenstrup, 1875). Hydrobiologia 2014, 725, 23–32. [Google Scholar] [CrossRef]
- Sabirov, R.M. Reproductive system in males of Cephalopoda. III. Spermatophores. Proc. Kazan Univ. Nat. Sci. Ser. 2010, 152, 8–21. [Google Scholar]
- Lipiński, M.R.; Leslie, R.W. A new species of Sepia (Cephalopoda: Sepiidae) from south African waters with a re-description of Sepia dubia Adam et Rees, 1966. Folia Malacol. 2018, 26, 125–147. [Google Scholar] [CrossRef]
- Lipiński, M.R. Redescriptions of two species of Sepia (Cephalopoda: Sepiidae) from south African aaters: Sepia robsoni (Massy, 1927) and S. faurei Roeleveld, 1972. Folia Malacol. 2020, 28, 253–285. [Google Scholar] [CrossRef]
- Roeleveld, M.A.; Liltved, W.R. A new species of Sepia (Cephalopoda, Sepiidae) from South Africa. Ann. S. Afr. Mus. 1985, 96, 1–18. [Google Scholar]
- Filippova, Y.A.; Khromov, D.N. New data on the cuttlefish fauna (Sepiidae, Cephalopoda) from western Indian Ocean. Zool. Zhurnal. 1991, 70, 63–70. [Google Scholar]
- Steenstrup, J. Hemisepius, En Ny Slaegt of Sepia-Bläcksprutternes Familie, Bemaerkinger Om Sepia-Formerne i Almindelighed. K. Danske. Vidensk. Selsk. Skr. 1875, 10, 465–482. [Google Scholar]
- Leslie, R.W.; Richardson, A.J.; Lipiński, M.R. Detailed description and morphological assessment of Sepia typica (Steenstrup, 1875) (Cephalopoda: Sepiidae). Diversity 2022, 14, 1073. [Google Scholar] [CrossRef]
- Adam, W.; Cephalopoda, I.I. Révision Des Espèces Indo-Malaises Du Genre Sepia Linné, 1758 III–Révision Du Genre Sepiella (Gray) Steenstrup, 1880 Siboga-Expeditie. Résultats Des. Expéditions Zool. Bot. Océanographiques Et Entrep. Aux Indes Néerlandaises Orient. En 1899–1900 1939, 55b, 35–122. [Google Scholar]
- Homenko, L.P.; Khromov, D.N. A new species of the genus Sepia (Cephalopoda, Sepiidae) from the Arabian Sea. Zool. Zhurnal 1984, 63, 1150–1157. [Google Scholar]
- Khromov, D.N. A new species of the genus Sepia (Cephalopoda, Sepiidae) from the South-West Indian Ocean. Zool. Zhurnal 1982, 61, 137–139. [Google Scholar]
- Khromov, D.N. Two new species of the genus Sepia (Cephalopoda, Sepiidae) from the Sokotra Island (People’s Democratic Republic of Yemen) waters. Zool. Zhurnal 1988, 67, 785–790. [Google Scholar]
- Berry, S.S. Report on the Cephalopoda Obtained by the F.I.S. “Endeavour” in the Great Australian Bight and Other Southern Australian Localities. Biol. Results Fish. Exp. Carried By F.L.S. Endeav. 1909–1914 1918, 4, 201–298. [Google Scholar]
- Drew, G.A. Sexual activities of the squid Loligo pealii (Les.). II. The Spermatophore; its structure, ejaculation and formation. J. Morphol. 1919, 32, 379–435. [Google Scholar] [CrossRef] [Green Version]
- Sabirov, R.M. Spermatophorogenesis and Reproductive Strategy in Males of Ommastrephid Squids (Oegopsida: Ommastrephidae). Ph.D. Thesis, Institute of Evolutionary Morphology, Moscow, Russia, 1995. [Google Scholar]
- Nigmatullin, C.M.; Laptikhovsky, V.V.; Sabirov, R.M. Reproductive biology of the commander squid. In Fisheries Biology of the Commander Squid and Fishes in the Slope Communities of the Western Bering Sea; Elizarov, A.A., Ed.; VNIRO Publishing: Moscow, Russia, 1996; pp. 101–124. [Google Scholar]
- Nigmatullin, C.; Sabirov, R.; Zalygalin, V. Ontogenetic aspects of morphology, size, structure and production of spermatophores in ommastrephid squids: An Overview. Berliner. Paläobiol. Abh. 2003, 3, 225–240. [Google Scholar]
- Hoving, H.J.T.; Lipiński, M.R.; Dam, L. The male reproductive strategy of a deep-sea squid: Sperm allocation, continuous production, and long-term storage of spermatophores in Histioteuthis miranda. ICES J. Mar. Sci. 2010, 67, 1478–1486. [Google Scholar] [CrossRef] [Green Version]
- Sabirov, R.M.; Golikov, A.V.; Nigmatullin, C.M.; Lubin, P.A. Structure of the reproductive system and hectocotylus in males of lesser flying squid Todaropsis eblanae (Cephalopoda: Ommastrephidae). J. Nat. Hist. 2012, 46, 1761–1778. [Google Scholar] [CrossRef]
- Golikov, A.V.; Morov, A.R.; Sabirov, R.M.; Lubin, P.A.; Jørgensen, L.L. Functional morphology of reproductive system of Rossia Pplpebrosa (Cephalopoda, Sepiolida) in Barents Sea. Proc. Kazan Univ. Nat. Sci. Ser. 2013, 155, 116–129. [Google Scholar]
- Cuccu, D.; Mereu, M.; Agus, B.; Cau, A.; Culurgioni, J.; Sabatini, A.; Jereb, P. Male reproductive system and spermatophores production and storage in Histioteuthis bonnellii (Cephalopoda: Histioteuthidae): A look into deep-wea squids׳ reproductive strategy. Deep-Sea Res. Part I 2014, 91, 86–93. [Google Scholar] [CrossRef]
- Golikov, A.V.; Blicher, M.E.; Gudmundsson, G.; Manushin, I.E.; Poulsen, J.Y.; Zakharov, D.V.; Sabirov, R.M. Flapjack devilfish in the northern North Atlantic: Morphology, biology and ecology of Opisthoteuthis borealis (Cephalopoda, Octopoda, Cirrata). Mar. Biodivers. 2020, 50, 108. [Google Scholar] [CrossRef]
- Sabirov, R.M. Reproductive system in males of Cephalopoda. II. Spermatophoric complex of organs. Proc. Kazan Univ. Nat. Sci. Ser. 2009, 151, 34–50. [Google Scholar]
- Naud, M.J.; Shaw, P.W.; Hanlon, R.T.; Havenhand, J.N. Evidence for biased use of sperm sources in wild female giant cuttlefish (Sepia apama). Proc. R. Soc. B 2005, 272, 1047–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, T.; Takegaki, T.; Mori, T.; Natsukari, Y. Reproductive behavior of the japanese spineless cuttlefish Sepiella japonica. Venus (J. Malacol. Soc. Jpn.) 2006, 65, 221–228. [Google Scholar]
- Marian, J.E.A.R. Evolution of spermatophore transfer mechanisms in cephalopods. J. Nat. Hist. 2015, 49, 1423–1455. [Google Scholar] [CrossRef]
- Nateewathana, A. A new record of cuttlefish Metasepia tullbergi (appellof, 1886) (sepiidae: Cephalopoda), gulf of Thailand. Phuket Mar. Biol. Cent. Spec. Publ. 1998, 18, 323–330. [Google Scholar]
- Zuev, G.V. Cephalopods of the northwest part of the Indian Ocean; Naukova Dumka: Kyiv, Ukraine, 1971; 233p. [Google Scholar]




| Oocyte Diameter | Previtellogenesis | Early Vitellogenesis | Mid-Vitellogenesis | Late Vitellogenesis | Ripe | Ripe in Oviduct | Resorption |
|---|---|---|---|---|---|---|---|
| Absolute | 0.1–2.5 mm (0.9 ± 0.01 mm) | 0.3–3.9 (2.5 ± 0.02) | 2.3–5.1 mm (3.8 ± 0.02 mm) | 3.6–7.0 mm (4.8 ± 0.02 mm) | 3.6–5.8 mm (5.3 ± 0.08 mm) | 3.0–6.0 mm (4.9 ± 0.21 mm) | 0.2–5.7 mm (2.9 ± 0.03 mm) |
| In % of ML | 0.1–1.8% (0.6 ± 0.01%) | 0.2–2.8% (1.8 ± 0.01%) | 1.6–3.7% (2.7 ± 0.02%) | 2.6–4.2% (3.5 ± 0.02%) | 2.6–4.2% (3.8 ± 0.07%) | 2.1–4.2% (3.5 ± 0.15%) | 0.1–4.1% (2.1 ± 0.02%) |
| Measurements | Specimens Collected at the Beginning of Spawning Season | Specimens Collected at the End of Spawning Season | ||||
|---|---|---|---|---|---|---|
| Fundus | Central Portion | Penis | Fundus | Central Portion | Penis | |
| Spermatophore length | 13.0–18.0 mm (15.3 ± 0.03 mm) | 12.0–17.5 mm (14.7 ± 0.02 mm) | 12.0–20.0 mm (14.4 ± 0.06 mm) | 11.15–19.50 mm (14.6 ± 0.11 mm) | 11.0–20.0 mm (15.4 ± 0.03 mm) | 9.0–19.0 mm (14.3 ± 0.11 mm) |
| H = 291.6, p < 0.0001 | H = 291.6, p < 0.0001 | |||||
| Width of spermatophore | 0.5–0.7 mm (0.54 ± 0.01 mm) | 0.4–0.6 mm (0.53 ± 0.004 mm) | 0.4–0.6 mm (0.51 ± 0.01 mm) | 0.3–0.7 mm (0.45 ± 0.01 mm) | 0.3–0.9 mm (0.48 ± 0.01 mm) | 0.3–0.5 mm (0.40 ± 0.01 mm) |
| H = 7.49, p = 0.0116 | H = 32.77, p < 0.0001 | |||||
| Weight of spermatophore | 2.2–6.0 mg (3.83 ± 0.07 mg) | 2.5–4.7 mg (3.71 ± 0.04 mg) | 2.4–4.5 mg (3.51 ± 0.07 mg) | 1.1–6.4 mg (2.2 ± 0.13 mg) | 0.9–8.0 mg (2.83 ± 0.09 mg) | 0.9–4.7 mg (1.97 ± 0.15 mg) |
| H = 11.84, p = 0.0022 | H = 33.43, p < 0.0001 | |||||
| Length of head | 0.6–0.9 mm (0.78 ± 0.01 mm) | 0.6–1.0 mm (0.77 ± 0.01 mm) | 0.4–0.9 mm (0.78 ± 0.01 mm) | 0.3–1.1 mm (0.76 ± 0.03 mm) | 0.3–1.3 mm (0.76 ± 0.02 mm) | 0.4–1.8 mm (0.65 ± 0.04 mm) |
| H = 1.23, p = 0.49 | H = 13.19, p = 0.0012 | |||||
| Length of ejaculatory apparatus | 0.4–0.9 mm (0.63 ± 0.01 mm) | 0.4–1.1 mm (0.63 ± 0.01 mm) | 0.3–0.9 mm (0.65 ± 0.02 mm) | 0.2–2.3 mm (1.14 ± 0.09 mm) | 0.3–2.3 mm (0.95 ± 0.04 mm) | 0.2–2.9 mm (0.88 ± 0.09 mm) |
| H = 2.37, p = 0.27 | H = 3.98, p = 0.13 | |||||
| Length of cement body | 2.0–2.7 mm (2.3 ± 0.02 mm) | 1.8–2.7 mm (2.3 ± 0.02 mm) | 1.6–2.5 mm (2.1 ± 0.03 mm) | 0.9–3.9 mm (2.01 ± 0.09 mm) | 0.7–2.7 mm (1.94 ± 0.04 mm) | 0.8–3.0 mm (1.84 ± 0.09 mm) |
| H = 21.57, p < 0.0001 | H = 2.99, p = 0.22 | |||||
| Length of seminal reservoir | 9.5–11.8 mm (10.56 ± 0.07 mm) | 9.0–11.5 mm (10.38 ± 0.05 mm) | 8.9–11.0 mm (10.31 ± 0.10 mm) | 6.1–12.5 mm (9.16 ± 0.24 mm) | 4.3–14.0 mm (10.41 ± 0.13 mm) | 6.1–13.7 mm (9.67 ± 0.30 mm) |
| H = 4.40, p = 0.11 | H = 19.01, p < 0.0001 | |||||
| Width of seminal reservoir | 0.3–0.6 mm (0.44 ± 0.01 mm) | 0.3–0.6 mm (0.41 ± 0.01 mm) | 0.3–0.5 mm (0.42 ± 0.01 mm) | 0.1–0.6 mm (0.38 ± 0.02 mm) | 0.2–0.9 mm (0.45 ± 0.01 mm) | 0.2–0.5 mm (0.36 ± 0.01 mm) |
| H = 7.53, p = 0.0102 | H = 27.79, p < 0.0001 | |||||
| Length of posterior hollow part | 0.1–1.6 mm (0.68 ± 0.06 mm) | 0.1–2.0 mm (0.91 ± 0.04 mm) | 0.2–3.6 mm (0.84 ± 0.09 mm) | 0.1–3.5 mm (0.46 ± 0.08 mm) | 0.1–5.2 mm (0.61 ± 0.06 mm) | 0.1–3.4 mm (0.72 ± 0.13 mm) |
| H = 11.44, p = 0.0032 | H = 1.37, p = 0.47 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasova, E.V.; Sabirov, R.M.; Golikov, A.V. Reproductive Biology of the Golden Cuttlefish Sepia esculenta (Cephalopoda, Sepiida). Diversity 2023, 15, 455. https://doi.org/10.3390/d15030455
Vlasova EV, Sabirov RM, Golikov AV. Reproductive Biology of the Golden Cuttlefish Sepia esculenta (Cephalopoda, Sepiida). Diversity. 2023; 15(3):455. https://doi.org/10.3390/d15030455
Chicago/Turabian StyleVlasova, Elizaveta V., Rushan M. Sabirov, and Alexey V. Golikov. 2023. "Reproductive Biology of the Golden Cuttlefish Sepia esculenta (Cephalopoda, Sepiida)" Diversity 15, no. 3: 455. https://doi.org/10.3390/d15030455
APA StyleVlasova, E. V., Sabirov, R. M., & Golikov, A. V. (2023). Reproductive Biology of the Golden Cuttlefish Sepia esculenta (Cephalopoda, Sepiida). Diversity, 15(3), 455. https://doi.org/10.3390/d15030455







