Abstract
Managing olive mill wastewater (OMW) is a major environmental problem. We followed two methods for OMW bioremediation: one with the white-rot fungus Pleurotus ostreatus alone and one with the fungus plus the microalga Chlorella vulgaris. To evaluate the potential use of both final products as soil amendments, in a pot experiment, we applied treated OMW to soil cultivated with Lactuca sativa, and we studied their short-term effects on the soil nematode community in terms of trophic and functional structure, metabolic footprint, genera composition, and interaction networks. We also applied non-treated OMW and simply water (control). The addition of non-treated OMW significantly reduced the abundance of all nematodes, and the network of interactions was the most fragmented and the least robust against future disturbance. The effect on trophic group abundances was similar but less pronounced when OMW was previously detoxified either by the fungus alone or by its combination with the alga. In the latter case, the phytoparasites were suppressed but the bacterivorous nematodes were not affected. However, the most cohesive and robust nematode network was formed in the soil that received the fungal-treated OMW. None of our OMW applications significantly changed community composition, none improved the already degraded status of the soil food web—which is attributed to the sandy texture of our soil—and none affected the growth of lettuce plants, perhaps because of the short duration of the experiment (30 days). Thus, our future research will aim to estimate the long-term impact of OMW.
1. Introduction
Even though olive oil production is one of the most successful and profitable industries in the Mediterranean [1], managing olive mill wastewater (OMW), i.e., the liquid effluent generated from the olive oil production process, is a major environmental problem. Among the OMW’s main characteristics are the high organic content, high suspended solids content, extremely high biological (BOD) and chemical oxygen demand (COD), low pH, high salinity, and high concentrations of total phenols, carbohydrates, polysaccharides, fatty acids, polyalcohols, pectins, and tannins [2,3]. Polluting compounds of OMW, especially the resistant polymeric phenols [4], have been reported as responsible for stress on soil biota [5], phytotoxic effects [6], and groundwater pollution [7]. Furthermore, disposal of OMW induces soil salinization [8], water repellency [9], immobilization of available nitrogen (N) [10], and changes in soil water and solute infiltration rates [11].
However, OMW’s application could be beneficial either as a biofertilizer, particularly in degraded agricultural soils or even as a botanical against plant pathogens [12]. Among the most effective strategies for OMW degradation and detoxification is the biological treatment of OMW with various microorganisms such as fungi, bacteria, and algae [13,14]. For example, white-rot fungi are capable of degrading organic compounds with complex structures [15]. Especially species of the genus Pleurotus have been used for the bioremediation of OMW by reducing the phenolic load and leading to the decolorization of the effluent [16,17,18,19,20]. Apart from fungi, algae have been proposed for use in sewage treatment [21] and can remove phenols from wastewater [22].
In the work presented here, we followed two methods for OMW bioremediation: one where OMW was treated with fungi and one where OMW was treated by a fungus plus algae combination. The two methods were compared on the basis of the physicochemical characteristics of the final product, and then both treated OMWs were used as soil amendments in pots with lettuce seedlings. To examine the potential use of these products as soil amendments, we focused on the community of soil nematodes. Since soil nematodes occur at multiple levels of the soil food web, the study of their community, both in terms of trophic types and life strategies, gives a better insight into the functionality of the food web when compared to other soil biota [23,24]. Due to their important role in significant soil processes, such as the decomposition of organic materials, nutrient cycling, and nitrogen mineralization, their community has been used as a bioindicator of soil quality in a plethora of studies. As far as we know, there is a lack of information regarding the responses of soil nematodes to the application of OMWs, especially the microbially treated ones. The few relevant studies examine mainly the ability of OMW to suppress pathogenic nematodes [12,25].
This study aimed to investigate (1) the efficacy of the white-rot fungus Pleurotus ostreatus alone and in combination with the microalga Chlorella vulgaris in detoxifying OMW and (2) the short-term effects of these pre-treated OMWs on the soil nematode community by analyzing its trophic and functional structure, the metabolic footprint, as well as its composition in terms of nematode genera. We also employed network analysis techniques to evaluate changes in the web of interactions among nematode genera. For this purpose, we mapped the nematode communities in different treatments as networks, where the nodes represent nematode genera, while the ties connecting these nodes represent the joint occurrence of genera in samples. Moreover, we analyzed the structure of nematode networks by estimating certain metrics provided by the mathematical graph theory for the assessment of network connectedness or the structural importance of certain nodes/genera.
For the needs of our experiment, we used nutrient-poor sandy soil collected from an agricultural field. Generally, sandy soils are considered marginal; they exhibit low values of soil organic matter and clay, poor water-holding capacity, and an inability to store nutrients, while also being structureless [26]. However, as land resources are under pressure, these poor-quality soils have gained increasing interest, especially in the Mediterranean region, where most soils are of low fertility due to erosion and extensive agriculture.
2. Materials and Methods
2.1. Preparation of Treatments
2.1.1. Initial Preparation of Olive Mill Wastewaters
Fresh OMW was collected from a three-phase olive mill in the Peloponnese. The OMW was centrifuged at 4000 rpm, 16 °C for 30 min and were subsequently filtered via vacuum-assisted filtration through filter paper to remove any suspended solids and ensure the uniformity of the material. This procedure is in accordance with the current Greek legislation [27] regarding the waste’s pretreatment before its disposal. After filtration, the OMW was centrifuged again at 8000 rpm, 10 °C for 30 min, and the supernatant was removed. The OMW was maintained at −20 °C, until its use.
2.1.2. Selection of Fungal Strains
We tested seven fungal strains: three strains of Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm., two strains of Pleurotus pulmonarius (Fr.) Quel., and two strains of Ganoderma applanatum (Pers.) Pat. deposited at the Hellenic Agricultural Organization—Dimitra/ITAP/Laboratory of Edible Fungi culture collection with the accession numbers AMRL 133, 147, 151-5, 182, 183, 340, and 341. All strains were routinely preserved on potato dextrose agar (PDA). The fungal strains were assessed for their potential to grow in the presence of OMW. Each strain was cultivated in Petri dishes containing 25% (v/v) OMW in 23 g L−1 agar [20,28]. After inoculation, the cultures were incubated in the dark at 25 °C and examined for fungal growth. The fungal strains P. ostreatus AMRL 133, P. ostreatus AMRL 147, and P. pulmonarius AMRL 182 showed higher growth based on the diameter of growing mycelia and were selected for further investigation.
In order to further select the best fungal strain for OMW detoxification, 1 cm of the PDA agar cultures of the three selected fungal strains was transferred with a sterilized cutter into a 250 mL Erlenmeyer flask containing 100 mL of the basal medium (g L−1): glucose, 30; yeast extract, 3; KH2PO4, 1; MgSO4, 0.5; CaCO3, 2; and NaNO3, 0.25. After ten days of incubation in a rotary shaker at 150 rpm and 25 °C, 5 mL of culture was transferred into a new flask containing the basal medium supplemented with 10% OMW. Before heat sterilization at 121 °C for 20 min, the initial pH of the medium was adjusted to 6.0 with the addition of 1 M NaOH. At certain time intervals, samples were withdrawn and centrifuged (5000 rpm, 5 min), and the supernatant was tested for OMW discoloration and determination of glucose consumption and total phenol removal. Fungal biomass was separated from the culture medium by centrifugation at 4000× g for 10 min, freeze-dried, and weighed.
The total phenolic removal, decolorization, and glucose consumption were evaluated over time (Supplementary Figure S1). Out of the three fungal strains, P. ostreatus AMRL 133 was chosen as the most suitable for OMW detoxification. After 14 days, the total phenolic reduction for this strain was 82.62%, while the degree of decolorization reached 84.26%; the fungal biomass reached values of 20 g/L, whereas the total sugar concentration was 12 g/L.
2.1.3. Chlorella vulgaris
Chlorella vulgaris is a single-celled green alga with a high growth rate that was selected for our experiment because it has been effectively utilized for treating wastewater and biofuel production [29]. C. vulgaris was preserved in Bold Basal Medium (BBM) containing the following chemicals (g L−1): NaNO3, 0.228; K2HPO4·3H2O, 0.175; MgSO4·7H2O, 0.075; CaCl2·2H2O, 0.025; KH2PO4, 0.175; NaCl, 0.025; and 12 mL trace element solution. Trace elements (1 L) include Na2EDTA· 2H2O, 0.830 g; FeCl3·6H2O, 97 mg; MnCl2·4H2O, 41 mg; ZnCl2 4 mg; Na2MoO4·2H2O, 4.5 mg; and CoCl2·6H2O, 2 mg. Microalgae cultures were grown in 250 mL Erlenmeyer flasks with BBM on a light incubator under constant illumination at 35 μmole × m−2 × s−1, using cool/warm white LED light in a ratio 1:1, constant agitation at 110 rpm, and in a temperature of 23 ± 1 °C.
2.1.4. OMW Fermentation by Pleurotus ostreatus AMRL 133 and Chlorella vulgaris in Bioreactors
Two stirred tank bioreactors (3.5 L, Ralph Bioengineering) were charged with the basal medium (described in Section 2.1.2), and the OMW was diluted at 10% v/v in the medium. We selected the above dilution for OMW since high concentrations of phenols in OMW might induce toxicity and could lead to growth inhibition of microalgae [30]. Pleurotus strain’s liquid culture (5% v/v) was used alone as inoculum for the first bioreactor and combined with C. vulgaris (5% v/v) for the second one. The final volume in the bioreactors was 2 L. The agitation rate was 200 rpm, and aeration was 1 vvm. The initial pH was 6. The addition of antifoam was required only in the bioreactor inoculated with fungal culture on the second day after inoculation. Samples were collected at specific time intervals. After centrifugation, the samples’ supernatant was used to evaluate total phenols and glucose consumption to check the fermentation process. After 14 days, total bioreactor content was collected. The biomass was separated by centrifugation at 5000× g for 10 min, freeze-dried, and weighed, while the supernatant was stored at −20 °C.
2.2. Pot Experiment
To set up the pot experiment, we collected soil samples from a previously cultivated field in the Redestos region, located in the eastern part of Thessaloniki, Greece (40.521° N, 23.043° E). Lactuca sativa seeds were placed in a seed container that was filled with sieved soil (2 mm mesh size). They grew for 45 days in outdoor conditions. When they reached the four leaves stage, lettuce plants were transplanted into 0.5 L pots (one plant per pot) containing non-sterilized sieved soil. Afterward, 80 mL of each treated material (non-treated OMW diluted at 10% v/v, OMW treated with Pleurotus strain alone, and OMW treated with both Pleurotus strain and C. vulgaris) were added into the soil. The amount of applied material was selected to prevent its flow outside the pot. Control soils without OMW were also prepared by adding distilled water instead of OMW. The physicochemical characteristics of treated OMW after the bioreactor process are presented in Table 1, together with those of non-treated OMW. We should note that the pH of non-treated OMW was adjusted from 5.4 (raw OMW) to 6 using CaO, in order to be at the same value as treated OMW.

Table 1.
Physicochemical properties of olive mill wastewaters.
The pots were arranged in a completely randomized design with five replicates per treatment. The experiment was conducted outdoors under natural light and temperature conditions with an average of 16 °C during November 2019. The pots were weighted daily to calculate water loss and watered regularly throughout the experiment to keep moisture content at 10% w/w, which imposes no limitation on plant growth [31]. No fertilizers were added to the pots. A destructive sampling was conducted 30 days after the application of treatments to test their short-term effect on plant growth and soil nematodes. Lactuca sativa is usually harvested 30 days after transplantation, while the short lifespan of soil nematodes allows the study of their response to treatments after this time period. The soil was collected from the rhizosphere of the plants by shaking the soil attached to the roots after the plants’ extraction and was stored at 4 °C before processing. The total plant biomass was estimated after oven-drying (48 h at 70 °C) the plant shoots and roots. Table 2 presents the soil’s physicochemical properties before and after treatments. The soil was characterized as sandy, with low organic carbon and nitrogen content. After the end of the experiment, pH values showed a tendency to decrease in OMW treatments in relation to the control, while those of soil organic carbon tended to increase. Total nitrogen remained approximately stable.

Table 2.
Properties of the soil used in the experiment before and after treatments, estimated from n = 5 soil samples.
2.3. Estimation of OMW and Soil Physicochemical Properties
The pH and electrical conductivity (mS/cm) were estimated using a Crison GLP 21 pH meter and a Hanna HI-8733 conductivity meter, respectively. Soil pH was measured in a 1:1 aqueous suspension of soil and water. The dinitrosalicylic acid (DNSA) method was used to assess glucose consumption [32]. Total phenolic content was determined with the Folin–Ciocalteu method [33]. Phenol concentration was expressed in mg/mL of caffeic acid equivalents using the appropriate calibration curve. Absorbance was measured at 725 nm. OMW discoloration was assayed spectrophotometrically by measuring the absorbance of inoculated and non-inoculated OMW samples at 395 nm [34]. OMW’s organic carbon content was determined by heating the sample at 550 °C for 4 h, while soil organic carbon was estimated at 375 for 16 h [35]. The Kjeldahl method was performed for nitrogen determination. Soil texture was estimated by the Bouyoucos method [36], and soil water content was determined gravimetrically at 105 °C according to ISO 11456 [37].
2.4. Nematode Extraction, Identification, and Estimation of Nematode Functional Indices
Nematodes were extracted from 150 mL of each soil sample. The soil was gently mixed by hand, and soil aggregates were broken up. For extraction, we used the modified Cobb’s sieving and decanting method described by S’Jacob and van Bezooijen [38], according to which a cotton wool filter is used in the last step. After counting the total abundance of nematodes under the stereoscope, we heat-killed them and fixed them with 4% formaldehyde. Subsequently, approximately 100 randomly selected nematodes were identified to genus level under the microscope, using the identification key of Bongers [39], based on their morphological characteristics, such as the stomodeum, reproductive organs, and tail. Nematode taxa were assigned to trophic groups according to Yeates et al. [40], classified along the colonization–persistence gradient (c–p values) following Bongers [41] and Bongers and Bongers [42], and assigned to functional guilds (portions of particular trophic groups exhibiting the same c–p value) according to Ferris et al. [43] and Bongers and Bongers [42].
The nematode functional indices that we estimated reflect different attributes of the nematode community. The maturity index (MI) for the free-living nematodes and the plant parasitic index (PPI) for the plant-feeding nematodes were calculated according to Bongers [41]. The maturity index (MI) reflects the level of disturbance, with lower values indicating more disturbed soil [40,44]. PPI is a maturity index for plant feeding taxa. The enrichment index (EI), the channel index (CI), and the structure index (SI), which indicate the functional structure of the food web, were calculated according to the weighted faunal analysis proposed by Ferris et al. [43]. The EI indicates soil enrichment with organic material, mirroring the increases in enrichment opportunists, which respond rapidly to increases in food. The CI indicates the degree of fungal participation in the decomposition pathway, while the SI is an indicator of nematodes with high longevity, body size, and disruption sensitivity. The SI shows whether the soil ecosystem is structured with more trophic links or degraded with fewer trophic links [43]. The metabolic footprint (MF) was calculated according to Ferris [45], using the automated calculation system for nematode-based biological monitoring (NINJA) [46]; it is an index of carbon utilization by nematodes and corresponds to the sum of the lifetime amount of carbon gained, partitioned into growth, egg production, and respiration.
2.5. Data Analysis
2.5.1. Statistical Analysis
In order to evaluate the effect of treatments on the trophic and functional structure of the nematode community, the abundances of nematode trophic groups, as well as the values of nematode functional indices and metabolic footprint were analyzed by one-way ANOVA using treatment as an independent variable. Data were first examined to assess compliance with the ANOVA assumptions (normality, homogeneity of variance, etc.). Then, a least square differences (LSD) test was further performed for significant effects. Kruskal–Wallis was used in the case where the prerequisites of normality were not met even after transformations. All procedures were carried out using STATISTICA (StatSoft Inc., Tulsa, OK, USA, 2001).
2.5.2. Network Analysis
Network analysis aims to represent and quantify structures, borrowing metrics from graph theory, a field of mathematics analyzing the patterns of relations among nodes [47]. In the present study, nodes stand for nematode genera, while the ties connecting these nodes represent the joint occurrence of genera in samples.
The abundance data of the nematode genera were used to construct community matrices on the basis of their joint occurrence. The probability of joint occurrence was calculated according to the niche overlap index of MacArthur and Levins [48]:
where n is the number of samples, pik and pjk are the proportional abundances of genera i and j in the sample k, respectively. Thus, Mij estimates the extent to which the niche space of genus i overlaps that of genus j. Since the effect of genus i on genus j did not have the same magnitude compared to the effect of j on i, the community matrix was not symmetrical. For all the networks, the estimated threshold values above which the relations would not be considered negligible were up to 20% of the community’s maximum value, which was used as a general rule [49]. Thus, if the Mij value was below 20% of the community’s maximum, the effect of genus i on genus j was considered negligible, and the corresponding entry in the matrix was set to zero.
The network map represents how the associative spatial relationships are formed. Community matrices were constructed separately for the nematode communities of each treatment and were further analyzed and visualized with UCINET 6 [50]. We estimated variables referring to cohesion for each network, such as density, compactness, and fragmentation. Density accounts for the total strength of relations divided by the number of possible ties. It assesses the extent of the connectedness of a network [51,52] or indicates the probability of two nodes being directly connected. UCINET used the binary version of the network to estimate compactness. From the centralization metrics, we evaluated the degree of centralization that accounts for the importance of a node within the network [53]. For each node, the degree is calculated as the sum of the values of its ties expressed as a percentage. Network centralization is the sum of node centralities. A few dominant nodes characterize highly centralized networks, which are vulnerable to fragmentation if these nodes are damaged.
Finally, we assessed modularity and the small-world index (SW). Modularity was evaluated according to the Girvan–Newman algorithm. The value of modularity > 0.4 shows that the network consists of discrete factions; the number of links between the members of each faction is higher than the number of links between the members of the different factions. Further, the small-world index (SW) estimated the shortest path (L) and clustering coefficient (CL) calculated from experimental data (Lreal and CLreal, respectively) and a set of 999 simulated random networks with the same number of ties and density (Lrand and Clrand, respectively). It was estimated as follows: SW = (Clreal/Clrand)/(Lreal/Lrand). If SW > 1, the actual network was considered small-world [54].
3. Results
3.1. Trophic and Functional Structure of the Soil Nematode Community
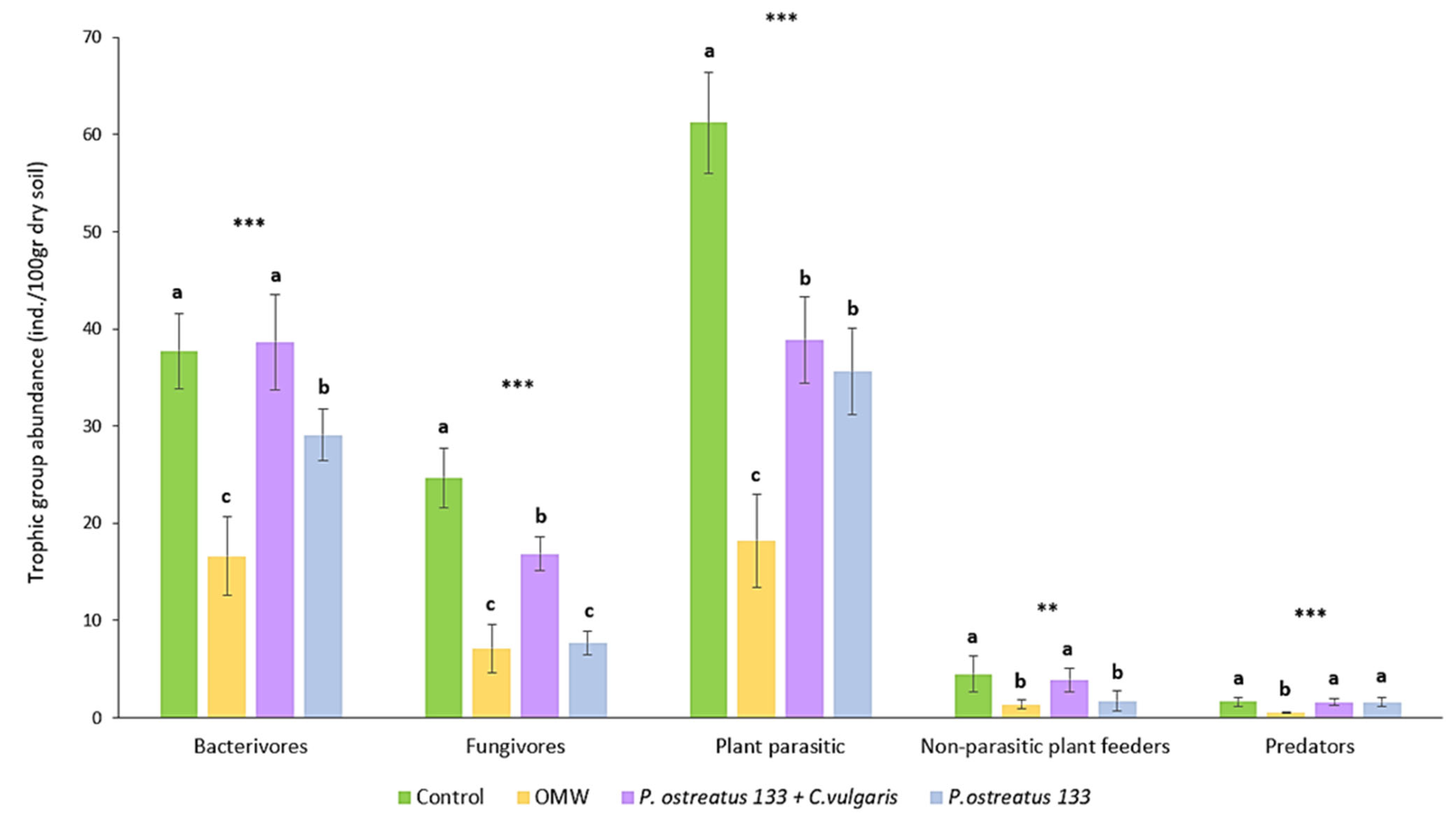
The abundance of the nematode trophic groups in different treatments is presented in Figure 1. No omnivorous genera were recorded. The addition of non-treated OMW to soil significantly reduced the abundance of all trophic groups. More specifically, the abundance of bacterial feeders was decreased by 55.9%, while fungivores, plant parasites, and non-parasitic showed a decline of around 70%. Predators’ reduction reached 66.9%. The addition of OMW treated with P. ostreatus AMRL 133 reduced the nematode abundance of all trophic groups compared to the control, apart from predators. Especially regarding fungal feeders and non-parasitic plant feeders, this decline was similar to that observed after the application of non-treated OMW. The combined treatment effect was significant only in the case of fungivores and plant parasites. Regarding the latter, the reduction in plant parasite abundance in the combined treatment was similar to the one recorded in the P. ostreatus AMRL133-treated pots.
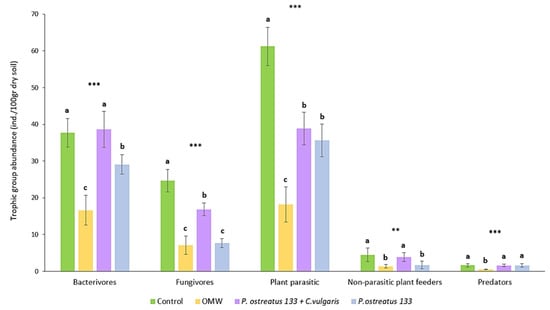
Figure 1.
Mean abundance (±SE) of nematode trophic groups (individuals/100 gr dry soil), 30 days after application of treatments. Different letters (a, b, and c) indicate statistically significant differences between treatments revealed by one-way ANOVA and LSD test. (**, p < 0.01; ***, p < 0.001).
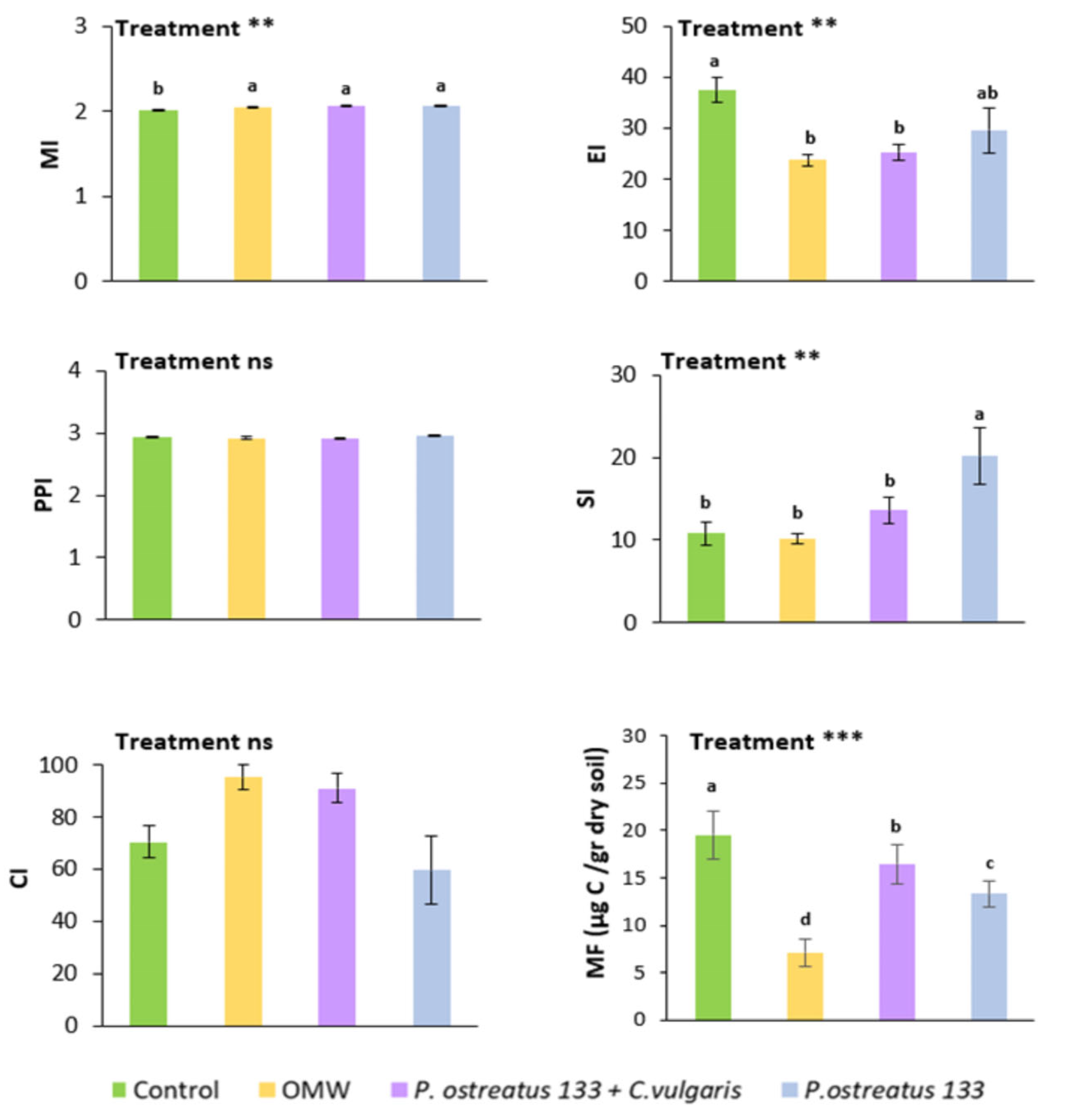
The response of nematode indices to treatments is depicted in Figure 2. The maturity index (MI) was higher in all OMW treatments compared to the control, but did not vary among them. All OMW treatments resulted in lower values of the enrichment index (EI) and the metabolic footprint, but the result was more pronounced in the case of non-treated OMW. The highest values of the structure index were recorded in samples with P. ostreatus AMRL 133-treated OMW. The other treatments did not differ significantly concerning the SI values.
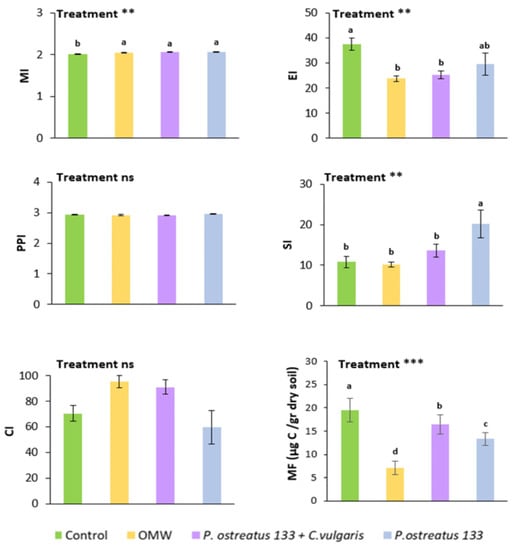
Figure 2.
Mean values (±SE) of the maturity index (MI), plant parasitic index (PPI), enrichment index (EI), structure index (SI), channel index (CI), and the metabolic footprint (MF), 30 days after application of different treatments. Different letters (a, b, c, and d) indicate statistically significant differences between treatments revealed by one-way ANOVA and Fisher’s LSD test. Kruskal–Wallis was used in the case of CI (**, p < 0.01; ***, p < 0.001).
3.2. Community Composition
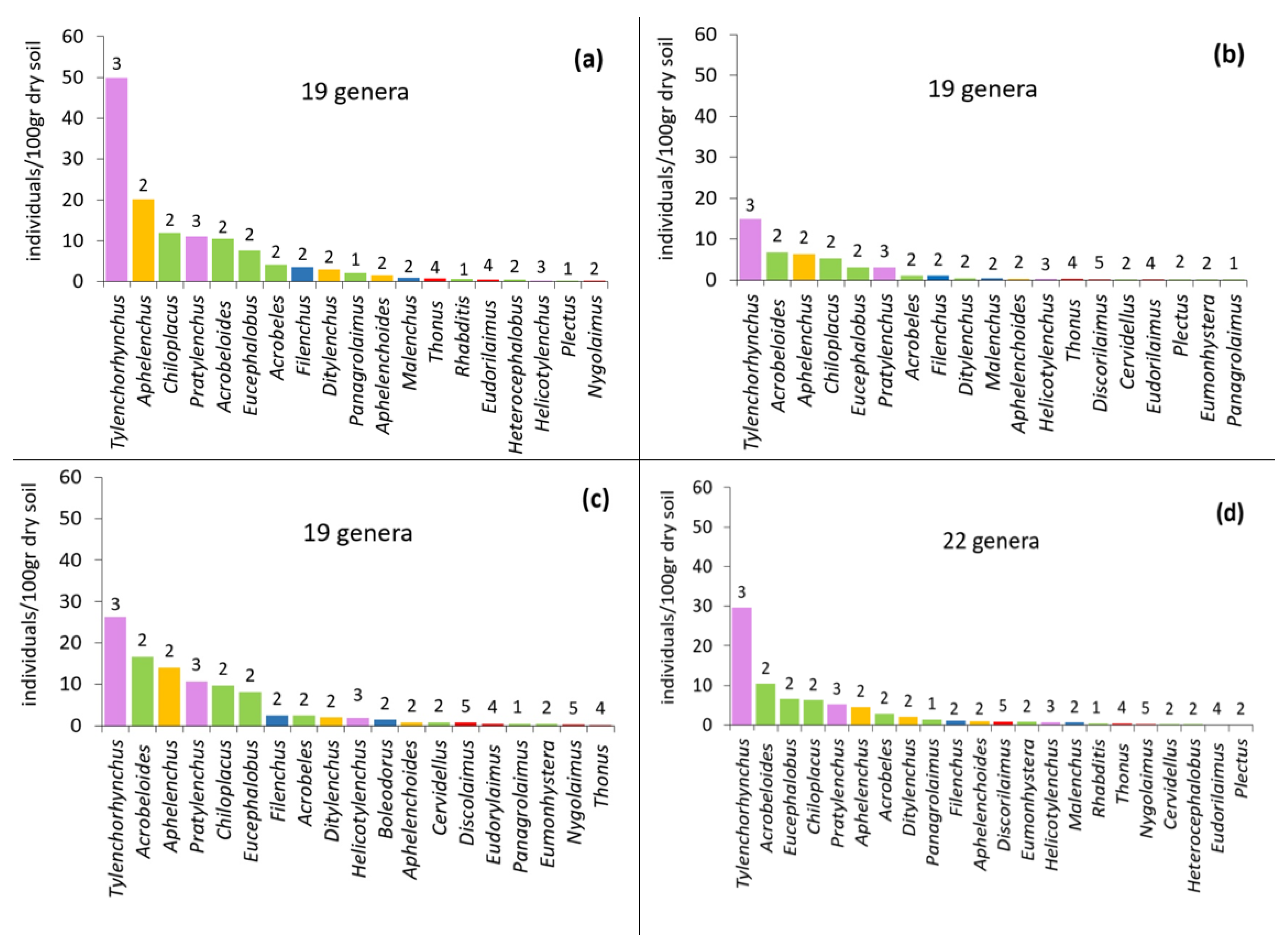
The composition of the nematode community under different treatments is given in the form of rank abundance graphs (Figure 3). Twenty-three nematode genera were recorded in our study. Ten genera were bacterial feeders, three were fungal feeders, three were plant-parasitic, three were non-parasitic plant feeders, and four were predators.
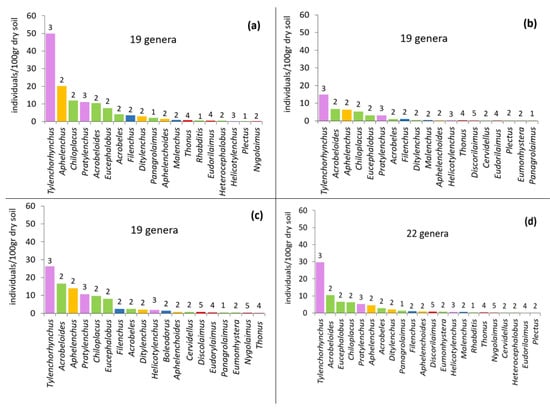
Figure 3.
Relative abundance of nematode genera after the application of different OMWs: (a) control, (b) non-treated OMW, (c) P. ostreatus AMRL 133 + C. vulgaris, (d) P. ostreatus AMRL 133. The number above the bar indicates the life strategy ranking (c–p value), while the different colors correspond to trophic guilds (purple: plant-parasites; green: bacterivores; yellow: fungivores; blue: non-parasitic plant feeders; and red: predators).
The highest number of nematode genera was recorded in soil samples where fungus-treated OMW was applied (22 genera). Changes in community composition did not relate to the abundant genera, since the cp-2 bacterivores Acrobeloides, Chiloplacus, Eucephalobus, the cp-3 phytoparasites Tylenchorhynchus and Pratylenchus, and the cp-2 fungivore Aphelenchus were the most abundant in all treatments. The phytoparasite Tylenchorhynchus was the dominant genus everywhere. However, the abundance of Tylenchorhynchus was lowest in the non-treated OMW soil samples, followed by OMW fungus-treated soil samples and OMW fungus–algae-treated ones. In the control pots, the abundance of Tylenchorhynchus was the highest.
3.3. Nematode Networks
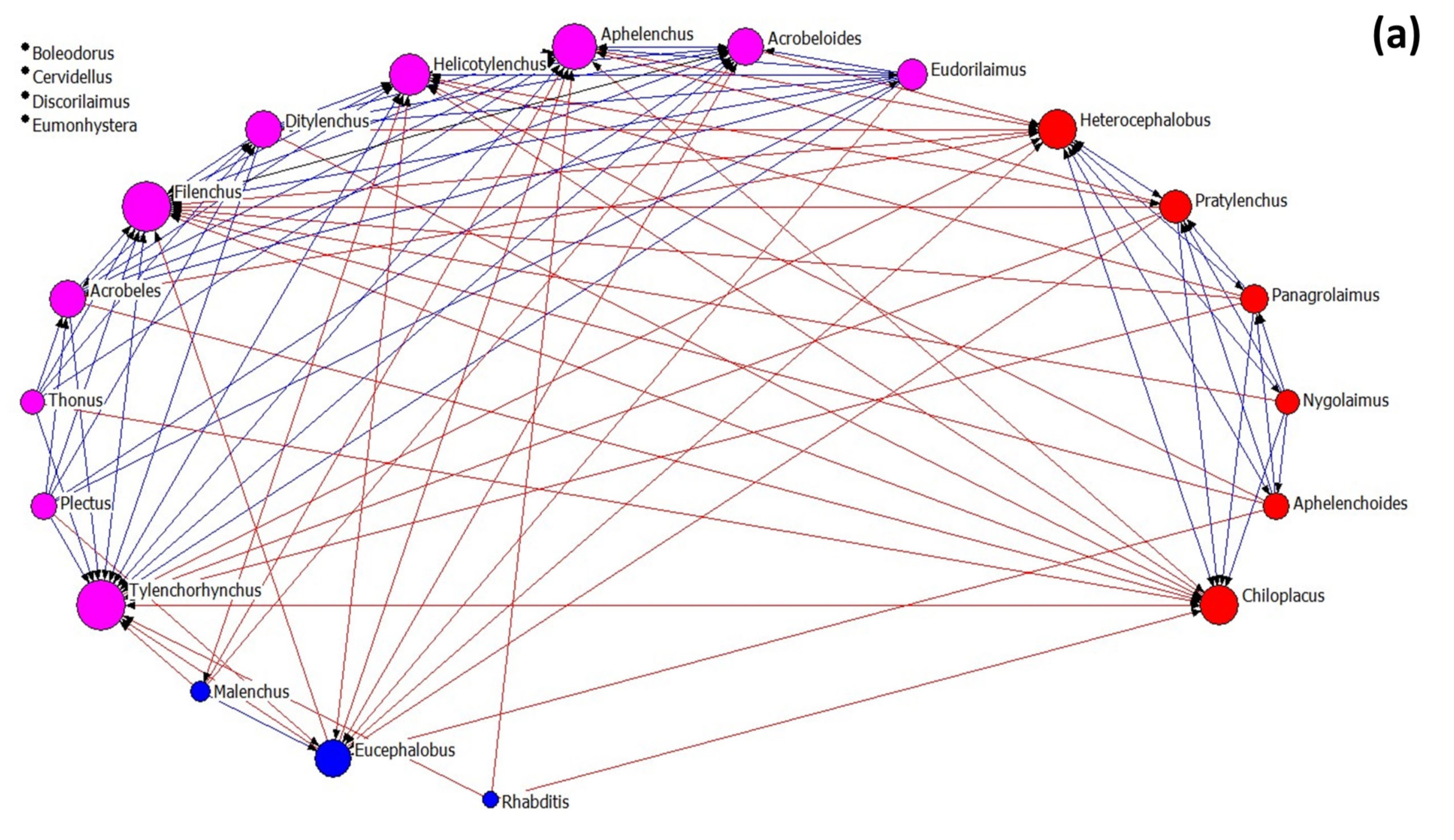
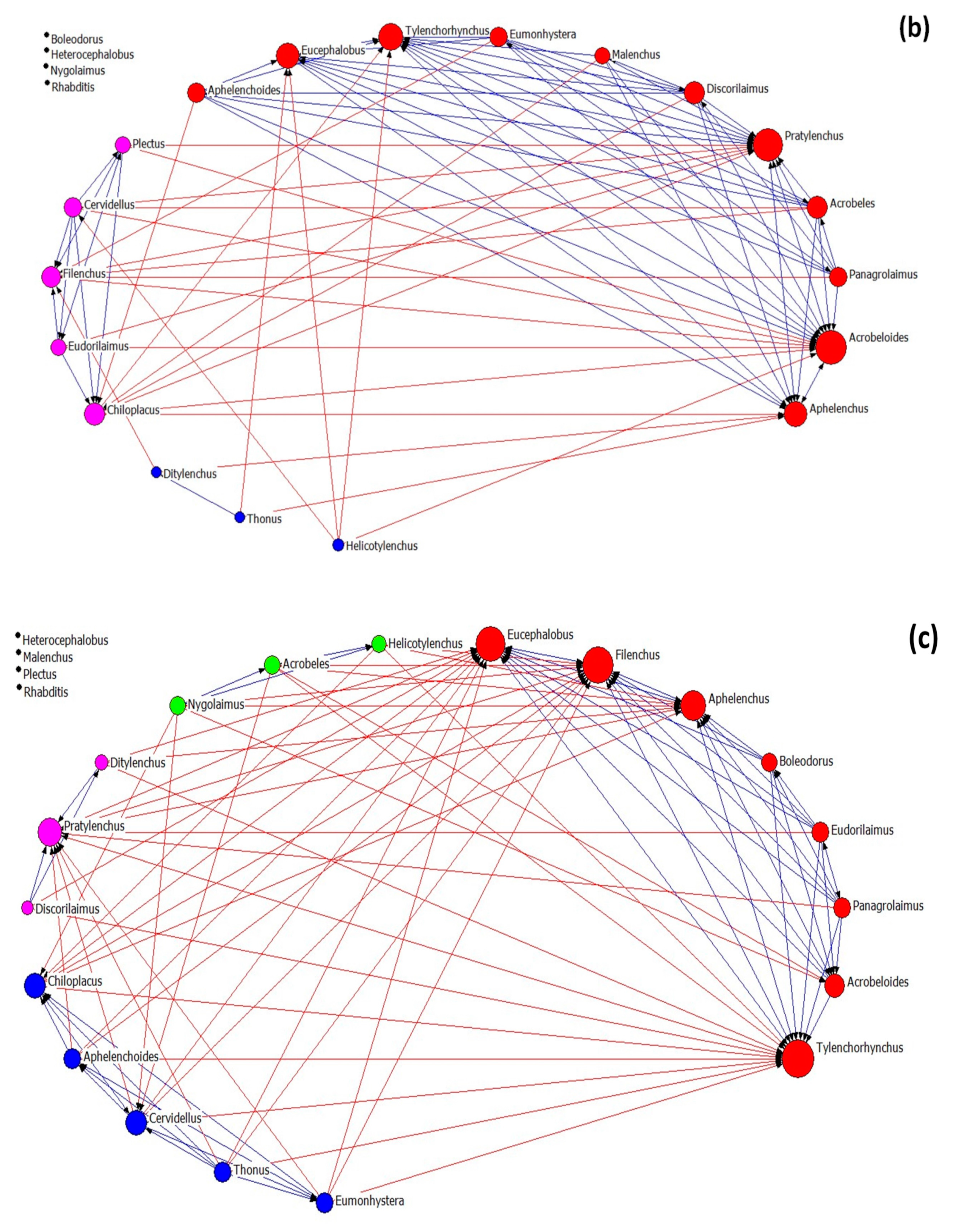
Nematode community networks in soils subjected to different treatments are shown in Figure 4, while data further describing the networks’ attributes are given in Table 3. As regards network architecture in control samples (Figure 4a), the nematode genera (nodes) are classified into two main factions (red and pink nodes). In these factions, there are more links between their nodes than between the nodes belonging to the same faction, as it seems by the modularity value that is below 0.4. In the first faction, there are two fungivore (Filenchus and Aphelenchus) and one plant-parasitic (Tylenchorhynchus) nematodes that exhibit the higher average in-degree centrality (many other genera influenced them). On the other hand, the bacterivorous genera Chiloplacus and Heterocephalobus showed the highest number of links in the second faction.
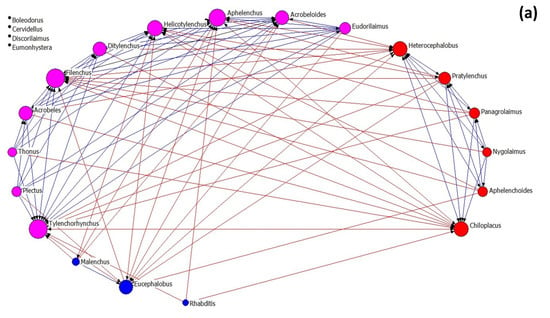
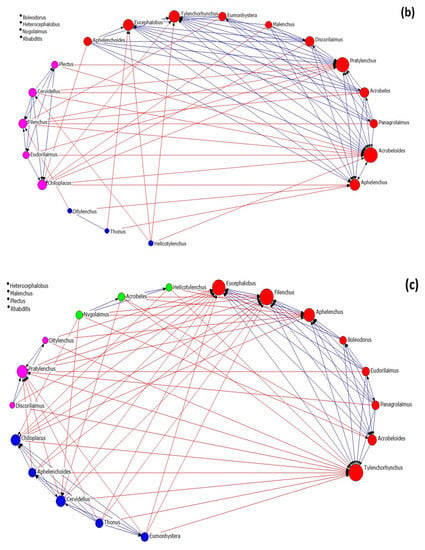
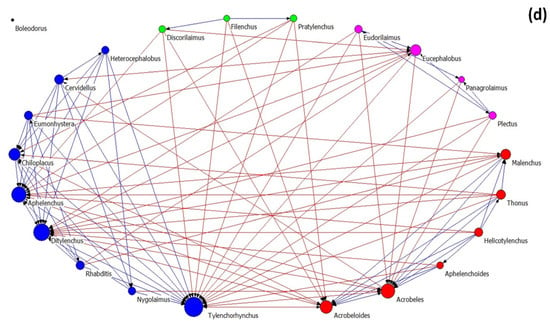
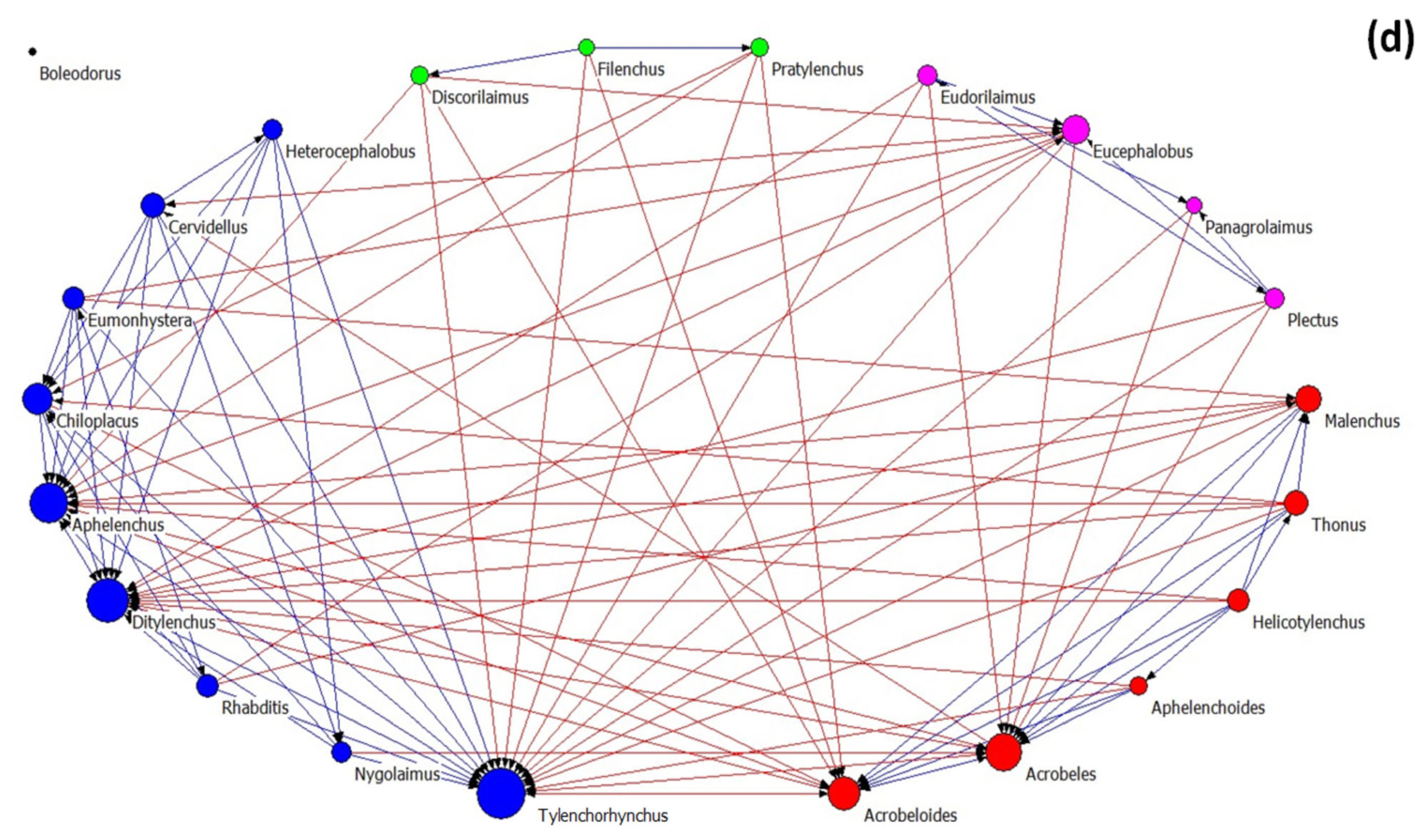
Figure 4.
The network of interactions between nematode genera under different treatments: (a) control, (b) OMW, (c) P. ostreatus AMRL 133 + C. vulgaris, and (d) P. ostreatus AMRL 133. Nodes of different colors correspond to different factions. The higher the size of a node the higher its degree centrality value. Red-colored ties represent links between nodes of different factions, while blue-colored ties represent links between nodes of the same faction. Arrows indicate the direction of the relationship.

Table 3.
Properties of nematode networks in different treatments.
In soils that received non-treated OMW (Figure 4b), the network consists of three nematode factions: one larger faction and two smaller ones. In the main faction, the bacterivore Acrobeloides, the fungivore Aphelenchus, and the plant-parasitic Pratylenchus had the higher number of links. In soils receiving jointly treated OMW (Figure 4c), the network consists of four factions: one primary and three smaller ones. The genera Tylenchorhynchus, Aphelenchus, Filenchus, and Eucephalobus exhibited higher in-degree centrality in the main group. Finally, in soils where the fungus-treated OMW was applied, the network consists of one main group and three smaller ones (Figure 4d). In this main group, the genera mainly influenced by others are Tylenchorhynchus, Ditylenchus, and Aphelenchus.
Regarding the architectural attributes of the networks, the network of the control samples is the most coherent; it had the highest density and compactness and the lowest fragmentation values. It also exhibited the lowest degree of centralization, meaning that the strength of interactions is more equally shared between nodes than in the rest of the networks. Further, this network revealed the lowest values of the small-world index. In all networks, the SW values were > 1, indicating small-world properties. The values of the architectural attributes reported in networks with non-treated OMW were different from the control (highest fragmentation, lowest density, and average distance). By contrast, networks from the soils where the treated OMW was applied exhibited intermediate characteristics. Comparing the networks of nematodes in soils with fungus-treated and jointly treated OMW, that with the fungus was the most coherent (less fragmented), with the lowest SW index and the highest degree centralization (unequal sharing of strength among nodes). In all networks, modularity was less than 0.4, indicating a non-modular architecture. For more information about the network metrics used herein, see Supplementary Table S1.
3.4. Growth of Lettuce Plants
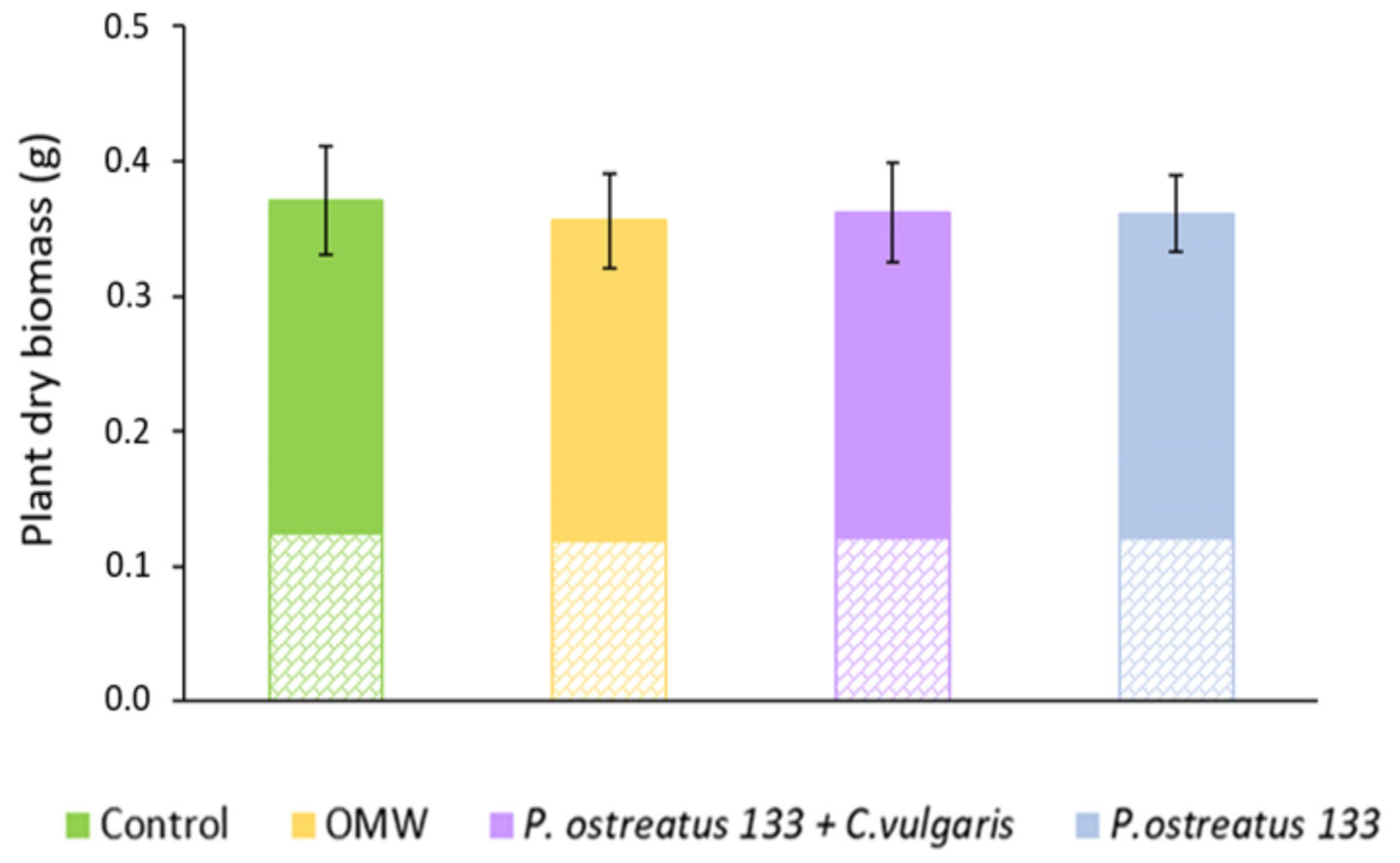
The effects of treatments on the total dry biomass of the lettuce plants are shown in Figure 5. Above- and below-ground dry biomass are indicated by different patterns. In all cases, the root dry biomass represents almost one-third of the total and half of the above-ground biomass. Regarding the total biomass, ANOVA revealed no statistically significant differences between treatments. When ANOVA was performed separately on values of above- and below-ground biomass, the same results were obtained.
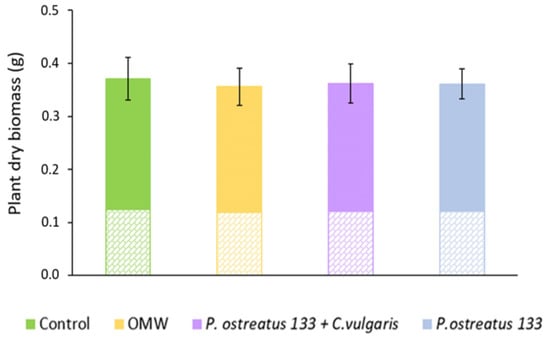
Figure 5.
Mean values (±SE) of total dry biomass (g) of Lactuca sativa plants grown under different treatments. The solid color represents the above-ground dry biomass, while the cross-brick pattern represents the below-ground dry biomass.
4. Discussion
In a pot experiment, we used olive mill wastewaters as soil amendments in pots sown with lettuce and studied their effect on the soil nematode community. Four series of pots were set up: one where no OMW was used (control), one with non-treated OMW, and two where OMW had undergone a procedure for detoxification by micro-organisms. More specifically, we used the fungus P. ostreatus AMRL 133 (selection out of seven fungal strains) and a combination of the selected fungus with the alga C. vulgaris.
Although OMW is considered a toxic substrate for many microorganisms, the fungus P. ostreatus AMRL 133 and the alga C. vulgaris grew on these effluents, reducing the phenolic content in bioreactor cultures. A greater reduction in the phenolic content was achieved when treating OMW with the fungus only than with the combination of both agents. The reduced efficiency of phenol removal in the combined treatment could be due to the significant inhibitory effects of OMW phenols on algae, since light enhances the autoxidation processes and, consequently, the toxicity of simple phenols [55].
4.1. Nematode Community
The application of non-treated OMW reduced the abundance of all nematodes and their activity as shown by the reduction in their metabolic footprint. OMW contains high-molecular-weight substances, such as tannins or phenolic compounds [2], that are suggested to be responsible for nematode suppression [12,56]. Specifically, bioassays of Meloidogynejavanica on tomato plants demonstrated the presence of nematicidal substances in olive mill wastewaters [25]. Moreover, Cayuela et al. [12] reported similar results for Meloidogyne incognita after using pure water extracts of two-phase olive mill waste. However, the above-mentioned studies focused on the suppressive effects of OMW on plant-parasitic nematodes, while there are no studies investigating their impact on the total soil nematode community. It is unclear whether waste additives directly impact soil nematodes or whether this effect could be attributed to changes in soil microflora [56,57]. It is well known that incorporating organic matter in the soil increases microbial abundance and, consequently, increases microbial activity. During the decomposition process, soil microorganisms release metabolic by-products, such as toxic proteins, enzymes, and small-molecule metabolites, which may be nematicidal. [25,57,58].
Incorporating P. ostreatus-treated OMW into soil had a similar but less pronounced effect on the trophic structure of the community, compared to that of the non-treated OMW. One possible reason could be the effluent’s lower amount of phenolic content compared to the non-treated OMW. Moreover, the observed changes may also be attributed to the ability of P. ostreatus AMRL 133 to produce potent toxins. Several species of Pleurotus produce toxins [59,60,61], enzymes [62], and fatty acids [63] with nematicidal activity (or suppress nematodes through their culture filtrate) [64,65]. Most studies regarding the toxins with nematicidal activity produced by white-rot fungi emphasized their effects on plant-parasitic nematodes since they impact crops and agricultural ecosystems. A study by Khan et al. [66] reported that plant-parasitic nematodes, such as those identified in the present study (Pratylenchus, Tylenchorhynchus, and Helicotylenchus), decreased after the application of P. ostreatus extracts and components. However, since we studied the total nematode community, and our results indicated that after the application of P. ostreatus-treated OMW all nematode groups were negatively impacted, we assumed that this fungus has a more general effect on nematodes and not only on the plant-parasitic ones. One might expect that the application of fungus-treated OMW would enhance soil fungi and consequently their predators, i.e., the fungivorous nematodes or even that of the non-parasitic plant feeders, which also have fungal-feeding habits [40,67,68]. However, our results indicated a decline in these trophic groups at the same level as in the non-treated OMW. According to Kwok et al. [59], the toxin produced by P. ostreatus has negative impacts not only on nematodes but also on saprophytic fungi by modifying the properties of their cell membranes.
Regarding the effect of OMW treated with fungus plus microalga on nematodes, it was milder than that of fungus-treated OMW, especially on microbivores that are essential for nutrient cycling in soil. Fungivores were reduced but to a lower extent compared to all OMW treatments, while bacterivores were not affected at all. The time of sampling after treatment (30 days) might play an important role. Bacteria, at least the less sensitive ones, are known to recover quickly after disturbances, causing a bottom-up recovery of bacterivorous nematodes [69]. Moreover, the fungal and microalgal remains incorporated in the soil might favor different groups of microorganisms, providing varied and alternative food substrates for microflora and nematodes. Indeed, among the three OMW treatments, the higher values of the nematode metabolic footprint were recorded in the mixed treatment.
The weighted faunal analysis of soil in all treatments, including the control, yielded low values of EI and SI (<50) and high values of CI (>50). According to Ferris et al. (2001) [43], this faunal profile indicates a degraded food web condition, characteristic of a low-resource and stressful environment with a high fungal participation in the decomposition pathway. We should note that the soil used in our experiment was sandy, with low organic carbon and nitrogen. Sandy soils have low organic matter and clay values and cannot store nutrients [26]. These soils lead to low water holding capacity, weak structure, low fertility, and low values of aggregate stability [70]. None of our OMW treatments improved the food web condition. Perhaps the 10% dilution of OMW, the short duration of our experiment, and the small size of the lettuce seedlings resulted in a low amount of labile food sources for soil decomposers.
Based on nematode rank abundance graphs, we recorded no significant difference in community composition between the treatments. The most common genus was Tylenchorhynchus, a cp-3 phytoparasite, which is among the most frequent plant-parasitic nematodes that are known to be associated with Lactuca sativa. The dominance of Tylenchorhynhus in all samples could also be attributed to soil type and texture [71]. Prasad and Rao [72] reported that sandy soils favored the growth of Tylenchorhynchus since they increased its mobility. Among the most abundant genera in our soil samples were the cp-2 bacterial feeders of the Cephalobidae family and the cp-2 fungal feeder Aphelenchus, belonging to the Aphelenchidae family. Ferris [43] suggested that the predominance of cp-2 nematodes that belong to these families is indicative of a basal food web under stressed conditions, characterized by the limitation of resources and unfavorable environmental conditions.
An interesting finding of our study was the higher values of SI in the pots that received the fungus-treated OMW. Higher SI values suggest more linkages in the food web [43]. Interestingly, network analysis also confirmed this outcome, as adding P. ostreatus-treated OMW led to the formation of a more connected nematode network with relevant unequal sharing of strength among genera. By contrast, the networks after non-treated OMW disposal in soils were the most fragmented and with a higher value of the small-world index. Such characteristics indicate a degraded network structure. Previous studies also revealed the degradation of networks because of disturbance [73,74]. Based on Peng et al. [75], there is a reverse relationship between robustness and small-worldness. According to those mentioned above, the highest SW in a network of soils treated with non-treated OMW indicates less robustness against subsequent disturbance than the rest of the treatments. Therefore, there is a greater possibility for the soil nematode community grown in soils with non-treated OMW to transition from stability to instability. By contrast, in soils under fungus-treated OMW application, the change from a stable to an unstable condition is less likely to happen, according to the relatively low SW value, fragmentation, and highest compactness value.
4.2. Plants
Our results showed that none of our treatments significantly affected plant biomass at the end of the sampling period. Similar findings were reported by Vouyoukalou and Stefanoudaki [25] regarding the fresh plant weight in an 80 d plant experiment. Other studies have reported that using OMW as a fertilizer enhanced plant growth but at lower levels than that under conventional fertilizer and potable water [11,76]. On the contrary, Aggelis et al. [17] observed a decrease in lettuce yields after non-treated OMW irrigation, due to the elevated EC values found in the soils, which caused difficulties in the water and nutrient uptake by the plants [77]. However, OMW dilutions at a greater level minimized the decrease in lettuce yields. In our study, plants of Lactuca sativa in pots with 10% diluted non-treated OMW showed a delay in plant development the first seven days after treatment application. However, after 30 days, the final dry biomass values did not differ significantly between the treatments at the end of the experiment. One possible reason is that adverse effects may disappear with time due to the degradation of phenolic compounds [78]. Furthermore, the fact that the soil used in the present study was degraded, combined with the high C/N of wastes in all treatments, must be considered with regard to plant growth. At an elevated C/N ratio, soil nitrogen becomes immobilized and unavailable to plants undergoing stress.
5. Conclusions
In a pot experiment, we used treated OMW as soil amendments and studied the effects on the soil nematode community and plant growth. Although the joint treatment of OMW with the fungus P. ostreatus and the alga C. vulgaris contributed effectively to its dephenolization, OMW treated only with P. ostreatus displayed the highest decrease in phenol levels. Incorporating non-treated OMW into soil resulted in reduction of all nematodes. The effect was milder when OMW was previously detoxified either by the fungus alone or by its combination with the alga. In the latter case, the plant-parasitic nematodes were suppressed but the bacterivorous nematodes that are essential for nutrient cycling were not affected. However, the most cohesive and robust nematode network was formed in the soil that received the fungal-treated OMW. None of our OMW applications improved the already degraded status of the soil food web and none affected the growth of lettuce plants. Since food webs are dynamic features, and the response of nematodes under different practices might not be direct, our future research will aim to estimate the long-term impact of OMW. Further research will provide complete and accurate information regarding the effects of OMW on soil communities and, consequently, their use as resources in the context of a circular bioeconomy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15040497/s1, Figure S1. The effect of the three selected fungal strains on reducing the medium’s s total phenolics, decolorization, and glucose consumption during a 14 d incubation period in 10% v/v OMW; Table S1. A layout of the properties and metrics referring to the architecture of the network.
Author Contributions
Conceptualization, N.M. and E.M.P.; formal analysis. M.D.D.; investigation, M.D.D.; methodology, M.D.D. and P.K.; supervision, N.M. and E.M.P.; writing—original draft, M.D.D.; writing—review and editing, E.M.P., N.M., P.K., P.A.D., M.D.A. and M.D.D. All authors have read and agreed to the published version of the manuscript.
Funding
The implementation of the doctoral thesis was co-financed by Greece and the European Union (European Social Fund-ESF) through the Operational Program “Human Resources Development, Education and Lifelong Learning” in the context of the Act “Enhancing Human Resources Research Potential by undertaking a Doctoral Research” Sub-action 2: IKY Scholarship Program for Ph.D. candidates at Greek Universities.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Acknowledgments
We would like to thank Haralambos Stamatis, head of the Laboratory of Biotechnology, Department of Biological Applications and Technologies, University of Ioannina, who allowed M.D.D. to work with his group.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gholamzadeh, N.; Peyravi, M.; Jahanshahi, M. Study on Olive Mill Wastewater Treatment: Nanotechnology Impact. Nanotechnol. J. Water Environ. Nanotechnol. 2016, 1, 145–161. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Navarro, D.; Maunier, S.; Sigoillot, J.C.; Lorquin, J.; Delattre, M.; Simon, J.L.; Asther, M.; Labat, M. Simple Phenolic Content in Olive Oil Residues as a Function of Extraction Systems. Food Chem. 2001, 75, 501–507. [Google Scholar] [CrossRef]
- Azbar, N.; Bayram, A.; Filibeli, A.; Muezzinoglu, A.; Sengul, F.; Ozer, A. A Review of Waste Management Options in Olive Oil Production. Crit. Rev. Environ. Sci. Technol. 2004, 34, 209–247. [Google Scholar] [CrossRef]
- Hamdi, M. Future Prospects and Constraints of Olive Mill Wastewaters Use and Treatment: A Review. Bioprocess Eng. 1993, 8, 209–214. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive Mill Wastes: Biochemical Characterizations and Valorization Strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Mekki, A.; Dhouib, A.; Sayadi, S. Changes in Microbial and Soil Properties Following Amendment with Treated and Untreated Olive Mill Wastewater. Microbiol. Res. 2006, 161, 93–101. [Google Scholar] [CrossRef]
- Omer, A.M. Production of Organic Biofertilizer from Olive Mill Waste Water. Aust. J. Basic Appl. Sci. 2012, 6, 654–663. [Google Scholar]
- Paredes, C.; Cegarra, J.; Roig, A.; Sánchez-Monedero, M.A.; Bernal, M.P. Characterization of Olive Mill Wastewater (Alpechin) and Its Sludge for Agricultural Purposes. Bioresour. Technol. 1999, 67, 111–115. [Google Scholar] [CrossRef]
- Mahmoud, M.; Janssen, M.; Haboub, N.; Nassour, A.; Lennartz, B. Impact of Olive Mill Wastewater Application on Flow and Transport Properties in Soils. Soil Tillage Res. 2010, 107, 36–41. [Google Scholar] [CrossRef]
- Sierra, J.; Martí, E.; Garau, M.A.; Cruañas, R. Effects of the Agronomic Use of Olive Oil Mill Wastewater: Field Experiment. Sci. Total Environ. 2007, 378, 90–94. [Google Scholar] [CrossRef]
- Barbera, A.C.; Maucieri, C.; Cavallaro, V.; Ioppolo, A.; Spagna, G. Effects of Spreading Olive Mill Wastewater on Soil Properties and Crops, a Review. Agric. Water Manag. 2013, 119, 43–53. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Millner, P.D.; Meyer, S.L.F.; Roig, A. Potential of Olive Mill Waste and Compost as Biobased Pesticides against Weeds, Fungi, and Nematodes. Sci. Total Environ. 2008, 399, 11–18. [Google Scholar] [CrossRef]
- Brozzoli, V.; Crognale, S.; Sampedro, I.; Federici, F.; Annibale, A.D.; Petruccioli, M. Bioresource Technology Assessment of Olive-Mill Wastewater as a Growth Medium for Lipase Production by Candida cylindracea in Bench-Top Reactor. Bioresour. Technol. 2009, 100, 3395–3402. [Google Scholar] [CrossRef]
- Abrunhosa, L.; Oliveira, F.; Dantas, D.; Gonçalves, C.; Belo, I. Lipase Production by Aspergillus ibericus Using Olive Mill Wastewater. Bioprocess Biosyst. Eng. 2013, 36, 285–291. [Google Scholar] [CrossRef]
- Díaz, A.I.; Laca, A.; Díaz, M. Fungal Treatment of an Effluent from Sewage Sludge Digestion to Remove Recalcitrant Organic Matter. Biochem. Eng. J. 2021, 172, 108056. [Google Scholar] [CrossRef]
- Blánquez, P.; Caminal, G.; Sarrà, M.; Vicent, M.T.; Gabarrell, X. Olive Oil Mill Waste Waters Decoloration and Detoxification in a Bioreactor by the White Rot Fungus Phanerochaete flavido-alba. Biotechnol. Prog. 2002, 18, 660–662. [Google Scholar] [CrossRef]
- Aggelis, G.; Iconomou, D.; Christou, M.; Bokas, D.; Kotzailias, S.; Christou, G.; Tsagou, V.; Papanikolaou, S. Phenolic Removal in a Model Olive Oil Mill Wastewater Using Pleurotus ostreatus in Bioreactor Cultures and Biological Evaluation of the Process. Water Res. 2003, 37, 3897–3904. [Google Scholar] [CrossRef]
- Ntougias, S.; Baldrian, P.; Ehaliotis, C.; Nerud, F.; Antoniou, T.; Merhautová, V.; Zervakis, G.I. Biodegradation and Detoxification of Olive Mill Wastewater by Selected Strains of the Mushroom Genera Ganoderma and Pleurotus. Chemosphere 2012, 88, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Olivier, G.; Russo, M.E.; Giardina, P.; Marzocchella, A.; Sannia, G.; Salatino, P. Strategies for Dephenolization of Raw Olive Mill Wastewater by Means of Pleurotus ostreatus. J. Ind. Microbiol. Biotechnol. 2012, 39, 719–729. [Google Scholar] [CrossRef]
- Diamantis, I.; Melanouri, E.M.; Dedousi, M.; Panagopoulou, I.; Papanikolaou, S.; Stoforos, N.G.; Diamantopoulou, P. Sustainable and Eco-Friendly Conversions of Olive Mill Wastewater-Based Media by Pleurotus pulmonarius Cultures. Fermentation 2022, 8, 129. [Google Scholar] [CrossRef]
- Oswald, W.; Gotaas, H. Photosynthesis in Sewage Treatment. Trans. Am. Soc. Civ. Eng. 1957, 122, 73–105. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Chen, P.; Min, M.; Zhou, W.; Martinez, B.; Zhu, J.; Ruan, R. Bioresource Technology Characterization of a Microalga Chlorella sp. Well Adapted to Highly Concentrated Municipal Wastewater for Nutrient Removal and Biodiesel Production. Bioresour. Technol. 2011, 102, 5138–5144. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.; Venette, R.C.; Lau, S.S. Dynamics of Nematode Communities in Tomatoes Grown in Conventional and Organic Farming Systems, and Their Impact on Soil Fertility. Appl. Soil Ecol. 1996, 3, 161–175. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T. Nematode Indicators of Organic Enrichment. J. Nematol. 2006, 38, 3–12. [Google Scholar] [PubMed]
- Vouyoukalou, E.; Stefanoudaki, E. Nematicidal activity of wastewater from olive oil mills. Nematol. Mediterr. 1998, 26, 157–160. [Google Scholar]
- Rutkowska, A.; Piku, D. Effect of crop rotation and nitrogen fertilization on the quality and quantity of soil organic matter. In Soil Processes and Current Trends in Quality Assessment; IntechOpen: London, UK, 2013; Volume 25, pp. 249–257. [Google Scholar] [CrossRef]
- Valta, K.; Aggeli, E.; Papadaskalopoulou, C.; Panaretou, V.; Sotiropoulos, A.; Malamis, D.; Moustakas, K.; Haralambous, K.J. Adding Value to Olive Oil Production through Waste and Wastewater Treatment and Valorisation: The Case of Greece. Waste Biomass Valorization 2015, 6, 913–925. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Gardeli, C.; Papanikolaou, S. Impact of Olive Mill Wastewaters on the Physiological Behavior of a Wild-Type New Ganoderma resinaceum Isolate. Environ. Sci. Pollut. Res. 2021, 28, 20570–20585. [Google Scholar] [CrossRef]
- Randrianarison, G.; Ashraf, M.A. Microalgae Plant (Chlorella sp.) for Wastewater Treatment and Energy Production. Ekoloji 2018, 27, 1455–1465. [Google Scholar]
- Lindner, A.V.; Pleissner, D. Removal of Phenolic Compounds from Olive Mill Wastewater by Microalgae Grown under Dark and Light Conditions. Waste Biomass Valorization 2021, 13, 525–534. [Google Scholar] [CrossRef]
- Troelstra, S.R.; Wagenaar, R.; Smant, W.; Peters, B.A.M. Interpretation of Bioassays in the Study of Interactions between Soil Organisms and Plants: Involvement of Nutrient Factors. New Phytol. 2001, 150, 697–706. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Box, J.D. Investigation of the Folin-Ciocalteau Phenol Reagent for the Determination of Polyphenolic Substances in Natural Waters. Water Res. 1983, 17, 511–525. [Google Scholar] [CrossRef]
- Sayadi, S.; Ellouz, R. Roles of Lignin Peroxidase and Manganese Peroxidase from Phanerochaete chrysosporium in the Decolorization of Olive Mill Wastewaters. Appl. Environ. Microbiol. 1995, 61, 1098–1103. [Google Scholar] [CrossRef]
- Kelling, P.S. Some experiments in the low-temperature removal of carbonaceous material from clay. Clay Min. Bull. 1962, 28, 155–158. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle and size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- ISO 11465; Soil Quality: Determination of Dry Matter and Water Content on a Mass Basis: Gravimetric Method. International Organization for Standardization: Geneva, Switzerland, 1993.
- S’Jacob, J.J.; van Bezooijen, J. A Manual for Practical Work in Nematology; Department of Nematology, Wageningen Agricultural University: Wageningen, The Netherlands, 1984. [Google Scholar]
- Bongers, T. De Nematoden van Nederland; Koninklijke Nederlandse Natuurhistorische Vereniging: Utrecht, The Netherlands, 1994. [Google Scholar]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding Habits in Soil Nematode Families and Genera—An Outline for Soil Ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar] [PubMed]
- Bongers, T. The Maturity Index: An Ecological Measure of Environmental Disturbance Based on Nematode Species Composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; De Goede, R.G.M. A Framework for Soil Food Web Diagnostics: Extension of the Nematode Faunal Analysis Concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Bongers, T.; Ferris, H. Nematode Community Structure as a Bioindicator in Environmental Monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H. Form and Function: Metabolic Footprints of Nematodes in the Soil Food Web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Sieriebriennikov, B.; Ferris, H.; de Goede, R.G.M. NINJA: An Automated Calculation System for Nematode-Based Biological Monitoring. Eur. J. Soil Biol. 2014, 61, 90–93. [Google Scholar] [CrossRef]
- Butts, C.T. A Relational Event Framework for Social Action. Sociol. Methodol. 2008, 38, 155–200. [Google Scholar] [CrossRef]
- Macarthur, R.; Levins, R. The limiting similarity convergence and divergence of coexisting species. Am. Nat. 1967, 101, 377–385. [Google Scholar] [CrossRef]
- Stamou, G.P.; Argyropoulou, M.D.; Rodriguez-Polo, I.; Boutsis, G.; Kapagianni, P.; Papatheodorou, E.M. A Case Study of Nematode Communities’ Dynamics along Successional Paths in the Reclaimed Landfill. Diversity 2020, 12, 274. [Google Scholar] [CrossRef]
- Borgatti, S.P.; Everett, G.; Freeman, L.C. UCINET 5.0, Version 1.00; Computer Manual; Analytech Technologies: Natick, MA, USA, 1999.
- Huisman, M.; Van Duijn, M.A.J. Software for social network analysis. In Models and Methods in Social Network Analysis; Carrington, P.J., Scott, J., Wasserman, S., Eds.; Cambridge University Press: New York, NY, USA, 2005; pp. 270–316. [Google Scholar]
- O’Malley, A.J.; Marsden, P.V. The Analysis of Social Networks. Health Serv. Outcomes Res. Methodol. 2008, 8, 222–269. [Google Scholar] [CrossRef]
- Rampelotto, P.H.; Barboza, A.D.M.; Pereira, A.B.; Triplett, E.W.; Schaefer, C.E.G.R.; Camargo, F.A.D.O.; Roesch, L.F.W. Distribution and Interaction Patterns of Bacterial Communities in an Ornithogenic Soil of Seymour Island, Antarctica. Microb. Ecol. 2015, 69, 684–694. [Google Scholar] [CrossRef]
- Humphries, M.D.; Gurney, K. Network “Small-World-Ness”: A Quantitative Method for Determining Canonical Network Equivalence. PLoS ONE 2008, 3, e0002051. [Google Scholar] [CrossRef]
- Nakai, S.; Inoue, Y.; Hosomi, M. Algal Growth Inhibition Effects and Inducement Modes by Plant-Producing Phenols. Water Res. 2001, 35, 1855–1859. [Google Scholar] [CrossRef]
- Chitwood, D.J. Phytochemical Based Strategies for Nematode Control. Annu. Rev. Phytopathol. 2002, 40, 221–249. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Kabana, R.; Morgan-Jones, G.; Chet, I. Biological control of nematodes: Soil amendments and microbial antagonists. Plant Soil 1987, 100, 237–247. [Google Scholar] [CrossRef]
- Akhtar, M.; Malik, A. Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: A review. Biores. Technol. 2000, 74, 35–47. [Google Scholar] [CrossRef]
- Kwok, O.C.H.; Plattner, R.; Weisleder, D.; Wicklow, D.T. A Nematicidal Toxin from Pleurotus ostreatus NRRL 3526. J. Chem. Ecol. 1992, 18, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Nordbring-Hertz, B.; Jansson, H.-B.; Friman, E.; Persson, Y.; Dackman, C.; Hard, T.; Poloczek, E.; Feldmann, R. Nematophagous Fungi; Film no. V 1851; Institut für den Wissenschaftlichen Film: Göttingen, Germany, 1995. [Google Scholar]
- Satou, T.; Kaneko, K.; Li, W.; Koike, K. The Toxin Produced by Pleurotus ostreatus Reduces the Head Size of Nematodes. Biol. Pharm. Bull. 2008, 31, 574–576. [Google Scholar] [CrossRef]
- Genier, H.L.A.; Soares, F.E.F.; Queiroz, J.H.; Gouveia, A.S.; Araújo, J.V.; Braga, F.R.; Pinheiro, I.R.; Kasuya, M.C.M. Activity of the fungus Pleurotus ostreatus and of its proteases on Panagrellus sp. larvae. Afr. J. Biotechnol. 2015, 14, 1496–1503. [Google Scholar] [CrossRef]
- Li, G.; Zhang, K.; Xu, J.; Dong, J.; Liu, Y. Nematicidal Substances from Fungi. Recent Pat. Biotechnol. 2008, 1, 212–233. [Google Scholar] [CrossRef]
- Samsam-Shariat, H.; Farid, H.; Kavianpour, M. A Study of the Anthelmintic Activity of Aqueous Extract of Pleurotus eryngii on Syphacia obvelata and Hymenolepis nana. J. Sci. Islam. Repub. Iran 1994, 5, 19–22. [Google Scholar]
- Palizi, P.; Goltapeh, E.M.; Pourjam, E.; Safaie, N. Potential of Oyster Mushrooms for the Biocontrol of Sugar Beet Nematode (Heterodera schachtii). J. Plant Prot. Res. 2009, 49, 27–33. [Google Scholar] [CrossRef]
- Khan, A.; Iqbal, M.; Hussain, S.; Pakhtunkhwa, K.; Pakhtunkhwa, K. Organic Control of Phytonematodes with Pleurotus Species. J. Nematol. 2014, 32, 155–161. [Google Scholar]
- Okada, H.; Harada, H.; Kadota, I. Fungal-feeding habits of six nematode isolates in the genus Filenchus. Soil Biol. Biochem. 2005, 37, 1113–1120. [Google Scholar] [CrossRef]
- Monokrousos, N.; Charalampidis, G.; Boutsis, G.; Sousanidou, V.; Papatheodorou, E.M.; Argyropoulou, M.D. Plant-Induced Differentiation of Soil Variables and Nematode Community Structure in a Mediterranean Serpentine Ecosystem. Soil Res. 2014, 52, 593–603. [Google Scholar] [CrossRef]
- Kapagianni, P.D.; Boutsis, G.; Argyropoulou, M.D.; Papatheodorou, E.M.; Stamou, G.P. The network of interactions among soil quality variables and nematodes: Short-term responses to disturbances induced by chemical and organic disinfection. Appl. Soil Ecol. 2010, 44, 67–74. [Google Scholar] [CrossRef]
- Ibrahim, A.; Usman, A.R.A.; Al-Wabel, M.I.; Nadeem, M.; Ok, Y.S.; Al-Omran, A. Effects of Conocarpus Biochar on Hydraulic Properties of Calcareous Sandy Soil: Influence of Particle Size and Application Depth. Arch. Agron. Soil Sci. 2017, 63, 185–197. [Google Scholar] [CrossRef]
- Van Diepeningen, A.D.; De Vos, O.J.; Korthals, G.W.; Van Bruggen, A.H.C. Effects of Organic versus Conventional Management on Chemical and Biological Parameters in Agricultural Soils. Appl. Soil Ecol. 2006, 31, 120–135. [Google Scholar] [CrossRef]
- Prasad, J.S.; Rao, Y.S. Influence of edaphic factors on the build-up of the root lesion nematode, Pratylenchus indicus Das, 1960 in rice. I. Effect of type, texture, porosity, and moisture of the soil. Eur. J. Soil Biol. 1980, 17, 173–179. [Google Scholar]
- Stamou, G.P.; Argyropoulou, M.D.; Tsiafouli, M.A.; Monokrousos, N.; Sgardelis, S.P.; Papatheodorou, E.M. The Study of Secondary Successional Patterns in Soil Using Network Analysis: The Case of Conversion from Conventional to Organic Farming. Pedobiologia 2011, 54, 253–259. [Google Scholar] [CrossRef]
- Stamou, G.P.; Monokrousos, N.; Gwynn-Jones, D.; Whitworth, D.E.; Papatheodorou, E.M. A Polyphasic Approach for Assessing Ecosystem Connectivity Demonstrates That Perturbation Remodels Network Architecture in Soil Microcosms. Microb. Ecol. 2019, 78, 949–960. [Google Scholar] [CrossRef]
- Peng, G.S.; Tan, S.Y.; Wu, J.; Holme, P. Trade-Offs between Robustness and Small-World Effect in Complex Networks. Sci. Rep. 2016, 6, 37317. [Google Scholar] [CrossRef]
- Rusan, M.J.M.; Albalasmeh, A.A.; Zuraiqi, S.; Bashabsheh, M. Evaluation of Phytotoxicity Effect of Olive Mill Wastewater Treated by Different Technologies on Seed Germination of Barley (Hordeum vulgare L.). Environ. Sci. Pollut. Res. 2015, 22, 9127–9135. [Google Scholar] [CrossRef]
- Black, C.A. Soil–Plant Relationships, 2nd ed.; Wiley: New York, NY, USA, 1968; pp. 230–238+372–404. [Google Scholar]
- Kurtz, M.P.; Dag, A.; Zipori, I.; Laor, Y.; Buchmann, C.; Saadi, I.; Medina, S.; Raviv, M.; Zchori-Fein, E.; Schaumann, G.E.; et al. Toward Balancing the Pros and Cons of Spreading Olive Mill Wastewater in Irrigated Olive Orchards. Processes 2021, 9, 780. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).