Living Taxa and Their Importance in Understanding the Extinct Diversity: A Look at Polypterid Pinnules
Abstract
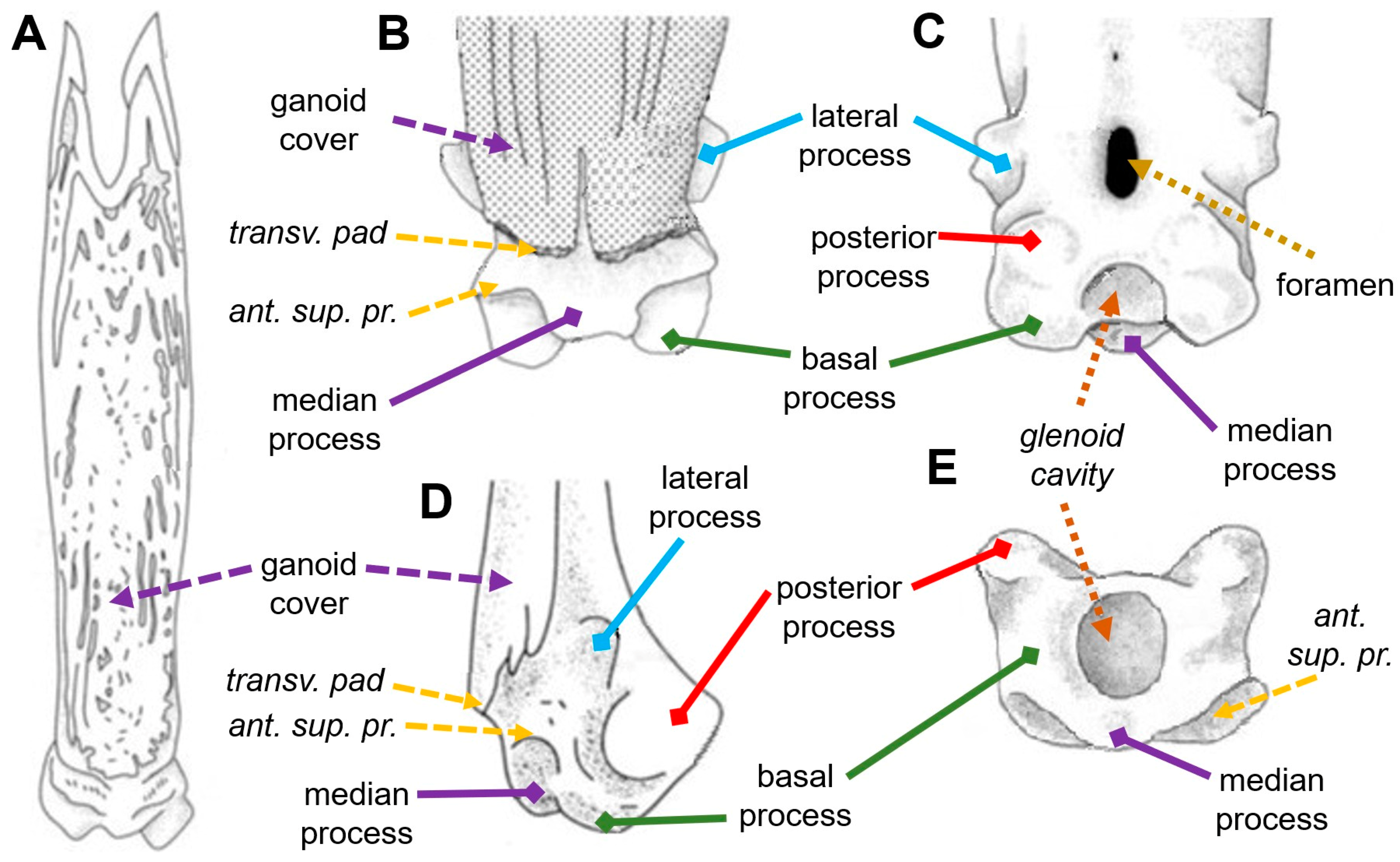
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A




















References
- Lacépède, B.G.E. Histoire Naturelle des Poissons; Massan: Paris, France, 1803; Volume 5, p. 803. [Google Scholar]
- Daget, J.; Gayet, M.; Meunier, F.J.; Sire, J.Y. Major discoveries on the dermal skeleton of fossil and recent polypteriforms: A review. Fish Fish 2001, 2, 113–124. [Google Scholar] [CrossRef]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V. Fishes of the World, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 116–117. [Google Scholar]
- Gayet, M.; Meunier, F.J.; Werner, C. Strange Polypteriformes from the upper Cretaceous of in Becetem (Niger) and Wadi milk formation (Sudan). Geobios 1997, 30, 249–255. [Google Scholar] [CrossRef]
- Meunier, F.J.; Gayet, M. Comparative morphology of the finlet spines of the extant Polypteridae (Osteichthyes; Cladistia). Systematic interest. Cybium 2020, 44, 19–37. [Google Scholar]
- Gayet, M.; Meunier, F.J.; Levrat-Calviac, V. Mise en évidence des plus anciens Polypteridae dans le gisement sénonien, d’In Becetem (Niger). In Comptes Rendus de l’Académie des Sciences, Série 2, Mécanique, Physique, Chimie, Sciences de l’univers, Sciences de la Terre; Gauthier Villars: Paris, France, 1988; pp. 205–210. [Google Scholar]
- Gayet, M.; Meunier, F.J. Nouveaux Polypteriformes du gisement coniacien-sénonien d’In Becetem (Niger). In Comptes Rendus de l’Académie des Sciences. Série 2. Sciences de la Terre et des Planètes; Elsevier: Paris, France, 1996; Volume 322, pp. 701–707. [Google Scholar]
- Werner, C.; Gayet, M. New fossil Polypteridae from the Cenomanian of Sudan. An evidence of their high diversity in the early Late Cretaceous. Cybium 1997, 21, 67–81. [Google Scholar]
- Greenwood, P.H. Fish remains from Miocene deposits of Rusinga island and Kavirondo Province, Kenya. Ann. Mag. Nat. 1951, 4, 1192–1201. [Google Scholar] [CrossRef]
- Greenwood, P.H. New fish fossils from the late Miocene of Wadi Natrum, Egypt. J. Zool. 1972, 168, 503–519. [Google Scholar] [CrossRef]
- Greenwood, P.H. Fish fossils from the late Miocene of Tunisia. Notes Serv. Geol. 1973, 37, 41–72. [Google Scholar]
- Van Neer, W.; Gayet, M. Etude des poissons en provenance des sites holocènes du bassin de Taoudenni- Araouane (Mali). Bull. Mus. Natl. Hist. Nat. 1988, 10, 343–353. [Google Scholar]
- Stewart, K.M. A Late Miocene fish fauna from Lothagam, Kenya. J. Vertebr. Paleontol. 1994, 14, 592–594. [Google Scholar] [CrossRef]
- Stewart, K.M. The freshwater fish of Neogene Africa (Miocene-Pleistocene): Systematics and biogeography. Fish Fish. 2001, 2, 177–230. [Google Scholar] [CrossRef]
- Stewart, K.M. Fossils fish remains from the Pliocene Kanapoi site, Kenya. Los Angeles County Mus. Contr. Sci. 2003, 498, 21–38. [Google Scholar] [CrossRef]
- Murray, A.M. Review of Palaeozoic, Mesozoic and Early Cenozoic fishes of Africa. Fish Fish 2000, 1, 111–145. [Google Scholar] [CrossRef]
- Murray, A.M.; Cook, P.; Attia, Y.; Chatrath, P.; Simons, E. A freshwater ichthyofauna from the late Eocene Birket Qarun Formation, Fayum, Egypt. J. Vertebr. Paleontol. 2010, 30, 665–680. [Google Scholar] [CrossRef]
- Otero, O.; Pinton, A.; Mackaye, H.T.; Likius, A.; Vignaud, P.; Brunet, M. First description of a Pliocene ichthyofauna from Central Africa (site KL2, Kolle area, Eastern Djurab, Chad): What do we learn? Afr. J. Earth Sci. 2009, 54, 62–74. [Google Scholar] [CrossRef]
- Otero, O.; Pinton, A.; Mackaye, H.T.; Likius, A.; Vignaud, P.; Brunet, M. The fish assemblage associated with the Late Miocene Chadian hominid (Toros-Menalla, Western Djurab) and its palaeoenvironmental significance. Paleontogr. Abt. A 2010, 3, 21–51. [Google Scholar] [CrossRef]
- Otero, O.; Pinton, A.; Cappetta, H.; Adnet, S.; Valentin, X.; Salem, M.; Jaeger, J.J. A fish assemblage from the Middle Eocene from Libya (Dur At-Talah) and the earliest record of modern African fish genera. PLoS ONE 2015, 10, e0144358. [Google Scholar] [CrossRef]
- Sewertzoff, A.N. The development of the dorsal fin of Polypterus delhezi. J. Morphol. 1924, 38, 551–580. [Google Scholar] [CrossRef]
- Moritz, T.; Britz, R. Revision of the extant Polypteridae (Actinopterygii: Cladistia). Ichthyol. Explor. Freshw. 2019, 29, 97–192. [Google Scholar]
- Coelho, M.V.; Cupello, C.; Brito, P.M. Morphological variations in the dorsal fin finlets of extant polypterids raise questions about their taxonomical validity. PeerJ 2018, 6, e5083. [Google Scholar] [CrossRef]
- Near, T.J.; Dornburg, A.; Tokita, M.; Suzuki, D.; Brandley, M.C.; Friedman, M. Boom and bust: Ancient and recent diversification in bichirs (Polypteridae: Actinopterygii), a relictual lineage of rayfinned fishes. Evolution 2014, 68, 1014–1026. [Google Scholar] [CrossRef] [Green Version]
- Gayet, M.; Meunier, F.J. Polyptériformes (pisces, cladistia) du maastrichtien et du paléocène de Bolivie. Geobios 1992, 25, 159–168. [Google Scholar] [CrossRef]
- Grande, L. Categorizing various classes of morphological variation, and the importance of this to vertebrate paleontology. In Mesozoic Fishes 3-Systematics, Paleoenvironments and Biodiversity, 1st ed.; Arratia, G., Tintori, A., Eds.; Verlag Dr Friedrich Pfeil: Munchen, Germany, 2004; pp. 123–136. [Google Scholar]


| Taxon | Features with Intraspecific Variation | Probably Reliable Features | |||
|---|---|---|---|---|---|
| Shape of the Head of the Pinnula | Degree of Individualization of the Processes | Ganoin Ornamentation | Open Glenoid Cavity or Basal Foramen | Overall Shape of the Processes | |
| Bartschichthys | 🗸 | 🗸 | 🗸 | ||
| Bartschichthys arnoulti | 🗸 | 🗸 | 🗸 | 🗸 | |
| Bartschichthys napatensis | 🗸 | 🗸 | |||
| Bartschichthys tubularis | 🗸 | 🗸 | |||
| Polypterus dageti | 🗸 | 🗸 | |||
| Polypterus sudanensis | 🗸 | ||||
| Saharichthys africanus | 🗸 | 🗸 | |||
| Saharichthys nigeriensis | 🗸 | 🗸 | |||
| Sainthilairia | 🗸 | 🗸 | |||
| Sainthilairia beccusiformes | 🗸 | ||||
| Sainthilairia elongata | 🗸 | ||||
| Sainthilairia falcifomis | 🗸 | 🗸 | |||
| Sainthilairia grandis | 🗸 | 🗸 | 🗸 | ||
| Sainthilairia intermedia | 🗸 | ||||
| Sudania | 🗸 | 🗸 | |||
| Sudania gracilis | 🗸 | 🗸 | |||
| Sudania oblonga | 🗸 | 🗸 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, M.V.; Cupello, C.; Brito, P.M.; Otero, O. Living Taxa and Their Importance in Understanding the Extinct Diversity: A Look at Polypterid Pinnules. Diversity 2023, 15, 517. https://doi.org/10.3390/d15040517
Coelho MV, Cupello C, Brito PM, Otero O. Living Taxa and Their Importance in Understanding the Extinct Diversity: A Look at Polypterid Pinnules. Diversity. 2023; 15(4):517. https://doi.org/10.3390/d15040517
Chicago/Turabian StyleCoelho, Marcos Vinícius, Camila Cupello, Paulo M. Brito, and Olga Otero. 2023. "Living Taxa and Their Importance in Understanding the Extinct Diversity: A Look at Polypterid Pinnules" Diversity 15, no. 4: 517. https://doi.org/10.3390/d15040517
APA StyleCoelho, M. V., Cupello, C., Brito, P. M., & Otero, O. (2023). Living Taxa and Their Importance in Understanding the Extinct Diversity: A Look at Polypterid Pinnules. Diversity, 15(4), 517. https://doi.org/10.3390/d15040517







