Effects of Plant-Growth-Promoting Rhizobacteria on Soil Bacterial Community, Soil Physicochemical Properties, and Soil Enzyme Activities in the Rhizosphere of Alfalfa under Field Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Experimental Design
2.3. Plant and Rhizosphere Soil Sampling
2.4. Biolog Ecoplate Method
2.5. 16S rRNA Gene High-Throughput Sequencing
2.6. Determination of Soil Physicochemical Properties and Soil Enzyme Activities
2.7. Bioinformatics Analysis
2.8. Statistical Analysis
3. Results
3.1. Alfalfa Biomass
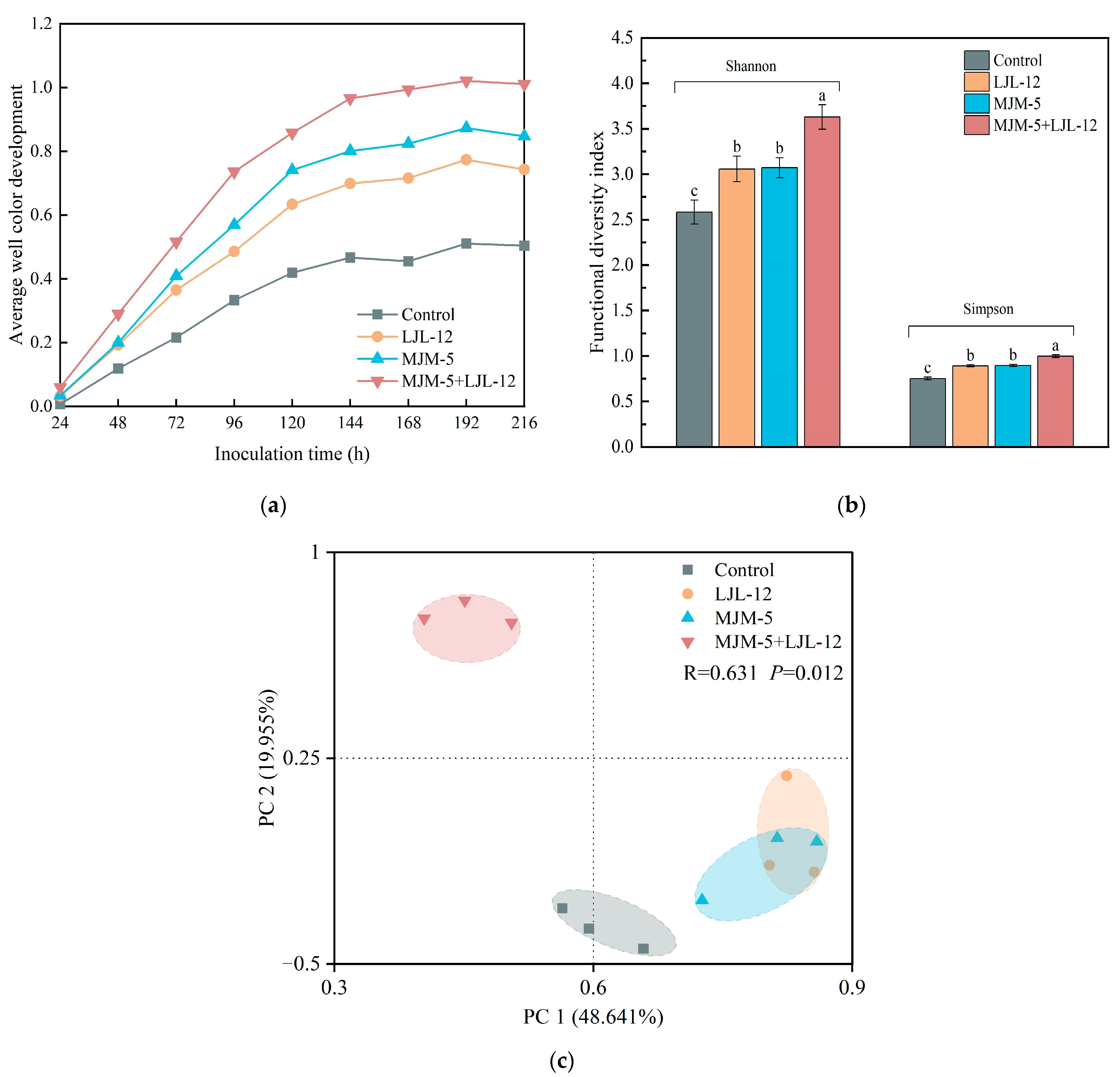
3.2. Carbon Source Metabolic Activity of Alfalfa Rhizosphere Soil
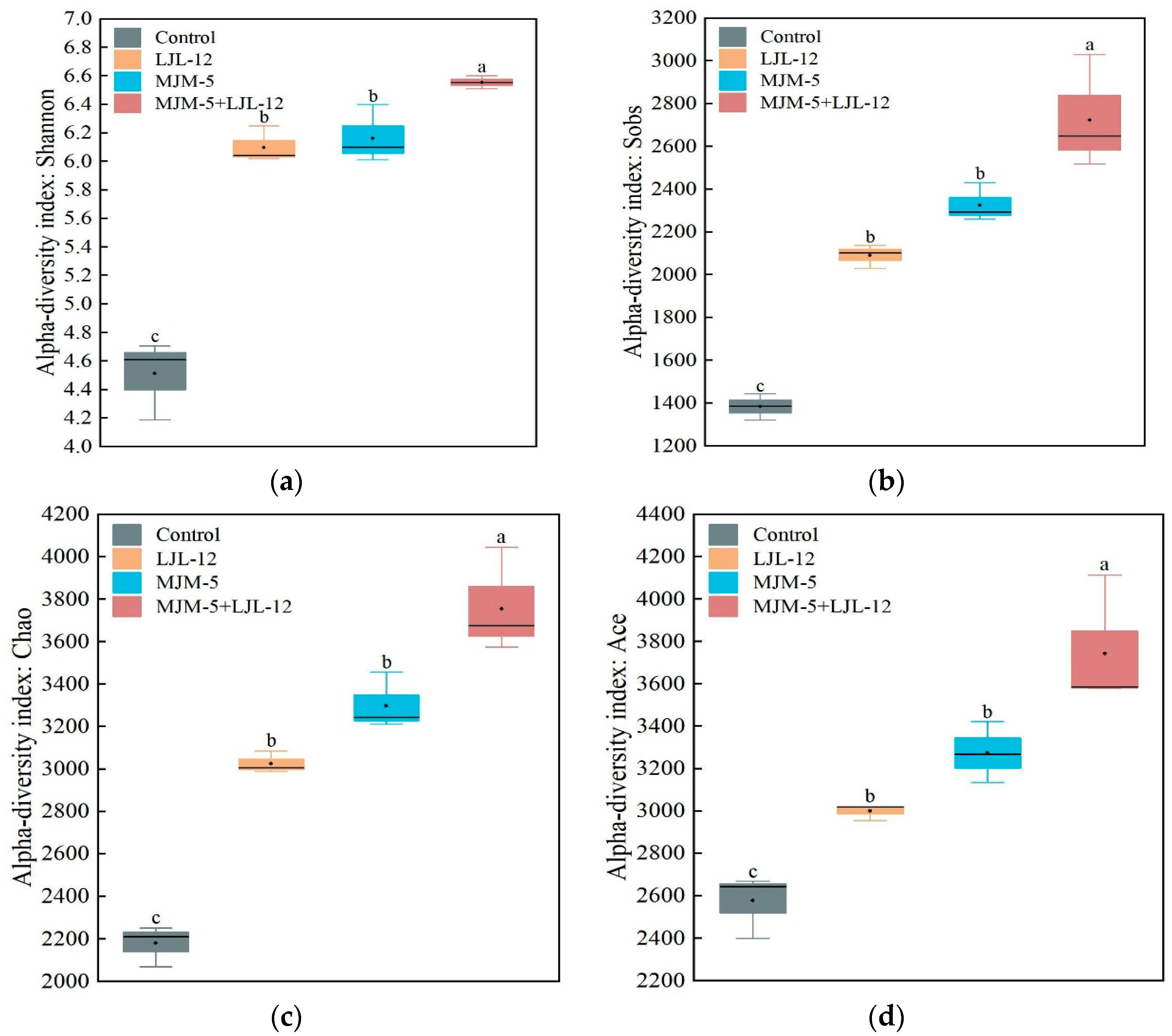
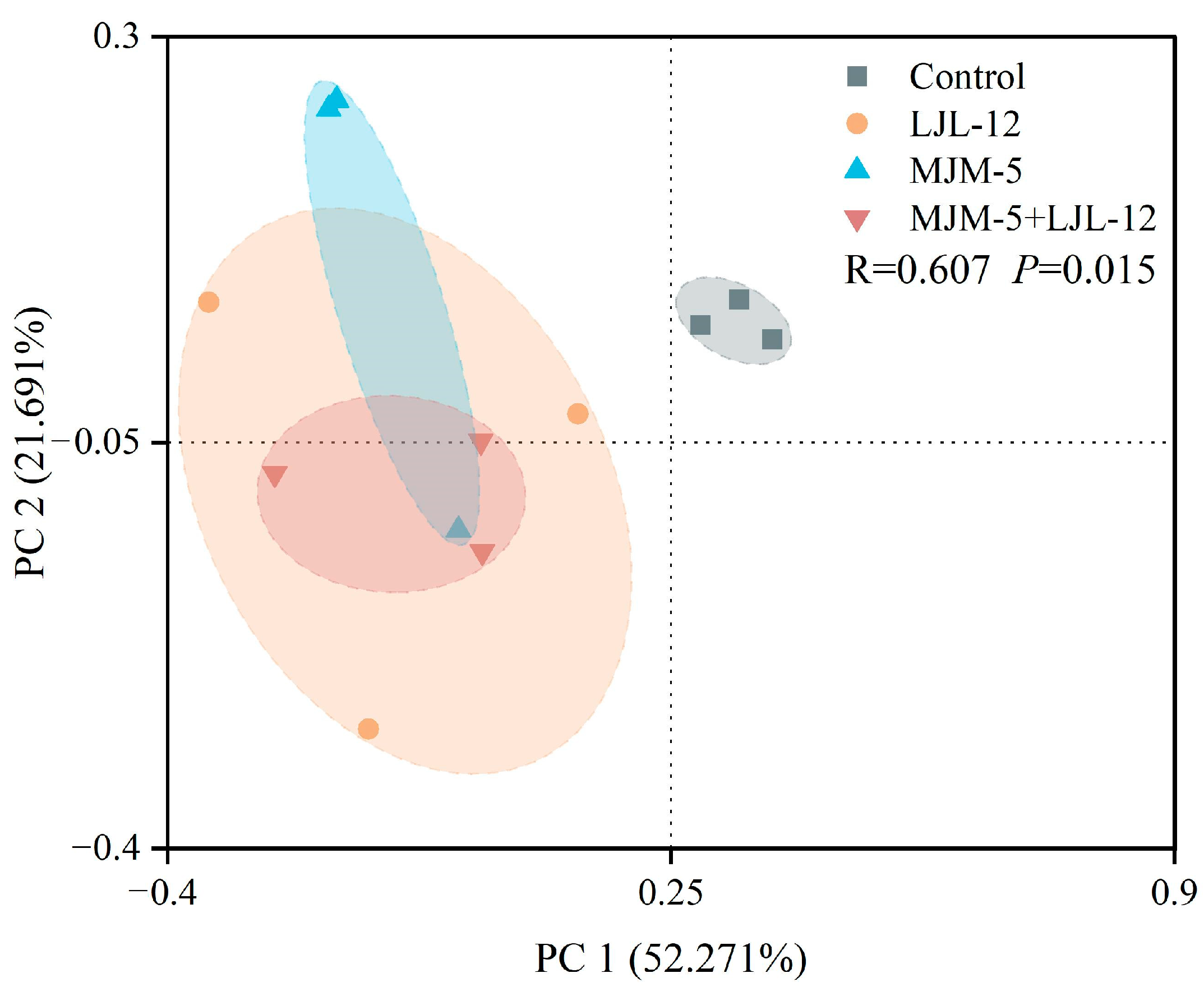
3.3. Alpha- and Beta-Diversity of Bacterial Communities in the Rhizosphere Soil of Alfalfa
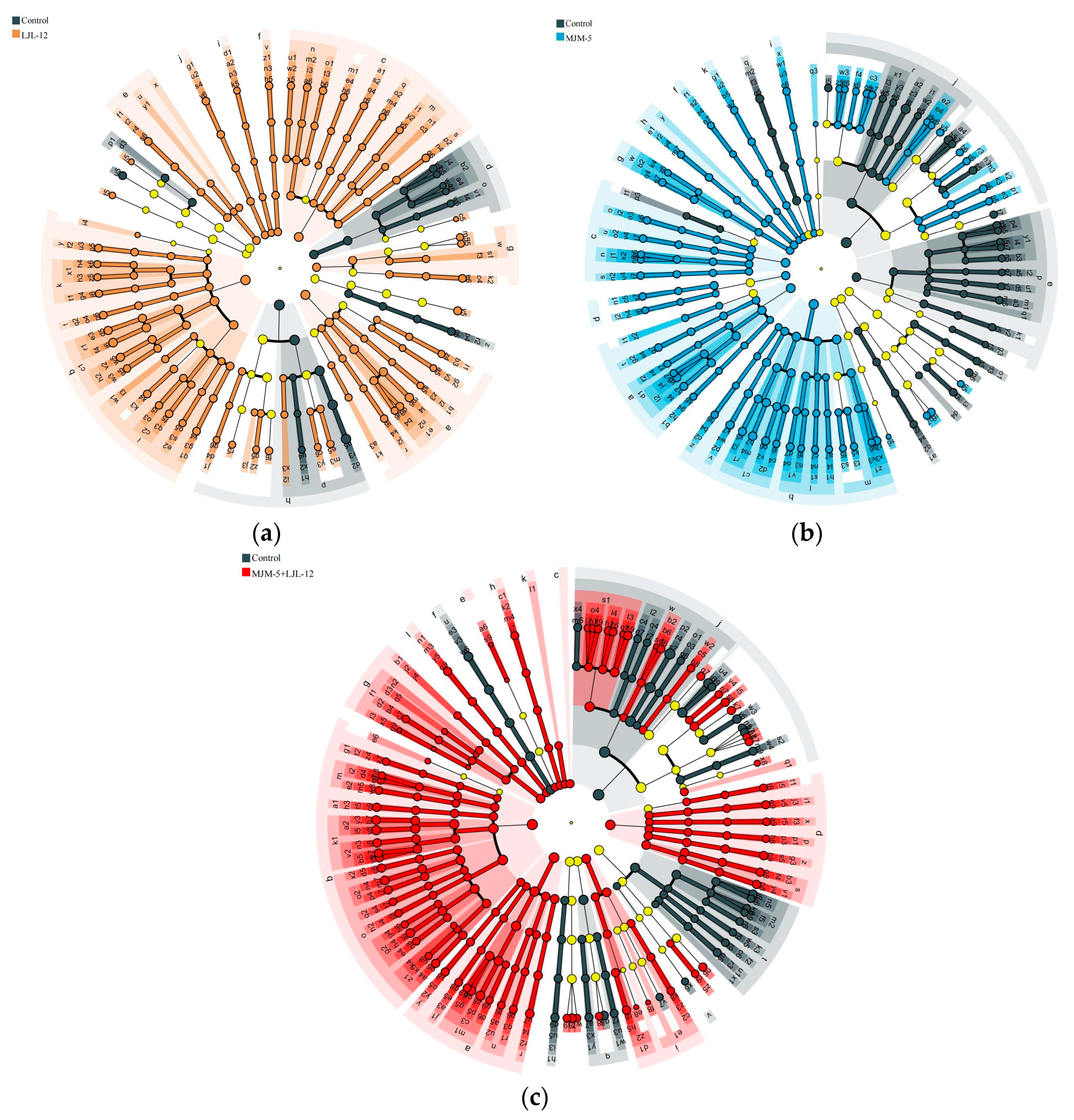
3.4. Bacterial Community Composition in the Rhizosphere Soil of Alfalfa
3.5. Taxonomy of Key Bacteria in the Rhizosphere Soil of Alfalfa
3.6. Soil Physicochemical Properties and Enzyme Activities in the Rhizosphere of Alfalfa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, D.E.; Singh, B.P.; Dalal, R.C. Soil Health Indicators Under Climate Change: A Review of Current Knowledge. In Soil Health and Climate Change; Springer: Berlin/Heidelberg, Germany, 2011; pp. 25–45. [Google Scholar]
- Hirokazu, T.; Tanabe, A.S.; Hirotoshi, S. Network hubs in root-associated fungal metacommunities. Microbiome 2018, 6, 116. [Google Scholar]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Zhu, Y.G.; Chu, H. Biodiversity of key-stone phylotypes determines crop production in a 4-decade fertilization experiment. ISME J. 2021, 15, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; Heijden, M. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef] [Green Version]
- Alkorta, I.; Aizpurua, A.; Riga, P.; Albizu, I.; Amézaga, I.; Garbisu, C. Soil enzyme activities as biological indicators of soil health. Rev. Environ. Health 2003, 18, 65–73. [Google Scholar] [CrossRef]
- Chandini, K.R.; Kumar, R.; Prakash, O. The impact of chemical fertilizers on our environment and ecosystem. In Research Trends in Environmental Sciences, 2nd ed.; AkiNik Publications: New Delhi, India, 2019; Chapter 5; pp. 69–86. [Google Scholar]
- Jimenez-Ballesta, R.; Bravo, S.; Amoros, J.A.; Perez-de-los-Reyes, C.; Garcia-Pradas, J.; Sanchez, M.; Garcia-Navarro, F.J. Occurrence of some rare earth elements in vineyard soils under semiarid Mediterranean environmen. Environ. Monit. Assess. 2021, 194, 341. [Google Scholar]
- Gupta, G.; Snehi, S.K.; Singh, V. Role of PGPR in Biofilm Formations and Its Importance in Plant Health. Biofilms Plant Soil Health 2017, 2, 27–42. [Google Scholar]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; De-Bashan, L.E. How the plant growth-promoting bacterium Azospirillum promotes plant growth—A critical assessment. Adv. Agron. 2010, 108, 77–136. [Google Scholar]
- Liu, J.; Tang, L.; Gao, H.; Zhang, M.; Guo, C. Enhancement of alfalfa yield and quality by plant growth-promoting rhizobacteria under saline-alkali conditions. J. Sci. Food Agric. 2019, 99, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Bechtaoui, N.; Raklami, A.; Benidire, L.; Tahiri A-i Göttfert, M.; Oufdou, K. Effects of PGPR co-inoculation on growth, phosphorus nutrition and phosphatase/phytase activities of faba bean under different phosphorus availability conditions. Pol. J. Environ. Stud. 2020, 29, 1557–1565. [Google Scholar] [CrossRef]
- Chen, L.; Hao, Z.; Li, K.; Sha, Y.; Wang, E.; Sui, X.; Mi, G.; Tian, C.; Chen, W. Effectsof growth-promoting rhizobacteria on maize growth and rhizosphere microbial community under conservation tillage in Northeast China. Microb. Biotechnol. 2020, 14, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, R.L.; Romagnoli, E.M.; Taketani, R.G.; Santos, S.N.; Zucchi, T.D.; Melo, I.S. Impact of Inoculation with Pseudomonas aestus CMAA 1215T on the Non-target Resident Bacterial Community in a Saline Rhizosphere Soil. Curr. Microbiol. 2021, 78, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Kim, H.S.; Sang, M.K.; Song, J.; Weon, H.-Y. Effect of Bacillus mesonae H20-5 treatment on rhizospheric bacterial community of tomato plants under salinity stress. Plant Pathol. J. 2021, 37, 662. [Google Scholar] [CrossRef]
- Ju, W.; Liu, L.; Fang, L.; Cui, Y.; Duan, C.; Wu, H. Impact of co-inoculation with plant-growth-promoting rhizobacteria and rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotoxicol. Environ. Saf. 2019, 167, 218–226. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Nan, X.; Sun, G.; Sun, M.; Cai, D.; Gu, S. Alkalinity and salinity tolerance during seed germination and early seedling stages of three alfalfa (Medicago sativa L.) cultivars. Legume Res. 2017, 40, 853–858. [Google Scholar] [CrossRef] [Green Version]
- Yost, M.A.; Russelle, M.P.; Coulter, J.A.; Bolstad, P.V. Alfalfa stand length and subsequent crop patterns in the upper midwestern USA. Agron. J. 2014, 106, 1697–1708. [Google Scholar] [CrossRef]
- Bremner, J. Total nitrogen. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; The American Society of Agronomy: Madison, WI, USA, 1965; Volume 9, pp. 1149–1178. [Google Scholar]
- Busse, M.D.; Ratcliff, A.W.; Shestak, C.J.; Powers, R.F. Glyphosate toxicity and the effects of long-term vegetation control on soil microbial communities. Soil Biol. Biochem. 2001, 33, 1777–1789. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shidan, B. Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Guan, S.; Zhang, D.; Zhang, Z. Soil Enzyme and Its Research Methods; China Agricultural Press: Beijing, China, 1986; pp. 274–297. [Google Scholar]
- Huse, S.M.; Mark Welch, D.B.; Voorhis, A.; Shipunova, A.; Morrison, H.G.; Eren, A.M.; Sogin, M.L. VAMPS: A website for visualization and analysis of microbial population structures. BMC Bioinform. 2014, 15, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Chang, F.; Xie, P.; Zhang, Y.; Duan, L.; Li, H.; Zhang, X.; Zhang, Y.; Li, D.; Zhang, H. Microbiota assembly patterns and diversity of nine plateau lakes in Yunnan, southwestern China. Chemosphere 2023, 314, 137700. [Google Scholar] [CrossRef]
- Sánchez, A.C.; Gutiérrez, R.T.; Santana, R.C.; Urrutia, A.R.; Fauvart, M.; Michiels, J.; Vanderleyden, J. Effects of co-inoculation of native Rhizobium and Pseudomonas strains on growth parameters and yield of two contrasting Phaseolus vulgaris L. genotypes under Cuban soil conditions. Eur. J. Soil Biol. 2014, 62, 105–112. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Chang, S.X.; Jiang, X.; Song, Y. Biochar increases soil microbial biomass but has variable effects on microbial diversity: A meta-analysis. Sci. Total Environ. 2020, 749, 141593. [Google Scholar] [CrossRef]
- Muñiz, S.; Lacarta, J.; Pata, M.P.; Jiménez, J.; Navarro, E. Analysis of the Diversity of Substrate Utilisation of Soil Bacteria Exposed to Cd and Earthworm Activity Using Generalised Additive Models. PLoS ONE 2014, 9, e85057. [Google Scholar] [CrossRef]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z. Monitoring the changes in a bacterial community in petroleum-polluted soil bioaugmented with hydrocarbon-degrading strains. Appl. Soil Ecol. 2016, 105, 76–85. [Google Scholar] [CrossRef]
- Tribedi, P.; Sil, A.K. Bioaugmentation of polyethylene succinate-contaminated soil with Pseudomonas sp. AKS2 results in increased microbial activity and better polymer degradation. Environ. Sci. Pollut. Res. 2013, 20, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, M.Y.A.; Milani, K.M.L.; Miras-Moreno, B.; Lucini, L.; Valentinuzzi, F.; Mimmo, T.; Pii, Y.; Cesco, S.; Rodrigues, E.P.; de Olivira, A.l.M. Inoculation with plant growth-promoting bacteria alters the rhizosphere functioning of tomato plants. Appl. Soil Ecol. 2021, 158, 103784. [Google Scholar] [CrossRef]
- Campbell, C.; Grayston, S.; Hirst, D. Use of rhizosphere carbon sources in sole carbon source tests to discriminate soil microbial communities. J. Microbiol. Methods 1997, 30, 33–41. [Google Scholar] [CrossRef]
- Wang, H.-W.; Zhu, Y.-X.; Xu, M.; Cai, X.-Y.; Tian, F. Co-application of spent mushroom substrate and PGPR alleviates tomato continuous cropping obstacle by regulating soil microbial properties. Rhizosphere 2022, 23, 100563. [Google Scholar] [CrossRef]
- Beckers, B.; Beeck, M.; Weyens, N.; Boerjan, W.; Vangronsveld, J. Structural variability and niche differentiation in the rhizosphere and endosphere bacterial microbiome of field-grown poplar trees. Microbiome 2017, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, Y.X.; Zhang, N.; Hu, B.; Jin, T.; Xu, H.; Qin, Y.; Yan, P.; Zhang, X.; Guo, X.; et al. NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 2019, 37, 676–684. [Google Scholar] [CrossRef]
- Fu, L.; Penton, C.R.; Ruan, Y.; Shen, Z.; Xue, C.; Li, R.; Shen, Q. Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol. Biochem. 2017, 104, 39–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Shen, Z.; Zhu, C.; Jiao, Z.; Li, R.; Shen, Q. Pre-colonization of PGPR triggers rhizosphere microbiota succession associated with crop yield enhancement. Plant Soil 2019, 439, 553–567. [Google Scholar] [CrossRef]
- Saleem, M.; Hu, J.; Jousset, A. More than the sum of its parts: Microbiome biodiversity as a driver of plant growth and soil health. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 145–168. [Google Scholar] [CrossRef]
- Kielak, A.M.; Cipriano, M.A.; Kuramae, E.E. Acidobacteria strains from subdivision 1 act as plant growth-promoting bacteria. Arch. Microbiol. 2016, 198, 987–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stassinos, P.M.; Rossi, M.; Borromeo, I.; Capo, C.; Beninati, S.; Forni, C. Amelioration of salt stress tolerance in rapeseed (Brassica napus) cultivars by seed inoculation with Arthrobacter globiformis. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2022, 156, 370–383. [Google Scholar] [CrossRef]
- Asaf, S.; Khan, A.L.; Khan, M.A.; Al-Harrasi, A.; Lee, I.-J. Complete genome sequencing and analysis of endophytic Sphingomonas sp. LK11 and its potential in plant growth. 3 Biotech 2018, 8, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinnaswamy, A.; Coba de la Peña, T.; Stoll, A.; de la Peña Rojo, D.; Bravo, J.; Rincón, A.; Lucas, M.M.; Pueyo, J.J. A nodule endophytic Bacillus megaterium strain isolated from Medicago polymorpha enhances growth, promotes nodulation by Ensifer medicae and alleviates salt stress in alfalfa plants. Ann. Appl. Biol. 2018, 172, 295–308. [Google Scholar] [CrossRef]
- Cordovez, V.; Carrion, V.J.; Etalo, D.W.; Mumm, R.; Zhu, H.; Van Wezel, G.P.; Raaijmakers, J.M. Diversity and functions of volatile organic compounds produced by Streptomyces from a disease-suppressive soil. Front. Microbiol. 2015, 6, 1081. [Google Scholar] [CrossRef] [Green Version]
- Palaniyandi, S.; Damodharan, K.; Yang, S.; Suh, J. Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of ‘Micro Tom’tomato plants. J. Appl. Microbiol. 2014, 117, 766–773. [Google Scholar] [CrossRef]
- Patel, P.; Gajjar, H.; Joshi, B.; Krishnamurthy, R.; Amaresan, N. Inoculation of Salt-Tolerant Acinetobacter sp. (RSC9) Improves the Sugarcane (Saccharum sp. Hybrids) Growth Under Salinity Stress Condition. Sugar Tech. 2022, 24, 494–501. [Google Scholar] [CrossRef]
- Caravaca, F.; Figueroa, D.; ROLDÁn, A.; Azcon-Aguilar, C. Alteration in rhizosphere soil properties of afforested Rhamnus lycioides seedlings in short-term response to mycorrhizal inoculation with Glomus intraradices and organic amendment. Environ. Manag. 2003, 31, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Thornton, S.F.; Fenton, O.; Szara, E. Restoration of soil quality using biochar and brown coal waste: A review. Sci. Total Environ. 2020, 722, 137852. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, W.; Shen, J.; Li, S.; Liang, G. Soil quality assessment of Albic soils with different productivities for eastern China. Soil Tillage Res. 2014, 140, 74–81. [Google Scholar] [CrossRef]
- Song, X.; Tao, B.; Guo, J.; Li, J.; Chen, G. Changes in the microbial community structure and soil chemical properties of vertisols under different cropping systems in Northern China. Front. Environ. Sci. 2018, 6, 132. [Google Scholar] [CrossRef] [Green Version]
- Madhaiyan, M.; Poonguzhali, S.; Kang, B.G.; Lee, Y.J.; Chung, J.B.; Sa, T.M. Effect of co-inoculation of methylotrophic Methylobacterium oryzae with Azospirillum brasilense and Burkholderia pyrrocinia on the growth and nutrient uptake of tomato, red pepper and rice. Plant Soil 2010, 328, 71–82. [Google Scholar] [CrossRef]
- Ren, H.; Qin, X.; Huang, B.; Fernández-García, V.; Lv, C. Responses of soil enzyme activities and plant growth in a eucalyptus seedling plantation amended with bacterial fertilizers. Arch. Microbiol. 2020, 202, 1381–1396. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Sergio, S.; Vito, R.; Paolo, R.; Rosa, A.M.; Francesco, S.; Dario, G.; Alfonso, S.F.; Federico, M. Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front. Plant Sci. 2015, 6, 815. [Google Scholar]


| Treatment | Optimized Sequence | Effective Sequence | Base Number | Mean Length |
|---|---|---|---|---|
| Control | 32,756.667 | 19,693.333 | 12,972,184.670 | 396.014 |
| LJL-12 | 41,055.333 | 20,677.333 | 16,285,595.330 | 396.643 |
| MJM-5 | 43,178.000 | 32,247.000 | 16,557,306.000 | 383.500 |
| MJM-5+LJL-12 | 44,978.667 | 30,579.000 | 17,567,318.000 | 390.174 |
| Treatment | MC (%) | BD (g·cm−3) | OM (g·kg−1) | AN (mg·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) |
|---|---|---|---|---|---|---|
| Control | 20.5 ± 1.9 c | 1.3 ± 0.1 a | 29.1 ± 1.1 c | 119.6 ± 3.6 c | 27.1 ± 2.8 c | 202.8 ± 10.2 c |
| LJL-12 | 23.8 ± 1.1 b | 1.2 ± 0.1 b | 32.6 ± 1.4 b | 140.6 ± 4.4 b | 32.8 ± 2.2 b | 231.6 ± 10.2 b |
| MJM-5 | 23.9 ± 1.3 b | 1.2 ± 0.1 b | 31.7 ± 0.9 b | 142.3 ± 3.6 b | 33.4 ± 2.7 b | 234.4 ± 9.4 b |
| MJM-5+LJL-12 | 26.4 ± 0.9 a | 1.2 ± 0.1 b | 34.9 ± 0.8 a | 150.5 ± 3.5 a | 38.0 ± 1.7 a | 255.4 ± 9.4 a |
| Treatment | Urease Activity mg (NH3-N)·g−1·24 h−1 | Sucrase Activity mg·g−1·24 h−1 | Dehydrogenase Activity mg·TPF·g−1·24 h−1 | Protease Activity NH2-N mg·g−1·24 h−1 |
|---|---|---|---|---|
| Control | 16.0 ± 1.1 c | 3.1 ± 0.1 c | 2.0 ± 0.1 c | 5.5 ± 0.2 c |
| LJL-12 | 18.7 ± 1.1 b | 3.6 ± 0.1 b | 2.2 ± 0.1 b | 6.1 ± 0.2 b |
| MJM-5 | 18.9 ± 1.3 b | 3.6 ± 0.1 b | 2.2 ± 0.1 b | 6.2 ± 0.2 b |
| MJM-5+LJL-12 | 21.3 ± 0.8 a | 3.9 ± 0.1 a | 2.4 ± 0.1 a | 6.6 ± 0.2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Shi, Y.; Zhang, Y.; Yang, D.; Guo, C. Effects of Plant-Growth-Promoting Rhizobacteria on Soil Bacterial Community, Soil Physicochemical Properties, and Soil Enzyme Activities in the Rhizosphere of Alfalfa under Field Conditions. Diversity 2023, 15, 537. https://doi.org/10.3390/d15040537
Tang L, Shi Y, Zhang Y, Yang D, Guo C. Effects of Plant-Growth-Promoting Rhizobacteria on Soil Bacterial Community, Soil Physicochemical Properties, and Soil Enzyme Activities in the Rhizosphere of Alfalfa under Field Conditions. Diversity. 2023; 15(4):537. https://doi.org/10.3390/d15040537
Chicago/Turabian StyleTang, Lu, Yimeng Shi, Yilu Zhang, Dihe Yang, and Changhong Guo. 2023. "Effects of Plant-Growth-Promoting Rhizobacteria on Soil Bacterial Community, Soil Physicochemical Properties, and Soil Enzyme Activities in the Rhizosphere of Alfalfa under Field Conditions" Diversity 15, no. 4: 537. https://doi.org/10.3390/d15040537
APA StyleTang, L., Shi, Y., Zhang, Y., Yang, D., & Guo, C. (2023). Effects of Plant-Growth-Promoting Rhizobacteria on Soil Bacterial Community, Soil Physicochemical Properties, and Soil Enzyme Activities in the Rhizosphere of Alfalfa under Field Conditions. Diversity, 15(4), 537. https://doi.org/10.3390/d15040537





