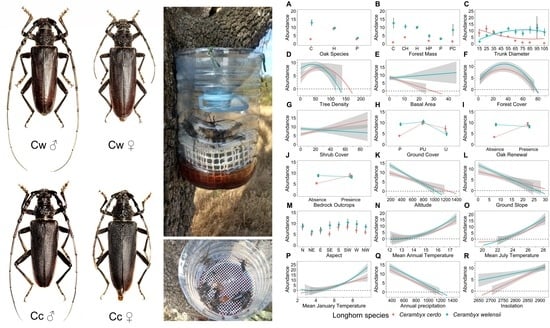
Cerambyx cerdo and Cerambyx welensii Oak-Living Sympatric Populations Exhibit Species-Specific Responses to Face Ecological Factors in the Wild
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Species
2.3. Abundance Estimates: Feeding Traps
2.4. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Médail, F.; Quézel, P. Biodiversity hotspots in the Mediterranean Basin: Setting global conservation priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Blondel, J. The ‘design’ of Mediterranean landscapes: A millennial story of humans and ecological systems during the historic period. Hum. Ecol. 2006, 34, 713–729. [Google Scholar] [CrossRef]
- Moreno, G.; Pulido, F.J. The functioning, management and persistence of dehesas. In Agroforestry in Europe; Rigueiro-Rodríguez, A., McAdam, J., Mosquera-Losada, M.R., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 127–160. [Google Scholar] [CrossRef]
- Bugalho, M.N.; Caldeira, M.C.; Pereira, J.S.; Aronson, J.; Pausas, J.G. Mediterranean cork oak savannas require human use to sustain biodiversity and ecosystem services. Front. Ecol. Environ. 2011, 9, 278–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Hernández, A.; Micó, E.; Marcos-García, M.A.; Brustel, H.; Galante, E. The “dehesa”, a key ecosystem in maintaining the diversity of Mediterranean saproxylic insects (Coleoptera and Diptera: Syrphidae). Biodivers. Conserv. 2014, 23, 2069–2087. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Mendiola-Díaz, F.J.; López, R.; Sánchez, Á.; Ponce, F.; Fernández, F.; Zugasti, C.; de-Juan, J.M.; Echevarría-León, E.; Cáceres, Y.; et al. Distribución actualizada del género Cerambyx Linnaeus, 1758 (Coleoptera: Cerambycidae) en Extremadura: Desde los registros históricos al muestreo a escala regional. Graellsia 2022, 78, e169. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Mendiola-Díaz, F.J.; Moral-García, F.J.; Canelo, T. Large-scale geostatistical mapping and occupancy-abundance patterns of Cerambyx species threatening SW Spain oak forests. Eur. J. For. Res. 2022, 141, 1045–1057. [Google Scholar] [CrossRef]
- CEC [Council of the European Communities]. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora [Habitats Directive]. Off. J. Eur. Communities 1992, 35, 7–50. [Google Scholar]
- Thomas, F.M.; Blank, R.; Hartmann, G. Abiotic and biotic factors and their Interactions as causes of oak decline in Central Europe. For. Pathol. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent insects, pathogens and drought shape changing patterns in oak decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205. [Google Scholar] [CrossRef]
- Manion, P.D. Evolution of concepts in forest pathology. Phytopathology 2003, 93, 1052–1055. [Google Scholar] [CrossRef] [Green Version]
- Torres-Vila, L.M.; Echave-Sanabria, A.C.; Mendiola-Díaz, F.J.; Moral-García, F.J. Mapping oak shoot browning in SW Spain using online imagery as virtual prospecting tool. Ann. For. Sci 2019, 76, 32. [Google Scholar] [CrossRef] [Green Version]
- Evans, H.F.; Moraal, L.G.; Pajares, J.A. Biology, ecology and economic importance of Buprestidae and Cerambycidae. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Lieutier, F., Day, K.R., Battisti, A., Grégoire, J.-C., Evans, H.F., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 447–474. [Google Scholar] [CrossRef]
- Sallé, A.; Nageleisen, L.M.; Lieutier, F. Bark and wood boring insects involved in oak declines in Europe: Current knowledge and future prospects in a context of climate change. For. Ecol. Manag. 2014, 328, 79–93. [Google Scholar] [CrossRef]
- Picard, F. Coléoptères: Cerambycidae. Faune de France 20; P. Lechevalier: Paris, France, 1929; 166p. [Google Scholar]
- Villiers, A. Faune des Coléoptères de France 1. Cerambycidae. Encyclopédie Entomologique 42; Lechevalier: Paris, France, 1978; 612p. [Google Scholar]
- Vives, E. Coleoptera Cerambycidae. Fauna Ibérica 12; Museo Nacional de Ciencias Naturales (CSIC): Madrid, Spain, 2000; 715p. [Google Scholar]
- Miroshnikov, A.I. Review of the longicorn beetles genus Cerambyx Linnaeus, 1758 (Coleoptera, Cerambycidae) of the Caucasus [in Russian]. Moscow State For. Univ. Bull. (Lesnoy Vestnik) 2009, 5, 43–55. [Google Scholar]
- Escherich, K. Die Forstinsekten Mitteleuropas 2; P. Parey: Berlin, Germany, 1923; 663p. [Google Scholar]
- Buse, J.; Schröder, B.; Assmann, T. Modelling habitat and spatial distribution of an endangered longhorn beetle–a case study for saproxylic insect conservation. Biol. Conserv. 2007, 137, 281–372. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Zugasti-Martínez, C.; Mendiola-Díaz, F.J.; De-Juan-Murillo, J.M.; Sánchez-González, Á.; Conejo-Rodríguez, Y.; Ponce-Escudero, F.; Fernández-Moreno, F. Larval assemblages of large saproxylic cerambycids in Iberian oak forests: Wood quality and host preference shape resource partitioning. Pop. Ecol. 2017, 59, 315–328. [Google Scholar] [CrossRef]
- Speight, M.C.D. Saproxylic Invertebrates and Their Conservation. Nature and Environment Series 42; Council of Europe: Strasbourg, France, 1989; 79p. [Google Scholar]
- Grove, S.J. Saproxylic insect ecology and the sustainable management of forests. Annu. Rev. Ecol. Syst. 2002, 33, 1–23. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Buse, J.; Ranius, T.; Assmann, T. An endangered longhorn beetle associated with old oaks and its possible role as an ecosystem engineer. Conserv. Biol. 2008, 22, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Saint-Germain, M.; Drapeau, P.; Buddle, C.M. Host-use patterns of saproxylic phloeophagous and xylophagous Coleoptera adults and larvae along the decay gradient in standing dead black spruce and aspen. Ecography 2007, 30, 737–748. [Google Scholar] [CrossRef]
- Davies, Z.G.; Tyler, C.; Stewart, G.B.; Pullin, A.S. Are current management recommendations for saproxylic invertebrates effective? A systematic review. Biodivers. Conserv. 2008, 17, 209–234. [Google Scholar] [CrossRef] [Green Version]
- Regnery, B.; Paillet, Y.; Couvet, D.; Kerbiriou, C. Which factors influence the occurrence and density of tree microhabitats in Mediterranean oak forests? For. Ecol. Manag. 2013, 295, 118–125. [Google Scholar] [CrossRef]
- Micó, E.; García-López, A.; Sánchez, A.; Juárez, M.; Galante, E. What can physical, biotic and chemical features of a tree hollow tell us about their associated diversity? J. Insect Conserv. 2015, 19, 141–153. [Google Scholar] [CrossRef]
- Martín, J.; Cabezas, J.; Buyolo, T.; Patón, D. The relationship between Cerambyx spp. damage and subsequent Biscogniauxia mediterranum infection on Quercus suber forests. For. Ecol. Manag. 2005, 216, 166–174. [Google Scholar] [CrossRef]
- Domínguez, L.; López-Pantoja, G.; Cremades, D.; Paramio, A.; Hidalgo, P.J.; Sánchez-Osorio, I. Incidence of large wood borers in the conservation of dehesa islands forests in southwestern Spain. Forests 2022, 13, 413. [Google Scholar] [CrossRef]
- Duque-Lazo, J.; Navarro-Cerrillo, R.M. What to save, the host or the pest? The spatial distribution of xylophage insects within the Mediterranean oak woodlands of Southwestern Spain. For. Ecol. Manag. 2017, 392, 90–104. [Google Scholar] [CrossRef]
- Torres-Vila, L.M. Reproductive biology of the great capricorn beetle, Cerambyx cerdo (Coleoptera: Cerambycidae): A protected but occasionally harmful species. Bull. Entomol. Res. 2017, 107, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vila, L.M.; Bonal, R. DNA barcoding of large oak-living cerambycids: Diagnostic tool, phylogenetic insights and natural hybridization between Cerambyx cerdo and Cerambyx welensii (Coleoptera: Cerambycidae). Bull. Entomol. Res. 2019, 109, 583–594. [Google Scholar] [CrossRef]
- Schoener, T.W. Resource partitioning in ecological communities. Science 1974, 185, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, A.A.; Ackerly, D.D.; Adler, F.R.; Arnold, A.E.; Cáceres, C.; Doak, D.F.; Post, E.; Hudson, P.J.; Maron, J.; Mooney, K.A.; et al. Filling key gaps in population and community ecology. Front. Ecol. Environ. 2007, 5, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Yoshida, T.; Koyama, S.; Yamagami, A.; Takata, M.; Doi, H.; Kurachi, T.; Hayashi, S.; Hirobe, T.; Hata, Y. Resource partitioning based on body size contributes to the species diversity of wood-boring beetles and arboreal nesting ants. Insect Conserv. Divers. 2016, 9, 4–12. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; López-Calvo, R.; Sánchez-González, Á.; Mendiola-Díaz, F.J. Ecology of Oobius rudnevi, egg parasitoid of Cerambyx cerdo and Cerambyx welensii in oak forests. Entomol. Exp. Appl. 2021, 169, 646–656. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- AEMET. Guía Resumida del Clima en España (1981–2010), AEMET [Agencia Estatal de Meteorología]. Available online: https://www.aemet.es/es/conocermas/recursos_en_linea/publicaciones_y_estudios/publicaciones/detalles/guia_resumida_2010 (accessed on 15 January 2023).
- Torres-Vila, L.M.; Sánchez-González, Á.; Ponce-Escudero, F.; Martín-Vertedor, D.; Ferrero-García, J.J. Assessing mass trapping efficiency and population density of Cerambyx welensii Küster by mark-recapture in dehesa open woodlands. Eur. J. For. Res. 2012, 131, 1103–1116. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Sánchez-González, Á.; Merino-Martínez, J.; Ponce-Escudero, F.; Conejo-Rodríguez, Y.; Martín-Vertedor, D.; Ferrero-García, J.J. Mark-recapture of Cerambyx welensii in dehesa woodlands: Dispersal behaviour, population density, and mass trapping efficiency with low trap densities. Entomol. Exp. Appl. 2013, 149, 273–281. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Mendiola-Díaz, F.J.; Conejo-Rodríguez, Y.; Sánchez-González, Á. Reproductive traits and number of matings in males and females of Cerambyx welensii (Coleoptera: Cerambycidae) an emergent pest of oaks. Bull. Entomol. Res. 2016, 106, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vila, L.M.; Mendiola-Díaz, F.J.; Sánchez-González, Á. Dispersal differences of a pest and a protected Cerambyx species (Coleoptera: Cerambycidae) in oak open woodlands: A mark–recapture comparative study. Ecol. Entomol. 2017, 42, 18–32. [Google Scholar] [CrossRef]
- Drag, L.; Cižek, L. Radio-tracking suggests high dispersal ability of the great capricorn beetle (Cerambyx cerdo). J. Insect Behav. 2018, 31, 138–143. [Google Scholar] [CrossRef]
- MITECO. Mapa Forestal de España (MFE) de máxima actualidad [Extremadura], MITECO [Ministerio para la Transición Ecológica y el Reto Demográfico]. Available online: https://www.miteco.gob.es/es/cartografia-y-sig/ide/descargas/biodiversidad/mfe.aspx (accessed on 15 January 2023).
- CNIG-IGN. MDT05: Modelo Digital del Terreno con Paso de Malla 5m (SRG: ETRS89), CNIG-IGN [Centro Nacional de Información Geográfica, Instituto Geográfico Nacional]. Available online: http://centrodedescargas.cnig.es/CentroDescargas/catalogo.do?Serie=LIDAR (accessed on 15 January 2023).
- Brooks, M.E.; Kristensen, K.; Darrigo, M.R.; Rubim, P.; Uriarte, M.; Bruna, E.; Bolker, B.M. Statistical modeling of patterns in annual reproductive rates. Ecology 2019, 100, e02706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; 574p. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; 2630p. [Google Scholar]
- Southwood, T.R.E. Ecological Methods with Particular Reference to the Study of Insect Populations; Chapman and Hall: London, UK, 1978; 524p. [Google Scholar]
- Seber, G. The Estimation of Animal Abundance; Macmillan Publishing Co.: New York, NY, USA, 1982; 654p. [Google Scholar]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Modell. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Rudnev, D.F. Der grosse Eichenbock, Cerambyx cerdo L., seine Lebensweise, wirtschaftliche Bedeutung und Bekämpfung. Z. Angew. Entomol. 1936, 22, 61–96. [Google Scholar] [CrossRef]
- Heyrovský, L. Fauna ČSR, Svazek 5. Tesaříkovití- Cerambycidae (Řád brouci.—Coleoptera); Nakladatelství Československé Akademie: Prague, Czech Republic, 1955; 346p. [Google Scholar]
- Săvulescu, N. Cîteva observații ecologice asupra speciilor din Republica Socialistă România ale genului Cerambyx L. (Col. Cerambycidae). In Comunicări de Zoologie, Prima Consfătuire Naţională de Entomologie, II-a; Societatea de Ştiinţe Biologice din RSR: Bucureşti, Romania, 1969; pp. 281–290. [Google Scholar]
- Strojny, W. Badania nad biologia kozioroga debosza, Cerambyx cerdo L. (Coleoptera, Cerambycidae) zasiedlajacego deby szypulkowe, Quercus robur L. na Swojcu iw Wilczycach pod Wroclawiem w latach 1973–1976. Pol. Pis. Entomol. 1977, 47, 727–746. [Google Scholar]
- Albert, J.; Platek, M.; Cižek, L. Vertical stratification and microhabitat selection by the great capricorn beetle (Cerambyx cerdo) (Coleoptera: Cerambycidae) in open-grown, veteran oaks. Eur. J. Entomol. 2012, 109, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Platek, M.; Sebek, P.; Hauck, D.; Cižek, L. When is a tree suitable for a veteran tree specialist? Variability in the habitat requirements of the great capricorn beetle (Cerambyx cerdo) (Coleoptera: Cerambycidae). Eur. J. Entomol. 2019, 116, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Valbuena-Carabaña, M.; Gil, L. Centenary coppicing maintains high levels of genetic diversity in a root resprouting oak (Quercus pyrenaica Willd.). Tree Genet. Genomes 2017, 13, 28. [Google Scholar] [CrossRef]
- May, M.R.; Provance, M.C.; Sanders, A.C.; Ellstrand, N.C.; Ross-Ibarra, J. A Pleistocene clone of Palmer’s oak persisting in southern California. PLoS ONE 2009, 4, e8346. [Google Scholar] [CrossRef] [PubMed]
- Heggenstaller, D.J.; Zenner, E.K.; Brose, P.H.; Peck, J.E. How much older are Appalachian oaks below-ground than above-ground? North. J. Appl. For. 2012, 29, 155–157. [Google Scholar] [CrossRef]
- Ratzeburg, J.T.C. Die Waldverderber und ihre Feinde: Ein Handbuch für Forstmänner, Landwirthe, Gärtner und alle mit Waldbäumen Beschäftigte; Nicolaische Verlags-Buchhandlung: Berlin, Germany, 1876; 523p. [Google Scholar]
- López-Pantoja, G.; Domínguez Nevado, L.; Sánchez-Osorio, I. Mark-recapture estimates of the survival and recapture rates of Cerambyx welensii Küster (Coleoptera cerambycidae) in a cork oak dehesa in Huelva (Spain). Cent. Eur. J. Biol. 2008, 3, 431–441. [Google Scholar] [CrossRef]
- Reitter, E. Fauna Germanica. Die Käfer des Deutschen Reiches 4; K.G. Lutz: Stuttgard, Germany, 1912; 236p. [Google Scholar]
- Vodka, S.; Konvicka, M.; Cižek, L. Habitat preferences of oak-feeding xylophagous beetles in a temperate woodland: Implications for forest history and management. J. Insect. Conserv. 2009, 13, 553–562. [Google Scholar] [CrossRef]
- Starzyk, J.R. Kozioróg dębosz Cerambyx cerdo L. w Puszczy Niepołomickiej. Chrońmy Przyr. Ojczystą 1973, 29, 22–30. [Google Scholar]
- Strojny, W. Sędziwe dęby czy kozioróg dębosz? Wszechświat 1981, 1981, 32–36. [Google Scholar]
- Mulsant, É. Histoire Naturelle des Coléoptères de France: Longicornes; Magnin, Blanchard et Cie.: Paris, France, 1862–1863; 590p. [Google Scholar]
- Gardiner, L.M. Survival of wood borers (Coleoptera: Cerambycidae, Buprestidae) in water-stored logs. For. Chron. 1962, 38, 459–462. [Google Scholar] [CrossRef]
- Hanks, L.M.; Millar, J.G.; Paine, T.D. Evaluation of cold temperatures and density as mortality factors of the Eucalyptus Longhorned Borer (Coleoptera: Cerambycidae) in California. Environ. Entomol. 1991, 20, 1653–1658. [Google Scholar] [CrossRef] [Green Version]
- Morales-Rodríguez, C.; Sánchez-González, Á.; Conejo-Rodríguez, Y.; Torres-Vila, L.M. First record of Beauveria bassiana (Balsamo) Vuillemin (Ascomycota: Clavicipitaceae) infecting Cerambyx welensii (Coleoptera: Cerambycidae) and pathogenicity tests using a new bioassay method. Biocontrol Sci. Technol. 2015, 25, 1213–1219. [Google Scholar] [CrossRef]
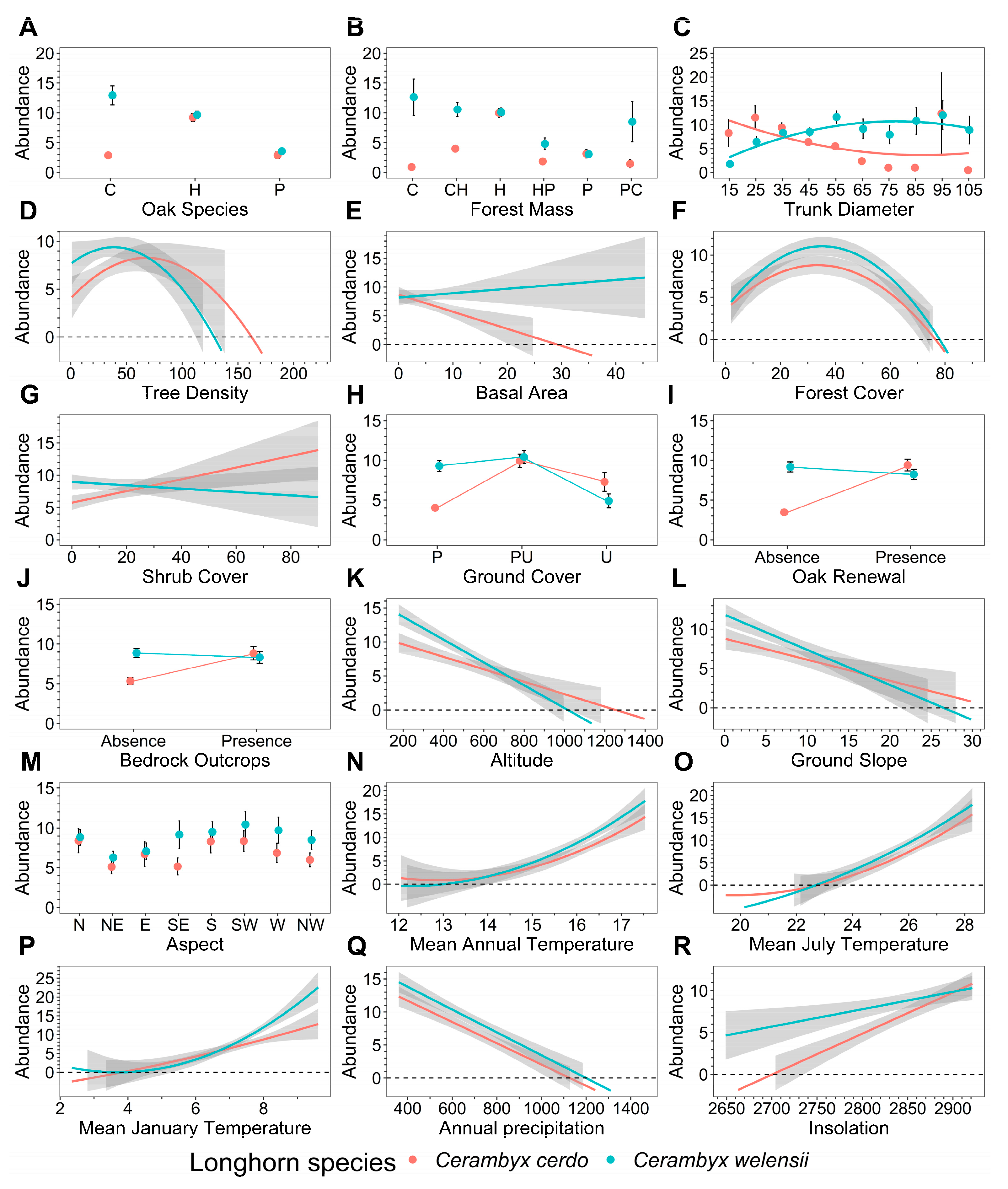
| Ref. | Predictor Variable | Variable Nature 1 | Variable Type 2 | Classes/[Units]/Definition | Data Souce 3 |
|---|---|---|---|---|---|
| 1 | Oak Species | B | C | n = 3 classes: holm oak (H), cork oak (C), pyrenean oak (P) [main species in the forest mass] | t.s. |
| 2 | Forest Mass | B | C | n = 6 classes: holm oak pure mass (H), cork oak pure mass (C), pyrenean oak pure mass (P), cork-holm oak mixed mass (CH), holm-pyrenean oak mixed mass (HP), pyrenean-cork oak mixed mass (PC) | t.s. |
| 3 | Trunk Diameter | B | SQ | n = 10 classes (10-cm DBH classes): from 10–20 cm (young oaks) to 100–110 cm (old/veteran oaks); DBH: Diameter at Breast Height [cm] | t.s. |
| 4 | Tree Density | B | Q | [trees ha−1] | t.s. |
| 5 | Basal Area | B | Q | [m2 ha−1], cross-sectional area of trees at breast height in square meters per ha | t.s. |
| 6 | Forest Cover | B | Q | [%], surface covered by tree canopies per surface unit (=crown cover) | [49] |
| 7 | Shrub Cover | B | Q | [%], surface covered by shrubs per surface unit | [49] |
| 8 | Ground Cover | B | C | n = 3 classes: pastureland (P), pastureland-undergrowth (PU), undergrowth (U) | t.s. |
| 9 | Oak Renewal | B | C | n = 2 classes: presence or absence of oak root resprouting | t.s. |
| 10 | Bedrock Outcrops | A | C | n = 2 classes: presence or absence | t.s. |
| 11 | Altitude | A | Q | [m], elevation in meters above sea level | [50] |
| 12 | Ground Slope | A | Q | [%] | [50] |
| 13 | Aspect | A | C | n = 8 classes: N, NE, E, SE, S, SW, W, NW | [50] |
| 14 | Mean Annual Temperature | A | Q | Tm [°C] | [43] |
| 15 | Mean July Temperature | A | Q | Tm7 [°C], mean temperature of the warmest month | [43] |
| 16 | Mean January Temperature | A | Q | Tm1 [°C], mean temperature of the coldest month | [43] |
| 17 | Annual Precipitation | A | Q | P [mm], mean annual precipitation | [43] |
| 18 | Insolation | A | Q | [h], mean number of sunshine hours per year | [43] |
| Ref. | Predictor Variable 1 | GLMM 2 | Cerambyx Species Effect | Predictor Effect [Quadratic Effect] 1 | Predictor x Species Effect | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chi2 | df | p | Chi2 | df | p | Chi2 | df | p | |||
| 1 | Oak Species | GP | 59.71 | 1 | <0.001 | 168.12 | 2 | <0.001 | 97.00 | 2 | <0.001 |
| 2 | Forest Mass | P | 39.56 | 1 | <0.001 | 195.50 | 5 | <0.001 | 96.46 | 5 | <0.001 |
| 3 | Trunk Diameter | P | 43.54 | 1 | <0.001 | 5.91 | 1 | <0.05 | 114.58 | 1 | <0.001 |
| 4 | Tree Density | P | 50.31 | 1 | <0.001 | 7.51 | 1 | <0.01 | 11.57 | 1 | <0.001 |
| Tree Density ^2 | [18.18] | 1 | [<0.001] | ||||||||
| 5 | Basal Area | GP | 62.75 | 1 | <0.001 | 0.08 | 1 | 0.77 ns | 64.76 | 1 | <0.001 |
| 6 | Forest Cover | P | 50.46 | 1 | <0.001 | 40.44 | 1 | <0.001 | 4.79 | 1 | <0.05 |
| Forest Cover ^2 | [57.51] | 1 | [<0.001] | ||||||||
| 7 | Shrub Cover | P | 50.47 | 1 | <0.001 | 2.48 | 1 | 0.12 ns | 5.10 | 1 | <0.05 |
| 8 | Ground Cover | GP | 61.98 | 1 | <0.001 | 66.70 | 2 | <0.001 | 55.94 | 2 | <0.001 |
| 9 | Oak Renewal | GP | 64.09 | 1 | <0.001 | 0.11 | 1 | 0.74 ns | 109.54 | 1 | <0.001 |
| 10 | Bedrock Outcrops | GP | 61.72 | 1 | <0.001 | 2.06 | 1 | 0.15 ns | 22.93 | 1 | <0.001 |
| 11 | Altitude | P | 50.58 | 1 | <0.001 | 251.51 | 1 | <0.001 | 0.05 | 1 | 0.83 ns |
| 12 | Ground Slope | GP | 60.22 | 1 | <0.001 | 113.10 | 1 | <0.001 | 1.30 | 1 | 0.25 ns |
| 13 | Aspect | GP | 60.90 | 1 | <0.001 | 9.10 | 7 | 0.25 ns | 5.43 | 7 | 0.25 ns |
| 14 | Mean Annual Temp. | P | 50.37 | 1 | <0.001 | 11.94 | 1 | <0.001 | 5.56 | 1 | <0.05 |
| Mean Annual Temp. ^2 | [7.20 | 1 | <0.01] | ||||||||
| 15 | Mean July Temp. | P | 50.33 | 1 | <0.001 | 30.64 | 1 | <0.001 | 7.24 | 1 | <0.01 |
| Mean July Temp. ^2 | [27.22 | 1 | <0.001] | ||||||||
| 16 | Mean January Temp. | P | 50.62 | 1 | <0.001 | 42.89 | 1 | <0.001 | 0.31 | 1 | 0.58 ns |
| Mean January Temp. ^2 | [22.58 | 1 | <0.001] | ||||||||
| 17 | Annual Precipitation | P | 46.52 | 1 | <0.001 | 341.39 | 1 | <0.001 | 21.55 | 1 | <0.001 |
| 18 | Insolation | P | 48.33 | 1 | <0.001 | 79.49 | 1 | <0.001 | 36.08 | 1 | <0.001 |
| Model | Cerambyx Species | |||||||
|---|---|---|---|---|---|---|---|---|
| C. cerdo | C. welensii | |||||||
| Effects | Chi2 | df | p | Effects | Chi2 | df | p | |
| Biotic | Forest Mass | 170.19 | 5 | <0.001 | Forest Mass | 78.70 | 5 | <0.001 |
| Trunk Diameter | 12.61 | 1 | <0.001 | Trunk Diameter | 46.64 | 1 | <0.001 | |
| Tree Density | 11.94 | 1 | <0.001 | Tree Density | 4.10 | 1 | <0.05 | |
| Forest Cover | 2.24 | 1 | 0.14 ns | Forest Cover | 58.39 | 1 | <0.001 | |
| Ground Cover | 10.75 | 2 | <0.01 | Forest Cover ^2 | 38.06 | 1 | <0.001 | |
| Oak Renewal | 21.50 | 1 | <0.001 | Ground Cover | 13.32 | 2 | <0.01 | |
| Forest Mass x Trunk Diameter | 18.70 | 5 | <0.01 | Forest Mass x Forest Cover | 16.76 | 5 | <0.01 | |
| Forest Mass x Forest Cover | 33.98 | 5 | <0.001 | Forest Mass x Ground Cover | 24.47 | 10 | <0.01 | |
| Forest Mass x Ground Cover | 34.27 | 10 | <0.001 | Trunk Diameter x Ground Cover | 6.99 | 2 | <0.05 | |
| Forest Mass x Oak Renewal | 18.98 | 5 | <0.01 | Forest Cover x Ground Cover | 5.99 | 2 | <0.05 | |
| Forest Cover x Ground Cover | 8.54 | 2 | <0.05 | |||||
| Abiotic | Bedrock Outcrops | 42.71 | 1 | <0.001 | Bedrock Outcrops | 3.34 | 1 | ~0.05 |
| Altitude | 11.95 | 1 | <0.001 | Altitude | 32.20 | 1 | <0.001 | |
| Aspect | 9.44 | 7 | 0.23 ns | Ground Slope | 6.25 | 1 | <0.05 | |
| Annual Precipitation | 47.65 | 1 | <0.001 | Aspect | 7.43 | 7 | 0.39 ns | |
| Insolation | 14.30 | 1 | <0.001 | Annual Precipitation | 34.44 | 1 | <0.001 | |
| Bedrock Outcrops x Altitude | 5.70 | 1 | <0.05 | Bedrock Outcrops x Altitude | 5.70 | 1 | <0.05 | |
| Aspect x Annual Precipitation | 21.01 | 7 | <0.01 | Bedrock Out. x Annual Prec. | 9.99 | 1 | <0.01 | |
| Altitude x Aspect | 19.24 | 7 | <0.01 | |||||
| Pooled | Forest Mass | 33.38 | 5 | <0.001 | Forest Mass | 52.33 | 5 | <0.001 |
| Trunk Diameter | 5.25 | 1 | <0.05 | Trunk Diameter | 47.95 | 1 | <0.001 | |
| Tree Density | 13.08 | 1 | <0.001 | Tree Density | 3.42 | 1 | ~0.05 | |
| Forest Cover | 0.11 | 1 | 0.74 ns | Forest Cover | 59.96 | 1 | <0.001 | |
| Ground Cover | 13.50 | 2 | <0.01 | Forest Cover^2 | 33.06 | 1 | <0.001 | |
| Oak Renewal | 16.22 | 1 | <0.001 | Ground Cover | 9.36 | 2 | <0.01 | |
| Bedrock Outcrops | 13.58 | 1 | <0.001 | Bedrock Outcrops | 2.90 | 1 | ~0.05 | |
| Aspect | 5.74 | 7 | 0.57 ns | Altitude | 26.72 | 1 | <0.001 | |
| Annual Precipitation | 113.22 | 1 | <0.001 | Annual Precipitation | 52.14 | 1 | <0.001 | |
| Forest Mass x Trunk Diameter | 16.67 | 5 | <0.01 | Forest Mass x Forest Cover | 10.61 | 5 | ~0.05 | |
| Forest Mass x Forest Cover | 21.01 | 5 | <0.001 | Trunk Diameter x Ground Cover | 6.04 | 2 | <0.05 | |
| Forest Cover x Ground Cover | 13.95 | 2 | <0.001 | Forest Cover x Ground Cover | 11.11 | 2 | <0.01 | |
| Aspect x Annual Precipitation | 19.40 | 7 | <0.01 | Bedrock Outcrops x Altitude | 4.78 | 1 | <0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Vila, L.M.; Mendiola-Díaz, F.J.; Canelo, T. Cerambyx cerdo and Cerambyx welensii Oak-Living Sympatric Populations Exhibit Species-Specific Responses to Face Ecological Factors in the Wild. Diversity 2023, 15, 545. https://doi.org/10.3390/d15040545
Torres-Vila LM, Mendiola-Díaz FJ, Canelo T. Cerambyx cerdo and Cerambyx welensii Oak-Living Sympatric Populations Exhibit Species-Specific Responses to Face Ecological Factors in the Wild. Diversity. 2023; 15(4):545. https://doi.org/10.3390/d15040545
Chicago/Turabian StyleTorres-Vila, Luis M., F. Javier Mendiola-Díaz, and Tara Canelo. 2023. "Cerambyx cerdo and Cerambyx welensii Oak-Living Sympatric Populations Exhibit Species-Specific Responses to Face Ecological Factors in the Wild" Diversity 15, no. 4: 545. https://doi.org/10.3390/d15040545