Obtaining Sustainable Population Structures for the Management of Red Deer
Abstract
:1. Introduction
2. Materials and Methods
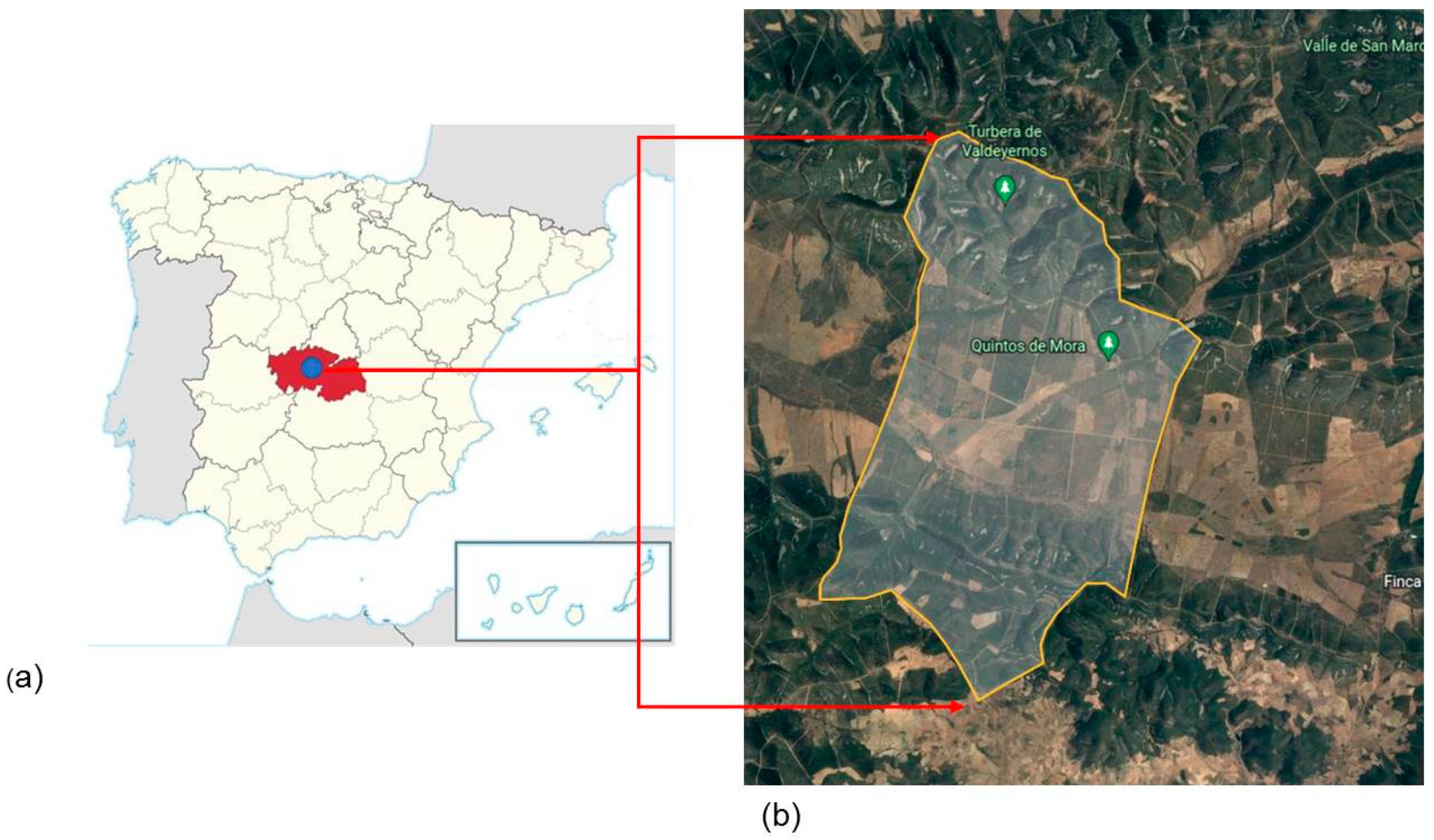
2.1. The Study Area
2.2. The Model
3. Results
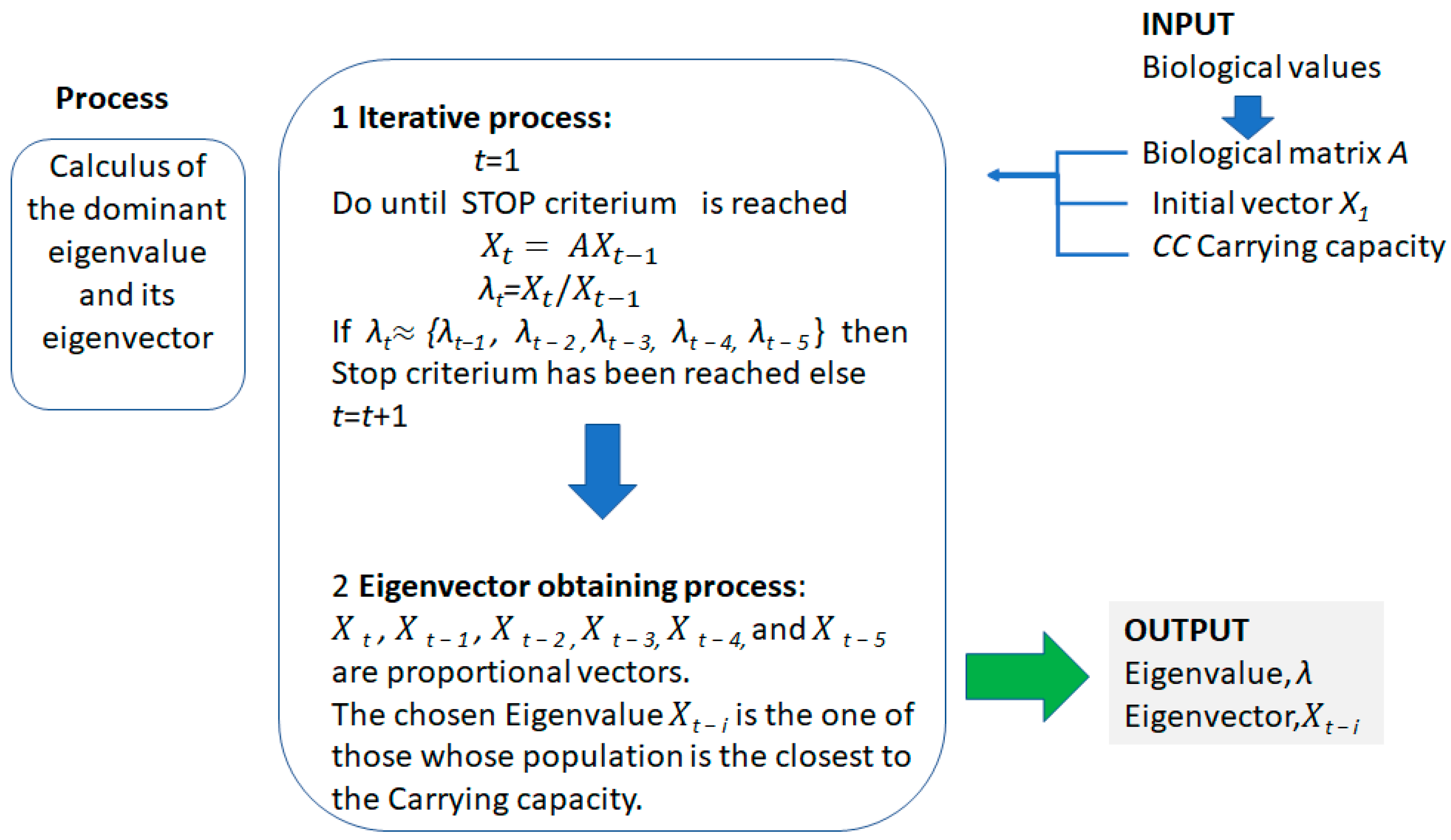
- The manager must obtain the birth and death rates by age and sex classes to obtain the matrix A, the initial population distribution by age and sex classes X1, and the optimum carrying capacity of the preserve.
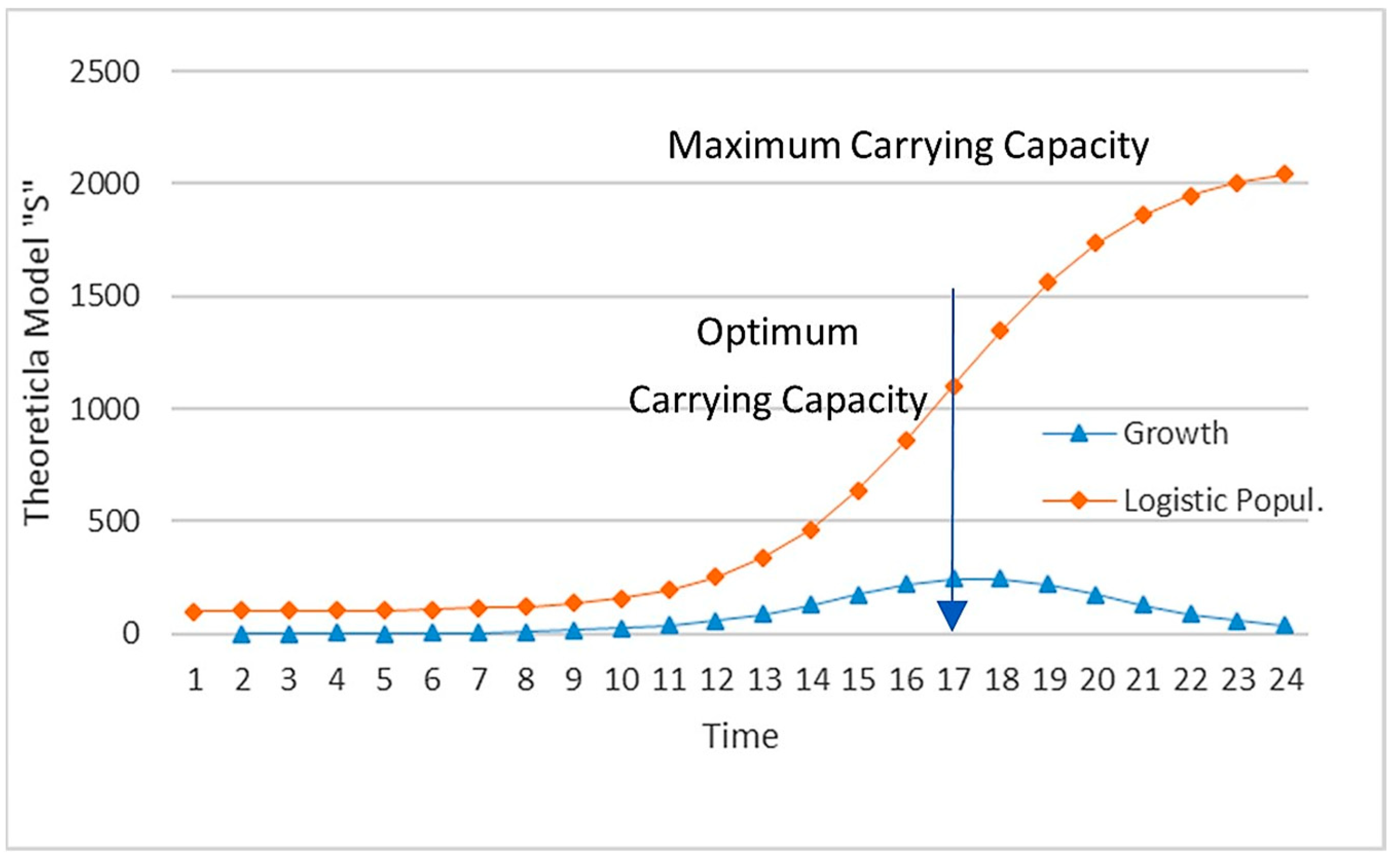
- The eigenvalue can be obtained in a fast iterative process, (software such as Excel can be used) that ends when this value is the same as the ones obtained in the previous five iterations (see Figure 7).
- The chosen eigenvector Xt−i is the one from Xt, Xt−1, Xt−2, Xt−3, Xt−4, and Xt−5 whose total population is the closest to the optimum carrying capacity.
- From year t−i onwards, managers will remove some individuals from the population to maintain the optimum carrying capacity value.
- The management culling to lower the population structure should be proportional to the eigenvector that was calculated in the stabilization process. The sum of the components of this vector must be lower than and close to the OCC value.
- For the following periods, the culling quotas by age classes should stabilize the population according to the dominant lambda value.
- Stabilize the population structure and quantity and make them sustainable, with a management plan that will be updated periodically and with statistical sampling of abundance by the age classes in matrix A.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Calculation of the Carrying Capacity
| Vegetation Types | Minimum Production of Dry Matter per ha and Year in kg | Maximum Production of Dry Matter per ha and Year in kg | Food Units per 1 kg of Dry Matter | Surface in ha | Total Food Units |
|---|---|---|---|---|---|
| 0.-Unproductive areas | |||||
| Built-up areas, coastlines, bodies of water, waterlogged areas, rocky areas, sandy areas, and recently logged areas. | 0 | 0 | 0 | 71.4 | 0.0 |
| 1.-SCRUB: | |||||
| 1.1.-Dense Scrub | 300 | 400 | 0.6 | 2069.6 | 434,609.7 |
| 2.-CROPS: | |||||
| 2.1.-Dry Crop | 200 | 800 | 0.48 | 1037.6 | 249,028.8 |
| 4.-FOREST: | |||||
| 4.1.-Dense Conifer Forest | 50 | 100 | 0.1 | 21.9 | 164.0 |
| 5.-DENSE SCRUB AND FOREST: | |||||
| 5.1.-Dense Scrub and Conifer Forest | 150 | 300 | 0.33 | 1137.9 | 84,489.1 |
| 5.2.-Dense Scrub and Quercus and Conifer Forest | 250 | 275 | 0.6 | 104.2 | 16,416.2 |
| 5.3.-Dense Scrub and other Deciduous Forest | 250 | 300 | 0.52 | 392.0 | 56,060.3 |
| 6.-SPARSE SCRUB AND WOODLAND | |||||
| 6.1.-Sparse Scrub and Quercus and Conifer Woodland. | 200 | 300 | 0.48 | 60.2 | 7224.0 |
| 6.2.-Sparse Scrub and Sparse Woodland | 250 | 300 | 0.6 | 600.5 | 99,082.5 |
| 7.-PASTURE AND WOODLAND: | |||||
| 7.1.-Dense Pasture and Dense Conifer Forest | 75 | 300 | 0.2 | 22.1 | 829.9 |
| 8.-PASTURE AND OTHER VEGETATION | 150 | 200 | 0.33 | 1105.9 | 63,864.6 |
| TOTALS | 6623.4 | 1,011,769.1 |
- Equation (A1) calculates the total food units for a vegetation type i:ADMi is the average production of dry matter per ha and year in kg of type of vegetation i.fui is the number of food units per 1 kg of dry matter of the type of vegetation i.Si is the area in ha of type of vegetation i in Quintos de Mora preserve.
- The total food units in the study area are the following:
References
- Mysterud, A. Still walking on the wild side? Management actions as steps towards ‘semi-domestication’ of hunted ungulates. J. Appl. Ecol. 2010, 47, 920–925. [Google Scholar] [CrossRef]
- Carpio, A.J.; Apollonio, M.; Acevedo, P. Wild ungulate overabundance in Europe: Contexts, causes, monitoring and management recommendations. Mamm. Rev. 2020, 51, 95–108. [Google Scholar] [CrossRef]
- Laguna, E.; Carpio, A.J.; Vicente, J.; Barasona, J.A.; Triguero-Ocaña, R.; Jiménez-Ruiz, S.; Gómez-Manzaneque, A.; Acevedo, P. The spatial ecology of red deer under different land use and management scenarios: Protected areas, mixed farms and fenced hunting preserves. Sci. Total Environ. 2021, 786, 47124. [Google Scholar] [CrossRef]
- Hard, J.J.; Mills, L.S.; Peek, J.M. Genetic implications of reduced survival of male red deer Cervus elaphus under harvest. Wildl. Biol. 2006, 12, 427–441. [Google Scholar] [CrossRef]
- Webb, S.L.; DeYoung, R.W.; Demarais, S.; Strickland, B.K.; Gee, K.L. Testing a Local Inbreeding Hypothesis as a Cause of Observed Antler Characteristics in Managed Populations of White-Tailed Deer. Diversity 2021, 13, 116. [Google Scholar] [CrossRef]
- Apollonio, M.; Andersen, R.; Putman, R. European Ungulates and their Management in the 21st Century; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Valente, A.M.; Acevedo, P.; Tableueiredo, A.M.; Fonseca, C.; Torres, R.T. Overabundant wild ungulate populations in Europe: Management with consideration of socio-ecological consequences. Mamm. Rev. 2020, 50, 353–366. [Google Scholar] [CrossRef]
- Ebert, C.; Sandrini, J.; Welter, B.; Thiele, B.; Hohmann, U. Estimating red deer (Cervus elaphus) population size based on non-invasive genetic sampling. Eur. J. Wildl. Res. 2021, 67, 27. [Google Scholar] [CrossRef]
- Austin, Z.; Raffaelli, D.; White, P. Interactions between ecological and social drivers in determining and managing biodiversity impacts of deer. Biol. Conserv. 2013, 158, 214–222. [Google Scholar] [CrossRef]
- Kavčić, K.; Palencia, P.; ·Apollonio, M.; Vicente, J.; Šprem, N. Random encounter model to estimate density of mountain-dwelling ungulate. Eur. J. Wildl. Res. 2021, 67, 87. [Google Scholar] [CrossRef]
- Nugent, G.; McShea, W.; Parkes, J.; Woodley, S.; Waithaka, J.; Moro, J.; Gutierrez, R.; Azorit, C.; Guerrero, F.M.; Flueck, W. Policies and management of overabundant deer (native or exotic) in protected areas. Anim. Prod. Sci. 2011, 51, 384–389. [Google Scholar] [CrossRef]
- Massei, G.; Quy, R.J.; Gurney, J.; Cowan, D.P. Can translocations be used to mitigate human–wildlife conflicts? Wildl. Res. 2010, 37, 428–439. [Google Scholar] [CrossRef]
- Boulanger, J.R.; Curtis, P.D.; Cooch, E.G.; DeNicola, A.J. Sterilization as an alternative deer control technique: A review. Hum.-Wildl. Interact. 2012, 6, 273–282. [Google Scholar]
- Ritchie, E.G.; Elmhagen, B.; Glen, A.S.; Letnic, M.; Ludwig, G.; McDonald, R.A. Ecosystem restoration with teeth: What role for predators? Trends Ecol. Evol. 2012, 27, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Ver Cauteren, K.C.; Lavelle, M.J.; Hygnstrom, S. From the field: Fences and deer-damage management: A review of designs and efficacy. Wildl. Soc. Bull. 2006, 34, 191–200. [Google Scholar] [CrossRef]
- Gortázar, C.; Vicente, J.; Boadella, M.; Ballesteros, C.; Galindo, R.C.; Garrido, J.; Aranaz, A.; De La Fuente, J. Progress in the control of bovine tuberculosis in Spanish wildlife. Vet. Microbiol. 2011, 151, 170–178. [Google Scholar] [CrossRef]
- Putman, R.; Staines, B. Supplementary winter feeding of wild red deer Cervus elaphus in Europe and North America: Justifications, feeding practice and effectiveness. Mamm. Rev. 2004, 34, 285–306. [Google Scholar] [CrossRef]
- Johnson, H.E.; Mills, L.S.; Stephenson, T.R.; Wehausen, J.D. Population-specific vital rate contributions influence management of an endangered ungulate. Ecol. Appl. 2010, 20, 1753–1765. [Google Scholar] [CrossRef]
- Lwin, N.; Linkie, M.; Harihar, A.; Aung, S.S.; Lin, A.K.; Momberg, F. Initiating conservation of a newly discovered population of the Endangered hog deer Axis porcinus in Myanmar. Oryx 2016, 52, 126–133. [Google Scholar] [CrossRef]
- Macmillan, D.C.; Phillip, S. Consumptive and non-consumptive values of wild mammals in Britain. Mamm. Rev. 2008, 38, 189–204. [Google Scholar] [CrossRef]
- Smart, J.C.R.; White, P.C.L.; Termansen, M. Modelling conflicting objectives in the management of a mobile ecological resource: Red deer in the Scottish Highlands. Ecol. Econ. 2008, 64, 881–892. [Google Scholar] [CrossRef]
- Pfeffer, S.E.; Dressel, S.; Wallgren, M.; Bergquist, J.; Kalén, C. Browsing Damage on Scots Pine: Direct and Indirect Effects of Landscape Characteristics, Moose and Deer Populations. Diversity 2022, 14, 734. [Google Scholar] [CrossRef]
- Côté, S.D.; Rooney, T.P.; Tremblay, J.P.; Dussault, C.; Waller, D.M. Ecological Impacts of Deer Overabundance. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 113–147. Available online: https://www.jstor.org/stable/30034112 (accessed on 15 September 2022). [CrossRef]
- Marques, F.F.C.; Buckland, S.T.; Goffin, D.; Dixon, C.E.; Borchers, D.L.; Mayle, B.A.; Peace, A.J. Estimating deer abundance from line transect surveys of dung: Sika deer in southern Scotland. J. Appl. Ecol. 2001, 38, 349–363. [Google Scholar] [CrossRef]
- Stankovski, V.; Debeljak, M.; Bratko, I.; Adamič, M. Modelling the population dynamics of red deer (Cervus elaphus L.) with regard to forest development. Ecol. Modell. 1998, 108, 145–153. [Google Scholar] [CrossRef]
- Newman, K.B.; Buckland, S.T.; Lindley, S.T.; Thomas, l.; Fernández, C. Hidden Process Models for Animal Population Dynamics. Ecol. Appl. 2006, 16, 74–86. [Google Scholar] [CrossRef]
- Refoyo-Román, P.; Olmedo-Salinas, C.; Muñoz-Araújo, B. La utilidad de los modelos de distribución de especies en la gestión cinegética de los ungulados silvestres. Anim. Biodivers. Conserv. 2014, 37, 165–176. [Google Scholar] [CrossRef]
- Jensen, A.L. Sex and age structured matrix model applied to harvesting a white tailed deer population. Ecol. Modell. 2000, 128, 245–249. [Google Scholar] [CrossRef]
- Sommer, R.S.; Zachos, F.E.; Street, M.; Jöris, O.; Skog, A.; Benecke, N. Late Quaternary distribution dynamics and phylogeography of the red deer (Cervus elaphus) in Europe. Quat. Sci. Rev. 2008, 27, 714–733. [Google Scholar] [CrossRef]
- Carranza, J. Cervus elaphus Linnaeus, 1758. In Atlas y Libro Rojo de los Mamíferos Terrestres de España; Palomo, I.J., Gisbert, J., Blanco, J.L., Eds.; Dirección General para la Biodiversidad-SECEM-SECEMU: Madrid, Spain, 2007; pp. 352–355. [Google Scholar]
- Meiri, M.; Lister, A.M.; Higham, T.F.G.; Stewart, J.R.; Straus, L.G.; Obermaier, H.; González-Morales, M.R.; Marín-Arroyo, A.B.; Barnes, I. Late-glacial recolonization and phylogeography of European red deer Cervus elaphus L.). Mol. Ecol. 2013, 22, 4711–4722. [Google Scholar] [CrossRef]
- Inventarios Nacionales. MITECO Publishing PhysicsWeb. Available online: https://www.miteco.gob.es/ (accessed on 15 September 2022).
- Caballero García de Arévalo, R. Hábitat y Alimentación del Ciervo en Ambiente Mediterráneo; Instituto Nacional para la Conservación de la Naturaleza: Madrid, Spain, 1985. [Google Scholar]
- Caswell, H. Matrix Population Models: Construction, Analysis, and Interpretation; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- McLeod, S.R.; Saunders, G.R. Improving management strategies for the red fox by using projection matrix analysis. Wildl. Res. 2001, 28, 333–340. [Google Scholar] [CrossRef]
- Gertsev, V.I.; Gertseva, V.V. Classification of mathematical models in ecology. Ecol. Modell. 2004, 178, 329–334. [Google Scholar] [CrossRef]
- Logofet, D.O. Projection matrices in variable environments: λ1 in theory and practice. Ecol. Modell. 2013, 251, 307–311. [Google Scholar] [CrossRef]
- Leslie, P.H. On the Use of Matrices in Certain Population Mathematics. Biometrika 1945, 33, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Hanley, B.; Dennis, B. Analytical expressions for the eigenvalues, demographic quantities, and extinction criteria arising from a three-stage wildlife population matrix. Nat. Resour. Model. 2019, 32, e12207. [Google Scholar] [CrossRef]
- Roughan, M. 2017 Leslie Matrices. Leslie Matrix. Calculator. Available online: https://www.adelaide.edu.au/ (accessed on 12 December 2022).
- Stubben, C.J.; Milligan, B.G. Estimating and Analyzing Demographic Models Using the popbio Package in R. J. Stat. Softw. 2007, 22, 1–23. [Google Scholar] [CrossRef]
- Forchhammer, M.C.; Stenseth, N.C.; Post, E.; Langvatn, R. Population dynamics of Norwegian red deer: Density-dependence and climatic variation. Proc. R. Soc. Lond. Ser. B Biol. 1998, 265, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Millas-Prendergast, F. Aplicación de un Modelo Cuantitativo para Garantizar la Sostenibilidad en la Ordenación Cinegética del Ciervo (Cervus elaphus L.) en Los Quintos de Mora (Toledo). Master’s Dissertation, Universidad Politécnica de Madrid, Madrid, Spain, July 2015. [Google Scholar]
- Montoya-Oliver, J.M. El ciervo y el Monte: Manejo y Conservación; Mundi-Prensa: Madrid, Spain, 1999. [Google Scholar]
- San Miguel, A.; Rodríguez-Vigal, C.; Perea García-Calvo, R. Los Quintos de Mora. Gestión integral del monte mediterráneo. In Pastos, Paisajes Culturales Entre Tradición y Nuevos Paradigmas del siglo XXI. Visitas de Campo; López-Carrasco, C., Rodríguez, M.P., San Miguel, A., Fernández, F., Roig, S., Eds.; Sociedad Española para el Estudio de los Pastos: Madrid, Spain, 2011; pp. 57–93. [Google Scholar]
- Soriguer, R.C.; Fandos, P.; Bernáldez, E.; Delibes, J.R. El ciervo en Andalucía; Junta de Consejería de Agricultura y Pesca: Sevilla, Spain, 1994. [Google Scholar]
- Landete-Castillejos, T.; García, A.; Gómez, J.A.; Gallego, L. Lactation under food constraints in Iberian red deer Cervus elaphus hispanicus. Wildl. Biol. 2003, 9, 131–139. [Google Scholar] [CrossRef]
- Rodriguez-Hidalgo, P.; Gortázar, C.; Tortosa, F.S.; Rodriguez-Vigal, C.; Fierro, Y.; Vicente, J. Effects of density, climate, and supplementary forage on body mass and pregnancy rates of female red deer in Spain. Oecologia 2010, 164, 389–398. [Google Scholar] [CrossRef]
- Organismo Autónomo Parques Nacionales. Plan Técnico de Caza y Aprovechamientos de Quintos de Mora; Ministerio para la transición Ecológica y Reto Demográfico: Madrid, Spain, 2019. [Google Scholar]
- López, I.; Ortuño, S.F.; Martín, A.J.; Fullana, C. Estimating the sustainable harvesting and the stable diameter distribution of European beech with projection matrix models. Ann. For. Sci. 2007, 64, 593–599. [Google Scholar] [CrossRef]
- Martínez, A.; Martín, A.J. A matrix system using quality classes can be applied for managing sustainable wild ungulates populations: Convergence below optimum capacity. Ecol. Eng. 2017, 108, 10–16. [Google Scholar] [CrossRef]
- Lopatin, V.N.; Rosolovsky, S.V. Evaluation of the state and productivity of moose populations using Leslie Matrix analyses. Alces 2002, 38, S77. [Google Scholar]
- Walters, S. Landscape pattern and productivity effects on source-sink dynamics of deer populations. Ecol. Modell. 2001, 143, 17–32. [Google Scholar] [CrossRef]
- Millspaugh, J.J.; Skalski, J.R.; Townsend, R.L.; Diefenbach, D.R.; Boyce, M.S.; Hansen, L.P.; Kammermeyer, K. An Evaluation of Sex-Age-Kill (SAK) Model Performance. J. Wildl. Manag. 2009, 73, 442–451. Available online: http://www.jstor.org/stable/40208548 (accessed on 15 September 2022). [CrossRef]
- Gilbert, B.A.; Raedeke, K.J.; Skalski, J.R.; Stringer, A.B. Modeling Black-Tailed Deer Population Dynamics Using Structured and Unstructured Approaches. J. Wildl. Manag. 2007, 71, 144–154. [Google Scholar] [CrossRef]
- Jensen, A.L. Matrix population model with density-dependent recruitment for assessment of age-structured wildlife populations. Bull. Math. Biol. 1997, 59, 255–262. [Google Scholar] [CrossRef]
- Khurram, N.; Entao, C.; Ying, Z. A Novel Hierarchical Multinomial Approach to Modeling Age-Specific Harvest. In Quantitative Methods in Environmental and Climate Research; Cameletti, M., Finazzi, F., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 35–54. [Google Scholar] [CrossRef]
- Hanley, B.; Connelly, P.; Dennis, B. Another look at the eigenvalues of a population matrix model. PeerJ 2019, 7, e8018. [Google Scholar] [CrossRef]
- Zipkin, E.F.; Royle, J.A.; Dawson, D.K.; Bates, S. Multi-species occurrence models to evaluate the effects of conservation and management actions. Biol. Conserv. 2010, 143, 479–484. [Google Scholar] [CrossRef]
- Torres-Porras, J.; Carranza, J.; Pérez-González, J.; Mateos, C.; Alarcos, S. The tragedy of the commons: Unsustainable population structure of Iberian red deer in hunting preserves. Eur. J. Wildl. Res. 2014, 6, 351–357. [Google Scholar] [CrossRef]
- Beddington, J.R. Age structure, sex-ratio and population-density in harvesting of natural animal populations. J. Appl. Ecol. 1974, 11, 915–924. [Google Scholar] [CrossRef]
- Ginsberg, J.R.; Milner-Gulland, E.J. Sex-biased harvesting and population dynamics in ungulates: Implications for conservation and sustainable use. Conserv. Biol. 1994, 8, 157–166. [Google Scholar] [CrossRef]
- Hagen, R.; Haydn, A.; Suchant, R. Estimating red deer (Cervus elaphus) population size in the Southern Black Forest: The role of hunting in population control. Eur. J. Wildl. Res. 2018, 64, 42. [Google Scholar] [CrossRef]
- McGowan, C.P.; Allan, N.; Servoss, J.; Hedwall, S.; Wooldridge, B. Incorporating population viability models into species status assessment and listing decisions under the U.S. Endangered Species Act. Glob. Ecol. Conserv. 2017, 12, 119–130. [Google Scholar] [CrossRef]
- Pérez-Barbería, F.J.; Duff, E.I.; Brewer, M.J.; Guinness, F.E. Assessing the age of Scottish red deer. J. Zool. 2014, 294, 180–189. [Google Scholar] [CrossRef]
- Forsyth, D.M.; Allen, R.B.; Marburg, A.E.; MacKenzie, D.I.; Douglas, M.J.W. Population dynamics and resource use of red deer after release from harvesting in New Zealand. N. Z. J. Ecol. 2010, 34, 277–287. [Google Scholar]
- Torres-Porras, J.; Carranza, J.; Pérez-González, J. Selective culling of Iberian red deer stags (Cervus elaphus hispanicus) by selective montería in Spain. Eur. J. Wildl. Res. 2009, 55, 117–123. [Google Scholar] [CrossRef]
- Smith, G.C.; Trout, R.C. Using Leslie matrices to determine wild rabbit population growth and the potential for control. J. Appl. Ecol. 1994, 31, 223–230. [Google Scholar] [CrossRef]
- Buckland, S.T.; Newman, K.B.; Thomas, L.; Koesters, N.B. State-space models for the dynamics of wild animal populations. Ecol. Modell. 2004, 171, 157–175. [Google Scholar] [CrossRef]
- Trenkel, V.M.; Elston, D.A.; Buckland, S.T. Fitting population dynamics models to count and cull data using sequential importance sampling. J. Am. Stat. Assoc. 2000, 95, 363–374. [Google Scholar] [CrossRef]




| Rate | Value |
|---|---|
| Birth rate (Calves birth/reproductive female) | 0.564 |
| Birth sex ratio (M:F) | 1:1 |
| Females calves death rate (Less than 1 year old) | 0.12 |
| Males calves death rate (Less than 1 year old) | 0.15 |
| Young females death rate (From 1 to 2 years old) | 0.06 |
| Young males death rate (From 1 to 2 years old) | 0.1 |
| Adult females death rate (From 2 to 10 years old) | 0.03 |
| Adult males death rate (From 2 to 10 years old) | 0.05 |
| Females | Males | Total | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| X0 | 10 | 10 | 8 | 8 | 8 | 6 | 0 | 0 | 0 | 0 | 10 | 10 | 8 | 8 | 8 | 6 | 0 | 0 | 0 | 0 | 100 |
| X17 | 133 | 101 | 81 | 68 | 57 | 47 | 39 | 33 | 28 | 95 | 133 | 97 | 75 | 62 | 50 | 41 | 34 | 27 | 22 | 47 | 1271 |
| X18 | 155 | 117 | 95 | 79 | 66 | 55 | 46 | 38 | 32 | 119 | 155 | 113 | 88 | 72 | 58 | 48 | 39 | 32 | 26 | 66 | 1478 |
| Stable Distribution (%) | TOTAL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | |
| 10.5 | 7.9 | 6.4 | 5.3 | 4.5 | 3.7 | 3.1 | 2.6 | 2.2 | 7.5 | |
| M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | |
| 10.5 | 7.7 | 5.9 | 4.8 | 4.0 | 3.2 | 2.6 | 2.2 | 1.8 | 3.7 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Fernández, Á.J.; Ortuño, S.; Martín-Fernández, S. Obtaining Sustainable Population Structures for the Management of Red Deer. Diversity 2023, 15, 612. https://doi.org/10.3390/d15050612
Martín-Fernández ÁJ, Ortuño S, Martín-Fernández S. Obtaining Sustainable Population Structures for the Management of Red Deer. Diversity. 2023; 15(5):612. https://doi.org/10.3390/d15050612
Chicago/Turabian StyleMartín-Fernández, Ángel J., Sigfredo Ortuño, and Susana Martín-Fernández. 2023. "Obtaining Sustainable Population Structures for the Management of Red Deer" Diversity 15, no. 5: 612. https://doi.org/10.3390/d15050612
APA StyleMartín-Fernández, Á. J., Ortuño, S., & Martín-Fernández, S. (2023). Obtaining Sustainable Population Structures for the Management of Red Deer. Diversity, 15(5), 612. https://doi.org/10.3390/d15050612






