Updating the National Baseline of Non-Indigenous Species in Spanish Marine Waters
Abstract
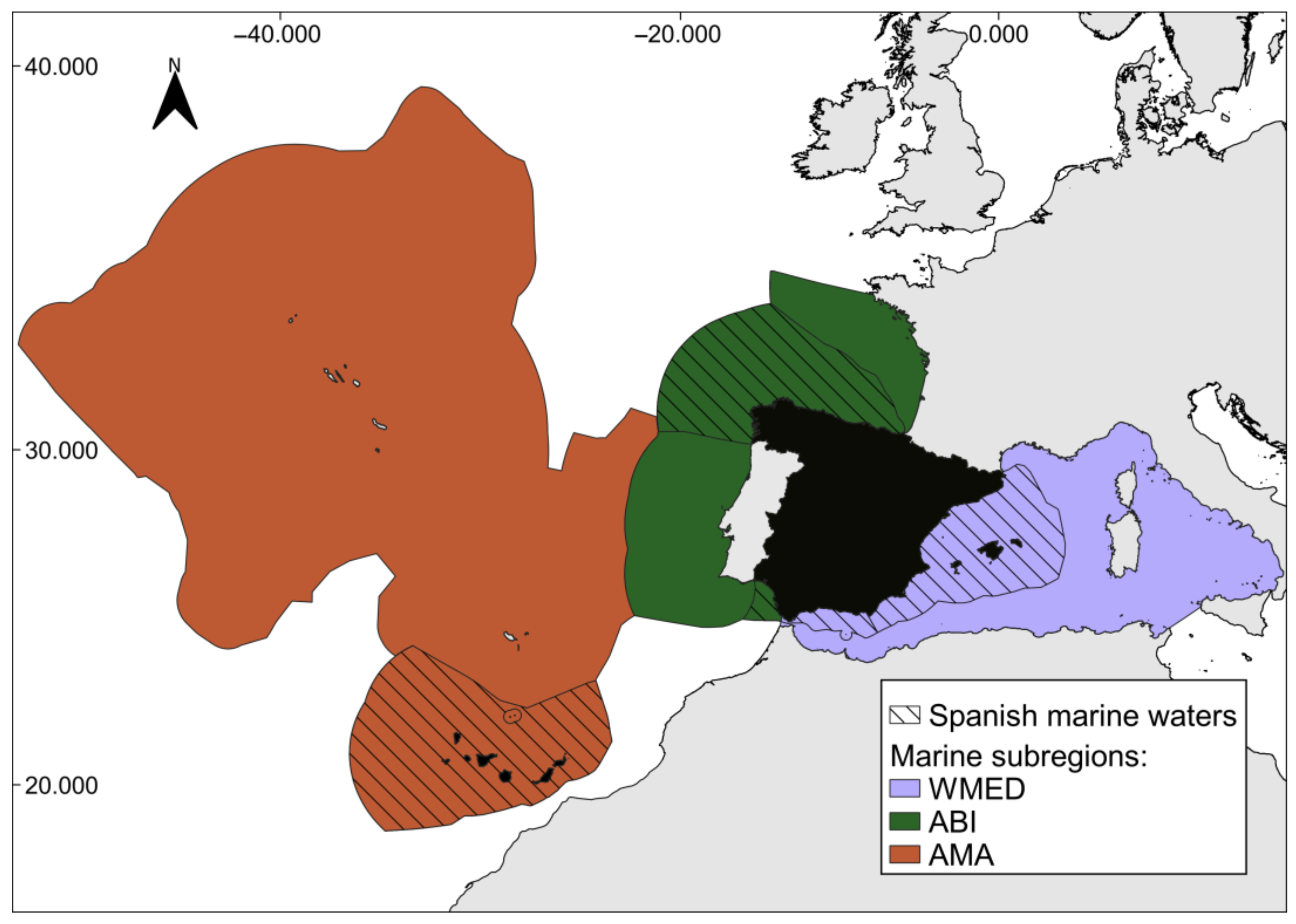
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nunes, A.L.; Katsanevakis, S.; Zenetos, A.; Cardoso, A.C. Gateways to alien invasions in the European seas. Aquat. Invasions 2014, 9, 133–144. [Google Scholar] [CrossRef]
- Campbell, M.L.; King, S.; Heppenstall, L.D.; van Gool, E.; Martin, R.; Hewitt, C.L. Aquaculture and urban marine structures facilitate native and non-indigenous species transfer through generation and accumulation of marine debris. Mar. Pollut. Bull. 2017, 123, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Molnar, J.L.; Gamboa, R.L.; Revenga, C.; Spalding, M.D. Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ. 2008, 6, 485–492. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of invasive alien marine species on ecosystem services and biodiversity: A pan-European review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Giakoumi, S.; Katsanevakis, S.; Albano, P.G.; Azzurro, E.; Cardoso, A.C.; Cebrian, E.; Deidun, A.; Edelist, D.; Francour, P.; Jimenez, C.; et al. Management priorities for marine invasive species. Sci. Total Environ. 2019, 688, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Bédry, R.; de Haro, L.; Bentur, Y.; Senechal, N.; Galil, B.S. Toxicological risks on the human health of populations living around the Mediterranean Sea linked to the invasion of non-indigenous marine species from the Red Sea: A review. Toxicon 2021, 191, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Ramus, A.P.; Silliman, B.R.; Thomsen, M.S.; Long, Z.T. An invasive foundation species enhances multifunctionality in a coastal ecosystem. Proc. Natl. Acad. Sci. USA 2017, 114, 8580–8585. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Rilov, G.; Edelist, D. Impacts of marine invasive alien species on European fisheries and aquaculture—Plague or boon? In Engaging Marine Scientists and Fishers to Share Knowledge and Perceptions—Early Lessons; CIESM Workshop Monograph n° 50; Briand, F., Ed.; CIESM Publisher: Monaco City, Monaco; Paris, France, 2018; pp. 125–132. [Google Scholar]
- Mancinelli, G.; Chainho, P.; Cilenti, L.; Falco, S.; Kapiris, K.; Katselis, G.; Ribeiro, F. On the Atlantic blue crab (Callinectes sapidus Rathbun 1896) in southern European coastal waters: Time to turn a threat into a resource? Fish. Res. 2017, 194, 1–8. [Google Scholar] [CrossRef]
- Vimercati, G.; Kumschick, S.; Probert, A.F.; Volery, L.; Bacher, S. The importance of assessing positive and beneficial impacts of alien species. NeoBiota 2020, 62, 525–545. [Google Scholar] [CrossRef]
- Tsirintanis, K.; Azzurro, E.; Crocetta, F.; Dimiza, M.; Froglia, C.; Gerovasileiou, V.; Langeneck, J.; Mancinelli, G.; Rosso, A.; Stern, N.; et al. Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat. Invasions 2022, 17, 308–352. [Google Scholar] [CrossRef]
- Castro, N.; Carlton, J.T.; Costa, A.C.; Marques, C.; Hewitt, C.L.; Cacabelos, E.; Gizzi, F.; Gestoso, I.; Monteiro, J.G.; Costa, J.L.; et al. Diversity and patterns of marine non-native species in the archipelagos of Macaronesia. Divers. Distrib. 2022, 28, 667–684. [Google Scholar] [CrossRef]
- Galanidi, M.; Zenetos, A. Data-driven recommendations for establishing threshold values for the NIS trend indicator in the Mediterranean Sea. Diversity 2022, 14, 57. [Google Scholar] [CrossRef]
- Zenetos, A.; Tsiamis, K.; Galanidi, M.; Carvalho, N.; Bartilotti, C.; Canning-Clode, J.; Castriota, L.; Chainho, P.; Comas-González, R.; Costa, A.C.; et al. Status and trends in the rate of introduction of marine non-indigenous species in European seas. Diversity 2022, 14, 1077. [Google Scholar] [CrossRef]
- Tsiamis, K.; Palialexis, A.; Stefanova, K.; Gladan, Ž.N.; Skejić, S.; Despalatović, M.; Cvitković, I.; Dragičević, B.; Dulčić, J.; Vidjak, O.; et al. Non-indigenous species refined national baseline inventories: A synthesis in the context of the European Union’s Marine Strategy Framework Directive. Mar. Pollut. Bull. 2019, 145, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Tsiamis, K.; Palialexis, A.; Connor, D.; Antoniadis, S.; Bartilotti, C.; Bartolo, A.G.; Berggreen, U.C.; Boschetti, S.; Buschbaum, C.; Canning-Clode, J.; et al. Marine Strategy Framework Directive- Descriptor 2, Non-Indigenous Species, Delivering Solid Recommendations for Setting Threshold Values for Non-Indigenous Species Pressure on European Seas; Publication Office of the European Union: Luxembourg, 2021; Available online: https://data.europa.eu/doi/10.2760/035071 (accessed on 21 March 2023).
- World Register of Marine Species. Available online: https://www.marinespecies.org (accessed on 21 March 2023).
- European Commission—Joint Research Centre—European Alien Species Information Network (EASIN). Available online: https://easin.jrc.ec.europa.eu/ (accessed on 21 March 2023).
- UNEP/MAP. Baseline for the IMAP Common Indicator 6 Related to Non-Indigenous Species (UNEP/MED WG.520/5); UNEP: Nairobi, Kenya, 2022. [Google Scholar]
- Convention on Biological Diversity. Pathways of Introduction of Invasive Species, Their Prioritization and Management. (UNEP/CBD/SBSTTA/18/9/Add.1). Subsidiary Body on Scientific, Technical and Technological Advice, Montreal. 2014. Available online: https://www.cbd.int/doc/meetings/sbstta/sbstta-18/official/sbstta-18-09-add1-en.pdf (accessed on 21 March 2023).
- Pergl, J.; Brundu, G.; Harrower, C.A.; Cardoso, A.C.; Genovesi, P.; Katsanevakis, S.; Lozano, V.; Perglová, I.; Rabitsch, W.; Richards, G.; et al. Applying the Convention on Biological Diversity Pathway Classification to alien species in Europe. NeoBiota 2020, 62, 333–363. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org (accessed on 21 March 2023).
- Posit Team. RStudio: Integrated Development Environment for R; Posit Software: Boston, MA, USA, 2023; Available online: http://www.posit.co (accessed on 21 March 2023).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; López Garcia, E.; Stern, N.; Tsiamis, K.; Galanidi, M. Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Mediterr. Mar. Sci. 2022, 23, 196–212. [Google Scholar] [CrossRef]
- Massé, C.; Viard, F.; Humbert, S.; Antajan, E.; Auby, I.; Bachelet, G.; Bernard, G.; Bouchet, V.M.P.; Burel, T.; Dauvin, J.-C.; et al. An Overview of Marine Non-Indigenous Species Found in Three Contrasting Biogeographic Metropolitan French Regions: Insights on Distribution, Origins and Pathways of Introduction. Diversity 2023, 15, 161. [Google Scholar] [CrossRef]
- Ferrario, J.; Caronni, S.; Occhipinti-Ambrogi, A.; Marchini, A. Role of commercial harbours and recreational marinas in the spread of non-indigenous fouling species. Biofouling 2017, 33, 651–660. [Google Scholar] [CrossRef]
- Falcón, J.M. Ictiofauna de las Islas Canarias. Análisis Biogeográfico. Ph.D. Thesis, Universidad de La Laguna, Santa Cruz de Tenerife, Spain, 2015. [Google Scholar]
- Pajuelo, J.G.; González, J.A.; Triay-Portella, R.; Martín, J.A.; Ruiz-Díaz, R.; Lorenzo, J.M.; Luque, A. Introduction of non-native marine fish species to the Canary Islands waters through oil platforms as vectors. J. Mar. Syst. 2016, 163, 23–30. [Google Scholar] [CrossRef]
- Brito, A.; Clemente, S.; Herrera, R. On the occurrence of the African hind, Cephalopholis taeniops, in the Canary Islands (eastern subtropical Atlantic): Introduction of large-sized demersal littoral fishes in ballast water of oil platforms? Biol. Invasions 2011, 13, 2185–2189. [Google Scholar] [CrossRef]
- Falcón, J.M.; Herrera, R.; Ayza, O.; Brito, A. New species of tropical litoral fish found in Canarian waters. Oil platforms as a central introduction vector. Rev. Acad. Canar. Cienc. 2015, 27, 67–82. [Google Scholar]
- Falcón, J.M.; Brito, A.; Herrera, R.; Monterroso, O.; Rodríguez, M.; Álvarez, O.; Ramos, E.; Miguel, A. New records of tropical littoral fishes from the Canary Islands as a result of two driving forces: Natural expansion and introduction by oil platforms. Rev. Acad. Canar. Cienc. 2018, 30, 39–56. [Google Scholar]
- López, C.; Clemente, S.; Moreno, S.; Ocaña, O.; Herrera, R.; Moro, L.; Monterroso, O.; Rodríguez, A.; Brito, A. Invasive Tubastraea spp. and Oculina patagonica and other introduced scleractinians corals in the Santa Cruz de Tenerife (Canary Islands) harbor: Ecology and potential risks. Reg. Stud. Mar. Sci. 2019, 29, 100713. [Google Scholar] [CrossRef]
- Triay-Portella, R.; Martín, J.A.; Luque, L.; Pajuelo, J.G. Relevance of feeding ecology in the management of invasive species: Prey variability in a novel invasive crab. Estuar. Coast. Shelf Sci. 2022, 274, 107949. [Google Scholar] [CrossRef]
- Png-Gonzalez, L.; Andrade, C.; Abramic, A.; Nogueira, N. Analysis of the Aquaculture Industry in Macaronesia under MSFD. Technical Report for PLASMAR Project. 2019. Available online: http://hdl.handle.net/10553/55195 (accessed on 20 March 2023).
- Bianchi, C.; Caroli, F.; Guidetti, P.; Morri, C. Seawater warming at the northern reach for southern species: Gulf of Genoa, NW Mediterranean. J. Mar. Biolog. Assoc. UK 2018, 98, 1–12. [Google Scholar] [CrossRef]
- Fortič, A.; Al-Sheikh Rasheed, R.; Almajid, Z.; Badreddine, A.; Báez, J.C.; Belmonte-Gallegos, A.; Bettoso, N.; Borme, D.; Camisa, F.; Caracciolo, D.; et al. New records of introduced species in the Mediterranean Sea (April 2023). Mediterr. Mar. Sci. 2023, 24, 182–202. [Google Scholar] [CrossRef]
- Korpinen, S.; Klančnik, K.; Peterlin, M.; Nurmi, M.; Laamanen, L.; Zupančič, G.; Popit, A.; Murray, C.; Harvey, T.; Andersen, J.H.; et al. Multiple Pressures and Their Combined Effects in Europe’s Seas; European Topic Centre on Inland, Coastal and Marine Waters: Magdeburg, Germany, 2019. [Google Scholar]
- Corsini-Foka, M.; Zenetos, A.; Crocetta, F.; Cinar, M.E.; Kocak, F.; Golani, D.; Katsanevakis, S.; Tsiamis, K.; Cook, E.; Froglia, C.; et al. Inventory of alien and cryptogenic species of the Dodecanese (Aegean Sea, Greece): Collaboration through COST action training school. Manag. Biol. Invasions 2015, 6, 351–366. [Google Scholar] [CrossRef]
- Staehr, P.A.; Jakobsen, H.H.; Hansen, J.L.; Andersen, P.; Christensen, J.; Göke, C.; Thomsen, M.S.; Stebbing, P.D. Trends in records and contribution of non-indigenous and cryptogenic species to marine communities in Danish waters: Potential indicators for assessing impact. Aquat. Invasions 2020, 15, 127–244. [Google Scholar] [CrossRef]
- Díaz-Tapia, P.; Bárbara, I.; Cremades, J.; Verbruggen, H.; Maggs, C.A. Three new cryptogenic species in the tribes Polysiphonieae and Streblocladieae (Rhodomelaceae, Rhodophyta). Phycologia 2017, 56, 605–623. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Tsirintanis, K.; Tsaparis, D.; Doukas, D.; Sini, M.; Athanassopoulou, F.; Kolygas, M.N.; Tontis, D.; Koutsoubas, D.; Bakopoulos, V. The cryptogenic parasite Haplosporidium pinnae invades the Aegean Sea and causes the collapse of Pinna nobilis populations. Aquat. Invasions 2019, 14, 150–164. [Google Scholar] [CrossRef]
- Haydar, D. What is natural? The scale of cryptogenesis in the North Atlantic Ocean. Divers. Distrib. 2012, 18, 101–110. [Google Scholar] [CrossRef]
- Zibrowius, H. Oculina patagonica, Scléractiniaire hermatypique introduit en Méditerranée. Helgol. Wissenschaftliche Meeresunters. 1974, 26, 153–173. [Google Scholar] [CrossRef]
- Fine, M.; Zibrowius, H.; Loya, Y. Oculina patagonica: A non-lessepsian scleractinian coral invading the Mediterranean Sea. Mar. Biol. 2001, 138, 1195–1203. [Google Scholar] [CrossRef]
- Salomidi, M.; Katsanevakis, S.; Issaris, Y.; Tsiamis, K.; Katsiaras, N. Anthropogenic disturbance of coastal habitats promotes the spread of the introduced scleractinian coral Oculina patagonica in the Mediterranean Sea. Biol. Invasions 2013, 15, 1961–1971. [Google Scholar] [CrossRef]
- Zibrowius, H.; Ramos-Esplá, A.A. Oculina patagonica, scléractiniaire exotique en Méditerranée—nouvelles observations dans le Sud-Est de l’Espagne. Rapp. CIESM 1983, 28, 297–301. [Google Scholar]
- Serrano, E.; Ribes, M.; Coma, R. Demographics of the zooxanthellate coral Oculina patagonica along the Mediterranean Iberian coast in relation to environmental parameters. Sci. Total Environ. 2018, 634, 1580–1592. [Google Scholar] [CrossRef]
- Leydet, K.P.; Hellberg, M.E. The invasive coral Oculina patagonica has not been recently introduced to the Mediterranean from the western Atlantic. BMC Evol. Biol. 2015, 15, 79. [Google Scholar] [CrossRef]
- Zenetos, A.; Çinar, M.E.; Crocetta, F.; Golani, D.; Rosso, A.; Servello, G.; Shenkar, N.; Turon, X.; Verlaque, M. Uncertainties and validation of alien species catalogues: The Mediterranean as an example. Estuar. Coast. Shelf Sci. 2017, 191, 171–187. [Google Scholar] [CrossRef]
- Brito, A.; López, C.; Ocaña, O.; Herrera, R.; Moro, L.; Monterroso, O.; Rodríguez, A.; Clemente, S.; Sánchez, J.J. Colonization and expansion of two potentially invasive coral species in the Canary Islands introduced through oil platforms. Vieraea 2017, 45, 65–82. [Google Scholar] [CrossRef]
- Gofas, S.; Luque, A.A.; Templado, J.; Salas, C. A national checklist of marine Mollusca in Spanish waters. Sci. Mar. 2017, 81, 241–254. [Google Scholar] [CrossRef]
- Bañón, R.; Rolán, E.; García-Tasende, M. First record of the purple dye murex Bolinus brandaris (Gastropoda: Muricidae) and a revised list of non native molluscs from Galician waters (Spain, NE Atlantic). Aquat. Invasions 2008, 3, 331–334. [Google Scholar] [CrossRef]
- Brito, A.; Pascual, P.J.; Falcón, J.M.; Sancho, A.; González, G. Peces de las Islas Canarias. Catálogo Comentado e Ilustrado; Francisco Lemus Editor: Santa Cruz de Tenerife, Spain, 2002; 419p. [Google Scholar]
- González Lorenzo, G.; Brito, A.; Barquín, J. Impacts of the escapees from mariculture cage in Canary Islands. Vieraea 2005, 33, 449–454. [Google Scholar]
- Toledo Guedes, K.; Sánchez-Jerez, P.; González-Lorenzo, G.; Brito Hernández, A. Detecting the degree of establishment of a non-indigenous species in coastal ecosystems: Sea bass Dicentrarchus labrax escapes from sea cages in Canary Islands (Northeastern Central Atlantic). Hydrobiologia 2009, 623, 203–212. [Google Scholar] [CrossRef]
- Toledo-Guedes, K.; Sanchez-Jerez, P.; Mora-Vidal, J.; Girard, D.; Brito, A. Escaped introduced sea bass (Dicentrarchus labrax) infected by Sphaerospora testicularis (Myxozoa) reach maturity in coastal habitats off Canary Islands. Mar. Ecol. 2012, 33, 26–31. [Google Scholar] [CrossRef]
- Brito, A.; Moreno-Borges, S.; Escánez, A.; Falcón, J.M.; Herrera, R. New records of Actinopterygian fishes from the Canary Islands: Tropicalization (range expansion) as the most important driving force increasing fish diversity. Rev. Acad. Canar. Cienc. 2017, 29, 31–44. [Google Scholar]
- Triay-Portella, R.; Pajuelo, J.G.; Manent, P.; Espino, F.; Ruiz-Díaz, R.; Lorenzo, J.M.; González, J.A. New records of non-indigenous fishes (Perciformes and Tetraodontiformes) from the Canary Islands (north-eastern Atlantic). Cybium 2015, 39, 163–174. [Google Scholar] [CrossRef]
- Galanidi, M.; Zenetos, A.; Bacher, S. Assessing the socio-economic impacts of priority marine invasive fishes in the Mediterranean with the newly proposed SEICAT methodology. Mediterr. Mar. Sci. 2018, 19, 107–123. [Google Scholar] [CrossRef]
- Buddo, D.S.A.; Steele, R.D.; Webber, M.K. Public health risks posed by the invasive Indo-Pacific green mussel, Perna viridis (Linnaeus, 1758) in Kingston Harbour, Jamaica. BioInvasions Rec. 2012, 1, 171–178. [Google Scholar] [CrossRef]
- Javidpour, J.; Molinero, J.C.; Ramírez-Romero, E.; Roberts, P.; Larsen, T. Cannibalism makes invasive comb jelly, Mnemiopsis leidyi, resilient to unfavourable conditions. Commun. Biol. 2020, 3, 212. [Google Scholar] [CrossRef]
- Roohi, A.; Yasin, Z.B.; Kideys, A.E.; Hwai, A.T.; Khanari, A.G.; Eker-Develi, E. Impact of a new invasive ctenophore (Mnemiopsis leidyi) on the zooplankton community of the Southern Caspian sea. Mar. Ecol. 2008, 29, 421–434. [Google Scholar] [CrossRef]
- Kamakin, A.M.; Khodorevskaya, R.P. Impact of the Alien Species Mnemiopsis leidyi A. Agassiz, 1865 on Fish of the Caspian Sea. Inland Water Biol. 2018, 11, 173–178. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Poursanidis, D.; Yokes, M.B.; Mačić, V.; Beqiraj, S.; Kashta, L.; Sghaier, Y.R.; Zakhama-Sraieb, R.; Benamer, I.; Bitar, G.; et al. Twelve years after the first report of the crab Percnon gibbesi (H. Milne Edwards, 1853) in the Mediterranean: Current distribution and invasion rates. J. Biol. Res.-Thessalon. 2011, 16, 224–236. [Google Scholar]
- Félix-Hackradt, F.C.; Sanchis-Martínez, A.M.; Hackradt, C.W.; Treviño-Otón, J.; García-Charton, J.A. Distribution and ecological relations among the alien crab, Percnon gibbesi (H. Milne-Edwards 1853) and autochthonous species, in and out of an SW Mediterranean MPA. Hydrobiologia 2018, 806, 187–201. [Google Scholar] [CrossRef]
- Mateo-Ramírez, M.; Iñiguez, C.; Fernández-Salas, L.M.; Sánchez-Leal, R.F.; Farias, C.; Bellanco, M.J.; Gil, J.; Rueda, J.L. Healthy thalli of the invasive seaweed Rugulopteryx okamurae (Phaeophyceae) being massively dragged into deep-sea bottoms by the Mediterranean Outflow Water. Phycologia 2023, 62, 99–108. [Google Scholar] [CrossRef]
- García-Gómez, J.C.; Sempere-Valverde, J.; González, A.R.; Martínez-Chacón, M.; Olaya-Ponzone, L.; Sánchez-Moyano, E.; Ostalé-Valriberas, E.; Megina, C. From exotic to invasive in record time: The extreme impact of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in the strait of Gibraltar. Sci. Total Environ. 2020, 704, 135408. [Google Scholar] [CrossRef]
- Bernal-Ibáñez, A.; Chebaane, S.; Sempere-Valverde, J.; Faria, J.; Ramalhosa, P.; Kaufmann, M.; Florido, M.; Albert-Fonseca, A.; Canning-Clode, J.; Gestoso, I.; et al. A worrying arrival: The first record of brown macroalga Rugulopteryx okamurae in Madeira Island and its invasive risk. BioInvasions Rec. 2022, 11, 912–924. [Google Scholar] [CrossRef]
- García-Gómez, J.C.; Florido, M.; Olaya-Ponzone, L.; Rey Díaz de Rada, J.; Donázar-Aramendía, I.; Chacón, M.; Quintero, J.J.; Magariño, S.; Megina, C. Monitoring Extreme Impacts of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in El Estrecho Natural Park (Biosphere Reserve). Showing Radical Changes in the Underwater Seascape. Front. Ecol. Evol. 2021, 9, 639161. [Google Scholar] [CrossRef]
- Casal-Porras, I.; Zubía, E.; Brun, F.G. Dilkamural: A novel chemical weapon involved in the invasive capacity of the alga Rugulopteryx okamurae in the Strait of Gibraltar. Estuar. Coast. Shelf Sci. 2021, 257, 107398. [Google Scholar] [CrossRef]
- Cuevas, B.; Arroba, A.I.; de los Reyes, C.; Gómez-Jaramillo, L.; González-Montelongo, M.C.; Zubía, E. Diterpenoids from the Brown Alga Rugulopteryx okamurae and Their Anti-Inflammatory Activity. Mar. Drugs 2021, 19, 677. [Google Scholar] [CrossRef]
- Dittel, A.I.; Epifanio, C.E. Invasion biology of the Chinese mitten crab Eriocheir sinensis: A brief review. J. Exp. Mar. Biol. Ecol. 2009, 374, 79–92. [Google Scholar] [CrossRef]
- Garcia-de-Lomas, J.; Dana, E.D.; López-Santiago, J.; González, R.; Ceballos, G.; Ortega, F. Management of the Chinese mitten crab, Eriocheir sinensis (H. Milne Edwards, 1853) in the Guadalquivir Estuary (Southern Spain). Aquat. Invasions 2010, 5, 323–330. [Google Scholar] [CrossRef]
- Roy, H.E.; Bacher, S.; Essl, F.; Adriaens, T.; Aldridge, D.C.; Bishop, J.D.D.; Blackburn, T.M.; Branquart, E.; Brodie, J.; Carboneras, C.; et al. Developing a list of invasive alien species likely to threaten biodiversity and ecosystems in the European Union. Glob. Chang. Biol. 2019, 25, 1032–1048. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Png-Gonzalez, L.; Comas-González, R.; Calvo-Manazza, M.; Follana-Berná, G.; Ballesteros, E.; Díaz-Tapia, P.; Falcón, J.M.; García Raso, J.E.; Gofas, S.; González-Porto, M.; et al. Updating the National Baseline of Non-Indigenous Species in Spanish Marine Waters. Diversity 2023, 15, 630. https://doi.org/10.3390/d15050630
Png-Gonzalez L, Comas-González R, Calvo-Manazza M, Follana-Berná G, Ballesteros E, Díaz-Tapia P, Falcón JM, García Raso JE, Gofas S, González-Porto M, et al. Updating the National Baseline of Non-Indigenous Species in Spanish Marine Waters. Diversity. 2023; 15(5):630. https://doi.org/10.3390/d15050630
Chicago/Turabian StylePng-Gonzalez, Lydia, Robert Comas-González, Matías Calvo-Manazza, Guillermo Follana-Berná, Enric Ballesteros, Pilar Díaz-Tapia, Jesús M. Falcón, J. Enrique García Raso, Serge Gofas, Marcos González-Porto, and et al. 2023. "Updating the National Baseline of Non-Indigenous Species in Spanish Marine Waters" Diversity 15, no. 5: 630. https://doi.org/10.3390/d15050630
APA StylePng-Gonzalez, L., Comas-González, R., Calvo-Manazza, M., Follana-Berná, G., Ballesteros, E., Díaz-Tapia, P., Falcón, J. M., García Raso, J. E., Gofas, S., González-Porto, M., López, E., Ramos-Esplá, A. A., Velasco, E., & Carbonell, A. (2023). Updating the National Baseline of Non-Indigenous Species in Spanish Marine Waters. Diversity, 15(5), 630. https://doi.org/10.3390/d15050630







