Short-Term and Long-Term Predictions: Is the Green Crab Carcinus maenas a Threat to Antarctica and Southern South America under a Climate-Change Scenario?
Abstract
1. Introduction
2. Materials and Methods
2.1. Occurrences
2.2. Environmental Predictors
2.3. Species Distribution Modeling: Calibration, Evaluation, and Projection
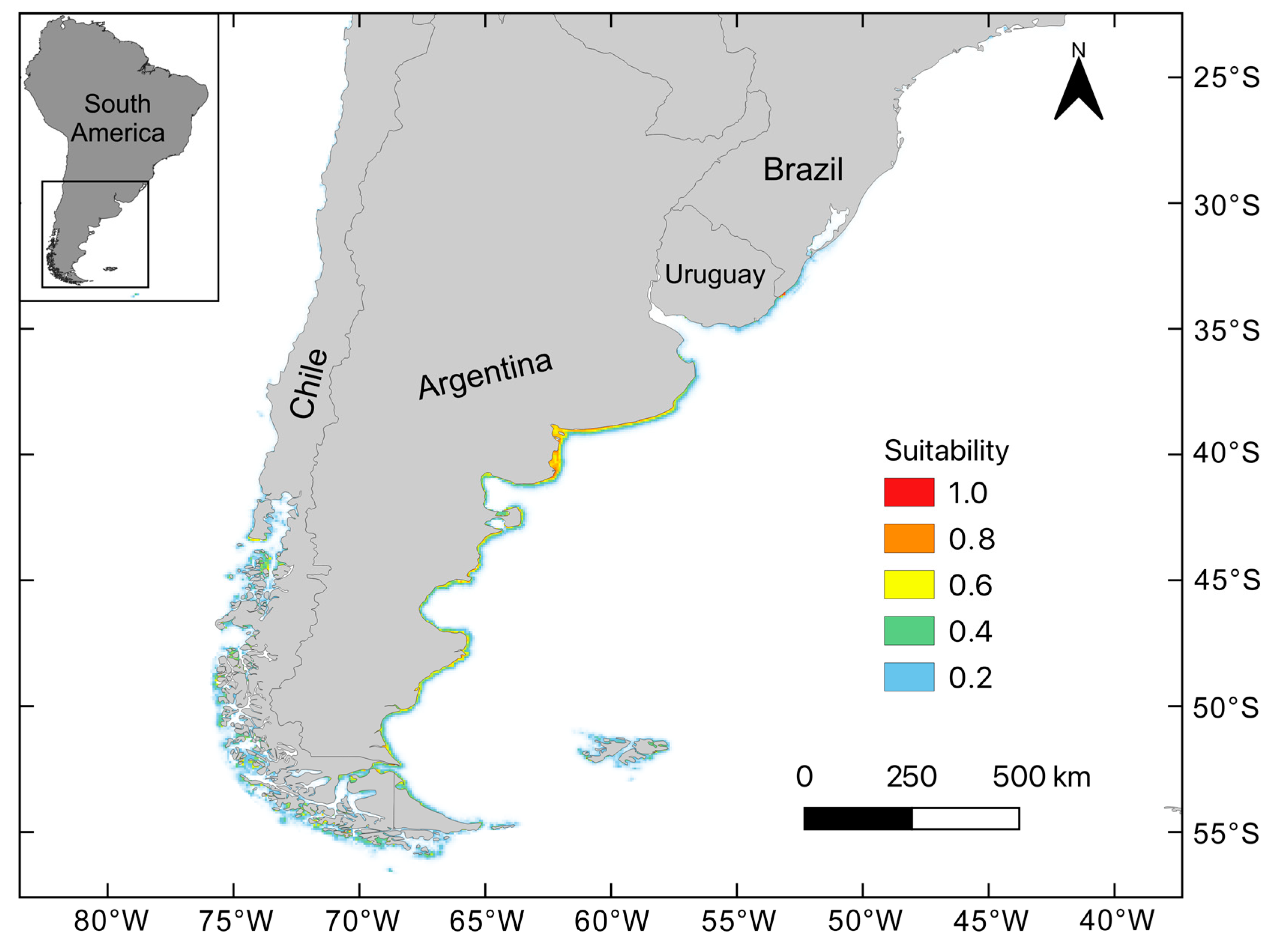
3. Results
3.1. Contemporary Habitat Suitability
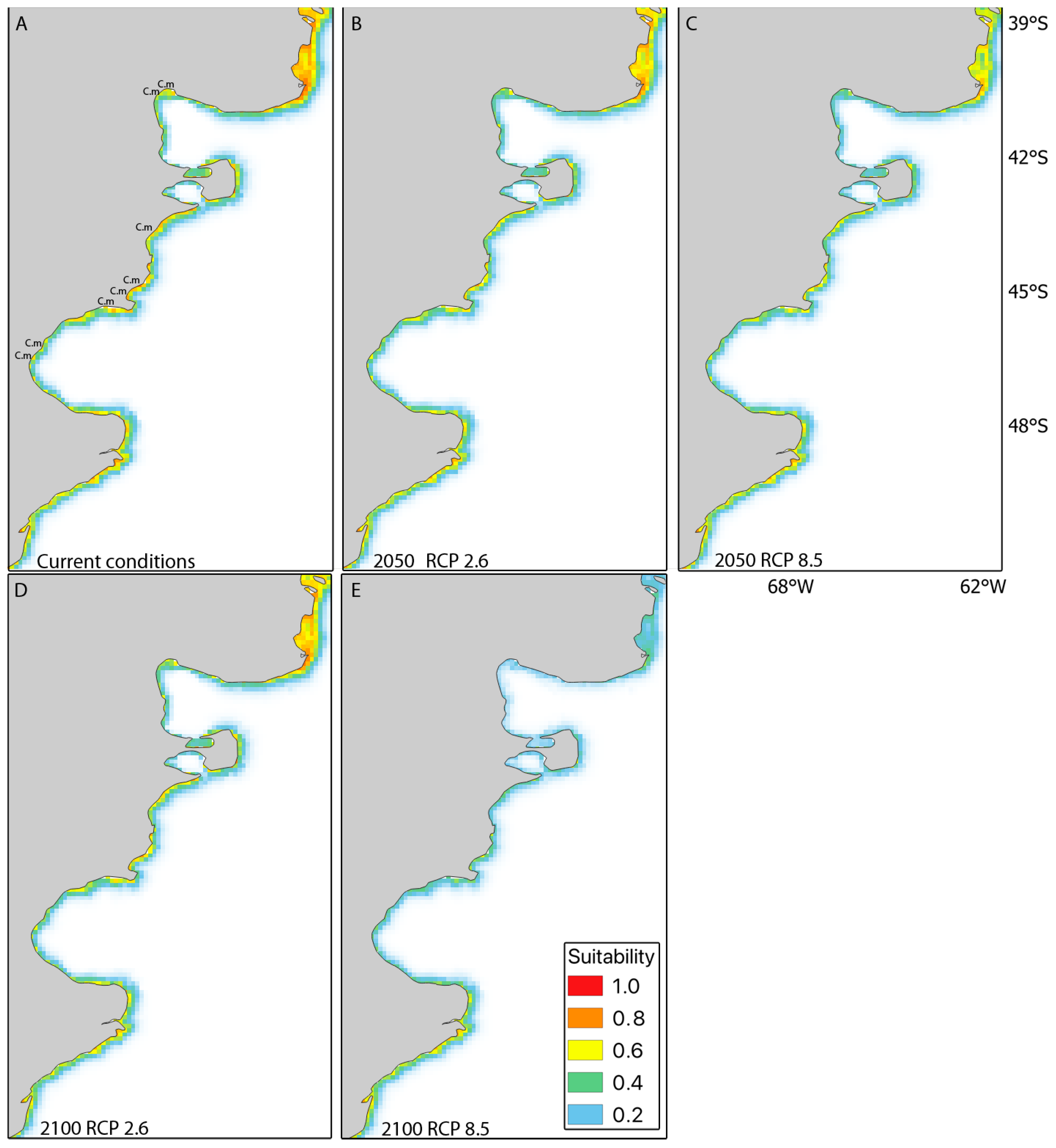
3.2. Short-Term Habitat Suitability (2050) and Long-Term Habitat Suitability (2100) under RCP2.6 and RCP8.5
4. Discussion
4.1. Crabs in Antarctica
4.2. Green Crab in Southern South America: Current Potential Distribution and Predictions by 2050 and 2100
4.3. Species Distribution Models and Invasive Species
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošík, V.; Wilson, J.R.; Richardson, D.M. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef]
- Lodge, D.M. Biological invasions: Lessons for ecology. Trends Ecol. Evol. 1993, 8, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Novoa, A.; Richardson, D.M.; Pyšek, P.; Meyerson, L.A.; Bacher, S.; Canavan, S.; Catford, J.A.; Čuda, J.; Essl, F.; Foxcroft, L.C.; et al. Invasion syndromes: A systematic approach for predicting biological invasions and facilitating effective management. Biol. Invasions 2020, 22, 1801–1820. [Google Scholar] [CrossRef]
- Heger, T.; Trepl, L. Predicting biological invasions. Biol. Invasions 2003, 5, 313–321. [Google Scholar] [CrossRef]
- Levine, J.M.; D’Antonio, C.M. Forecasting biological invasions with increasing international trade. Conserv. Biol. 2003, 17, 322–326. [Google Scholar] [CrossRef]
- Thuiller, W. Climate change and the ecologist. Nature 2007, 448, 550–552. [Google Scholar] [CrossRef]
- Stephens, A.E.; Stringer, L.D.; Suckling, D.M. Advance, retreat, resettle? Climate change could produce a zero-sum game for invasive species. Austral Entomol. 2016, 55, 177–184. [Google Scholar] [CrossRef]
- Mainka, S.A.; Howard, G.W. Climate change and invasive species: Double jeopardy. Integr. Zool. 2010, 5, 102–111. [Google Scholar] [CrossRef]
- Hellmann, J.J.; Byers, J.; Bierwagen, B.G.; Dukes, J. Five potential consequences of climate change for invasive species. Conserv. Biol. 2008, 22, 534–543. [Google Scholar] [CrossRef]
- Gallardo, B.; Aldridge, D.C. Evaluating the combined threat of climate change and biological invasions on endangered species. Biol. Conserv. 2013, 160, 225–233. [Google Scholar] [CrossRef]
- Raghavan, R.K.; Koestel, Z.; Ierardi, R.; Peterson, A.T.; Cobos, M.E. Climatic suitability of the eastern paralysis tick, Ixodes holocyclus, and its likely geographic distribution in the year 2050. Sci. Rep. 2021, 11, 15330. [Google Scholar] [CrossRef]
- Bradley, B.A.; Wilcove, D.S. When invasive plants disappear: Transformative restoration possibilities in the western United States resulting from climate change. Restor. Ecol. 2009, 17, 715–721. [Google Scholar] [CrossRef]
- Fraser, C.I.; Morrison, A.K.; Hogg, A.M.; Macaya, E.C.; van Sebille, E.; Ryan, P.G.; Padovan, A.; Jack, C.; Valdivia, N.; Waters, J.M. Antarctica’s ecological isolation will be broken by storm-driven dispersal and warming. Nat. Clim. Chang. 2018, 8, 704–708. [Google Scholar] [CrossRef]
- Cárdenas, L.; Leclerc, J.-C.; Bruning, P.; Garrido, I.; Détrée, C.; Figueroa, A.; Astorga, M.; Navarro, J.M.; Johnson, L.E.; Carlton, J.T.; et al. First mussel settlement observed in Antarctica reveals the potential for future invasions. Sci. Rep. 2020, 10, 5552. [Google Scholar] [CrossRef] [PubMed]
- Carlton, J.T. Community assembly and historical biogeography in the North Atlantic Ocean: The potential role of human-mediated dispersal vectors. In Migrations and Dispersal of Marine Organisms, Proceedings of the 37th European Marine Biology Symposium, Reykjavík, Iceland, 5–9 August 2002; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–8. [Google Scholar]
- Grosholz, E.D.; Ruiz, G.M. Predicting the impact of introduced marine species: Lessons from the multiple invasions of the European green crab Carcinus maenas. Biol. Conserv. 1996, 78, 59–66. [Google Scholar] [CrossRef]
- Compton, T.J.; Leathwick, J.R.; Inglis, G.J. Thermogeography predicts the potential global range of the invasive European green crab (Carcinus maenas). Divers. Distrib. 2010, 16, 243–255. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Barón, P.J.; Orensanz, J.M. A prediction come true: The green crab invades the Patagonian coast. Biol. Invasions 2005, 7, 547–552. [Google Scholar] [CrossRef]
- Darling, J.A.; Bagley, M.J.; Roman, J.; Tepolt, C.K.; Geller, J.B. Genetic patterns across multiple introductions of the globally invasive crab genus Carcinus. Mol. Ecol. 2008, 17, 4992–5007. [Google Scholar] [CrossRef]
- Frederich, M.; Sartoris, F.; Arntz, W.; Pörtner, H.-O. Haemolymph Mg(2+) regulation in decapod crustaceans: Physiological correlates and ecological consequences in polar areas. J. Exp. Biol. 2000, 203, 1383–1393. [Google Scholar] [CrossRef]
- Aronson, R.B.; Frederich, M.; Price, R.; Thatje, S. Prospects for the return of shell-crushing crabs to Antarctica. J. Biogeogr. 2015, 42, 1–7. [Google Scholar] [CrossRef]
- Holland, O.; Shaw, J.; Stark, J.S.; Wilson, K.A. Hull fouling marine invasive species pose a very low, but plausible, risk of introduction to East Antarctica in climate change scenarios. Divers. Distrib. 2021, 27, 973–988. [Google Scholar] [CrossRef]
- McCarthy, A.H.; Peck, L.S.; Aldridge, D.C. Ship traffic connects Antarctica’s fragile coasts to worldwide ecosystems. Proc. Natl. Acad. Sci. USA 2022, 119, e2110303118. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.T.; Soberon, J. Species distribution modeling and ecological niche modeling: Getting the concepts right. Nat. Conserv. 2012, 10, 102–107. [Google Scholar] [CrossRef]
- Miller, J. Species distribution modeling. Geogr. Compass 2010, 4, 490–509. [Google Scholar] [CrossRef]
- Randin, C.F.; Dirnböck, T.; Dullinger, S.; Zimmermann, N.E.; Zappa, M.; Guisan, A. Are niche-based species distribution models transferable in space? J. Biogeogr. 2006, 33, 1689–1703. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Stehfest, E.; den Elzen, M.G.; Kram, T.; van Vliet, J.; Deetman, S.; Isaac, M.; Klein Goldewijk, K.; Hof, A.; Mendoza Beltran, A.; et al. RCP2. 6: Exploring the possibility to keep global mean temperature increase below 2 C. Clim. Chang. 2011, 109, 95–116. [Google Scholar] [CrossRef]
- Schwalm, C.R.; Glendon, S.; Duffy, P.B. RCP8. 5 tracks cumulative CO2 emissions. Proc. Natl. Acad. Sci. USA 2020, 117, 19656–19657. [Google Scholar] [CrossRef]
- Olhoff, A.; Christensen, J.M. (Eds.) Emissions Gap Report 2020; UN Environment Programme; UNEP DTU Partnership: Copenhagen, Denmark, 2019. [Google Scholar]
- GBIF.org. GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.hubmph (accessed on 2 September 2021).
- Zhang, Z.; Mammola, S.; McLay, C.L.; Capinha, C.; Yokota, M. To invade or not to invade? Exploring the niche-based processes underlying the failure of a biological invasion using the invasive Chinese mitten crab. Sci. Total. Environ. 2020, 728, 138815. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Tyberghein, L.; Verbruggen, H.; Pauly, K.; Troupin, C.; Mineur, F.; De Clerck, O. Bio-ORACLE: A global environmental dataset for marine species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 272–281. [Google Scholar] [CrossRef]
- Assis, J.; Tyberghein, L.; Bosch, S.; Verbruggen, H.; Serrão, E.A.; De Clerck, O.; Tittensor, D. Bio-ORACLE v2.0: Extending marine data layers for bioclimatic modelling. Glob. Ecol. Biogeogr. 2018, 27, 277–284. [Google Scholar] [CrossRef]
- Sbrocco, E.J.; Barber, P.H. MARSPEC: Ocean climate layers for marine spatial ecology: Ecological Archives E094-086. Ecology 2013, 94, 979. [Google Scholar] [CrossRef]
- Derviche, P.; Saucsen, A.; Spier, D.; Lana, P. Distribution patterns and habitat suitability of the non-native brittle star Ophiothela mirabilis Verrill, 1867 along the Western Atlantic. J. Sea Res. 2021, 168, 101994. [Google Scholar] [CrossRef]
- López-Farrán, Z.; Guillaumot, C.; Vargas-Chacoff, L.; Paschke, K.; Dulière, V.; Danis, B.; Poulin, E.; Saucède, T.; Waters, J.; Gérard, K. Is the southern crab Halicarcinus planatus (Fabricius, 1775) the next invader of Antarctica? Glob. Chang. Biol. 2021, 27, 3487–3504. [Google Scholar] [CrossRef]
- Castro, K.L.; Battini, N.; Giachetti, C.B.; Trovant, B.; Abelando, M.; Basso, N.G.; Schwindt, E. Early detection of marine invasive species following the deployment of an artificial reef: Integrating tools to assist the decision-making process. J. Environ. Manag. 2021, 297, 113333. [Google Scholar] [CrossRef]
- Gimenez, L.; Rivera, R.; Brante, A. One step ahead of sea anemone invasions with ecological niche modeling: Potential distributions and niche dynamics of three successful invasive species. Mar. Ecol. Prog. Ser. 2022, 690, 83–95. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Anderson, D.; Burnham, K. Model Selection and Multi-Model Inference, 2nd ed.; Springer: New York, NY, USA, 2004; Volume 63, p. 10. [Google Scholar]
- Peterson, L.E.; Coleman, M.A. Machine learning-based receiver operating characteristic (ROC) curves for crisp and fuzzy classification of DNA microarrays in cancer research. Int. J. Approx. Reason. 2008, 47, 17–36. [Google Scholar] [CrossRef]
- Boyce, M.S.; Vernier, P.R.; Nielsen, S.; Schmiegelow, F.K. Evaluating resource selection functions. Ecol. Model. 2002, 157, 281–300. [Google Scholar] [CrossRef]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A.; et al. A standard protocol for reporting species distribution models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Sillero, N.; Barbosa, A.M. Common mistakes in ecological niche models. Int. J. Geogr. Inf. Sci. 2021, 35, 213–226. [Google Scholar] [CrossRef]
- Osorio-Olvera, L.; Lira-Noriega, A.; Soberón, J.; Peterson, A.T.; Falconi, M.; Contreras-Díaz, R.G.; Martínez-Meyer, E.; Barve, V.; Barve, N. ntbox: An r package with graphical user interface for modelling and evaluating multidimensional ecological niches. Methods Ecol. Evol. 2020, 11, 1199–1206. [Google Scholar] [CrossRef]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; d’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range?shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Team, R.C. R Core Team R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org (accessed on 27 January 2023).
- Association, Q. QGIS Geographic Information System; QGIS Association: Lucerne, Switzerland, 2021; Available online: http://www.qgis.org (accessed on 27 October 2022).
- Walton, W.C.; MacKinnon, C.; Rodriguez, L.F.; Proctor, C.; Ruiz, G.M. Effect of an invasive crab upon a marine fishery: Green crab, Carcinus maenas, predation upon a venerid clam, Katelysia scalarina, in Tasmania (Australia). J. Exp. Mar. Biol. Ecol. 2002, 272, 171–189. [Google Scholar] [CrossRef]
- Hollebone, A.L.; Hay, M.E. An invasive crab alters interaction webs in a marine community. Biol. Invasions 2008, 10, 347–358. [Google Scholar] [CrossRef]
- Hughes, K.A.; Pescott, O.L.; Peyton, J.; Adriaens, T.; Cottier-Cook, E.J.; Key, G.; Rabitsch, W.; Tricarico, E.; Barnes, D.K.; Baxter, N.; et al. Invasive non-native species likely to threaten biodiversity and ecosystems in the Antarctic Peninsula region. Glob. Chang. Biol. 2020, 26, 2702–2716. [Google Scholar] [CrossRef]
- Carlton, J.T.; Cohen, A.N. Episodic global dispersal in shallow water marine organisms: The case history of the European shore crabs Carcinus maenas and C. aestuarii. J. Biogeogr. 2020, 30, 1809–1820. [Google Scholar] [CrossRef]
- Cohen, A.N.; Carlton, J.T.; Fountain, M.C. Introduction, dispersal and potential impacts of the green crab Carcinus maenas in San Francisco Bay, California. Mar. Biol. 1995, 122, 225–237. [Google Scholar] [CrossRef]
- Villaseñor-Parada, C.; Pauchard, A.; Macaya, E.C. Ecología de invasiones marinas en Chile continental: ¿Qué sabemos y que nos falta por saber? Rev. Biol. Mar. Oceanogr. 2017, 52, 1–17. [Google Scholar] [CrossRef]
- Torres, P.J.; González-Pisani, X. Primer registro del cangrejo verde, Carcinus maenas (Linnaeus, 1758), en Golfo Nuevo, Argentina: Un nuevo límite norte de distribución en costas patagónicas. Ecol. Austral 2016, 26, 134–137. [Google Scholar] [CrossRef]
- Yorio, P.; Suárez, N.; Kasinsky, T.; Pollicelli, M.; Ibarra, C.; Gatto, A. The introduced green crab (Carcinus maenas) as a novel food resource for the opportunistic kelp gull (Larus dominicanus) in Argentine Patagonia. Aquat. Invasions 2020, 15, 140–159. [Google Scholar] [CrossRef]
- Broggio, M.F.; Garcia, C.A.E.; Silva, R.R. Evaluation of South Atlantic Thermohaline Properties from BESM-OA2. 5 and Three Additional Global Climate Models. Ocean. Coast. Res. 2021, 69. [Google Scholar] [CrossRef]
- Nakamura, R.; Mäll, M. Pseudo Global Warming Sensitivity Experiments of Subtropical Cyclone Anita (2010) Under RCP 8.5 Scenario. J. Geophys. Res. Atmos. 2021, 126, e2021JD035261. [Google Scholar] [CrossRef]
- Young, A.M.; Elliott, J.A. Life history and population dynamics of green crabs (Carcinus maenas). Fishes 2019, 5, 4. [Google Scholar] [CrossRef]
- Zimmermann, N.E.; Edwards, T.C., Jr.; Graham, C.H.; Pearman, P.B.; Svenning, J.C. New trends in species distribution modelling. Ecography 2010, 33, 985–989. [Google Scholar] [CrossRef]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J.; Gordon, A.; Kujala, H.; Lentini, P.E.; McCarthy, M.A.; Tingley, R.; Wintle, B.A. Is my species distribution model fit for purpose? Matching data and models to applications. Glob. Ecol. Biogeogr. 2015, 24, 276–292. [Google Scholar] [CrossRef]
- Mainali, K.P.; Warren, D.L.; Dhileepan, K.; McConnachie, A.; Strathie, L.; Hassan, G.; Karki, D.; Shrestha, B.B.; Parmesan, C. Projecting future expansion of invasive species: Comparing and improving methodologies for species distribution modeling. Glob. Chang. Biol. 2015, 21, 4464–4480. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Lafond, V.; Griess, V.C. Species distribution models (SDM): Applications, benefits and challenges in invasive species management. CABI Rev. 2019, 14, 1–13. [Google Scholar] [CrossRef]
- Beaury, E.M.; Fusco, E.J.; Jackson, M.R.; Laginhas, B.B.; Morelli, T.L.; Allen, J.M.; Pasquarella, V.J.; Bradley, B.A. Incorporating climate change into invasive species management: Insights from managers. Biol. Invasions 2020, 22, 233–252. [Google Scholar] [CrossRef]
- Leishman, M.R.; Gallagher, R.V. Will there be a shift to alien-dominated vegetation assemblages under climate change? Divers. Distrib. 2015, 21, 848–852. [Google Scholar] [CrossRef]
- Bezeng, B.S.; Morales-Castilla, I.; van der Bank, M.; Yessoufou, K.; Daru, B.H.; Davies, T.J. Climate change may reduce the spread of non-native species. Ecosphere 2017, 8, e01694. [Google Scholar] [CrossRef]
- Bradley, B.A.; Oppenheimer, M.; Wilcove, D.S. Climate change and plant invasions: Restoration opportunities ahead? Glob. Chang. Biol. 2009, 15, 1511–1521. [Google Scholar] [CrossRef]
- Teo, E.J.; Hailu, S.; Kelava, S.; Zalucki, M.P.; Furlong, M.J.; Nakao, R.; Barker, D.; Barker, S.C. Climatic requirements of the southern paralysis tick, Ixodes cornuatus, with a consideration of its host, Vombatus ursinus, and the possible geographic range of the tick up to 2090. Ticks Tick-Borne Dis. 2021, 12, 101758. [Google Scholar] [CrossRef]
- Anibaba, Q.A.; Dyderski, M.K.; Jagodziński, A.M. Predicted range shifts of invasive giant hogweed (Heracleum mantegazzianum) in Europe. Sci. Total Environ. 2022, 825, 154053. [Google Scholar] [CrossRef]
- Queiroga, H. Distribution and drift of the crab Carcinus maenas (L.)(Decapoda, Portunidae) larvae over the continental shelf off northern Portugal in April 1991. J. Plankton Res. 1996, 18, 1981–2000. [Google Scholar] [CrossRef]
- Malve, M.E.; Rivadeneira, M.; Gordillo, S. Northward range expansion of the European green crab Carcinus maenas in the SW Atlantic: A synthesis after ~20 years of invasion history. bioRxiv 2020. [Google Scholar] [CrossRef]
- Yin, X.; Martinez, A.S.; Sepúlveda, M.S.; Christie, M.R. Rapid genetic adaptation to recently colonized environments is driven by genes underlying life history traits. BMC Genom. 2021, 22, 269. [Google Scholar] [CrossRef] [PubMed]
- Tepolt, C.K.; Grosholz, E.D.; de Rivera, C.E.; Ruiz, G.M. Balanced polymorphism fuels rapid selection in an invasive crab despite high gene flow and low genetic diversity. Mol. Ecol. 2022, 31, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Dobrzycka-Krahel, A.; Kemp, J.A.; Hidalgo, M.L. Cold-tolerant traits that favour northwards movement and establishment of Mediterranean and Ponto-Caspian aquatic invertebrates. Aquat. Sci. 2022, 84, 47. [Google Scholar] [CrossRef]
- Meredith, M.P.; Brandon, M.A. Oceanography and sea ice in the Southern Ocean. In Sea Ice, 3rd ed.; David, T.N., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2017. [Google Scholar]
- Müller Baigorria, M.; Aguilar, A.; Cabrera Galeano, D.; Fraire, S.; Patocchi, A.; Sierra, C.; Sánchez, L.; Hünicken, L.; González, R.; Narvarte, M. Caracterización Demográfica del Cangrejo Verde Carcinus maenas en dos Sectores Costeros de Las Grutas, Río Negro, Argentina. Available online: http://rdi.uncoma.edu.ar/handle/uncomaid/16872 (accessed on 27 January 2023).
- Vinuesa, J.H. Distribución de crustáceos decápodos y estomatópodos del golfo San Jorge, Argentina. Rev. De Biol. Mar. Y Oceanogr. 2005, 40, 7–21. [Google Scholar] [CrossRef]
- Vinuesa, J.H. Molt and reproduction of the European green crab Carcinus maenas (Decapoda: Portunidae) in Patagonia, Argentina. Rev. De Biol. Trop. 2007, 55, 49–54. [Google Scholar] [CrossRef]
| Environmental Predictor (Unit) | Present | Future | Source |
|---|---|---|---|
| Depth (m) | - | Same as present | MARSPEC |
| Distance to shore (km) | - | Same as present | |
| Mean seafloor temperature (°C) | 2000–2014 | RCP 2.6 and 8.5 for 2050 and 2010 | Bio-Oracle |
| Min seafloor temperature (°C) | 2000–2014 | RCP 2.6 and 8.5 for 2050 and 2010 | |
| Max seafloor temperature (°C) | 2000–2014 | RCP 2.6 and 8.5 for 2050 and 2010 | |
| Mean seafloor salinity (PSS) | 2000–2014 | RCP 2.6 and 8.5 for 2050 and 2010 | |
| Min seafloor salinity (PSS) | 2000–2014 | RCP 2.6 and 8.5 for 2050 and 2010 | |
| Max seafloor salinity (PSS) | 2000–2014 | RCP 2.6 and 8.5 for 2050 and 2010 | |
| Mean surface primary productivity (g·m−3 day−1) | 2000–2014 | Same as present | |
| Mean seafloor current velocity (m−1) | 2000–2014 | RCP 2.6 and 8.5 for 2050 and 2010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera-Escalona, I.; Gimenez, L.H.; Brante, A. Short-Term and Long-Term Predictions: Is the Green Crab Carcinus maenas a Threat to Antarctica and Southern South America under a Climate-Change Scenario? Diversity 2023, 15, 632. https://doi.org/10.3390/d15050632
Vera-Escalona I, Gimenez LH, Brante A. Short-Term and Long-Term Predictions: Is the Green Crab Carcinus maenas a Threat to Antarctica and Southern South America under a Climate-Change Scenario? Diversity. 2023; 15(5):632. https://doi.org/10.3390/d15050632
Chicago/Turabian StyleVera-Escalona, Iván, Lucas H. Gimenez, and Antonio Brante. 2023. "Short-Term and Long-Term Predictions: Is the Green Crab Carcinus maenas a Threat to Antarctica and Southern South America under a Climate-Change Scenario?" Diversity 15, no. 5: 632. https://doi.org/10.3390/d15050632
APA StyleVera-Escalona, I., Gimenez, L. H., & Brante, A. (2023). Short-Term and Long-Term Predictions: Is the Green Crab Carcinus maenas a Threat to Antarctica and Southern South America under a Climate-Change Scenario? Diversity, 15(5), 632. https://doi.org/10.3390/d15050632








