Seasonal Changes in the Size Distribution of Copepods Is Affected by Coastal Upwelling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Sample Processing
2.3. Image Processing
2.4. Data Analysis
3. Results
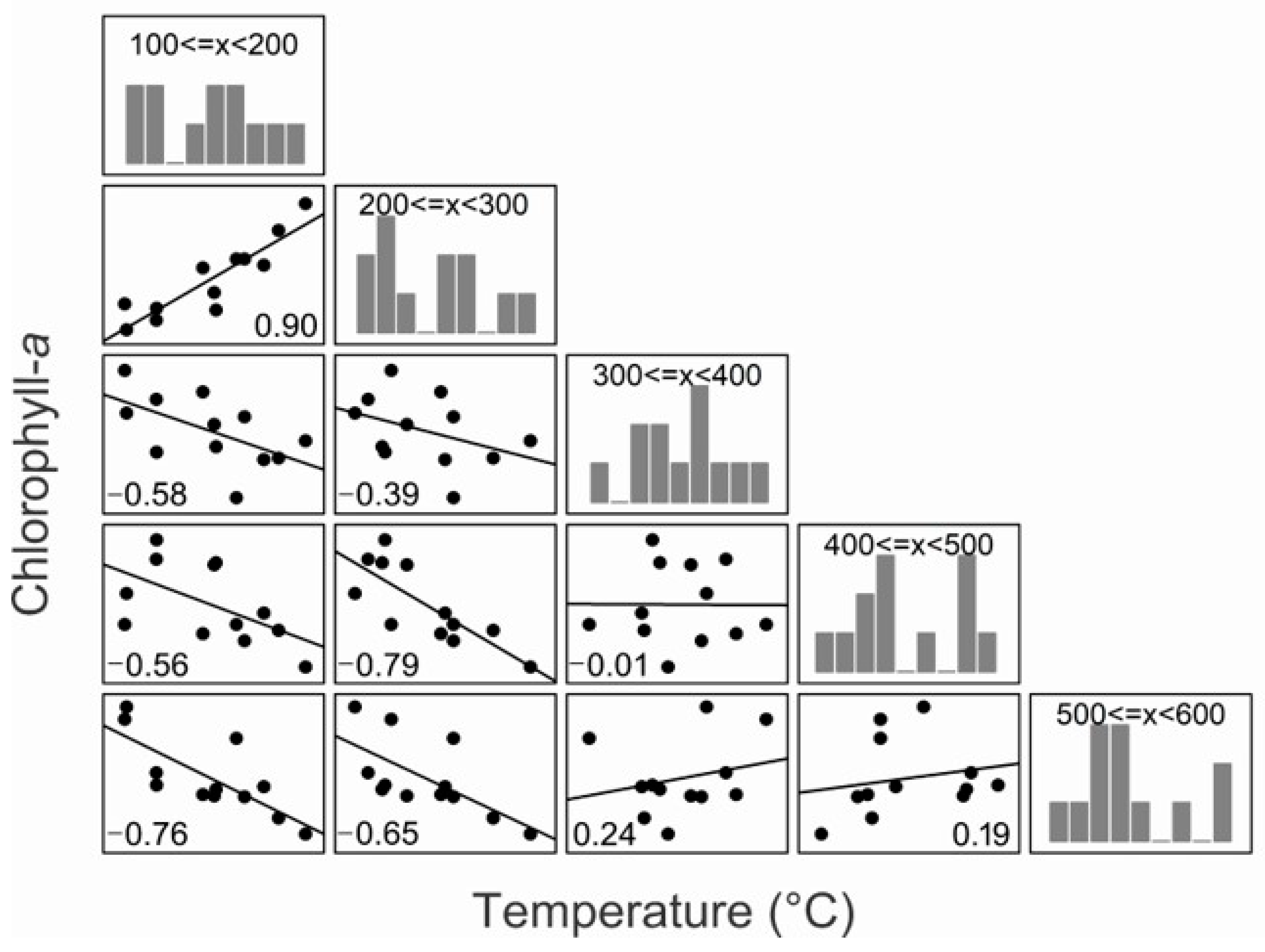
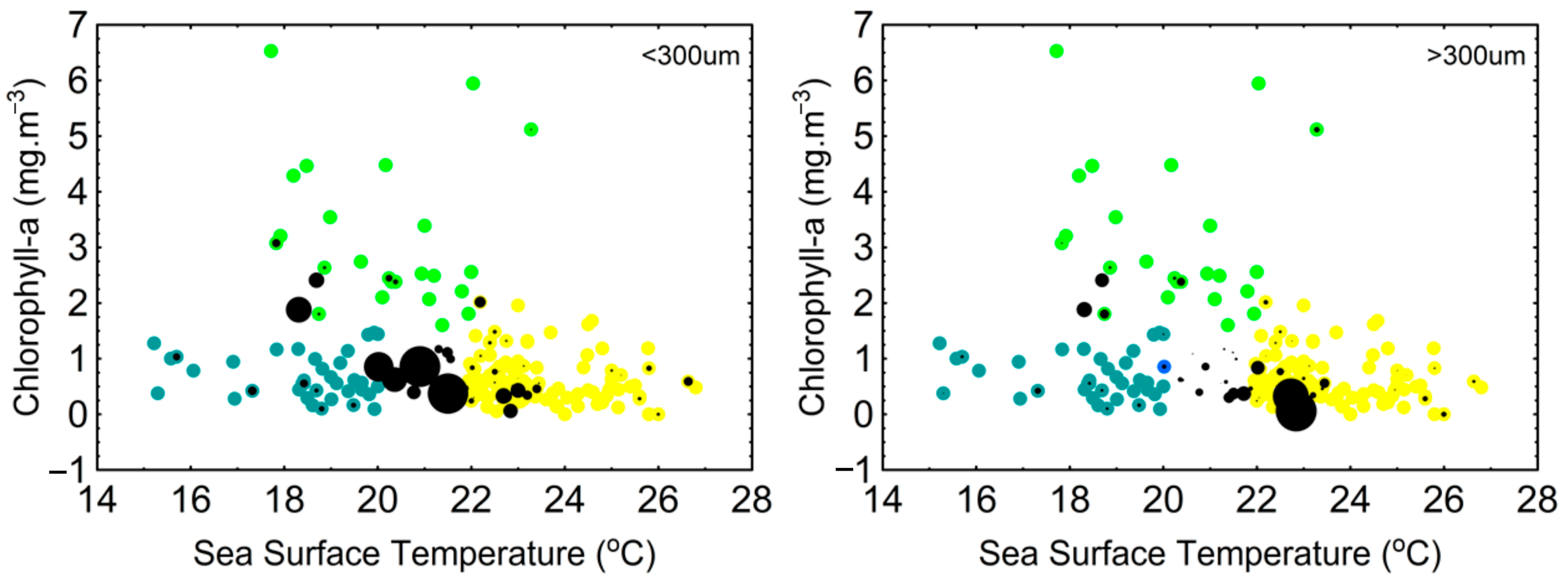
3.1. Temperature and Chlorophyll-a
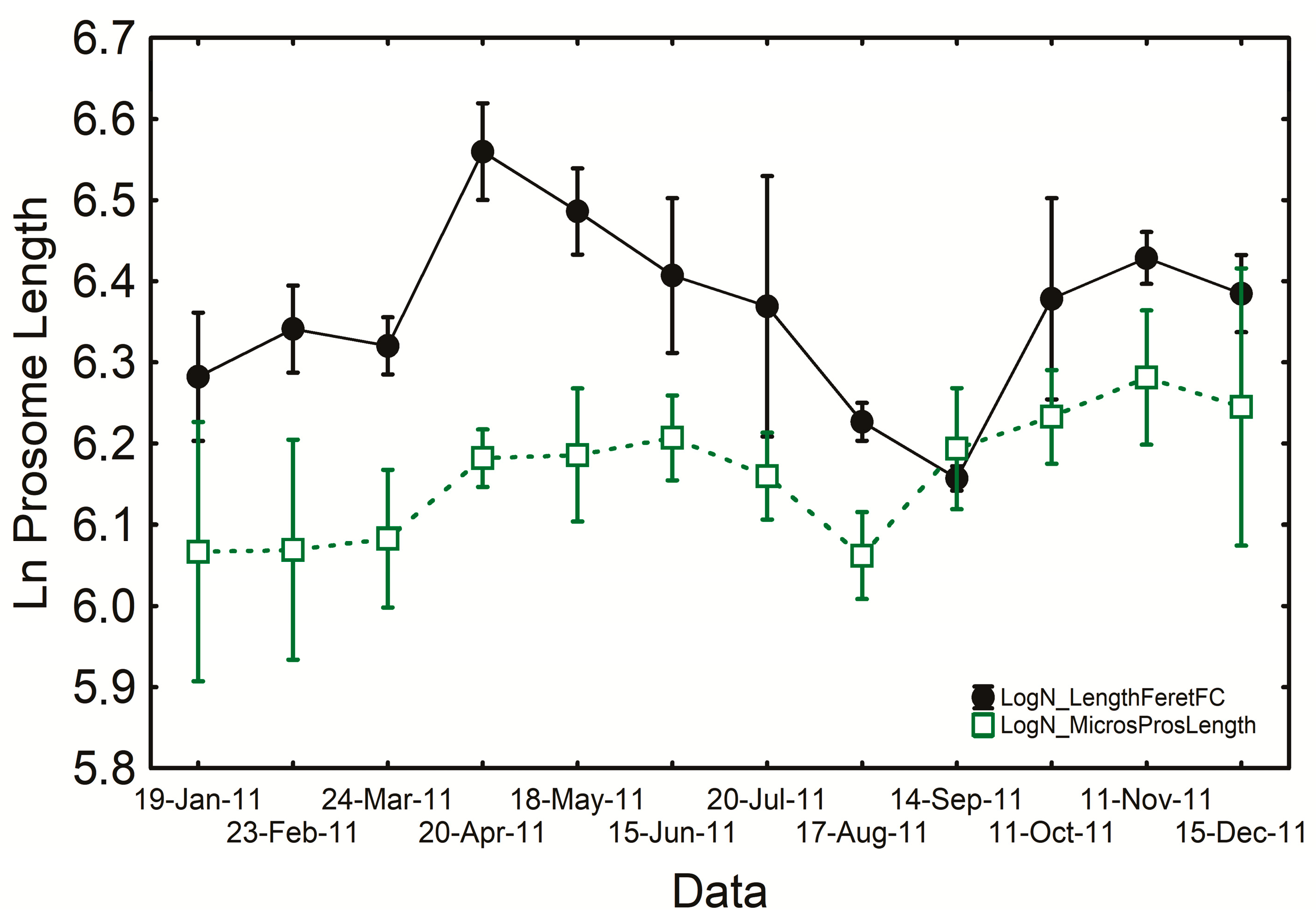
3.2. Copepod Measurements in ImageJ (IJ) and FlowCAM (FC)
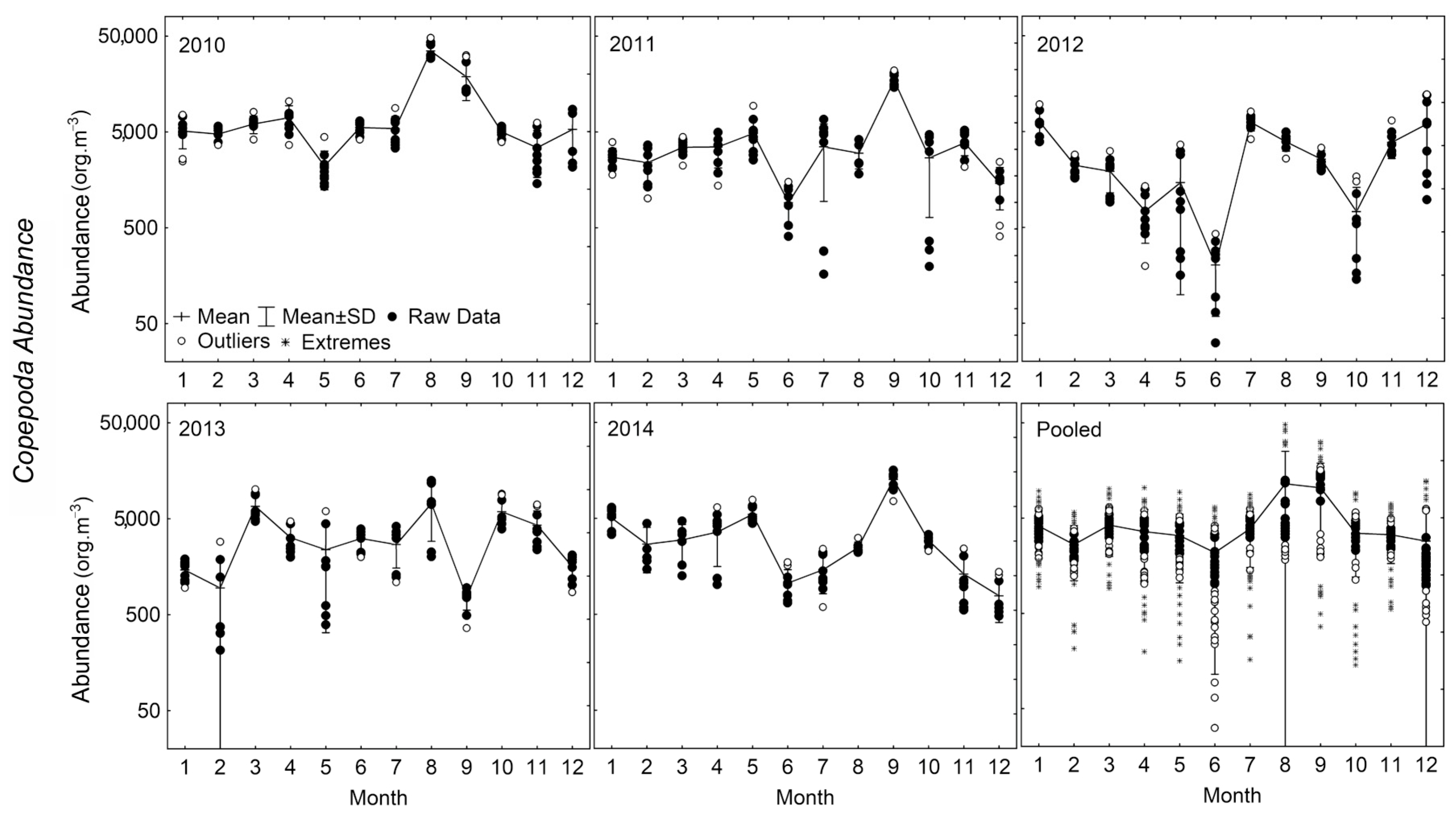
3.3. Copepod Assemblage
3.4. Sea Temperature Cycles’ Effect on Copepod Assemblage
4. Discussion
4.1. Temperature and Chlorophyll-a
4.2. Copepod Measurements in ImageJ (IJ) and FlowCAM (FC)
4.3. Copepod Assemblage and Sea Temperature Cycles on Copepod Assemblage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Décima, M. Zooplankton Trophic Structure and Ecosystem Productivity. Mar. Ecol. Prog. Ser. 2022, 692, 23–42. [Google Scholar] [CrossRef]
- Everett, J.; Heneghan, R.; Blanchard, J.; Suthers, I.; Pakhomov, E.; Sykes, P.; Schoeman, D.; Baird, M.; Basedow, S.L.; Błachowiak-Samołyk, K.; et al. Self-Organisation of Zooplankton Communities Produces Similar Food Chain Lengths throughout the Ocean; In Review; Research Square LLC: Durham, NA, USA, 2022. [Google Scholar]
- Fernandes, L.D.D.A.; Fagundes Netto, E.B.; Coutinho, R. Inter-Annual Cascade Effect on Marine Food Web: A Benthic Pathway Lagging Nutrient Supply to Pelagic Fish Stock. PLoS ONE 2017, 12, e0184512. [Google Scholar] [CrossRef]
- Hébert, M.P.; Beisner, B.E.; Maranger, R. Linking Zooplankton Communities to Ecosystem Functioning: Toward an Effect-Trait Framework. J. Plankton Res. 2017, 39, 3–12. [Google Scholar] [CrossRef]
- Kang, H.C.; Jeong, H.J.; Jang, S.H.; Lee, K.H. Feeding by Common Heterotrophic Protists on the Phototrophic Dinoflagellate Biecheleriopsis Adriatica (Suessiaceae) Compared to That of Other Suessioid Dinoflagellates. ALGAE 2019, 34, 127–140. [Google Scholar] [CrossRef]
- Burki, F.; Sandin, M.M.; Jamy, M. Diversity and Ecology of Protists Revealed by Metabarcoding. Curr. Biol. 2021, 31, R1267–R1280. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Kim, D.; Liu, H.; Kang, C.K. Variability in Copepod Trophic Levels and Feeding Selectivity Based on Stable Isotope Analysis in Gwangyang Bay of the Southern Coast of the Korean Peninsula. Biogeosciences 2018, 15, 2055–2073. [Google Scholar] [CrossRef]
- Dvoretsky, V.; Dvoretsky, A. Morphological Plasticity in the Small Copepod Oithona Similis in the Barents and White Seas. Mar. Ecol. Prog. Ser. 2009, 385, 165–178. [Google Scholar] [CrossRef]
- Skjoldal, H.R. Species Composition of Three Size Fractions of Zooplankton Used in Routine Monitoring of the Barents Sea Ecosystem. J. Plankton Res. 2021, 43, 762–772. [Google Scholar] [CrossRef]
- Fakhraee, M.; Planavsky, N.J.; Reinhard, C.T. The Role of Environmental Factors in the Long-Term Evolution of the Marine Biological Pump. Nat. Geosci. 2020, 13, 812–816. [Google Scholar] [CrossRef]
- Fernandes, L.D.D.A.; Quintanilha, J.; Monteiro-Ribas, W.; Gonzalez-Rodriguez, E.; Coutinho, R. Seasonal and Interannual Coupling between Sea Surface Temperature, Phytoplankton and Meroplankton in the Subtropical South-Western Atlantic Ocean. J. Plankton Res. 2012, 34, 236–244. [Google Scholar] [CrossRef]
- Oliveira, M.M.F.; Pereira, G.C.; Ebecken, N.F.F.; Oliveira, J.L.F. Multivariate Analysis of Extreme Physical, Biological and Chemical Patterns in the Dynamics of Aquatic Ecosystem. J. Environ. Prot. 2015, 6, 885–901. [Google Scholar] [CrossRef]
- Rosa, J.C.L.; Monteiro-Ribas, W.M.; Fernandes, L.D.A. Herbivorous Copepods with Emphasis on Dynamic Paracalanus Quasimodo in an Upwelling Region. Braz. J. Oceanogr. 2016, 64, 67–74. [Google Scholar] [CrossRef]
- Guenther, M.; Valentin, J.L. Bacterial and Phytoplankton Production in Two Coastal Systems Influenced By Distinct Eutrophication Processes. Oecologia Bras. 2008, 12, 172–178. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, E.; Valentin, J.L.; Andre, S.I.; Jacob, S.A. Upwelling and Downwelling at Cabo Frio (Brazil): Comparison of Biomass and Primary Production Responses. J. Plankton Res. 1992, 14, 289–306. [Google Scholar] [CrossRef]
- Hunt, B.P.V.; Carlotti, F.; Donoso, K.; Pagano, M.; D’Ortenzio, F.; Taillandier, V.; Conan, P. Trophic Pathways of Phytoplankton Size Classes through the Zooplankton Food Web over the Spring Transition Period in the North-West Mediterranean Sea. J. Geophys. Res. Ocean. 2017, 122, 6309–6324. [Google Scholar] [CrossRef]
- Chen, T.-C.; Ho, P.-C.; Gong, G.-C.; Tsai, A.-Y.; Hsieh, C. Finding Approaches to Exploring the Environmental Factors That Influence Copepod-Induced Trophic Cascades in the East China Sea. Diversity 2021, 13, 299. [Google Scholar] [CrossRef]
- Moe, S.; Hobæk, A.; Persson, J.; Skjelbred, B.; Løvik, J. Shifted Dynamics of Plankton Communities in a Restored Lake: Exploring the Effects of Climate Change on Phenology through Four Decades. Clim. Res. 2022, 86, 125–143. [Google Scholar] [CrossRef]
- Toruan, R.L.; Coggins, L.X.; Ghadouani, A. Response of Zooplankton Size Structure to Multiple Stressors in Urban Lakes. Water 2021, 13, 2305. [Google Scholar] [CrossRef]
- Rosa, J.C.L.; Batista, L.L.; Monteiro-ribas, W.M. Tracking of Spatial Changes in the Structure of the Zooplankton Community According to Multiple Abiotic Factors along a Hypersaline Lagoon. Nauplius 2020, 28, 1–10. [Google Scholar] [CrossRef]
- Bradford-Grieve, J.M.; Markhasena, E.L.; Rocha, C.E.F.; Abiahy, B. Copepoda. In South Atlantic Zooplankton; Boltovskoy, D., Ed.; Backhuys Publishers: Leiden, The Netherlands, 1999; pp. 869–1098. [Google Scholar]
- Sadaiappan, B.; PrasannaKumar, C.; Nambiar, V.U.; Subramanian, M.; Gauns, M.U. Meta-Analysis Cum Machine Learning Approaches Address the Structure and Biogeochemical Potential of Marine Copepod Associated Bacteriobiomes. Sci. Rep. 2021, 11, 3312. [Google Scholar] [CrossRef]
- Setubal, R.B.; Do Nascimento, R.A.; Bozelli, R.L. Zooplankton Secondary Production: Main Methods, Overview and Perspectives from Brazilian Studies. Int. Aquat. Res. 2020, 12, 85–99. [Google Scholar] [CrossRef]
- Evans, L.E.; Hirst, A.G.; Kratina, P.; Beaugrand, G. Temperature-mediated Changes in Zooplankton Body Size: Large Scale Temporal and Spatial Analysis. Ecography 2020, 43, 581–590. [Google Scholar] [CrossRef]
- Sha, Y.; Zhang, H.; Lee, M.; Björnerås, C.; Škerlep, M.; Gollnisch, R.; Herzog, S.D.; Ekelund Ugge, G.; Vinterstare, J.; Hu, N.; et al. Diel Vertical Migration of Copepods and Its Environmental Drivers in Subtropical Bahamian Blue Holes. Aquat. Ecol. 2021, 55, 1157–1169. [Google Scholar] [CrossRef]
- Valentin, J.L. Spatial Structure of the Zooplankton Community in the Cabo Frio Region (Brazil) Influenced by Coastal Upwelling. Hydrobiologia 1984, 113, 183–199. [Google Scholar] [CrossRef]
- Valentin, J.L.; Monteiro-Ribas, W.M.; Mureb, M.A. O Zooplâncton Das Águas Superficiais Costeiras Do Litoral Fluminense: Análise Multivariada. Ciênc. Cult. 1987, 39, 265–271. [Google Scholar]
- Harris, R.P.; Wiebe, P.H.; Lenz, J.; Skjoldal, H.R.; Huntley, M. Zooplankton Methodology Manual; Academic Press: Cambridge, MA, USA, 2000; ISBN 0-12-327645-4. [Google Scholar]
- Witty, L.M. Practical Guide to Identifying Freshwater Crustacean Zooplankton; Cooperative Freshwater Ecology Unit: Sudbury, ON, Canada, 2004. [Google Scholar]
- Álvarez, E.; Moyano, M.; López-Urrutia, Á.; Nogueira, E.; Scharek, R. Routine Determination of Plankton Community Composition and Size Structure: A Comparison between FlowCAM and Light Microscopy. J. Plankton Res. 2014, 36, 170–184. [Google Scholar] [CrossRef]
- Le Bourg, B.; Cornet-Barthaux, V.; Pagano, M.; Blanchot, J. FlowCAM as a Tool for Studying Small (80–1000 mm) Metazooplankton Communities. J. Plankton Res. 2015, 37, 666–670. [Google Scholar] [CrossRef]
- Sieracki, C.K.; Sieracki, M.E.; Yentsch, C.S. An Imaging-in-Flow System for Automated Analysis of Marine Microplankton. Mar. Ecol. Prog. Ser. 1998, 168, 285–296. [Google Scholar] [CrossRef]
- Álvarez, E.; López-Urrutia, Á.; Nogueira, E. Improvement of Plankton Biovolume Estimates Derived from Image-Based Automatic Sampling Devices: Application to FlowCAM. J. Plankton Res. 2012, 34, 454–469. [Google Scholar] [CrossRef]
- Billones, R.G.; Tackx, M.L.M.; Flachier, A.T.; Zhu, L.; Daro, M.H. Image Analysis as a Tool for Measuring Particulate Matter Concentrations and Gut Content, Body Size, and Clearance Rates of Estuarine Copepods: Validation and Application. J. Mar. Syst. 1999, 22, 179–194. [Google Scholar] [CrossRef]
- Detmer, T.M.; Broadway, K.J.; Potter, C.G.; Collins, S.F.; Parkos, J.J.; Wahl, D.H. Comparison of Microscopy to a Semi-Automated Method (FlowCAM®) for Characterization of Individual-, Population-, and Community-Level Measurements of Zooplankton. Hydrobiologia 2019, 838, 99–110. [Google Scholar] [CrossRef]
- Lalli, C.M.; Parsons, T.R. Biological Oceanography: An Introduction; Elsevier’s Science & Technology: Oxford, UK, 1997; ISBN 0-7506-3384-0. [Google Scholar]
- Valentin, J.L. Analyse Des Parametres Hydrobiologiques Dans La Remonte’e de Cabo Frio. Mar. Biol. 1984, 82, 259–276. [Google Scholar] [CrossRef]
- Boltovskoy, D.C. Atlas del Zooplancton del Atlântico Sudoccidental Metodos de Trabajo Com el Zooplancton Marine; National Institute for Fisheries Research and Development: Mar del Plata, Argentina, 1981. [Google Scholar]
- Chojnacki, J.; Hussein, M.M. Body Length and Weight of the Dominant Copepod Species in the Southern Baltic Sea. Zesz. Nauk. Akad. Roln. Szczec. 1983, 53–64. [Google Scholar]
- Viñas, M.D.; Diovisalvi, N.R.; Cepeda, G.D. Individual Biovolume of Some Dominant Copepod Species in Coastal Waters off Buenos Aires Province, Argentine Sea. Braz. J. Oceanogr. 2010, 58, 177–181. [Google Scholar] [CrossRef]
- Fernández Aráoz C Individual Biomass, Based on Body Measures, of Copepod Species Considered as Main Forage Items for Fishes of the Argentine Shelf. Oceanol. Acta 1991, 14, 575–580.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.r-project.org/ (accessed on 10 January 2023).
- Supriyadi, E.; Hidayat, R. Identification of Upwelling Area of the Western Territorial Waters of Indonesia from 2000 to 2017. IJG 2020, 52, 105. [Google Scholar] [CrossRef]
- Xiao, F.; Wu, Z.; Lyu, Y.; Zhang, Y. Abnormal Strong Upwelling off the Coast of Southeast Vietnam in the Late Summer of 2016: A Comparison with the Case in 1998. Atmosphere 2020, 11, 940. [Google Scholar] [CrossRef]
- Cohen, J.E.; Jonsson, T.; Carpenter, S.R. Ecological Community Description Using the Food Web, Species Abundance, and Body Size. Proc. Natl. Acad. Sci. USA 2003, 100, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- Sinistro, R. Top-down and Bottom-up Regulation of Planktonic Communities in a Warm Temperate Wetland. J. Plankton Res. 2010, 32, 209–220. [Google Scholar] [CrossRef]
- Tiselius, P.; Belgrano, A.; Andersson, L.; Lindahl, O. Primary Productivity in a Coastal Ecosystem: A Trophic Perspective on a Long-Term Time Series. J. Plankton Res. 2016, 38, 1092–1102. [Google Scholar] [CrossRef]
- Lynam, C.P.; Llope, M.; Möllmann, C.; Helaouët, P.; Bayliss-Brown, G.A.; Stenseth, N.C. Interaction between Top-down and Bottom-up Control in Marine Food Webs. Proc. Natl. Acad. Sci. USA 2017, 114, 1952–1957. [Google Scholar] [CrossRef] [PubMed]
- Gorsky, G.; Ohman, M.D.; Picheral, M.; Gasparini, S.; Stemmann, L.; Romagnan, J.-B.; Cawood, A.; Pesant, S.; García-Comas, C.; Prejger, F. Digital Zooplankton Image Analysis Using the ZooScan Integrated System. J. Plankton Res. 2010, 32, 285–303. [Google Scholar] [CrossRef]
- Herman, A.W. Design and Calibration of a New Optical Plankton Counter Capable of Sizing Small Zooplankton. Deep Sea Res. Part A Oceanogr. Res. Pap. 1992, 39, 395–415. [Google Scholar] [CrossRef]
- Jakobsen, H.; Carstensen, J. FlowCAM: Sizing Cells and Understanding the Impact of Size Distributions on Biovolume of planktonic Community Structure. Aquat. Microb. Ecol. 2011, 65, 75–87. [Google Scholar] [CrossRef]
- Wong, E.; Sastri, A.R.; Lin, F.S.; Hsieh, C.H. Modified FlowCAM Procedure for Quantifying Size Distribution of Zooplankton with Sample Recycling Capacity. PLoS ONE 2017, 12, e0175235. [Google Scholar] [CrossRef] [PubMed]
- Schulze, K.; Tillich, U.M.; Dandekar, T.; Frohme, M. PlanktoVision—an Automated Analysis System for the Identification of Phytoplankton. BMC Bioinform. 2013, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Forest, A.; Stemmann, L.; Picheral, M.; Burdorf, L.; Robert, D.; Fortier, L.; Babin, M. Size Distribution of Particles and Zooplankton across the Shelf-Basin System in Southeast Beaufort Sea: Combined Results from an Underwater Vision Profiler and Vertical Net Tows. Biogeosciences 2012, 9, 1301–1320. [Google Scholar] [CrossRef]
- Karnan, C.; Jyothibabu, R.; Kumar, T.M.M.; Jagadeesan, L.; Arunpandi, N. On the Accuracy of Assessing Copepod Size and Biovolume Using FlowCAM and Traditional Microscopy. Indian J. Geo Mar. Sci. 2017, 46, 1261–1264. [Google Scholar]
- Alcaraz, M.; Saiz, E.; Calbet, A.; Trepat, I.; Broglio, E. Estimating Zooplankton Biomass through Image Analysis. Mar. Biol. 2003, 143, 307–315. [Google Scholar] [CrossRef]
- Schultes, S.; Lopes, R.M. Laser Optical Plankton Counter and Zooscan Intercomparison in Tropical and Subtropical Marine Ecosystems. Limnol. Oceanogr. Methods 2009, 7, 771–784. [Google Scholar] [CrossRef]
- Fluid Imaging Technologies Inc. FlowCAM® Manual Version 3.2; Fluid Imaging Technologies Inc.: Yarmouth, ME, USA, 2012. [Google Scholar]
- Kydd, J.; Rajakaruna, H.; Briski, E.; Bailey, S. Examination of a High Resolution Laser Optical Plankton Counter and FlowCAM for Measuring Plankton Concentration and Size. J. Sea Res. 2018, 133, 2–10. [Google Scholar] [CrossRef]
- Mallard, F.; Bourlot, V.L.; Tully, T. An Automated Image Analysis System to Measure and Count Organisms in Laboratory Microcosms. PLoS ONE 2013, 8, e64387. [Google Scholar] [CrossRef] [PubMed]
- Rishi, N.R. Particle Size and Shape Analysis Using Imagej with Customized Tools for Segmentation of Particles. Int. J. Eng. Tech. Res. 2015, 4, 247–250. [Google Scholar] [CrossRef]
- Zarauz, L.; Irigoien, X.; Fernandes, J.A. Modelling the Influence of Abiotic and Biotic Factors on Plankton Distribution in the Bay of Biscay, during Three Consecutive Years (2004–06). J. Plankton Res. 2008, 30, 857–872. [Google Scholar] [CrossRef]
- Reynolds, R.A.; Stramski, D.; Wright, V.M.; Woźniak, S.B. Measurements and Characterization of Particle Size Distributions in Coastal Waters. J. Geophys. Res. Ocean. 2010, 115, 20. [Google Scholar] [CrossRef]
- Álvarez, E.; López-Urrutia, Á.; Nogueira, E.; Fraga, S. How to Effectively Sample the Plankton Size Spectrum? A Case Study Using FlowCAM. J. Plankton Res. 2011, 33, 1119–1133. [Google Scholar] [CrossRef]
- Ide, K.; Takahashi, K.; Kuwata, A.; Nakamachi, M.; Saito, H. A Rapid Analysis of Copepod Feeding Using FlowCAM. J. Plankton Res. 2008, 30, 275–281. [Google Scholar] [CrossRef]
- Barth-Jensen, C.; Daase, M.; Ormańczyk, M.R.; Varpe, Ø.; Kwaśniewski, S.; Svensen, C. High Abundances of Small Copepods Early Developmental Stages and Nauplii Strengthen the Perception of a Non-Dormant Arctic Winter. Polar Biol. 2022, 45, 675–690. [Google Scholar] [CrossRef]
- Valentin, J.L.; Coutinho, R. Modelling Maximum Chlorophyll in the Cabo Frio (Brazil) Upwelling: A Preliminary Approach. Ecol. Model. 1990, 52, 103–113. [Google Scholar] [CrossRef]
- van Someren Gréve, H.; Kiørboe, T.; Almeda, R. Bottom-up Behaviourally Mediated Trophic Cascades in Plankton Food Webs. Proc. R. Soc. B Biol. Sci. 2019, 286, 10. [Google Scholar] [CrossRef]
- Lopes, C.L. Variação Espaço-Temporal Do Ictioplâncton e Condições Oceanográficas Na Região de Cabo Frio (RJ). Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2006; p. 226. [Google Scholar]
- Brandini, F.P.; Spach, H.L.; Lopes, R.M.; Sassi, R. Planctologia Na Plataforma Continental Do Brasil. Diagnose e Revisão Bibliográfica; CEMAR/MMA/CIBM/FEMAR: Brasília, Brazil, 1997; 196p. [Google Scholar]
- Sant’Anna, E.E. Remains of the Protozoan Sticholonche Zanclea in the Faecal Pellets of Paracalanus Quasimodo, Parvocalanus Crassirostris, Temora Stylifera and Temora Turbinata (Copepoda, Calanoida) in Brazilian Coastal Waters. Braz. J. Oceanogr. 2013, 61, 73–76. [Google Scholar] [CrossRef]
- Moser, G.A.O.; Gianesella-Galvão, S.M.F. Biological and Oceanographic Upwelling Indicators at Cabo Frio (RJ). Braz. J. Oceanogr. 1997, 45, 11–23. [Google Scholar] [CrossRef]
- Hirst, A.G.; Bunker, A.J. Growth of Marine Planktonic Copepods: Global Rates and Patterns in Relation to Chlorophyll a, Temperature, and Body Weight. Limonol. Oceanogr. 2003, 48, 1988–2010. [Google Scholar] [CrossRef]
- Lin, K.Y.; Sastri, A.R.; Gong, G.C.; Hsieh, C.H. Copepod Community Growth Rates in Relation to Body Size, Temperature, and Food Availability in the East China Sea: A Test of Metabolic Theory of Ecology. Biogeosciences 2013, 10, 1877–1892. [Google Scholar] [CrossRef]
- Mayor, D.J.; Sommer, U.; Cook, K.B.; Viant, M.R. The Metabolic Response of Marine Copepods to Environmental Warming and Ocean Acidification in the Absence of Food. Sci. Rep. 2015, 5, 13690. [Google Scholar] [CrossRef]
- Pomati, F.; Shurin, J.B.; Andersen, K.H.; Tellenbach, C.; Barton, A.D. Interacting Temperature, Nutrients and Zooplankton Grazing Control Phytoplankton Size-Abundance Relationships in Eight Swiss Lakes. Front. Microbiol. 2020, 10, 3155. [Google Scholar] [CrossRef]
- Turner, J.T. The Importance of Small Pelagic Planktonic Copepods and Their Role in Pelagic Marine Food Webs. Zool. Stud. 2004, 43, 255–266. [Google Scholar]
- Horne, C.R.; Hirst, A.G.; Atkinson, D.; Neves, A.; Kiørboe, T. A Global Synthesis of Seasonal Temperature–Size Responses in Copepods. Glob. Ecol. Biogeogr. 2016, 25, 988–999. [Google Scholar] [CrossRef]
- Drits, A.V.; Belevich, T.A.; Ilyash, L.V.; Semenova, T.N.; Flint, M.V. Does Zooplankton Control Phytoplankton Development in White Sea Coastal Waters in Spring? Oceanology 2018, 58, 558–572. [Google Scholar] [CrossRef]





| Species | N | PL | PW | UL | TL | BV (106) |
|---|---|---|---|---|---|---|
| All grouped | 1490 | 474 ± 121 | 172 ± 53 | 182 ± 64 | 656 ± 161 | 9.10 ± 8.06 |
| Onychocorycaeus giesbrechti | ||||||
| Copepodite | 19 | 395 ± 80 | 192 ± 37 | 199 ± 58 | 595 ± 130 | 8.59 ± 4.76 |
| Female | 11 | 590 ± 81 | 265 ± 48 | 338 ± 56 | 927 ± 76 | 24.13 ± 10.31 |
| Male | 31 | 540 ± 44 | 249 ± 25 | 350 ± 44 | 890 ± 45 | 18.70 ± 4.64 |
| Pooled | 61 | 504 ± 99 | 234 ± 44 | 301 ± 85 | 805 ± 166 | 16.53 ± 8.26 |
| Onychocorycaeus ovalis | ||||||
| Copepodite | 25 | 342 ± 90 | 180 ± 45 | 169 ± 68 | 511 ± 146 | 7.08 ± 5.35 |
| Female | 13 | 503 ± 70 | 261 ± 53 | 253 ± 44 | 756 ± 107 | 20.12 ± 11.67 |
| Male | 16 | 527 ± 42 | 243 ± 38 | 364 ± 70 | 891 ± 72 | 17.70 ± 6.15 |
| Pooled | 54 | 435 ± 113 | 217 ± 57 | 247 ± 104 | 682 ± 203 | 13.23 ± 9.45 |
| Oncaea media | ||||||
| Copepodite | 41 | 281 ± 52 | 105 ± 31 | 136 ± 31 | 417 ± 76 | 1.95 ± 1.24 |
| Female | 200 | 366 ± 45 | 146 ± 30 | 200 ± 38 | 566 ± 77 | 4.48 ± 1.91 |
| Male | 59 | 286 ± 27 | 111 ± 15 | 138 ± 23 | 424 ± 46 | 1.95 ± 0.66 |
| Pooled | 300 | 339 ± 58 | 134 ± 33 | 179 ± 45 | 517 ± 99 | 3.64 ± 2.04 |
| Paracalanus quasimodo | ||||||
| Copepodite | 128 | 423 ± 71 | 129 ± 25 | 127 ± 28 | 550 ± 94 | 4.15 ± 2.24 |
| Female | 506 | 554 ± 69 | 176 ± 36 | 174 ± 27 | 728 ± 90 | 9.82 ± 7.97 |
| Male | 112 | 542 ± 71 | 167 ± 30 | 173 ± 28 | 716 ± 95 | 8.66 ± 3.70 |
| Pooled | 746 | 530 ± 85 | 167 ± 38 | 166 ± 32 | 696 ± 113 | 8.68 ± 7.08 |
| Temora stylifera | ||||||
| Copepodite | 47 | 410 ± 147 | 166 ± 54 | 163 ± 87 | 573 ± 218 | 8.17 ± 7.54 |
| Female | 4 | 578 ± 65 | 243 ± 36 | 270 ± 51 | 848 ± 78 | 19.18 ± 7.48 |
| Male | 4 | 637 ± 25 | 251 ± 11 | 276 ± 14 | 913 ± 35 | 21.74 ± 2.78 |
| Pooled | 55 | 439 ± 154 | 178 ± 58 | 179 ± 90 | 618 ± 231 | 9.95 ± 8.45 |
| Temora turbinata | ||||||
| Copepodite | 206 | 437 ± 117 | 191 ± 62 | 165 ± 70 | 601 ± 172 | 10.64 ± 8.17 |
| Female | 42 | 595 ± 90 | 258 ± 48 | 277 ± 78 | 872 ± 112 | 22.88 ± 9.74 |
| Male | 26 | 617 ± 62 | 254 ± 37 | 272 ± 45 | 889 ± 87 | 22.34 ± 7.86 |
| Pooled | 274 | 477 ± 131 | 207 ± 65 | 192 ± 84 | 669 ± 198 | 13.59 ± 9.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, J.d.C.L.d.; Matos, T.d.S.; da Silva, D.C.B.; Reis, C.; Dias, C.d.O.; Konno, T.U.P.; Fernandes, L.D.d.A. Seasonal Changes in the Size Distribution of Copepods Is Affected by Coastal Upwelling. Diversity 2023, 15, 637. https://doi.org/10.3390/d15050637
Rosa JdCLd, Matos TdS, da Silva DCB, Reis C, Dias CdO, Konno TUP, Fernandes LDdA. Seasonal Changes in the Size Distribution of Copepods Is Affected by Coastal Upwelling. Diversity. 2023; 15(5):637. https://doi.org/10.3390/d15050637
Chicago/Turabian StyleRosa, Judson da Cruz Lopes da, Thiago da Silva Matos, Débora Costa Brito da Silva, Carolina Reis, Cristina de Oliveira Dias, Tatiana Ungaretti Paleo Konno, and Lohengrin Dias de Almeida Fernandes. 2023. "Seasonal Changes in the Size Distribution of Copepods Is Affected by Coastal Upwelling" Diversity 15, no. 5: 637. https://doi.org/10.3390/d15050637






