The Influence of Roost Type and Diet on Energy Expenditure in Bats
Abstract
:1. Introduction
2. Methods
2.1. Data Collection
2.2. Phylogenetic Affiliations
2.3. Statistical Analysis
3. Results
3.1. Phylogeny of Bats
3.2. Ancestral States for Roost Type and Diet
3.3. The Effect of Roost Type on Physiological Variables
3.4. The Effect of Roost Type on the Area of the TRP
3.5. Estimates of Energy Expenditure of Species of Bats Using Different Roost Types
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McNab, B.K. The Physiological Ecology of Vertebrates: A View from Energetics; Cornell University Press: Ithaca, NY, USA, 2002. [Google Scholar]
- Terrien, J.; Perret, M.; Aujard, F. Behavioral thermoregulation in mammals: A review. Front. Biosci. 2011, 16, 1428–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozinovic, F.; Calosi, P.; Spicer, J.I. Physiological Correlates of Geographic Range in Animals. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 155–179. [Google Scholar] [CrossRef] [Green Version]
- Kunz, T.H. Ecology of Bats; Plenum Press: New York, NY, USA; London, UK, 1982. [Google Scholar]
- Kunz, T.H.; Lumsden, L.F.; Fenton, M.B. (Eds.) Bat Ecology; University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- Boyles, J.G.; Dunbar, M.B.; Storm, J.J.; Brack, V., Jr. Energy availability influences microclimate selection of hibernating bats. J. Exp. Biol. 2007, 210, 4345–4350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, A.C.; Broders, H.G. Distribution and Roost Selection of Bats on Newfoundland. Northeast. Nat. 2012, 19, 165–176. [Google Scholar] [CrossRef]
- Turbill, C. Roosting and thermoregulatory behaviour of male Gould’s long-eared bats, Nyctophilus gouldi: Energetic benefits of thermally unstable tree roosts. Aust. J. Zool. 2006, 54, 57–60. [Google Scholar] [CrossRef]
- Jacobs, D.; Kelly, E.; Mason, M.; Stoffberg, S. Thermoregulation in two free-ranging subtropical insectivorous bat species: Scotophilus species (Vespertilionidae). Can. J. Zool. 2007, 85, 883–890. [Google Scholar] [CrossRef]
- Raesly, R.L.; Edward, J. Winter Habitat Selection by North Temperate Cave Bats. Am. Midl. Nat. 1987, 118, 15–31. [Google Scholar] [CrossRef]
- Palmer, C.; Woinarski, J.C.Z. Seasonal roosts and foraging movements of the black flying fox (Pteropus alecto) in the Northern Territory: Resource tracking in a landscape mosaic. Wildl. Res. 1999, 26, 823–838. [Google Scholar] [CrossRef]
- Brunet, A.K.; Medellín, R.A. The species-area relationship in bat assemblages of tropical caves. J. Mammal. 2001, 82, 1114–1122. [Google Scholar] [CrossRef]
- Vonhof, M.J.; Barclay, R.M.R. Roost-site selection and roosting ecology of forest-dwelling bats in southern British Columbia. Can. J. Zool. 1996, 74, 1797–1805. [Google Scholar] [CrossRef]
- Perry, R.W. A review of factors affecting cave climates for hibernating bats in temperate North America. Environ. Rev. 2013, 21, 28–39. [Google Scholar] [CrossRef]
- Humphries, M.M.; Thomas, D.W.; Kramer, D.L. The Role of Energy Availability in Mammalian Hibernation: A Cost-Benefit Approach. Physiol. Biochem. Zool. 2003, 76, 165–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholander, P.F.; Hock, R.; Walters, V.; Johnson, F.; Irving, L. Heat regulation in some arctic and tropical mammals and birds. Biol. Bull. 1950, 99, 237–258. [Google Scholar] [CrossRef] [PubMed]
- McNab, B.K. A Statistical Analysis of Mammalian Rates of Metabolism. Funct. Ecol. 1992, 6, 672. [Google Scholar] [CrossRef]
- Cruz-Neto, A.P.; Garland, T.; Abe, A.S. Diet, phylogeny, and basal metabolic rate in phyllostomid bats. Zoology 2001, 104, 49–58. [Google Scholar] [CrossRef] [Green Version]
- McNab, B.K. On Estimating Thermal Conductance in Endotherms. Physiol. Zool. 1980, 53, 145–156. [Google Scholar] [CrossRef]
- Rezende, E.L.; Bacigalupe, L.D. Thermoregulation in endotherms: Physiological principles and ecological consequences. J. Comp. Physiol. B 2015, 185, 709–727. [Google Scholar] [CrossRef]
- Geiser, F. The Role of Torpor in the Life of Australian Arid Zone. Aust. Mammal. 2004, 26, 125–134. [Google Scholar] [CrossRef]
- Wojciechowski, M.S.; Jefimow, M. Is Torpor Only an Advantage? Effect of Thermal Environment on Torpor Use in the Siberian Hamsters (Phodopus sungorus). Can. J. Zool. 2006, 57, 83–92. [Google Scholar]
- Pretzlaff, I.; Kerth, G.; Dausmann, K.H. Communally breeding bats use physiological and behavioural adjustments to optimise daily energy expenditure. Sci. Nat. 2010, 97, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Ruf, T.; Geiser, F. Daily torpor and hibernation in birds and mammals. Biol. Rev. 2015, 90, 891–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiser, F. Reduction of metabolism during hibernation and daily torpor in mammals and birds: Temperature effect or physiological inhibition? J. Comp. Physiol. B 1988, 158, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Geiser, F. Metabolic Rate and Body Temperature Reduction During Hibernation and Daily Torpor. Annu. Rev. Physiol. 2004, 66, 239–274. [Google Scholar] [CrossRef] [Green Version]
- Menzies, A.K.; Webber, Q.M.; Baloun, D.E.; McGuire, L.P.; Muise, K.A.; Coté, D.; Willis, C.K. Metabolic rate, latitude and thermal stability of roosts, but not phylogeny, affect rewarming rates of bats. Physiol. Behav. 2016, 164, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Tyburec, J.; Chenger, J.; Snow, T.; Geiselman, C.; Member, B.B. BCI Bat Conservation and Management Workshop-Arizona; Bat Conservation International: Austin, TX, USA, 2011. [Google Scholar]
- Lausen, C.L.; Barclay, R.M.R. Thermoregulation and roost selection by reproductive female big brown bats (Eptesicus fuscus) roosting in rock crevices. J. Zool. 2003, 260, 235–244. [Google Scholar] [CrossRef]
- Willis, C.K.R.; Brigham, R.M.; Geiser, F. Deep, prolonged torpor by pregnant, free-ranging bats. Sci. Nat. 2006, 93, 80–83. [Google Scholar] [CrossRef]
- Chappell, M.A.; Roverud, R.C. Temperature effects on metabolism, ventilation, and oxygen extraction in a neotropical bat. Respir. Physiol. 1990, 81, 401–412. [Google Scholar] [CrossRef]
- Boyles, J.G.; Storm, J.J.; Brack, V., Jr. Thermal benefits of clustering during hibernation: A field test of competing hypotheses on Myotis sodalis. Funct. Ecol. 2008, 22, 632–636. [Google Scholar] [CrossRef]
- Willis, C.K.R.; Brigham, R.M. Social thermoregulation exerts more influence than microclimate on forest roost preferences by a cavity-dwelling bat. Behav. Ecol. Sociobiol. 2007, 62, 97–108. [Google Scholar] [CrossRef]
- Licht, P.; Leitner, P. Behavioral Responses to High Temperatures in Three Species of California Bats. J. Mammal. 1967, 48, 52–61. [Google Scholar] [CrossRef]
- Brooke, A.P. Tent selection, roosting ecology and social organization of the tent-making bat, Ectophylla alba. Costa Rica. J. Zool. 1990, 221, 11–19. [Google Scholar] [CrossRef]
- Agnarsson, I.; Zambrana-Torrelio, C.; Flores-Saldana, N.P.; May-Collado, L.J. A time-calibrated species-level phylogeny of bats (Chiroptera, Mammalia). PLoS Curr. 2011, 3, RRN1212. [Google Scholar] [CrossRef]
- Almeida, F.C.; Giannini, N.P.; Simmons, N.B.; Helgen, K.M. Each flying fox on its own branch: A phylogenetic tree for Pteropus and related genera (Chiroptera: Pteropodidae). Mol. Phylogenetics Evol. 2014, 77, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Flores-Abreu, I.; Trejo-Salazar, R.; Sánchez-Reyes, L.; Good, S.; Magallón, S.; García-Mendoza, A.; Eguiarte, L. Tempo and mode in coevolution of Agave sensu lato (Agavoideae, Asparagaceae) and its bat pollinators, Glossophaginae (Phyllostomidae). Mol. Phylogenetics Evol. 2019, 133, 176–188. [Google Scholar] [CrossRef]
- Lack, J.B.; Bussche, R.A.V.D. Identifying the confounding factors in resolving phylogenetic relationships in Vespertilionidae. J. Mammal. 2010, 91, 1435–1448. [Google Scholar] [CrossRef]
- Jones, K.E.; Purvis, A.; Maclarnon, A.; Bininda-Emonds, O.R.P.; Simmons, N.B. A phylogenetics supertree of the bats. pdf. Biol. Rev. 2002, 77, 223–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, K.E.; Bininda-Emonds, O.R.P.; Gittleman, J.L. Bats, clocks and rocks: Diversification patterns in Chiroptera. Evolution 2005, 59, 2243–2255. [Google Scholar] [CrossRef]
- Hassanin, A.; Bonillo, C.; Tshikung, D.; Shongo, C.P.; Pourrut, X.; Kadjo, B.; Nakouné, E.; Tu, V.T.; Prié, V.; Goodman, S.M. Phylogeny of African fruit bats (Chiroptera, Pteropodidae) based on complete mitochondrial genomes. J. Zool. Syst. Evol. Res. 2020, 58, 1395–1410. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Hedges, S.B. TimeTree: A Resource for Timelines, Timetrees, and Divergence Times. Mol. Biol. Evol. 2017, 34, 1812–1819. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. A Modular System for Evolutionary Analysis. Version 3.4. 2018.
- Revell, L.J. Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Muggeo, V.M.; Muggeo, M.V.M. Package “Segmented”. Biometrika 2017, 58, 516. [Google Scholar]
- Marom, S.; Korine, C.; Wojciechowski, M.S.; Tracy, C.R.; Pinshow, B. Energy Metabolism and Evaporative Water Loss in the European Free-Tailed Bat and Hemprich’s Long-Eared Bat (Microchiroptera): Species Sympatric in the Negev Desert. Physiol. Biochem. Zool. 2006, 79, 944–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midford, P.E. PDAP, Phenotypic Diversity Analysis Program. 2010. [Google Scholar]
- Grafen, A.; Vickerman, K. The phylogenetic regression. Philos. Trans. R Soc. Lond. B Biol. Sci. 1989, 326, 119–157. [Google Scholar] [CrossRef] [PubMed]
- McNab, B.K. The economics of temperature regulation in neotropical bats. Comp. Biochem. Physiol. 1969, 31, 227–268. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.E.; Graham, J.B. Physiological responses to temperature in the longnosed bat, Leptonycteris sanborni. Comp. Biochem. Physiol. 1967, 22, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Hosken, D.J.; Withers, P.C. Temperature regulation and metabolism of an Australian bat, Chalinolobus gouldii (Chiroptera: Vespertilionidae) when euthermic and torpid. J. Comp. Physiol. B 1997, 167, 71–80. [Google Scholar] [CrossRef]
- McNab, B.K.; O’Donnell, C. The behavioral energetics of New Zealand’s bats: Daily torpor and hibernation, a continuum. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2018, 223, 18–22. [Google Scholar] [CrossRef]
- Minnaar, I.A.; Bennett, N.C.; Chimimba, C.T.; McKechnie, A.E. Summit Metabolism and Metabolic Expansibility in Wahlberg’s Epauletted Fruit Bats (Epomophorus wahlbergi): Seasonal Acclimatisation and Effects of Captivity. J. Exp. Biol. 2014, 217, 1363–1369. [Google Scholar] [CrossRef] [Green Version]
- Herreid, C.F.; Schmidt-Nielsen, K. Oxygen consumption, temperature, and water loss in bats from different environments. Am. J. Physiol. Leg. Content 1966, 211, 1108–1112. [Google Scholar] [CrossRef]
- Cryan, P.M.; Wolf, B.O. Sex differences in the thermoregulation and evaporative water loss of a heterothermic bat, Lasiurus cinereus, during its spring migration. J. Exp. Biol. 2003, 206, 3381–3390. [Google Scholar] [CrossRef] [Green Version]
- Baudinette, R.V.; Churchill, S.K.; Christian, K.A.; Nelson, J.E.; Hudson, P.J. Energy, water balance and the roost microenvi-ronment in three Australian cave-dwelling bats (Microchiroptera). J. Comp. Physiol. B 2000, 170, 439–446. [Google Scholar] [CrossRef]
- Bell, G.P.; Bartholomew, G.A.; Nagy, K.A. The roles of energetics, water economy, foraging behavior, and geothermal refugia in the distribution of the bat, Macrotus californicus. J. Comp. Physiol. B 1986, 156, 441–450. [Google Scholar] [CrossRef]
- Maloney, S.K.; Bronner, G.N.; Buffenstein, R. Thermoregulation in the Angolan Free-Tailed Bat Mops condylurus: A Small Mammal That Uses Hot Roosts. Physiol. Biochem. Zool. 1999, 72, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, D.C.; McKechnie, A.E. Interspecific variation in thermoregulation among three sympatric bats inhabiting a hot, semi-arid environment. J. Comp. Physiol. B 2012, 182, 1129–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosken, D.J.; Withers, P.C. Metabolic Physiology of Euthermic and Torpid Lesser Long-Eared Bats, Nyctophilus geoffroyi (Chiroptera: Vespertilionidae). J. Mammal. 1999, 80, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Hosken, D.J. Thermal Biology and Metabolism of the Greater Long-eared Bat, Nyctophilus major (Chirop-tera: Vespertilionidae). Aust. J. Zool. 1997, 45, 145–156. [Google Scholar] [CrossRef]
- Muñoz-Garcia, A.; Larraín, P.; Ben-Hamo, M.; Cruz-Neto, A.; Williams, J.B.; Pinshow, B.; Korine, C. Metabolic rate, evapora-tive water loss and thermoregulatory state in four species of bats in the Negev desert. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 191, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Korine, C.; Arad, Z. Effect of Water Restriction on Temperature Regulation of the Fruit Bat Rousettus aegyptiacus. J. Comp. Phys. 1993, 163, 401–405. [Google Scholar] [CrossRef]
- Perry, R.W. Potential energy expenditure by litter-roosting bats associated with temperature under leaf litter during winter. J. Therm. Biol. 2013, 38, 467–473. [Google Scholar] [CrossRef]
- Tobe, S.S.; Kitchener, A.C.; Linacre, A.M.T. Reconstructing Mammalian Phylogenies: A Detailed Comparison of the Cyto-chrome b and Cytochrome Oxidase Subunit I Mitochondrial Genes. PLoS ONE 2010, 5, e14156. [Google Scholar] [CrossRef] [Green Version]
- Teeling, E.C.; Springer, M.S.; Madsen, O.; Bates, P.; O’Brien, S.J.; Murphy, W.J. A Molecular Phylogeny for Bats Illuminates Biogeography and the Fossil Record. Science 2005, 307, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.M.; Ralph, T.M.C.; Naidoo, T.; Taylor, P.J.; Ratrimomanarivo, F.; Stanley, W.T.; Goodman, S.M. Toward a Molecular Phylogeny for the Molossidae (Chiroptera) of the Afro-Malagasy Region. Acta Chiropterol. 2011, 13, 1–16. [Google Scholar] [CrossRef]
- Gharaibeh, B.M.; Qumsiyeh, M.B. Otonycteris hemprichii. Mamm. Species 1995, 514, 1–4. [Google Scholar] [CrossRef]
- Hoofer, S.R.; Van Den Bussche, R.A.; Horáček, I. Generic status of the American pipistrelles (vespertilionidae) with description of a new genus. J. Mammal. 2006, 87, 981–992. [Google Scholar] [CrossRef] [Green Version]
- Flinn, J. Winter Roosting Behavior of Red Bats (Lasiurus borealis): Habitat Use, Microclimate, and Effects of Ambient Temperature on Roost Choice. Master’s Thesis, Michigan State University, Lansing, MI, USA, 2009. [Google Scholar]
- Bronrier, G.N.; Maloney, S.K.; Buffenstein, R. Survival tactics within thermally-challenging roosts: Heat tolerance and cold sensitivity in the Angolan free-tailed bat, Mops condylurus. S. Afr. J. Zool. 1999, 34, 1–10. [Google Scholar] [CrossRef]
- Muñoz-Garcia, A.; Oelbaum, P.; Korine, C. Stress Physiology, Foraging, and Ecophysiology of Bats in Urban Environments. In What Makes an Urban Bat? Bat Biology, Ecology, and Interactions with Humans in Urban Environments; Moretto, L., Fenton, M.B., Patriquin, K., Coleman, J., Korine, C., Davy, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Boyles, J.G.; Robbins, L.W. Characteristics of Summer and Winter Roost Trees Used by Evening Bats (Nycticeius humeralis) in Southwestern Missouri. Am. Midl. Nat. 2006, 155, 210–220. [Google Scholar] [CrossRef]
- Mormann, B.M.; Robbins, L.W. Winter Roosting Ecology of Eastern Red Bats in Southwest Missouri. J. Wildl. Manag. 2007, 71, 213–217. [Google Scholar] [CrossRef]
- Geiser, F.; Brigham, R.M. The Other Functions of Torpor. In Living in a Seasonal World; Ruf, T., Bieber, C., Arnold, W., Millesi, E., Eds.; Springer: Berlin/Heidelberg, Gernany, 2012; pp. 109–121. [Google Scholar]
- McKechnie, A.E.; Wolf, B.O. Solar radiation and the energetic cost of rewarming from torpor. In Life in the Cold: Evolution, Mechanisms, Adaptation and Application. In Proceedings of the 12th International Hibernation Symposium, Vancouver, BC, USA, 25 July–1 August 2004; pp. 63–70. [Google Scholar]
- Sedgeley, J.A. Quality of cavity microclimate as a factor influencing selection of maternity roosts by a tree-dwelling bat, Chalinolobus tuberculatus, in New Zealand. J. Appl. Ecol. 2001, 38, 425–438. [Google Scholar] [CrossRef]
- Racey, P.A.; Swift, S.M. Variations in gestation length in a colony of pipistrelle bats (Pipistrellus pipistrellus) from year to year. Reproduction 1981, 61, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Wilde, C.J.; Knight, C.H.; Racey, P.A. Influence of torpor on milk protein composition and secretion in lactating bats. J. Exp. Zool. 1999, 284, 35–41. [Google Scholar] [CrossRef]
- Thomas, D.W.; Dorais, M.; Bergeron, J.-M. Winter Energy Budgets and Cost of Arousals for Hibernating Little Brown Bats, Myotis lucifugus. J. Mammal. 1990, 71, 475–479. [Google Scholar] [CrossRef]
- Brosset, A.; Charles-Dominique, P.; Cockle, A.; Cosson, J.-F.; Masson, D. Bat communities and deforestation in French Guiana. Can. J. Zool. 1996, 74, 1974–1982. [Google Scholar] [CrossRef]
- Law, B.; Anderson, J.; Chidel, M. Bat communities in a fragmented forest landscape on the south-west slopes of New South Wales, Australia. Biol. Conserv. 1999, 88, 333–345. [Google Scholar] [CrossRef]
- Jaberg, C.; Guisan, A. Modelling the distribution of bats in relation to landscape structure in a temperate mountain environment. J. Appl. Ecol. 2001, 38, 1169–1181. [Google Scholar] [CrossRef]
- Korine, C.; Pinshow, B. Guild Structure, Foraging Space Use, and Distribution in a Community of Insectivorous Bats in the Negev Desert. J. Zool. 2004, 262, 187–196. [Google Scholar] [CrossRef]


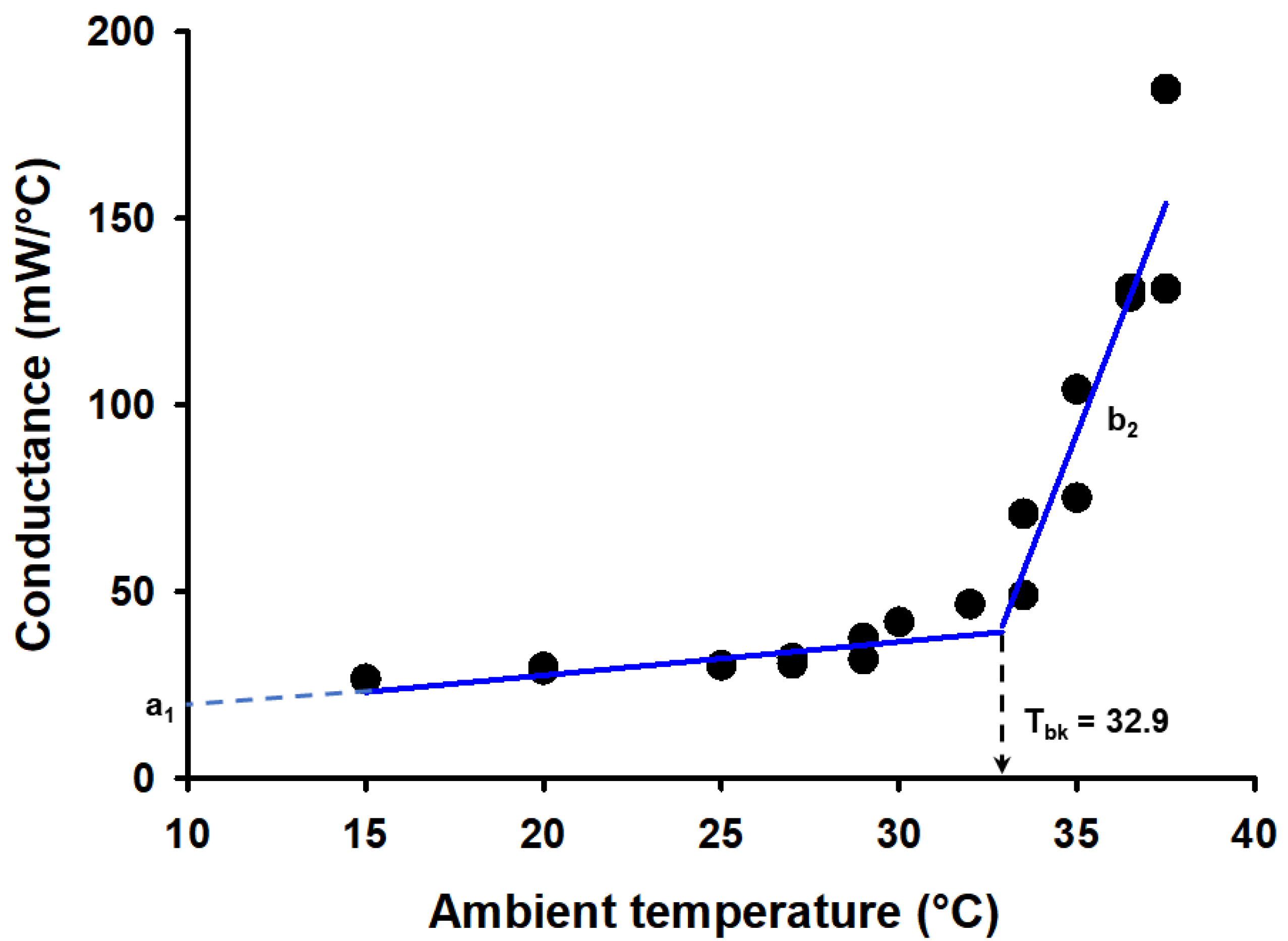
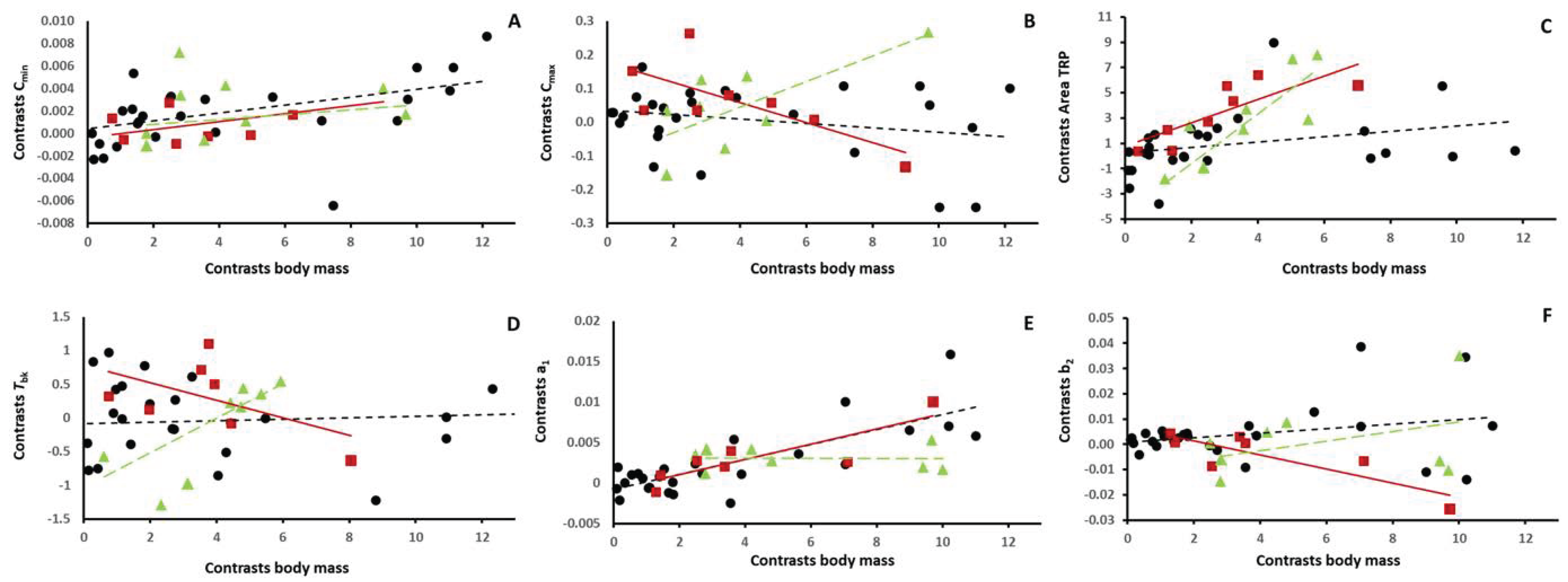
| Species | Roost Type | Diet | Mass (g) | Cmin | Cmax | TRP Area | Tbk | a1 | b2 |
|---|---|---|---|---|---|---|---|---|---|
| Anoura caudifer 1 | Buildings | Omnivorous | 11.5 | 0.0160 | 0.1330 | 17.174 | 24.5 | 0.0156 | 0.0073 |
| Artibeus concolor 1 | TFB | Frugivorous | 19.7 | 0.0230 | 0.2460 | 15.919 | 27.5 | 0.0281 | 0.0142 |
| Artibeus hirsutum 2 | Caves | Frugivorous | 48.0 | 0.0396 | 1.0009 | 30.836 | 29.4 | 0.0328 | 0.1137 |
| Artibeus jamaicensis 1 | TFB | Frugivorous | 45.2 | 0.0340 | 0.9300 | 37.418 | 29.5 | 0.0409 | 0.0477 |
| Artibeus literatus 1 | TFB | Frugivorous | 70.1 | 0.0370 | 0.9410 | 75.401 | 31.8 | 0.0462 | 0.0303 |
| Carollia perspicillata 1 | Caves | Frugivorous | 14.9 | 0.0250 | 0.4080 | 29.078 | 31.0 | 0.0254 | 0.0186 |
| Chalinolobus gouldii 3 | TCHT | Insectivorous | 17.5 | 0.0191 | 0.0278 | 6.844 | 29.7 | 0.0076 | 0.0401 |
| Chalinolobus tuberculatus 4 | TCHT | Insectivorous | 9.0 | 0.0050 | 0.0380 | 5.656 | - | - | - |
| Chrotopterus auritus 1 | Caves | Carnivorous | 96.1 | 0.0510 | 0.3490 | 58.196 | 26.0 | 0.0678 | 0.0106 |
| Desmodus rotundus 1 | Caves | Sanguinivorous | 29.4 | 0.0010 | 0.1670 | 40.996 | 28.6 | 0.0307 | 0.0068 |
| Diaemus youngi 1 | TCHT | Sanguinivorous | 36.6 | 0.0290 | 1.0230 | 13.821 | 28.5 | 0.0351 | 0.0133 |
| Diphylla ecaudata 1 | Caves | Sanguinivorous | 27.8 | 0.0220 | 0.1620 | 22.413 | 28.1 | 0.0293 | 0.0137 |
| Epomophorus wahlbergi 5 | MMS | Frugivorous | 84.1 | 0.0372 | 2.7400 | 29.580 | 32.7 | 0.0575 | 0.4765 |
| Eptesicus fuscus 6 | MMS | Insectivorous | 10.4 | 0.0174 | 0.3259 | 29.443 | 33.8 | 0.0245 | 0.0596 |
| Glossophaga soricina 1 | Caves | Omnivorous | 9.6 | 0.0170 | 1.3910 | 19.016 | 29.5 | 0.0165 | 0.0160 |
| Histiotus velatus 1 | Buildings | Insectivorous | 11.2 | 0.0070 | 0.3890 | 12.690 | 29.1 | 0.0151 | 0.0617 |
| Lasiurus cinereus 7 | TFB | Insectivorous | 32.5 | 0.0025 | 0.3268 | 51.308 | 33.0 | 0.0252 | 0.0380 |
| Leptonycteris sanborni 2 | Caves | Frugivorous | 22.0 | 0.0308 | 0.1223 | 18.959 | 30.8 | 0.0284 | 0.0138 |
| Macroderma gigas 8 | MMS | Carnivorous | 107.2 | 0.0613 | 0.8543 | 32.419 | 34.3 | 0.0392 | 0.1327 |
| Macrotus californicus 9 | Caves | Insectivorous | 11.7 | 0.0233 | 0.0641 | 11.742 | 30.0 | 0.0233 | 0.0068 |
| Miniopterus schreibersii 8 | Buildings | Insectivorous | 10.9 | 0.0311 | 0.3669 | 14.150 | 33.9 | 0.0217 | 0.0570 |
| Molossus molossus 1 | Buildings | Insectivorous | 15.6 | 0.0240 | 0.2090 | 11.296 | 32.0 | 0.0157 | 0.0159 |
| Mops condylurus 10 | Buildings | Insectivorous | 23.2 | 0.0234 | 0.1871 | 23.780 | 33.6 | 0.0180 | 0.0197 |
| Mystacina tuberculata 4 | TCHT | Omnivorous | 13.5 | 0.0020 | 0.2720 | 9.431 | 25.9 | 0.0102 | 0.0809 |
| Noctilio albiventris 1 | MMS | Insectivorous | 39.9 | 0.0490 | 0.7770 | 38.578 | 27.5 | 0.0474 | 0.0512 |
| Noctilio leporinus 1 | MMS | Carnivorous | 61.0 | 0.0065 | 1.2290 | 80.950 | 30.2 | 0.0552 | 0.0321 |
| Nycteris thebaica 11 | TCHT | Insectivorous | 11.7 | 0.0088 | 0.0513 | 13.131 | 31.6 | 0.0123 | 0.0047 |
| Nyctophilus geoffroyi 12 | TFB | Insectivorous | 8.0 | 0.0027 | 0.1941 | 14.309 | 29.4 | 0.0123 | 0.0313 |
| Nyctophilus timoriensis 13 | TFB | Insectivorous | 13.6 | 0.0171 | 0.0406 | 5.947 | 24.2 | 0.0147 | 0.0023 |
| Otonycteris hemprichii 14 | RC | Insectivorous | 25.4 | 0.0098 | 0.1846 | 19.080 | 32.9 | 0.0096 | 0.0233 |
| Phyllostomus discolor 1 | TCHT | Omnivorous | 33.5 | 0.0280 | 1.3950 | 48.069 | 34.7 | 0.0137 | 0.1655 |
| Phyllostomus hastatus 1 | TCHT | Omnivorous | 84.2 | 0.0440 | 0.6980 | 88.235 | 30.7 | 0.0658 | 0.0313 |
| Pipistrellus kuhlii 15 | Buildings | Insectivorous | 6.9 | 0.0007 | 0.0359 | 10.593 | 25.0 | 0.0141 | 0.0058 |
| Plattyrhinus lineatus 1 | TFB | Omnivorous | 21.9 | 0.0280 | 0.1090 | 15.594 | 28.3 | 0.0235 | 0.0091 |
| Rhinonycteris aurantius 8 | Caves | Insectivorous | 8.3 | 0.0216 | 0.2385 | 8.616 | 34.2 | 0.0170 | 0.0629 |
| Rhinophylla pumilio 1 | TFB | Frugivorous | 9.5 | 0.0040 | 0.5580 | 18.690 | 29.5 | 0.0032 | 0.0180 |
| Roussetus aegyptiacus 16 | Buildings | Frugivorous | 150.8 | 0.1362 | 2.1028 | 28.257 | 32.5 | 0.1698 | 0.3783 |
| Sauromys petrophilus 11 | RC | Insectivorous | 11.0 | 0.0076 | 0.0842 | 3.969 | 39.2 | 0.0062 | 0.0255 |
| Sturnira lilium 1 | TCHT | Frugivorous | 21.9 | 0.0150 | 1.2320 | 36.367 | 34.2 | 0.0114 | 0.0617 |
| Tadarida brasiliensis 6 | Buildings | Insectivorous | 10.4 | 0.0207 | 0.0512 | 3.309 | 30.0 | 0.0246 | 0.0049 |
| Tadarida teniotis 14 | RC | Insectivorous | 32.0 | 0.0229 | 0.5977 | 21.634 | 31.0 | 0.0398 | 0.0489 |
| Taphozous mauritianus 11 | Buildings | Insectivorous | 26.2 | 0.0153 | 2.7478 | 17.542 | 32.5 | 0.0221 | 0.0115 |
| Tonatia bidens 1 | Caves | Carnivorous | 27.4 | 0.0178 | 1.1390 | 4.863 | 32.7 | 0.0196 | 0.0268 |
| Buildings | Caves | MMSs | RCs | TCHTs | TFB | All | |
|---|---|---|---|---|---|---|---|
| Frugivorous | 1 | 3 (2) | 1 | 1 | 4 (6) | 10 (8) | |
| Omnivorous | 1 | 1 (8) | 3 (2) | 1 | 6 (10) | ||
| Insectivorous | 7 | 2 (15) | 2 | 3 | 3 (6) | 3 (1) | 20 (22) |
| Carnivorous | 2 | 2 | 4 | ||||
| Sanguinivorous | 2 (2) | 1 | 3 | ||||
| All | 9 | 10 (27) | 5 | 3 | 8 (8) | 8 (7) | 43 (42) |
| CLSR | PICs | |||||||
|---|---|---|---|---|---|---|---|---|
| Mass | Roost Type | Diet | INT | Mass | Roost Type | Diet | INT | |
| Cmin | <0.001 | - | 0.004 | 0.006 | 0.53 | 0.026 | ||
| <0.001 | 0.33 | 0.46 | 0.015 | 0.71 | 0.40 | |||
| Cmax | 0.002 | 0.79 | 0.78 | 0.17 | - | 0.038 | ||
| 0.005 | 0.62 | 0.088 | 0.25 | 0.22 | 0.29 | |||
| Area of TRP | <0.001 | - | <0.001 | 0.037 | - | 0.005 | ||
| <0.001 | - | 0.004 | 0.028 | 0.18 | 0.43 | |||
| Tbk | 0.58 | 0.23 | 0.088 | 0.63 | 0.44 | 0.088 | ||
| 0.34 | 0.37 | 0.87 | 0.77 | 0.90 | 0.22 | |||
| a1 | <0.001 | - | <0.001 | <0.001 | 0.52 | 0.061 | ||
| <0.001 | - | 0.032 | <0.001 | 0.99 | 0.98 | |||
| b2 | <0.001 | 0.56 | 0.10 | 0.33 | 0.23 | 0.055(0.014) * | ||
| <0.001 | 0.082 | 0.051 | 0.30 | 0.66 | 0.17 | |||
| Species | Body Mass (g) | Roost Type | Troost (°C) | Tb (°C) | Estimated MR (mW) | Estimated TEE (kJ) |
|---|---|---|---|---|---|---|
| Lasiurus borealis | 10 | Tree canopy | 0 | 36 | 358.1 | |
| 0 | 10 | 92.1 | ||||
| Leaf litter | 3.9 | 36 | 328.4 | |||
| 3.9 | 10 | 62.4 | ||||
| Scotophilus dinganii | 25.3 | Exterior building | 25 | 33 | 246.2 | |
| 20 | 18.5 | 76.8 | 3.12 | |||
| No roost | 25 | 36/18.5 | 3.49 | |||
| Scotophilus mhlanganii | 28.4 | Tree cavities | 25 | 36 | 314.5 | |
| 20 | 18.5 | 68.6 | 3.06 | |||
| No roost | 25 | 36/18.5 | 3.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marroquin, C.; Gerth, T.; Muñoz-Garcia, A. The Influence of Roost Type and Diet on Energy Expenditure in Bats. Diversity 2023, 15, 655. https://doi.org/10.3390/d15050655
Marroquin C, Gerth T, Muñoz-Garcia A. The Influence of Roost Type and Diet on Energy Expenditure in Bats. Diversity. 2023; 15(5):655. https://doi.org/10.3390/d15050655
Chicago/Turabian StyleMarroquin, Cynthia, Thomas Gerth, and Agustí Muñoz-Garcia. 2023. "The Influence of Roost Type and Diet on Energy Expenditure in Bats" Diversity 15, no. 5: 655. https://doi.org/10.3390/d15050655
APA StyleMarroquin, C., Gerth, T., & Muñoz-Garcia, A. (2023). The Influence of Roost Type and Diet on Energy Expenditure in Bats. Diversity, 15(5), 655. https://doi.org/10.3390/d15050655






