Sewage Pipe Waters Affect Colour Composition in Palaemon Shrimp from the Intertidal in the Canary Islands: A New Non-lethal Bioindicator of Anthropogenic Pollution
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analyses
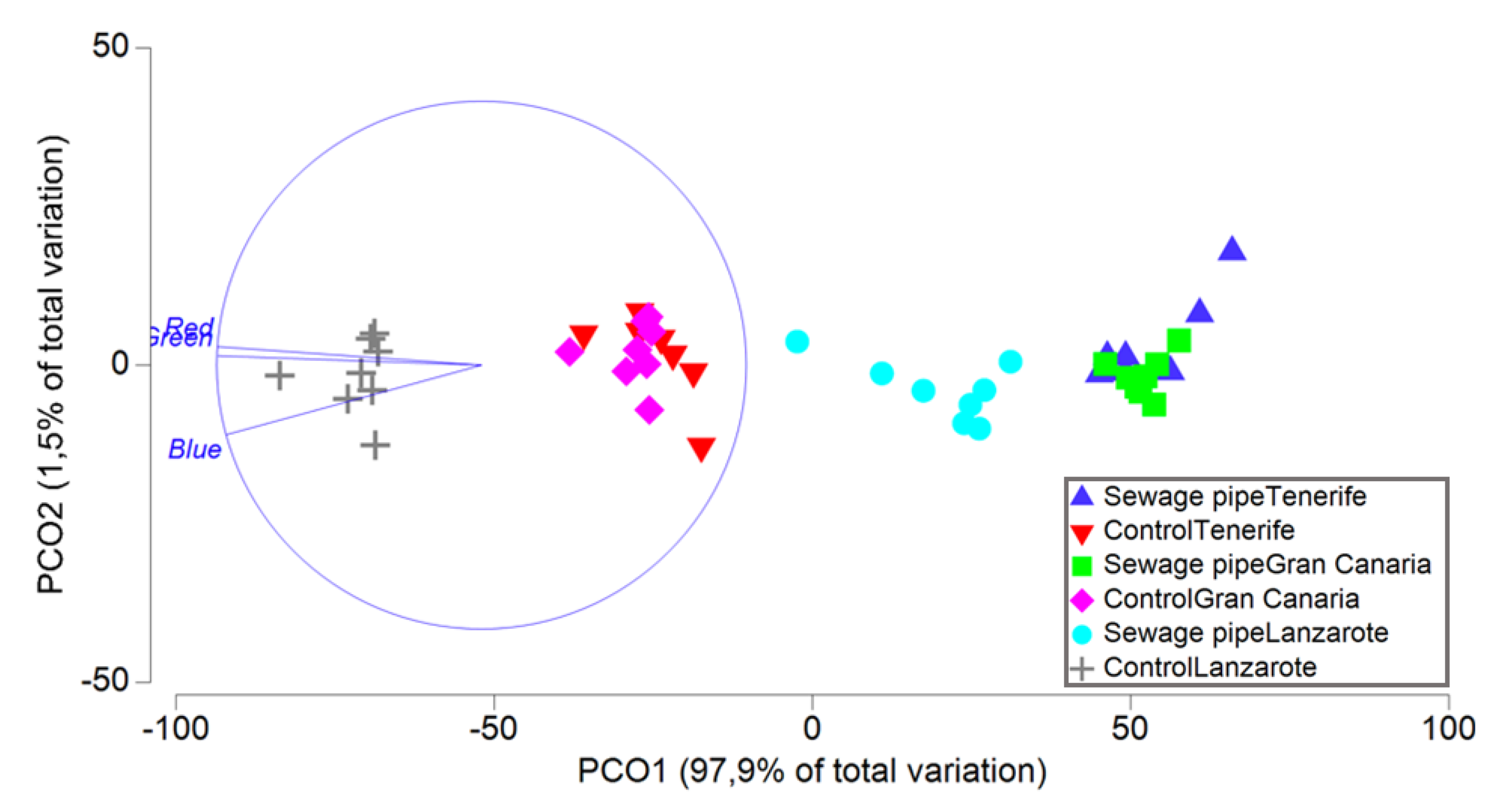
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Genthe, B.; Kapwata, T.; Le Roux, W.; Chamier, J.; Wright, C.Y. The reach of human health risks associated with metals/metalloids in water and vegetables along a contaminated river catchment: South Africa and Mozambique. Chemosphere 2018, 199, 1–9. Available online: http://www.sciencedirect.com/science/article/pii/S0045653518301772 (accessed on 15 February 2023). [CrossRef] [PubMed]
- Abdel Ghani, S.A. Trace metals in seawater, sediments and some fish species from Marsa Matrouh Beaches in north-western Mediterranean coast, Egypt. Egypt. J. Aquat. Res. 2015, 41, 145–154. Available online: http://www.sciencedirect.com/science/article/pii/S1687428515000199 (accessed on 23 January 2023). [CrossRef]
- Irabien, M.J.; Velasco, F. Heavy metals in Oka river sediments (Urdaibai National Biosphere Reserve, northern Spain): Lithogenic and anthropogenic effects. Environ. Geol. 1999, 37, 54–63. [Google Scholar] [CrossRef]
- Goutte, A.; Barbraud, C.; Herzke, D.; Bustamante, P.; Angelier, F.; Tartu, S.; Clément-Chastel, C.; Moe, B.; Bech, C.; Gabrielsen, G.W.; et al. Survival rate and breeding outputs in a high Arctic seabird exposed to legacy persistent organic pollutants and mercury. Environ. Pollut. 2015, 200, 1–9. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Alcázar-Treviño, J.; Fernández, J.J. Determination of δ15N in Anemonia sulcata as a pollution bioindicator. Ecol. Indic. 2018, 90, 179–183. [Google Scholar] [CrossRef]
- Ruilian, Y.; Xing, Y.; Zhao, Y.; Hu, G.; Tu, X. Heavy metal pollution in intertidal sediments from Quanzhou Bay, China. J. Environ. Sci. 2008, 20, 664–669. [Google Scholar]
- Laurent, O.; Bard, D.; Filleul, L.; Segala, C. Effect of socioeconomic status on the relationship between atmospheric pollution and mortality. J. Epidemiol. Community Health 2007, 61, 665–675. [Google Scholar] [CrossRef]
- Handoh, I.C.; Kawai, T. Modelling exposure of oceanic higher trophic-level consumers to polychlorinated biphenyls: Pollution ‘hotspots’ in relation to mass mortality events of marine mammals. Mar. Pollut. Bull. 2014, 85, 824–830. [Google Scholar] [CrossRef]
- Ferretti, F.; Worm, B.; Britten, G.L.; Heithaus, M.R.; Lotze, H.K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar] [CrossRef]
- Hipfner, J.M.; Galbraith, M.; Tucker, S.; Studholme, K.R.; Domalik, A.D.; Pearson, S.F.; Good, T.P.; Ross, P.S.; Hodum, P. Two forage fishes as potential conduits for the vertical transfer of microfibres in Northeastern Pacific Ocean food webs. Environ. Pollut. 2018, 239, 215–222. Available online: http://www.sciencedirect.com/science/article/pii/S0269749117351047 (accessed on 8 February 2023). [CrossRef]
- Lozano-Bilbao, E.; Lozano, G.; Gutiérrez, J.; Hardisson, A.; Rubio, C.; Paz, S.; Weller, D.G. The influence of the degassing phase of the Tagoro submarine volcano (Canary Islands) on the metal content of three species of cephalopods. Mar. Pollut. Bull. 2022, 182, 113964. Available online: https://www.sciencedirect.com/science/article/pii/S0025326X22006464 (accessed on 10 February 2023). [CrossRef] [PubMed]
- Bendell, L.I.; LeCadre, E.; Zhou, W. Use of sediment dwelling bivalves to biomonitor plastic particle pollution in intertidal regions; A review and study. PLoS ONE 2020, 15, e0232879. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.W.; Kim, P.J.; Kim, S.G.; Sun, C.I.; Koh, B.S.; Ryu, S.O.; Kim, T.H. Spatial distribution and pollution assessment of metals in intertidal sediments, Korea. Environ. Sci. Pollut. Res. 2019, 26, 19379–19388. [Google Scholar] [CrossRef]
- Frédou, F.L.; Frédou, T.; Gaertner, D.; Kell, L.; Potier, M.; Bach, P.; Travassos, P.; Hazin, F.; Ménard, F. Life history traits and fishery patterns of teleosts caught by the tuna longline fishery in the South Atlantic and Indian Oceans. Fish. Res. 2016, 179, 308–321. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Lozano, G.; Jiménez, S.; Jurado-Ruzafa, A.; Hardisson, A.; Rubio, C.; Weller, D.-G.; Paz, S.; Gutiérrez, J. Seasonal and ontogenic variations of metal content in the European pilchard (Sardina pilchardus) in northwestern African waters. Environ. Pollut. 2020, 266, 115113. Available online: http://www.sciencedirect.com/science/article/pii/S0269749120329407 (accessed on 10 February 2023). [CrossRef]
- Karunasagar, D.; Krishna, M.V.B.; Anjaneyulu, Y.; Arunachalam, J. Studies of mercury pollution in a lake due to a thermometer factory situated in a tourist resort: Kodaikkanal, India. Environ. Pollut. 2006, 143, 153–158. [Google Scholar] [CrossRef]
- Aydin-Önen, S.; Öztürk, M. Investigation of heavy metal pollution in eastern Aegean Sea coastal waters by using Cystoseira barbata, Patella caerulea, and Liza aurata as biological indicators. Environ. Sci. Pollut. Res. 2017, 24, 7310–7334. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Zhang, Z. Pollution haven or porter? The impact of environmental regulation on location choices of pollution-intensive firms in China. J. Environ. Manag. 2019, 248, 109248. [Google Scholar] [CrossRef]
- Anand, K.S.; Giraud-Carrier, F.C. Pollution regulation of competitive markets. Manag. Sci. 2020, 66, 4193–4206. [Google Scholar] [CrossRef]
- Neves, S.A.; Marques, A.C.; Patrício, M. Determinants of CO2 emissions in European Union countries: Does environmental regulation reduce environmental pollution? Econ. Anal. Policy 2020, 68, 114–125. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Giordano, M.E.; Pascariello, M.F.; Marinosci, L.; Schettino, T. Integrated use of biomarkers (acetylcholinesterase and antioxidant enzymes activities) in Mytilus galloprovincialis and Mullus barbatus in an Italian coastal marine area. Mar. Pollut. Bull. 2003, 46, 324–330. [Google Scholar] [CrossRef]
- Ding, J.; Sun, C.; He, C.; Li, J.; Ju, P.; Li, F. Microplastics in four bivalve species and basis for using bivalves as bioindicators of microplastic pollution. Sci. Total. Environ. 2021, 782, 146830. [Google Scholar] [CrossRef]
- Manickavasagam, S.; Sudhan, C.; Aanand, S. Bioindicators in aquatic environment and their significance. J. Aquac. Trop. 2019, 34, 73–79. [Google Scholar] [CrossRef]
- Thorne-Bazarra, T.; Lozano-Bilbao, E.; Triay-Portella, R.; Hardisson, A.; Paz, S.; Rubio-Armendariz, C.; Martín, V.; Gutiérrez, A.J. Metallic Study of the Invasive Species Cronius ruber—Assessment of Toxic Risk. Appl. Sci. 2022, 12, 3217. [Google Scholar] [CrossRef]
- Chandurvelan, R.; Marsden, I.D.; Glover, C.N.; Gaw, S. Assessment of a mussel as a metal bioindicator of coastal contamination: Relationships between metal bioaccumulation and multiple biomarker responses. Sci. Total. Environ. 2015, 511, 663–675. [Google Scholar] [CrossRef]
- Tokatli, C. Comparisons of diatoms and fishes as toxic metal bioindicator: A case study of an A-class wetland in northwest Turkey under effect of an intensive paddy cultivation stress. Environ. Sci. Pollut. Res. 2022, 29, 87231–87244. [Google Scholar] [CrossRef]
- McCauley, D.J.; Pinsky, M.L.; Palumbi, S.R.; Estes, J.A.; Joyce, F.H.; Warner, R.R. Marine defaunation: Animal loss in the global ocean. Science 2015, 347, 1255641. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Espinosa, J.M.; Lozano, G.; Hardisson, A.; Rubio, C.; González-Weller, D.; Gutiérrez, A.J. Determination of metals in Anemonia sulcata (Pennant, 1777) as a pollution bioindicator. Environ. Sci. Pollut. Res. 2020, 27, 21621–21627. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Herranz, I.; González-Lorenzo, G.; Lozano, G.; Hardisson, A.; Rubio, C.; González-Weller, D.; Paz, S.; Gutiérrez, A.J. Limpets as bioindicators of element pollution in the coasts of Tenerife (Canary Islands). Environ. Sci. Pollut. Res. 2021, 28, 42999–43006. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; González-Delgado, S.; Alcázar-Treviño, J. Use of survival rates of the barnacle Chthamalus stellatus as a bioindicator of pollution. Environ. Sci. Pollut. Res. 2021, 28, 1247–1253. [Google Scholar] [CrossRef]
- Amat, J.A.; Rendón, M.A. Flamingo coloration and its significance. In Flamingos, Behavior, Biology, and Relationship with Humans; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 77–95. [Google Scholar]
- Woolf, M.S.; Dignan, L.M.; Scott, A.T.; Landers, J.P. Digital postprocessing and image segmentation for objective analysis of colorimetric reactions. Nat. Protoc. 2021, 16, 218–238. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Braak, C.T. Permutation tests for multi-factorial analysis of variance. J. Stat. Comput. Simul. 2003, 73, 85–113. [Google Scholar] [CrossRef]
- Anderson, M.R. The Resource for the Power Industry Professional. Proc. ASME Power 2004, 32. [Google Scholar]
- Herrera, E.T.; Lozano-Bilbao, E.; Lozano, G.; Hardisson, A.; Rubio, C.; González-Weller, D.; Gutiérrez, Á.J. Influencia metálica de un emisario submarino de Punta del Hidalgo, en el norte de Tenerife, Islas Canarias, España. Majorensis 2020, 16, 12–19. [Google Scholar]
- González-Delgado, S.; Lozano-Bilbao, E.; Alcázar-Treviño, J. Habitat preference of Anemonia sulcata in intertidal pools in the North-East Atlantic. Majorensis 2018, 14, 7–11. [Google Scholar]
- Canary Islands Institute of Statistics (ISTAC). Available online: https://datos.canarias.es/catalogos/estadisticas/ (accessed on 15 February 2023).
- López, E.P.; García, F.C. Agrotourism, sustainable tourism and ultraperipheral areas: The case of Canary Islands. PASOS Rev. Tur. Patrim. Cult. 2006, 4, 85–97. [Google Scholar]
- Bianchi, R.V. Tourism restructuring and the politics of sustainability: A critical view from the European periphery (The Canary Islands). J. Sustain. Tour. 2004, 12, 495–529. [Google Scholar] [CrossRef]
- Carrillo, J.; González, A.; Pérez, J.C.; Expósito, F.J.; Díaz, J.P. Projected impacts of climate change on tourism in the Canary Islands. Reg. Environ. Chang. 2022, 22, 61. [Google Scholar] [CrossRef]
- García-Romero, L.; Carreira-Galbán, T.; Rodríguez-Báez, J.Á.; Máyer-Suárez, P.; Hernández-Calvento, L.; Yánes-Luque, A. Mapping Environmental Impacts on Coastal Tourist Areas of Oceanic Islands (Gran Canaria, Canary Islands): A Current and Future Scenarios Assessment. Remote. Sens. 2023, 15, 1586. [Google Scholar] [CrossRef]
- Martín Martín, J.M.; Guaita Martínez, J.M.; Molina Moreno, V.; Sartal Rodríguez, A. An analysis of the tourist mobility in the island of Lanzarote: Car rental vs. more sustainable transportation alternatives. Sustainability 2019, 11, 739. [Google Scholar] [CrossRef]
- Silvertown, J. A new dawn for citizen science. Trends Ecol. Evol. 2009, 24, 467–471. [Google Scholar] [CrossRef]
- Vohland, K.; Land-Zandstra, A.; Ceccaroni, L.; Lemmens, R.; Perelló, J.; Ponti, M.; Samson, R.; Wagenknecht, K. The Science of Citizen Science; Springer Nature: Berlin, Germany, 2021; 520p. [Google Scholar]
- Hof, A.E.V.T.; Campagne, P.; Rigden, D.J.; Yung, C.J.; Lingley, J.; Quail, M.A.; Saccheri, I.J. The industrial melanism mutation in British peppered moths is a transposable element. Nature 2016, 534, 102–105. [Google Scholar] [CrossRef]



| Colour | Control Zone | Sewage Pipe | |||||
|---|---|---|---|---|---|---|---|
| Gran Canaria | Lanzarote | Tenerife | Gran Canaria | Lanzarote | Tenerife | ||
| Red | Mean | 140 | 168 | 138 | 82 | 104 | 85 |
| SD | 3 | 4 | 7 | 2 | 7 | 3 | |
| Min | 134 | 161 | 127 | 81 | 97 | 80 | |
| Max | 147 | 176 | 149 | 87 | 115 | 88 | |
| Green | Mean | 132 | 158 | 130 | 85 | 103 | 85 |
| SD | 3 | 3 | 4 | 3 | 11 | 4 | |
| Min | 129 | 155 | 123 | 81 | 88 | 79 | |
| Max | 138 | 165 | 134 | 90 | 128 | 89 | |
| Blue | Mean | 106 | 127 | 105 | 76 | 92 | 72 |
| SD | 4 | 5 | 4 | 3 | 4 | 8 | |
| Min | 100 | 120 | 100 | 69 | 83 | 53 | |
| Max | 113 | 135 | 115 | 80 | 96 | 79 | |
| Gran Canaria | Lanzarote | Tenerife | |
|---|---|---|---|
| Sewage pipe vs. Control zone | 0.001 * F = 10.69 | 0.001 * F = 11.01 | 0.001 * F = 10.61 |
| Sewage Pipe | Control Zone | |
|---|---|---|
| Tenerife vs. Gran Canaria | 0.283 | 0.563 |
| Tenerife vs. Lanzarote | 0.003 * | 0.001 * |
| Gran Canaria vs. Lanzarote | 0.002 * | 0.002 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-Bilbao, E.; Alcázar-Treviño, J. Sewage Pipe Waters Affect Colour Composition in Palaemon Shrimp from the Intertidal in the Canary Islands: A New Non-lethal Bioindicator of Anthropogenic Pollution. Diversity 2023, 15, 658. https://doi.org/10.3390/d15050658
Lozano-Bilbao E, Alcázar-Treviño J. Sewage Pipe Waters Affect Colour Composition in Palaemon Shrimp from the Intertidal in the Canary Islands: A New Non-lethal Bioindicator of Anthropogenic Pollution. Diversity. 2023; 15(5):658. https://doi.org/10.3390/d15050658
Chicago/Turabian StyleLozano-Bilbao, Enrique, and Jesús Alcázar-Treviño. 2023. "Sewage Pipe Waters Affect Colour Composition in Palaemon Shrimp from the Intertidal in the Canary Islands: A New Non-lethal Bioindicator of Anthropogenic Pollution" Diversity 15, no. 5: 658. https://doi.org/10.3390/d15050658
APA StyleLozano-Bilbao, E., & Alcázar-Treviño, J. (2023). Sewage Pipe Waters Affect Colour Composition in Palaemon Shrimp from the Intertidal in the Canary Islands: A New Non-lethal Bioindicator of Anthropogenic Pollution. Diversity, 15(5), 658. https://doi.org/10.3390/d15050658







