Comparative Metabolome Profiles and Antioxidant Potential of Four Coffea arabica L. Varieties Differing in Fruit Color
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coffee Genetic Resources and Field Conditions
2.2. Determination of Total Flavonoid, Anthocyanin, and Phenolic Contents in the Peels of the four Coffee Varieties
2.3. Assessment of Antioxidant Activities in the Peels of the Four Coffee Varieties
2.4. Metabolome Profiling in the Peels of the Four Coffee Varieties by Ultra-High-Performance Liquid Chromatography-Mass Spectrometry
2.5. Statistical Analyses
3. Results
3.1. Levels of Bioactivities and Bioactive Secondary Metabolites in the Peels of Four Varieties of C. arabica
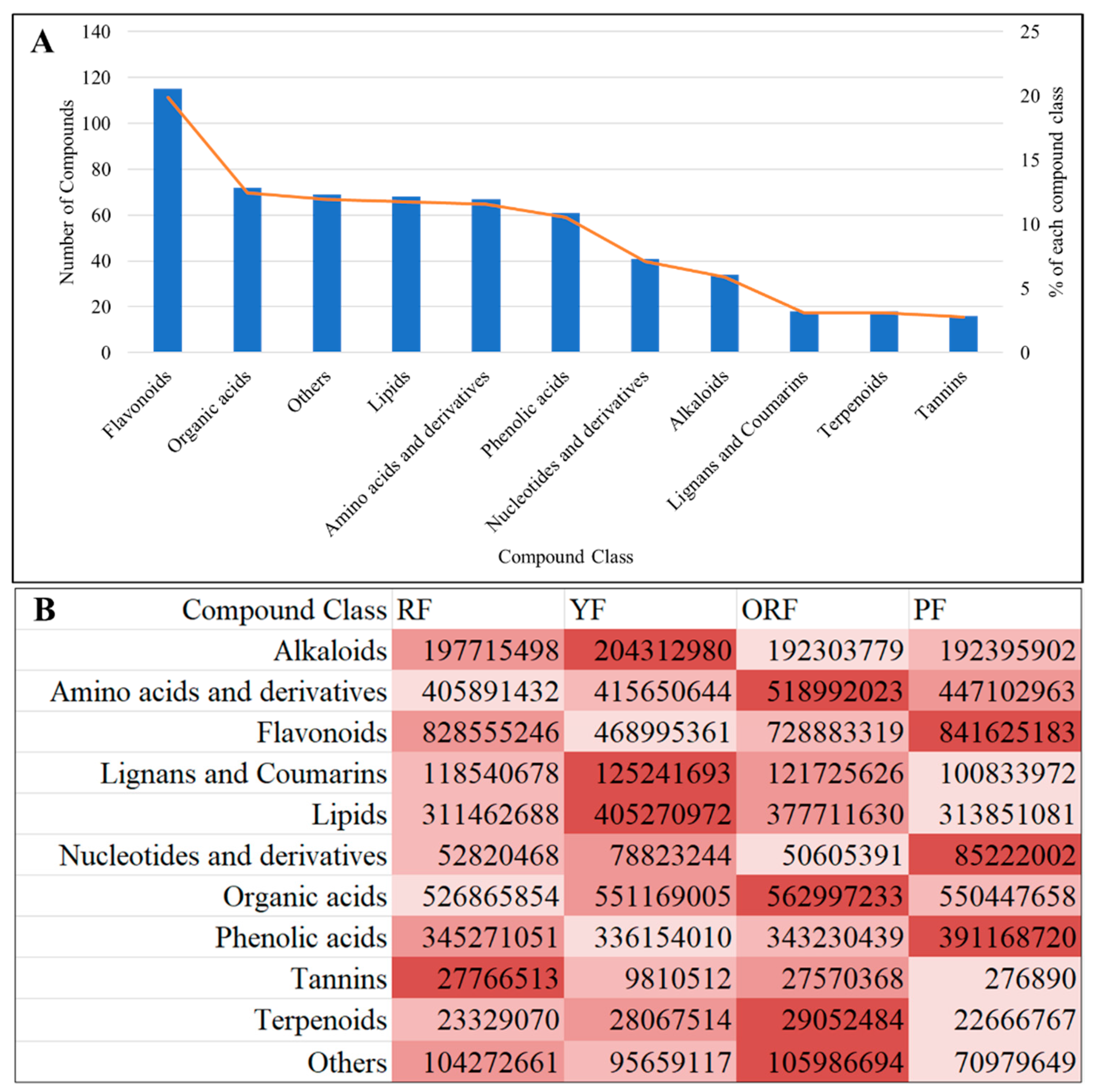
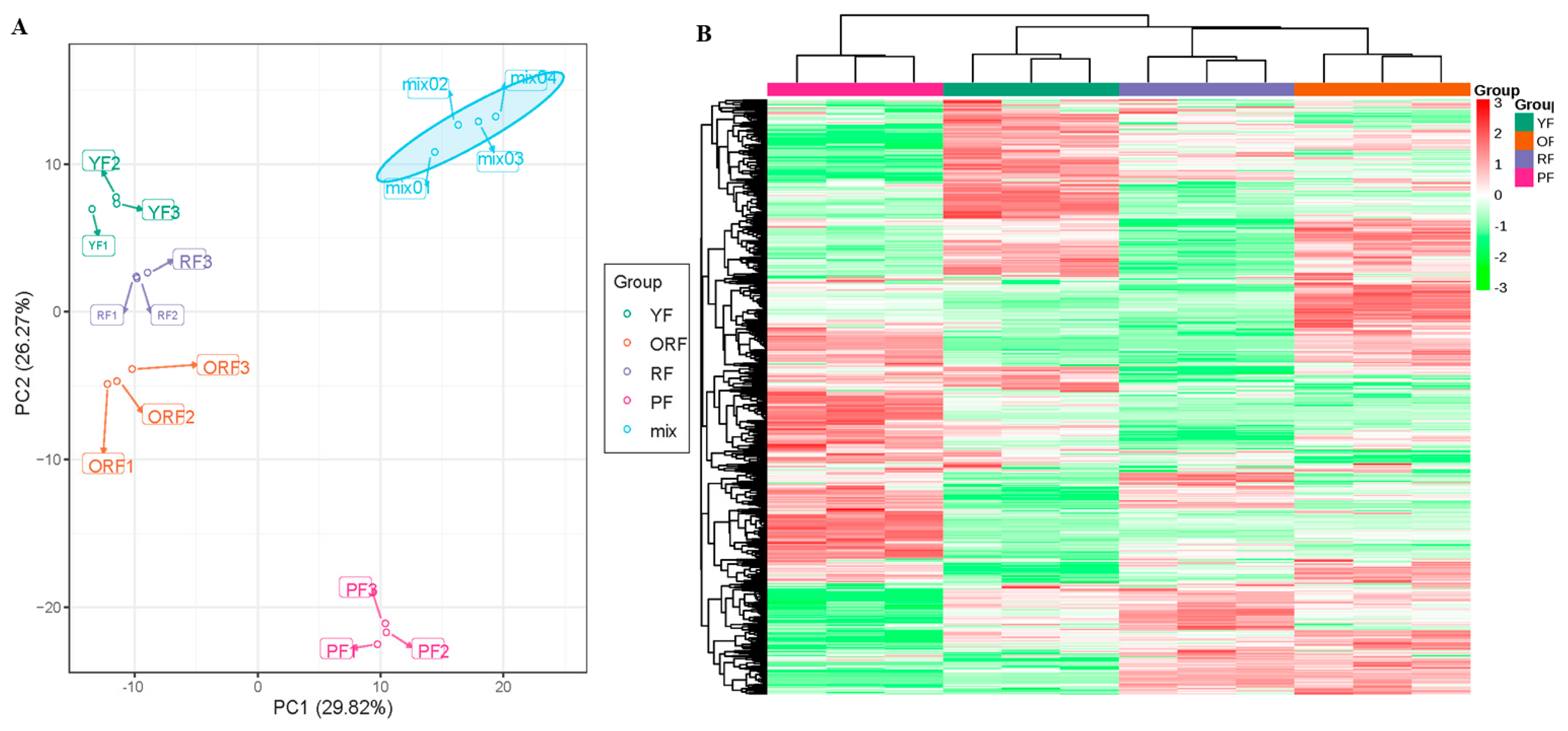
3.2. Overview of Metabolome Profiling among the Four Varieties of C. arabica
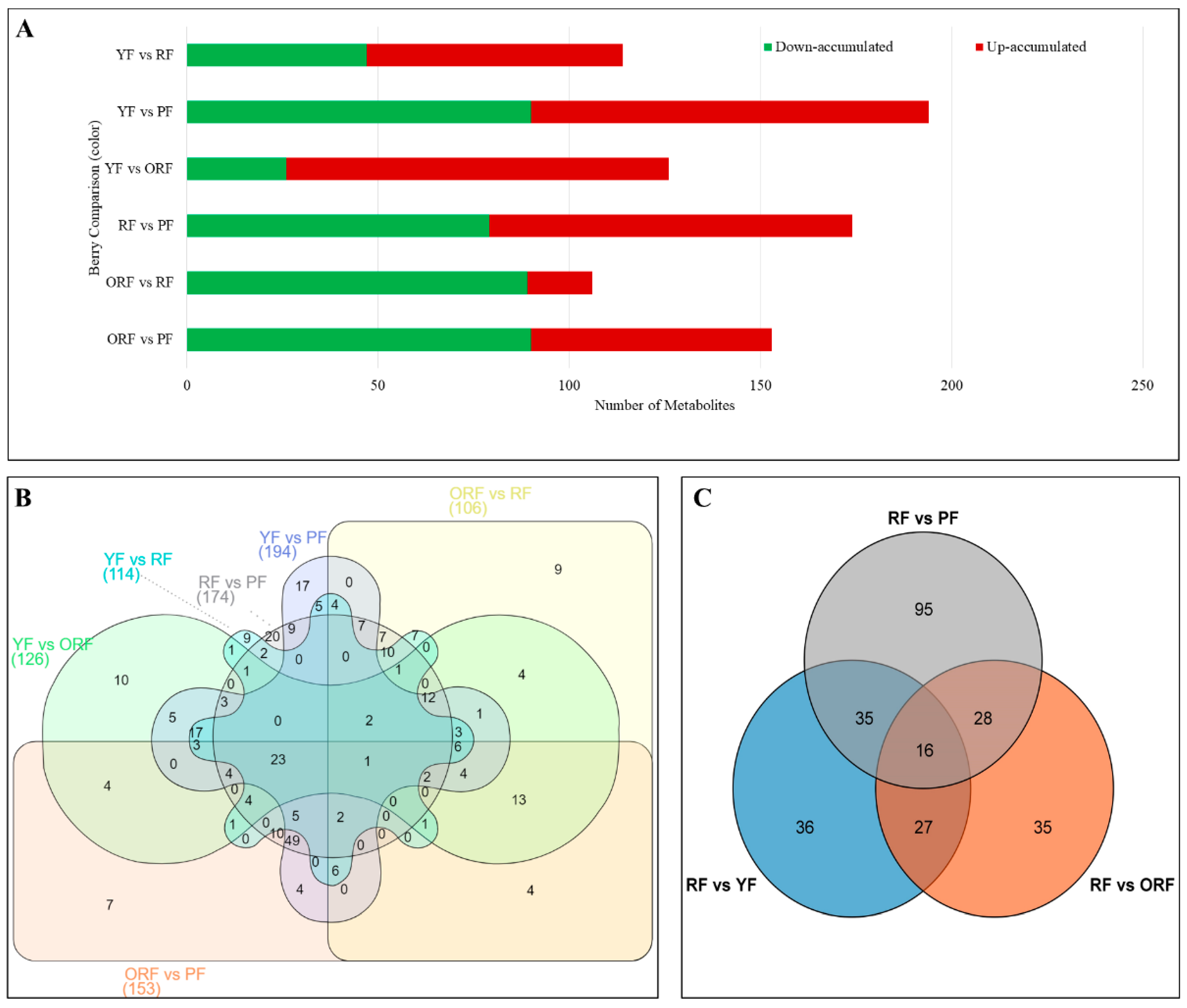
3.2.1. Comparative Analysis of Differentially Accumulated Metabolites among the Four Varieties of C. arabica
Variation in Flavonoid (and Anthocyanin) Contents in the Ripe Fruit Peels of Four C. arabica Varieties
Comparison of Phenolic Compounds Accumulated Differentially among the Ripe Fruit Peels of Four Varieties of C. arabica
Variation in Alkaloid and Lignan and Coumarin Compounds in RF Variety Relative to ORF, YF, and PF Varieties of C. arabica
Lipid and Terpenoid Compounds Showed Diverse Accumulation among the Peels of the Four Varieties of C. arabica
Composition of Organic Acid and Nucleotide and Derivative Compounds among the Ripe Fruit Peels of the Four Varieties of C. arabica
Amino Acid and Derivative, Tannin Compound, and Other (Saccharides and Alcohols, Stilbenes, and Vitamins) Variation among the Fruit Peels of the Four Varieties of C. arabica
4. Discussion
4.1. Coffee Berries with Different Colors Offer Different Antioxidant Potentials
4.2. Coffee Berries with Different Colors Have Different Metabolomic Profiles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guanyan-Tianxia. China’s Coffee Industry Development Status Analysis and Investment Trend Forecast Report (2022–2029); Guanyan Tianxia (Beijing) Information Consulting Co., Ltd.; Insight & Info Consulting Ltd.: Beijing, China, 2022. [Google Scholar]
- Zhang, H.; Li, J.; Zhou, H.; Chen, Z.; Song, G.; Peng, Z.; Pereira, A.; Silva, M.; Várzea, V. Arabica coffee production in the Yunnan Province of China. In Proceedings of the 24th International Conference on Coffee Science, San José, Costa Rica, 12–16 November 2012. [Google Scholar]
- Da Silva Taveira, J.H.; Borém, F.M.; Figueiredo, L.P.; Reis, N.; Franca, A.S.; Harding, S.A.; Tsai, C.-J. Potential markers of coffee genotypes grown in different Brazilian regions: A metabolomics approach. Food Res. Int. 2014, 61, 75–82. [Google Scholar] [CrossRef]
- Amalia, F.; Aditiawati, P.; Putri, S.P.; Fukusaki, E. Gas chromatography/mass spectrometry-based metabolite profiling of coffee beans obtained from different altitudes and origins with various postharvest processing. Metabolomics 2021, 17, 69. [Google Scholar] [CrossRef]
- De Melo Pereira, G.V.; de Carvalho Neto, D.P.; Júnior, A.I.M.; do Prado, F.G.; Pagnoncelli, M.G.B.; Karp, S.G.; Soccol, C.R. Chemical composition and health properties of coffee and coffee by-products. Adv. Food Nutr. Res. 2020, 91, 65–96. [Google Scholar]
- Sharma, H. A detail chemistry of coffee and its analysis. In Coffee—Production and Research; IntechOpen: London, UK, 2020; p. 79. [Google Scholar]
- Yusibani, E.; Putra, R.; Rahwanto, A.; Surbakti, M. Physical properties of Sidikalang robusta coffee beans medium roasted from various colors of coffee cherries. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK; p. 012046.
- Gonzalez-Rios, O.; Suarez-Quiroz, M.L.; Boulanger, R.; Barel, M.; Guyot, B.; Guiraud, J.-P.; Schorr-Galindo, S. Impact of “ecological” post-harvest processing on the volatile fraction of coffee beans: I. Green coffee. J. Food Compos. Anal. 2007, 20, 289–296. [Google Scholar] [CrossRef]
- Esquivel, P.; Jimenez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Montis, A.; Souard, F.; Delporte, C.; Stoffelen, P.; Stévigny, C.; Van Antwerpen, P. Targeted and Untargeted Mass Spectrometry-Based Metabolomics for Chemical Profiling of Three Coffee Species. Molecules 2022, 27, 3152. [Google Scholar] [CrossRef]
- Ságio, S.A.; Lima, A.A.; Barreto, H.G.; de Carvalho, C.H.S.; Paiva, L.V.; Chalfun-Junior, A. Physiological and molecular analyses of early and late Coffea arabica cultivars at different stages of fruit ripening. Acta Physiol. Plant. 2013, 35, 3091–3098. [Google Scholar] [CrossRef]
- Aswathi, K.; Shankar, S.; Seenivasan, K.; Prakash, I.; Murthy, P.S. Metagenomics and metabolomic profiles of Coffea canephora processed by honey/pulped natural technique. Innov. Food Sci. Emerg. Technol. 2022, 79, 103058. [Google Scholar] [CrossRef]
- De Castro, R.D.; Marraccini, P. Cytology, biochemistry and molecular changes during coffee fruit development. Braz. J. Plant Physiol. 2006, 18, 175–199. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, Q.; Wu, H.; Wang, S.; Zou, M. A comparative metabolomics analysis of guava (Psidium guajava L.) fruit with different colors. ACS Food Sci. Technol. 2020, 1, 96–106. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, Y.; Vainstein, A.; Chen, S.; Ma, H. Regulation of fig (Ficus carica L.) fruit color: Metabolomic and transcriptomic analyses of the flavonoid biosynthetic pathway. Front. Plant Sci. 2017, 8, 1990. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; He, N.; Umer, M.J.; Zhao, S.; Diao, W.; Zhu, H.; Dou, J.; Kaseb, M.O.; Kuang, H.; Lu, X. Comparative metabolomic profiling of Citrullus spp. fruits provides evidence for metabolomic divergence during domestication. Metabolites 2021, 11, 78. [Google Scholar]
- Li, Y.; Nie, J.; Shi, L.; Xie, Y.; Tan, D.; Yang, X.; Zhang, C.; Zheng, J. Transcriptomic and metabolomic profiling reveals the mechanisms of color and taste development in cherry tomato cultivars. LWT 2022, 167, 113810. [Google Scholar] [CrossRef]
- Hua, Q.; Chen, C.; Zur, N.T.; Wang, H.; Wu, J.; Chen, J.; Zhang, Z.; Zhao, J.; Hu, G.; Qin, Y. Metabolomic characterization of pitaya fruit from three red-skinned cultivars with different pulp colors. Plant Physiol. Biochem. 2018, 126, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shu, H.; Mumtaz, M.A.; Hao, Y.; Li, L.; He, Y.; Jin, W.; Li, C.; Zhou, Y.; Lu, X. Transcriptome and Metabolome Analysis of Color Changes during Fruit Development of Pepper (Capsicum baccatum). Int. J. Mol. Sci. 2022, 23, 12524. [Google Scholar] [CrossRef]
- Spence, C. On the relationship (s) between color and taste/flavor. Exp. Psychol. 2019, 66, 99–111. [Google Scholar] [CrossRef]
- Clydesdale, F.M. Color as a factor in food choice. Crit. Rev. Food Sci. Nutr. 1993, 33, 83–101. [Google Scholar] [CrossRef]
- Stroebele, N.; De Castro, J.M. Effect of ambience on food intake and food choice. Nutrition 2004, 20, 821–838. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Wang, Z.; Liu, Z.; Zhao, Z.; Zhou, G.; Liu, M.; Liu, P. Variations of the nutritional composition of jujube fruit (Ziziphus jujuba Mill.) during maturation stages. Int. J. Food Prop. 2020, 23, 1066–1081. [Google Scholar] [CrossRef]
- Li, Y.; Cui, W.; Qi, X.; Lin, M.; Qiao, C.; Zhong, Y.; Hu, C.; Fang, J. MicroRNA858 negatively regulates anthocyanin biosynthesis by repressing AaMYBC1 expression in kiwifruit (Actinidia arguta). Plant Sci. 2020, 296, 110476. [Google Scholar] [CrossRef] [PubMed]
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Aires, A.; Saavedra, M.J.; Gonçalves, B. Phenolic Profile and Bioactive Potential of Stems and Seed Kernels of Sweet Cherry Fruit. Antioxidants 2020, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.-J.; Zhang, H.; Ni, Y.-L.; Huang, B.; Zhang, J.; Feng, J.-Y.; Wang, S.-B.; Dunwell, J.M.; Zhang, Y.-M.; Wu, R. Methodological implementation of mixed linear models in multi-locus genome-wide association studies. Brief. Bioinform. 2018, 19, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kolde, R. Pheatmap: Pretty heatmaps. R Package Version 2012, 1, 747. [Google Scholar]
- Vanhaeren, H.; Nam, Y.-J.; De Milde, L.; Chae, E.; Storme, V.; Weigel, D.; Gonzalez, N.; Inzé, D. Forever young: The role of ubiquitin receptor DA1 and E3 ligase BIG BROTHER in controlling leaf growth and development. Plant Physiol. 2017, 173, 1269–1282. [Google Scholar] [CrossRef]
- Shan, M.; Liu, H.; Hao, Y.; Song, K.; Meng, T.; Feng, C.; Wang, Y.; Huang, Y. Metabolomic Profiling Reveals That 5-Hydroxylysine and 1-Methylnicotinamide Are Metabolic Indicators of Keloid Severity. Front. Genet. 2022, 12, 804248. [Google Scholar] [CrossRef]
- Bressani, A.P.P.; Martinez, S.J.; Batista, N.N.; Simão, J.B.P.; Schwan, R.F. Into the minds of coffee consumers: Perception, preference, and impact of information in the sensory analysis of specialty coffee. Food Sci. Technol. 2021, 41, 667–675. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Fernandes, I.; Marques, C.; Évora, A.; Faria, A.; Calhau, C.; Mateus, N.; de Freitas, V. Anthocyanins: Nutrition and Health. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1097–1133. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Muruganathan, N.; Dhanapal, A.R.; Baskar, V.; Muthuramalingam, P.; Selvaraj, D.; Aara, H.; Shiek Abdullah, M.Z.; Sivanesan, I. Recent Updates on Source, Biosynthesis, and Therapeutic Potential of Natural Flavonoid Luteolin: A Review. Metabolites 2022, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef]
- Martínez-Noguera, F.J.; Marín-Pagán, C.; Carlos-Vivas, J.; Rubio-Arias, J.A.; Alcaraz, P.E. Acute Effects of Hesperidin in Oxidant/Antioxidant State Markers and Performance in Amateur Cyclists. Nutrients 2019, 11, 1898. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and Anti-Inflammatory Effects of Citrus Flavonoid Hesperetin: Special Focus on Neurological Disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Celeghini, R.M.S.; Debien, I.C.N.; Nogueira, G.C.; Meireles, M.A.A. Phenolic Compounds in Coffee Compared to Other Beverages. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 137–142. [Google Scholar] [CrossRef]
- Wilson, A.; Johnson, J.B.; Naiker, M. Biotechnological Modification of Cider Brewing Processes for the Enhanced Production of 2-Phenylethanol. Beverages 2022, 8, 64. [Google Scholar] [CrossRef]
- Tieman, D.; Taylor, M.; Schauer, N.; Fernie, A.R.; Hanson, A.D.; Klee, H.J. Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde. Proc. Natl. Acad. Sci. USA 2006, 103, 8287–8292. [Google Scholar] [CrossRef]
- Wang, S.; Qiang, Q.; Xiang, L.; Fernie, A.R.; Yang, J. Targeted approaches to improve tomato fruit taste. Hortic. Res. 2023, 10, uhac229. [Google Scholar] [CrossRef]
- Selmar, D.; Bytof, G.; Knopp, S.-E. The Storage of Green Coffee (Coffea arabica): Decrease of Viability and Changes of Potential Aroma Precursors. Ann. Bot. 2008, 101, 31–38. [Google Scholar] [CrossRef]
- Vandeponseele, A.; Draye, M.; Piot, C.; Chatel, G. Subcritical water and supercritical carbon dioxide: Efficient and selective eco-compatible solvents for coffee and coffee by-products valorization. Green Chem. 2020, 22, 8544–8571. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef] [PubMed]
- Glabasnia, A.; Blank, I.; Mora, F.; Leloup, V.; Kerler, J. The multiple role of polyphenol chemistry in coffee associated with quality attributes. In Proceedings of the 24th ASIC Conference, San José, Costa Rica, 12–16 November 2012. [Google Scholar]
- Joanna, K. Introductory Chapter: Alkaloids—Their Importance in Nature and for Human Life. In Alkaloids; Joanna, K., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 1. [Google Scholar] [CrossRef]
- Ashihara, H.; Sano, H.; Crozier, A. Caffeine and related purine alkaloids: Biosynthesis, catabolism, function and genetic engineering. Phytochemistry 2008, 69, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Patay, É.B.; Bencsik, T.; Papp, N. Phytochemical overview and medicinal importance of Coffea species from the past until now. Asian Pac. J. Trop. Med. 2016, 9, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; An, H.; Feng, L.; Liu, Q.; Wang, S.; Zhang, T. Sinapine as an active compound for inhibiting the proliferation of Caco-2 cells via downregulation of P-glycoprotein. Food Chem. Toxicol. 2014, 67, 187–192. [Google Scholar] [CrossRef]
- Bastian, F.; Hutabarat, O.S.; Dirpan, A.; Nainu, F.; Harapan, H.; Emran, T.B.; Simal-Gandara, J. From Plantation to Cup: Changes in Bioactive Compounds during Coffee Processing. Foods 2021, 10, 2827. [Google Scholar] [CrossRef]
- Farias-Pereira, R.; Park, C.S.; Park, Y. Mechanisms of action of coffee bioactive components on lipid metabolism. Food Sci. Biotechnol. 2019, 28, 1287–1296. [Google Scholar] [CrossRef]
- Anagbogu, C.F.; Zhou, J.; Olasupo, F.O.; Baba Nitsa, M.; Beckles, D.M. Lipidomic and metabolomic profiles of Coffea canephora L. beans cultivated in Southwestern Nigeria. PLoS ONE 2021, 16, e0234758. [Google Scholar] [CrossRef]
- Deepak Kumar, D.; Chandra Kishore, T.; Anil Kumar, S.; Vaibhav, T. Revisiting the Medicinal Value of Terpenes and Terpenoids. In Revisiting Plant Biostimulants; Vijay Singh, M., Hanuman Prasad, P., Sunita Kumari, M., Eds.; IntechOpen: Rijeka, Croatia, 2022; Chapter 5. [Google Scholar] [CrossRef]
- Ali, A.; Zahid, H.F.; Cottrell, J.J.; Dunshea, F.R. A Comparative Study for Nutritional and Phytochemical Profiling of Coffea arabica (C. arabica) from Different Origins and Their Antioxidant Potential and Molecular Docking. Molecules 2022, 27, 5126. [Google Scholar] [CrossRef]
- Jham, G.N.; Fernandes, S.A.; Garcia, C.F.; Silva, A.A.d. Comparison of GC and HPLC for the quantification of organic acids in coffee. Phytochem. Anal. 2002, 13, 99–104. [Google Scholar] [CrossRef]
- Casal, S.; Mendes, E.; Oliveira, M.B.P.P.; Ferreira, M.A. Roast effects on coffee amino acid enantiomers. Food Chem. 2005, 89, 333–340. [Google Scholar] [CrossRef]
- Dong, W.; Tan, L.; Zhao, J.; Hu, R.; Lu, M. Characterization of Fatty Acid, Amino Acid and Volatile Compound Compositions and Bioactive Components of Seven Coffee (Coffea robusta) Cultivars Grown in Hainan Province, China. Molecules 2015, 20, 16687–16708. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Galappaththy, P. Health benefits of Ceylon cinnamon (Cinnamomum zeylanicum): A summary of the current evidence. Ceylon Med. J. 2016, 61, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cwiková, O.; Komprda, T.; Šottníková, V.; Svoboda, Z.; Simonová, J.; Slováček, J.; Jůzl, M. Effects of Different Processing Methods of Coffee Arabica on Colour, Acrylamide, Caffeine, Chlorogenic Acid, and Polyphenol Content. Foods 2022, 11, 3295. [Google Scholar] [CrossRef]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Fidrianny, I.; Ruslan, K. Antioxidant activities of Arabica green coffee from three regions using ABTS and DPPH assays. Asian J. Pharm. Clin. Res. 2016, 189–193. [Google Scholar]
- Çoklar, H.; Akbulut, M. Effect of sun, oven and freeze-drying on anthocyanins, phenolic compounds and antioxidant activity of black grape (Ekşikara)(Vitis vinifera L.). South Afr. J. Enol. Vitic. 2017, 38, 264–272. [Google Scholar] [CrossRef]
- Martínez, S.; Fuentes, C.; Carballo, J. Antioxidant Activity, Total Phenolic Content and Total Flavonoid Content in Sweet Chestnut (Castanea sativa Mill.) Cultivars Grown in Northwest Spain under Different Environmental Conditions. Foods 2022, 11, 3519. [Google Scholar] [CrossRef]
- Chairgulprasert, V.; Kongsuwankeeree, K. Preliminary phytochemical screening and antioxidant activity of robusta coffee blossom. Sci. Technol. Asia 2017, 22, 1–8. [Google Scholar]
- Mohanta, B.; Sen, D.J.; Mahanti, B.; Nayak, A.K. Antioxidant potential of herbal polysaccharides: An overview on recent researches. Sens. Int. 2022, 3, 100158. [Google Scholar] [CrossRef]
- Sung, S.H.; Kim, K.H.; Jeon, B.T.; Cheong, S.H.; Park, J.H.; Kim, D.H.; Kweon, H.J.; Moon, S.H. Antibacterial and antioxidant activities of tannins extracted from agricultural by-products. J. Med. Plants Res. 2012, 6, 3072–3079. [Google Scholar] [CrossRef]
- Ameca, G.M.; Cerrilla, M.E.O.; Córdoba, P.Z.; Cruz, A.D.; Hernández, M.S.; Haro, J.H. Chemical composition and antioxidant capacity of coffee pulp. Ciência E Agrotecnol. 2018, 42, 307–313. [Google Scholar] [CrossRef]
- Patay, É.B.; Sali, N.; Kőszegi, T.; Csepregi, R.; Balázs, V.L.; Németh, T.S.; Németh, T.; Papp, N. Antioxidant potential, tannin and polyphenol contents of seed and pericarp of three Coffea species. Asian Pac. J. Trop. Med. 2016, 9, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Zayed, A.; Sallam, I.E.; Abdelwareth, A.; Wessjohann, L.A. Metabolomics-based approach for coffee beverage improvement in the context of processing, brewing methods, and quality attributes. Foods 2022, 11, 864. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health effects, sources, utilization and safety of tannins: A critical review. Toxin Rev. 2021, 40, 432–444. [Google Scholar] [CrossRef]
- Strati, I.F.; Tataridis, P.; Shehadeh, A.; Chatzilazarou, A.; Bartzis, V.; Batrinou, A.; Sinanoglou, V.J. Impact of tannin addition on the antioxidant activity and sensory character of Malagousia white wine. Curr. Res. Food Sci. 2021, 4, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Izabel Luzia, M.; Marisa Alves Nogueira, D. Quinolines, Isoquinolines, Angustureine, and Congeneric Alkaloids—Occurrence, Chemistry, and Biological Activity. In Phytochemicals; Rao, A.V., Leticia, G.R., Eds.; IntechOpen: Rijeka, Croatia, 2015; Chapter 6. [Google Scholar] [CrossRef]
- Ashihara, H. Metabolism of alkaloids in coffee plants. Braz. J. Plant Physiol. 2006, 18, 1–8. [Google Scholar] [CrossRef]
- Perrois, C.; Strickler, S.R.; Mathieu, G.; Lepelley, M.; Bedon, L.; Michaux, S.; Husson, J.; Mueller, L.; Privat, I. Differential regulation of caffeine metabolism in Coffea arabica (Arabica) and Coffea canephora (Robusta). Planta 2015, 241, 179–191. [Google Scholar] [CrossRef]
- Muñoz, A.; Sojo, F.; Arenas, D.R.M.; Kouznetsov, V.V.; Arvelo, F. Cytotoxic effects of new trans-2,4-diaryl-r-3-methyl-1,2,3,4-tetrahydroquinolines and their interaction with antitumoral drugs gemcitabine and paclitaxel on cellular lines of human breast cancer. Chem.-Biol. Interact. 2011, 189, 215–221. [Google Scholar] [CrossRef]
- Qian, D.; Chen, J.; Lai, C.; Kang, L.; Xiao, S.; Song, J.; Xie, J.; Huang, L. Dicaffeoyl polyamine derivatives from bitter goji: Contribution to the bitter taste of fruit. Fitoterapia 2020, 143, 104543. [Google Scholar] [CrossRef]
- Roumani, M.; Duval, R.E.; Ropars, A.; Risler, A.; Robin, C.; Larbat, R. Phenolamides: Plant specialized metabolites with a wide range of promising pharmacological and health-promoting interests. Biomed. Pharmacother. 2020, 131, 110762. [Google Scholar] [CrossRef]
- Xiao, X.; Ren, W.; Zhang, N.; Bing, T.; Liu, X.; Zhao, Z.; Shangguan, D. Comparative Study of the Chemical Constituents and Bioactivities of the Extracts from Fruits, Leaves and Root Barks of Lycium barbarum. Molecules 2019, 24, 1585. [Google Scholar] [CrossRef] [PubMed]
- Mazur, W.; WÄHÄLÄ, K.; Rasku, S.; Makkonen, A.; Hase, T.; Adlercreutz, H. Lignans and Isoflavonoid Polyphenols in Tea and Coffee. J. Med. Food 1999, 2, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An important class of phytochemicals. Phytochem.-Isol. Characterisation Role Hum. Health 2015, 25, 533–538. [Google Scholar]
- Hano, C.F.; Dinkova-Kostova, A.T.; Davin, L.B.; Cort, J.R.; Lewis, N.G. Lignans: Insights into their biosynthesis, metabolic engineering, analytical methods and health benefits. Front. Plant Sci. 2021, 11, 630327. [Google Scholar] [CrossRef]
- Majnooni, M.B.; Fakhri, S.; Shokoohinia, Y.; Mojarrab, M.; Kazemi-Afrakoti, S.; Farzaei, M.H. Isofraxidin: Synthesis, Biosynthesis, Isolation, Pharmacokinetic and Pharmacological Properties. Molecules 2020, 25, 2040. [Google Scholar] [CrossRef]
- Pal, D.; Chandra, P.; Sachan, N. Sesame Seed in Controlling Human Health and Nutrition. In Nuts and Seeds in Health and Disease Prevention, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 183–210. [Google Scholar] [CrossRef]
- Hsueh, T.-P.; Tsai, T.-H. Preclinical Pharmacokinetics of Scoparone, Geniposide and Rhein in an Herbal Medicine Using a Validated LC-MS/MS Method. Molecules 2018, 23, 2716. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Dou, S.; Sun, W.; Wu, X.; Wang, P.; Wang, X. Metabolomics study on the hepatoprotective effect of scoparone using ultra-performance liquid chromatography/electrospray ionization quadruple time-of-flight mass spectrometry. Analyst 2013, 138, 353–361. [Google Scholar] [CrossRef]
- Du, X.; Huang, Q.; Guan, Y.; Lv, M.; He, X.; Fang, C.; Wang, X.; Sheng, J. Caffeine Promotes Conversion of Palmitic Acid to Palmitoleic Acid by Inducing Expression of fat-5 in Caenorhabditis elegans and scd1 in Mice. Front. Pharmacol. 2018, 9, 321. [Google Scholar] [CrossRef]
- Guerra-Vázquez, C.M.; Martínez-Ávila, M.; Guajardo-Flores, D.; Antunes-Ricardo, M. Punicic Acid and Its Role in the Prevention of Neurological Disorders: A Review. Foods 2022, 11, 252. [Google Scholar] [CrossRef]
- Luckose, F.; Pandey, M.C.; Radhakrishna, K. Effects of amino acid derivativeson physical, mental, and physiological activities. Crit. Rev. Food Sci. Nutr. 2015, 55, 1793–1807. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Tiwari, R.; Yatoo, M.I.; Karthik, K.; Michalak, I.; Dhama, K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health–a comprehensive review. Vet. Q. 2021, 41, 1–29. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Wang, J.; Han, S.; Ma, L.; Mo, X.; Li, M.; Hu, L.; Wang, L. Transcriptomic and Widely Targeted Metabolomic Approach Identified Diverse Group of Bioactive Compounds, Antiradical Activities, and Their Associated Genes in Six Sugarcane Varieties. Antioxidants 2022, 11, 1319. [Google Scholar] [CrossRef]
- Šentjurc, M.; Nemec, M.; Connor, H.D.; Abram, V. Antioxidant Activity of Sempervivum tectorum and Its Components. J. Agric. Food Chem. 2003, 51, 2766–2771. [Google Scholar] [CrossRef]
- Zhang, D.; Nie, S.; Xie, M.; Hu, J. Antioxidant and antibacterial capabilities of phenolic compounds and organic acids from Camellia oleifera cake. Food Sci. Biotechnol. 2019, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- GRAßMANN, J. Terpenoids as plant antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar] [PubMed]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and kahweol: A review on their bioactivities and pharmacological properties. Int. J. Mol. Sci. 2019, 20, 4238. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Song, G.; Liu, X.; Xu, M.; Li, Y. Nucleotides as optimal candidates for essential nutrients in living organisms: A review. J. Funct. Foods 2021, 82, 104498. [Google Scholar] [CrossRef]
- Wei, F.; Furihata, K.; Koda, M.; Hu, F.; Kato, R.; Miyakawa, T.; Tanokura, M. 13C NMR-based metabolomics for the classification of green coffee beans according to variety and origin. J. Agric. Food Chem. 2012, 60, 10118–10125. [Google Scholar] [CrossRef]



| Compounds | ORF_vs_RF | RF_vs_PF | YF_vs_RF | YF_vs_ORF | YF_vs_PF | ORF_vs_PF |
|---|---|---|---|---|---|---|
| Anthocyanins | ||||||
| Delphinidin-3-O-rutinoside-7-O-glucoside | 0.25 | 4.97 | ns | 3.60 | 4.43 | ns |
| Petunidin-3-O-(6″-O-p-coumaroyl)glucoside | 0.25 | 4.75 | ns | 6.11 | 7.13 | ns |
| Cyanidin-3-O-(6″-O-caffeoyl)glucoside | ns | 0.35 | ns | ns | ns | ns |
| Cyanidin-3-O-(6″-O-p-coumaroyl)glucoside | ns | 0.44 | ns | ns | 0.27 | ns |
| Cyanidin-3-O-glucoside (kuromanin) | 7.91 | ns | 257.07 | 32.50 | 202.24 | 6.22 |
| Cyanidin-3-O-rutinoside (keracyanin) | 4.17 | ns | 216.51 | 51.88 | 208.73 | 4.02 |
| Chalcones/dihydroflavones/flavonoids | ||||||
| Naringenin chalcone | ns | ns | 5.07 | 5.45 | 8.80 | ns |
| Naringenin (5,7,5′-trihydroxyflavanone) | ns | ns | 743.33 | 692.24 | 899.14 | ns |
| Naringenin-7-O-glucoside (prunin) | ns | ns | ns | ns | 2.31 | ns |
| Naringenin-4′-O-glucoside | ns | ns | 4.22 | 4.28 | 3.82 | ns |
| Hesperetin-5-O-glucoside | 0.35 | ns | ns | ns | ns | ns |
| Hesperetin-7-O-glucoside | 0.18 | 6.71 | ns | 10.97 | 13.17 | ns |
| Hesperetin-7-O-rutinoside (hesperidin) | ns | 4460.93 | ns | ns | 4460.93 | 4460.93 |
| Hesperetin-7-O-(6″-malonyl)glucoside | ns | ns | 0.44 | ns | 0.33 | ns |
| Luteolin-7-O-neohesperidoside (Lonicerin) | ns | 0.39 | ns | ns | ns | ns |
| Luteolin-7-O-rutinoside | ns | 0.41 | ns | ns | ns | ns |
| Luteolin-7-O-(2″-O-rhamnosyl)rutinoside | ns | 0.27 | ns | ns | 0.32 | ns |
| Luteolin-6-C-glucoside-7-O-(6″-feruloyl)arabinoside | ns | ns | 0.48 | ns | 0.50 | ns |
| Luteolin-8-C-glucoside-7-O-glucoside | ns | ns | ns | 2.11 | ns | ns |
| Luteolin-3′-O-glucoside | ns | ns | ns | ns | 0.39 | ns |
| Luteolin-4′-O-glucoside | ns | ns | ns | ns | 0.37 | ns |
| Dihydroquercetin(taxifolin) | 0.18 | ns | 3.57 | 19.72 | 3.38 | ns |
| Quercetin-3-O-xyloside (reynoutrin) | 0.36 | ns | ns | 2.91 | ns | 0.40 |
| Quercetin-3-O-arabinoside (guaijaverin) | 0.27 | ns | ns | 3.12 | ns | 0.44 |
| Quercetin-3-O-rhamnoside(quercitrin) | 0.23 | ns | ns | 5.93 | 2.50 | 0.42 |
| Quercetin-3-O-glucoside (isoquercitrin) | 0.38 | ns | ns | ns | ns | ns |
| Quercetin-3-O-sambubioside | 0.34 | ns | ns | 4.50 | 2.06 | 0.46 |
| Quercetin-3-O-apiosyl(1→2)galactoside | 0.35 | ns | ns | 4.47 | 2.07 | 0.46 |
| Quercetin-3-O-xylosyl(1→2)glucoside | 0.37 | ns | ns | 3.68 | ns | 0.50 |
| Quercetin-3-O-rutinoside (rutin) | 0.35 | ns | ns | 2.43 | ns | ns |
| Quercetin-3-O-rutinoside-7-O-rhamnoside | 0.31 | ns | ns | ns | ns | 0.44 |
| Azaleatin (5-O-methylquercetin) | 0.20 | 13.75 | ns | 9.72 | 27.18 | |
| Quercetin-7-O-(6″-malonyl)glucoside | ns | 2.58 | ns | ns | 2.06 | 2.22 |
| Quercetin-3-O-(6″-malonyl)galactoside | ns | 2.63 | ns | ns | ns | ns |
| Quercetin-3-O-(2″-galloyl)arabinoside | ns | 0.15 | 0.42 | 0.44 | 0.06 | 0.14 |
| Quercetin-3-O-neohesperidoside | ns | 20.09 | ns | ns | 31.83 | 24.16 |
| Quercetin-3-O-robinobioside | ns | 24.70 | ns | ns | 27.15 | 24.27 |
| 6-C-methylquercetin-3-O-rutinoside | ns | 59.26 | ns | 2.03 | 111.92 | 55.21 |
| Quercetin-3-O-rutinoside-7-O-rhamnoside | ns | ns | ns | 3.13 | ns | ns |
| Quercetin-3-O-(2‴-Caffeoyl)sophoroside | ns | ns | ns | ns | 0.40 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, F.; Bi, X.; Fu, X.; Li, Y.; Li, G.; Li, Y.; Liu, D.; Yang, Y.; Shi, R.; Dong, W. Comparative Metabolome Profiles and Antioxidant Potential of Four Coffea arabica L. Varieties Differing in Fruit Color. Diversity 2023, 15, 724. https://doi.org/10.3390/d15060724
Hu F, Bi X, Fu X, Li Y, Li G, Li Y, Liu D, Yang Y, Shi R, Dong W. Comparative Metabolome Profiles and Antioxidant Potential of Four Coffea arabica L. Varieties Differing in Fruit Color. Diversity. 2023; 15(6):724. https://doi.org/10.3390/d15060724
Chicago/Turabian StyleHu, Faguang, Xiaofei Bi, Xingfei Fu, Yanan Li, Guiping Li, Yaqi Li, Dexin Liu, Yang Yang, Rui Shi, and Wenjiang Dong. 2023. "Comparative Metabolome Profiles and Antioxidant Potential of Four Coffea arabica L. Varieties Differing in Fruit Color" Diversity 15, no. 6: 724. https://doi.org/10.3390/d15060724
APA StyleHu, F., Bi, X., Fu, X., Li, Y., Li, G., Li, Y., Liu, D., Yang, Y., Shi, R., & Dong, W. (2023). Comparative Metabolome Profiles and Antioxidant Potential of Four Coffea arabica L. Varieties Differing in Fruit Color. Diversity, 15(6), 724. https://doi.org/10.3390/d15060724





