Scorpions, Science and Folklore in Durango City
Abstract
:1. Introduction
1.1. Durango Society Coexists with Scorpions
1.2. Scorpions’ Responses to Light
1.3. Venom Extraction and Preparation
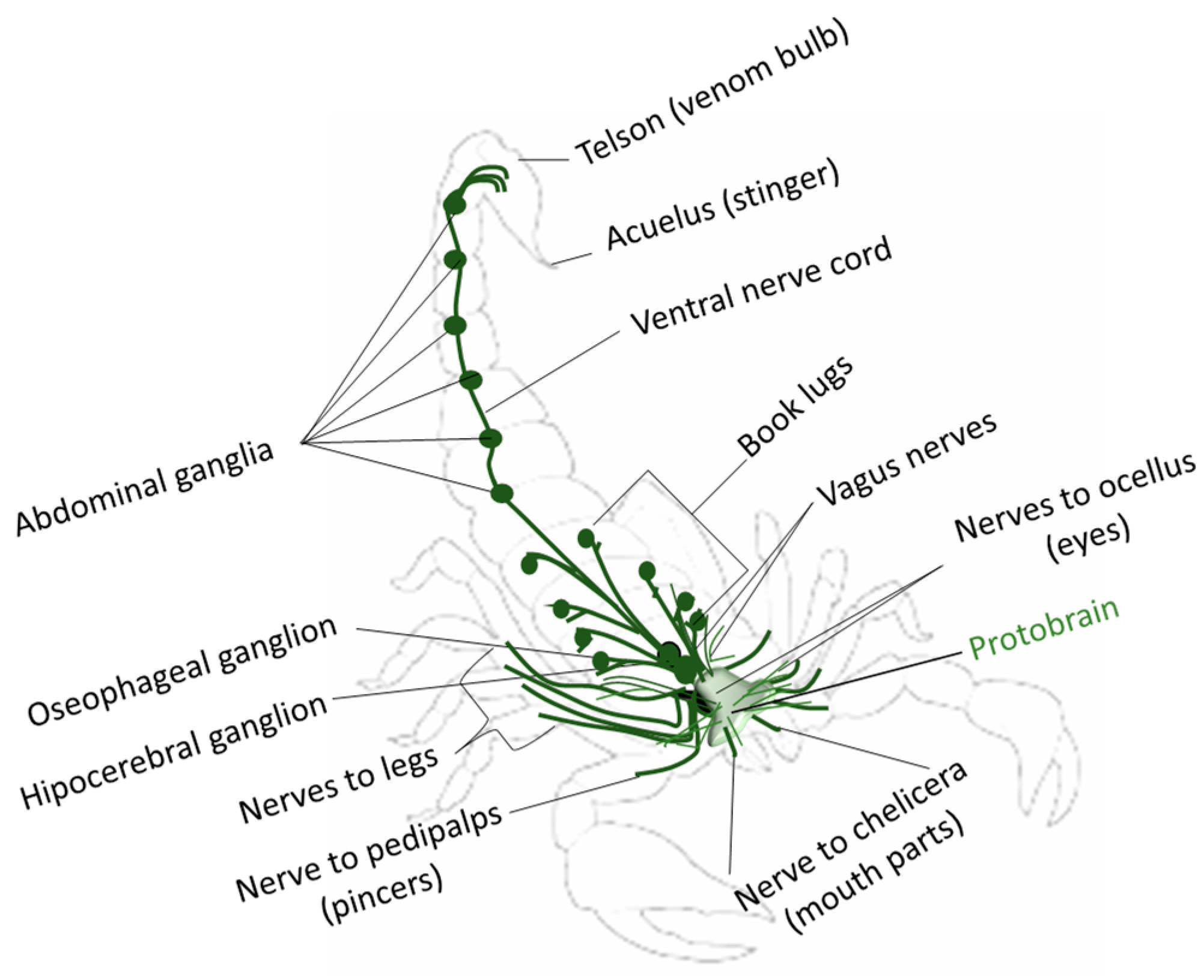
1.4. Biophysical, Biochemical, and Pharmacological Importance of the Centruroides suffusus Venom for Voltage-Gated Ion Channels
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wendru, A.J.; Babcock, L.E.; Wirkner, C.S.; Kluessendorf, J.; Mikulic, D.G. Open A Silurian ancestral scorpion with fossilised internal anatomy illustrating a pathway to arachnid terrestrialisation. Sci. Rep. 2020, 10, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellersztein, I.; Cohen, S.R.; Bar-on, B.; Wagner, H.D. Acta Biomaterialia The exoskeleton of scorpions pincers: Structure and micro-mechanical properties. Acta Biomater. 2019, 94, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, W.R.; Cloudsley-Thompson, J.L. The evolutionary significance of colour, colour patterns and fluorescence in scorpions. Rev. Suisse Zool. Hors Ser. 1996, 2, 449–458. [Google Scholar]
- Williams, S.C. Scorpion bionomics. Annu. Rev. Entomol. 1987, 32, 275–295. [Google Scholar] [CrossRef]
- Prendini, L. Order Scorpiones C.L. Koch, 1850. In: Zhang, Z.-Q. (Ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa 2011, 1850, 2005–2007. [Google Scholar] [CrossRef]
- Rein, J.O. The Scorpion Files. Norwegian University of Science and Technology. 2009. Available online: Http://www.Ub.Ntnu.No/Scorpion-Files (accessed on 9 January 2022).
- Santibáñez-lópez, C.E.; Francke, O.F.; Ureta, C.; Possani, L.D. Scorpions from Mexico: From Species Diversity to Venom Complexity. Toxins 2015, 8, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francke, O.F. Biodiversity of Arthropoda (Chelicerata: Arachnida ex Acari) in Mexico. Rev. Mex. Biodivers. 2014, 85, 408–418. [Google Scholar] [CrossRef] [Green Version]
- González-Santillán, E. Catálogo de Escorpiones de la Colección Nacional de Arácnidos (CNAN). Ph.D. Thesis, Facultad de Ciencias, Universidad Nacional Autónoma de México, Ciudad de México, Mexico, 2001. [Google Scholar]
- Lourenço, W.R.; Sissom, W.D. Scorpiones. Biodiversidad, Taxonomía y Biogeografía de Artrópodos de México: Hacia Una Síntesis de Su Conocimiento. Conabio 2000, 2, 115–135. [Google Scholar]
- Ponce-Saavedra, J.; Moreno-Barajas, R.J. El género Centruroides Marx 1890 (Scorpiones: Buthidae) en México. Biológicas 2005, 7, 42–51. [Google Scholar]
- Santibáñez-López, C.E.; Ponce-Saavedra, J. A new species of Centruroides (Scorpiones: Buthidae) from the northern mountain range of Oaxaca, Mexico. Rev. Mex. Biodivers. 2009, 80, 321–331. [Google Scholar]
- Jover, E.; Couraud, F.; Rochat, H. Two types of scorpion neurotoxins characterized by their binding to two separate receptor sites on rat brain synaptosomes. Biochem. Biophys. Res. Commun. 1980, 95, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Rochat, H.; Bernard, P.; Couraud, F. Scorpion toxins: Chemistry and mode of action. Adv. Cytopharmacol. 1979, 3, 325–334. [Google Scholar] [PubMed]
- Chippaux, J.P.; Celis, A.; Boyer, L.; Alagón, A. Factors involved in the resilience of incidence and decrease of mortality from scorpion stings in Mexico. Toxicon 2020, 188, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Abulude, F.O.; Ogunkoya, M.O.; Esiet, E.E.; Kayode, B.O.; Oni, J.O. Studies on Scorpion (Androctonus australis): Nutritional and Anti-nutritional Factors. J. Entomol. 2006, 3, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Goudarzi, H.R.; Salehi Najafabadi, Z.; Movahedi, A.; Noofeli, M. Bradykinin-Potentiating Factors of Venom from Iranian Medically Important Scorpions. Arch. Razi Inst. 2019, 74, 385–394. [Google Scholar]
- Martins, J.G.; Santos, G.C.; Procópio, R.E.D.L.; Arantes, E.C.; Bordon, K.D.C.F. Scorpion species of medical importance in the Brazilian Amazon: A review to identify knowledge gaps. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20210012. [Google Scholar] [CrossRef]
- Adams, A.M.; Marais, E.; Turner, J.S.; Prendini, L.; Pinshow, B. Similar burrow architecture of three arid-zone scorpion species implies similar ecological function. Sci. Nat. 2016, 103, 56. [Google Scholar] [CrossRef]
- Gaffin, D.D.; Muñoz, M.G.; Hoefnagels, M.H. Evidence of learning walks related to scorpion home burrow navigation. J. Exp. Biol. 2022, 225, jeb243947. [Google Scholar] [CrossRef]
- Smarandache-Wellmann, C.R. Arthropod neurons and nervous system. Curr. Biol. 2016, 26, R960–R965. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, G.; Hou, X.; Ma, X.; Edgecombe, G.D.; Strausfeld, N.J. Chelicerate neural ground pattern in a Cambrian great appendage arthropod. Nature 2013, 502, 364–367. [Google Scholar] [CrossRef]
- Prévost, E.D.; Stemme, T. Non-visual homing and the current status of navigation in scorpions. Anim. Cogn. 2020, 23, 1215–1234. [Google Scholar] [CrossRef]
- Loria, S.F.; Prendini, L. Homology of the lateral eyes of scorpiones: A six-ocellus model. PLoS ONE 2014, 9, e112913. [Google Scholar] [CrossRef] [PubMed]
- Locket, A. Scorpion Biology and Research; Brownell, P., Polis, G., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 79–106. [Google Scholar]
- Fleissner, G.; Fleissner, G. Night Vision in Desert Scorpions. In Scorpions 2001: In Memoriam Gary a Polis; British Arachnological Society: Burnham Beeches, UK, 2001; pp. 317–324. [Google Scholar]
- Giambelluca, F.L.; Osio, J.; Giambelluca, L.A.; Cappelletti, M.A. Novel scorpion detection system combining computer vision and fluorescence. arXiv 2021, arXiv:2108.04177. [Google Scholar]
- Darbaniyan, F.; Liu, L.; Sharma, F. Soft Matter Mechanics and the Mechanisms Underpinning the Infrared Vision of Snakes. 2020. Available online: https://ssrn.com/abstract=3606795 (accessed on 18 October 2022).
- Horn, A.C.M.; Chaval, A. The gross anatomy of the nervous system of Bothriurus bonariensis (L. C. KOCH, 1842) (Scorpiones, Bothriuridae). Braz. J. Biol. 2002, 62, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Ashfford, K.; Blankenship, R.; Carpenter, W.; Wheeler, I.; Gaffin, D. Response of the eastern sand scorpion, Paruroctonus utahensis, to air movement from a moth analog. J. Arachnol. 2018, 46, 226–230. [Google Scholar] [CrossRef]
- Pimenta Murayama, G.L.; Hirata Willemart, R. Are trichobothria used in terrestrial prey capture by the yellow scorpion Tityus serrulatus Lutz, Mello, 1922 (Buthidae)? Arachnology 2019, 18, 287–290. [Google Scholar] [CrossRef]
- Geethabali, K.P.R. A Metasomatic Neural Photoreceptor in the Scorpion. J. Exp. Biol. 1973, 58, 189–196. [Google Scholar]
- Lawrence, R.F. Fluorescence in Arthropoda. J. Entomol. Soc. S. Afr. 1954, 17, 167–170. [Google Scholar]
- Pavan, M. Studi sugli Scorpioni: I.-Una nuova caratteristica tipica del tegumento degli Scorpioni. Ital. J. Zool. 1954, 21, 283–291. [Google Scholar] [CrossRef]
- Graham, M.R. Malformed pedipalp finger dentition of the scorpion Superstitionia donensis (Scorpiones: Superstitioniidae). Euscorpius 2006, 42, 1–4. [Google Scholar] [CrossRef]
- Stahnke, H.L. Scorpion Nomenclature And Mensuration. Entomol. News 1970, 81, 297–316. [Google Scholar]
- Williams, S.C. Developmental anomalies in scorpion centruroides-sculpturatus (Scorpionida-buthidae). Pan-Pac. Entomol. 1971, 47, 76. [Google Scholar]
- Park, H.B.; Lam, Y.C.; Gaffney, J.P.; Weaver, J.C.; Krivoshik, S.R.; Hamchand, R.; Pieribone, V.; Gruber, D.F.; Crawford, J.M. Bright Green Biofluorescence in Sharks Derives from Bromo-Kynurenine Metabolism. IScience 2019, 19, 1291–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stachel, S.J.; Stockwell, S.A.; Van Vranken, D.L. The fluorescence of scorpions and cataractogenesis. Chem. Biol. 1999, 6, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloock, C.T.; Kubli, A.; Reynolds, R. Ultraviolet light detection: A function of scorpion fluorescence. J. Arachnol. 2010, 38, 441–445. [Google Scholar] [CrossRef]
- Lim, M.L.M.; Land, M.F.; Li, D. Sex-specific UV and fluorescence signals in jumping spiders. Science 2007, 315, 481. [Google Scholar] [CrossRef] [Green Version]
- Sparks, J.S.; Schelly, R.C.; Smith, W.L.; Davis, M.P.; Tchernov, D.; Pieribone, V.A.; Gruber, D.F. The Covert World of Fish Biofluorescence: A Phylogenetically Widespread and Phenotypically Variable Phenomenon. PLoS ONE 2014, 9, e83259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, V.L.; Van Hooijdonk, E.; Intrater, N.; Vigneron, J.P. Fluorescence in Insects. The Nature of Light: Light in Nature. Photo-Optical Instrumentation Engineers Photo-optical Instrumentation. Engineers 2012, 8480, 848004. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chiu, P.J.; Lee, C.C. Fluorescence and multilayer structure of the scorpion cuticle. In Optical Systems Design 2015: Illumination Optics IV; SPIE Press: Bellingham, WA, USA, 2015; Volume 9629, pp. 108–111. [Google Scholar]
- Cloudsley-Thompson, J.L.; Constantinou, C. Biological clocks in desert beetles (Tenebrionidae), with special reference to Erodius octocostatus Peyerimhof in Kuwait. J. Univ. Kuwait (Sci.) 1985, 12, 237–243. [Google Scholar]
- Frost, L.M.; Butler, D.R.; O’Dell, B.; Fet, V.A. A Coumarin as a Fluorescent Compound in Scorpion Cuticle. In Scorpions, In Memoriam Gary A. Polis; Fet, V., Selden, P.A., Eds.; British Arachnological Society: Burnham Beeches, UK, 2001; pp. 365–368. [Google Scholar]
- Yoshimoto, Y.; Tanaka, M.; Miyashita, M.; Abdel-Wahab, M.; Megaly, A.M.A.; Nakagawa, Y.; Miyagawa, H. A Fluorescent Compound from the Exuviae of the Scorpion, Liocheles australasiae. J. Nat. Prod. 2020, 83, 542–546. [Google Scholar] [CrossRef]
- Rodríguez-Ravelo, R.; Coronas, F.I.; Zamudio, F.Z.; González-Morales, L.; López, G.E.; Urquiola, A.R.; Possani, L.D. The Cuban scorpion Rhopalurus junceus (Scorpiones, Buthidae): Component variations in venom samples collected in different geographical areas. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucca, M.B.; Amorim, F.G.; Cerni, F.A.; de Castro Figueiredo Bordon, K.; Cardoso, I.A.; Anjolette, F.A.P.; Arantes, E.C. Influence of post-starvation extraction time and prey-specific diet in Tityus serrulatus scorpion venom composition and hyaluronidase activity. Toxicon 2014, 90, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnakone, P.; Cheah, J. Gwee MCE Black scorpion (Heterometrus longimanus) as a laboratory animal: Maintenance of a colony of scorpion for milking of venom for research, using a restraining device. Lab Anim. 1995, 29, 456–458. [Google Scholar] [CrossRef]
- van Cann, M.; Kuzmenkov, A.; Isensee, J.; Andreev-Andrievskiy, A.; Peigneur, S.; Khusainov, G.; Berkut, A.; Tytgat, J.; Vassilevski, A.; Hucho, T. Scorpion toxin MeuNaTxα-1 sensitizes primary nociceptors by selective modulation of voltage-gated sodium channels. Fed. Eur. Biochem. Soc. 2021, 288, 2418–2435. [Google Scholar] [CrossRef]
- Tobassum, S.; Tahir, H.M.; Zahid, M.T.; Gardner, Q.A.; Ahsan, M.M. Effect of Milking Method, Diet, and Temperature on Venom Production in Scorpions. J. Insect Sci. 2018, 18, 19. [Google Scholar] [CrossRef] [Green Version]
- Kamel, M.; Saile, R.; Tanane, O.; Kettani, A. The robotic scorpion venom extraction system. Rev. Rev. ’entrepreneuriat Et L’innovation 2022, IV, V4N14A2022. [Google Scholar]
- Ferreira, M.G.; Duarte, C.G.; Oliveira, M.S.; Castro, K.L.; Teixeira, M.S.; Reis, L.P.; Zambrano, J.A.; Kalapothakis, E.; Michel, A.F.; Soto-Blanco, B.; et al. Toxicity of crude and detoxified Tityus serrulatus venom in anti-venom-producing sheep. J. Vet. Sci. 2016, 17, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, A.K.; Kunnathodi, F.; Al Saadon, K.; Idris, M.M. Elemental analysis of scorpion venoms. J. Venom Res. 2016, 7, 16–20. [Google Scholar]
- Norma Oficial Mexicana NOM-062-ZOO-1999, Especificaciones Técnicas para la Producción, Cuidado y uso de los Animales de Laboratorio. Available online: http://www.sagarpa.gob.mx/Dgg/NOM/062zoo.pdf (accessed on 2 October 2022).
- Dehghani, R. Scorpions and Scorpion Sting (Biology, Ecology and Control of Them); Esfahan Beautiful Arts; Publications of Kashan University of Medical Sciences: Esfahan, Iran, 2006; p. 334. [Google Scholar]
- Dehghani, R.; Arani, M.G. Scorpion sting prevention and treatment in ancient Iran. J. Tradit. Complement. Med. 2015, 5, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Najmabadi, M. History of Medicine in Iran, 2nd ed.; Tehran University Press: Tehran, Iran, 1992. [Google Scholar]
- González, J.A.; Vallejo, J.R. The scorpion in Spanish folk medicine: A review of traditional remedies for stings and its use as a therapeutic resource. J. Ethnopharmacol. 2013, 146, 62–74. [Google Scholar] [CrossRef]
- Koppenhofer, E.; Schmidt, H. Incomplete sodium inactivation in nodes of ranvier treated with scorpion venom. Experientia 1967, 24, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Koppenhöfer, E.; Schmidt, H. Die Wirkung von Skorpiongift auf die Ionenströme des Ranvierschen Schnürrings. II. Unvollständiage Natrium-Inaktivierung Effect of scorpion venom on ionic currents of the node of Ranvier. II. Incomplete sodium inactivation. Pflug. Arch. 1968, 303, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.A. Physiological action of scorpion venom. Am. J. Trop. Med. Hyg. 1960, 9, 410–414. [Google Scholar] [CrossRef] [PubMed]
- d’Ajello, V.; Zlotkin, E.; Miranda, F.; Lissitzky, S.; Bettini, S. The effect of scorpion venom and pure toxins on the cockroach central nervous system. Toxicon 1972, 10, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, P.L.; Fletcher, M.; Fainter, L.K.; Terrian, D.M. Action of new world scorpion venom and its neurotoxins in secretion. Toxicon 1996, 34, 1399–1411. [Google Scholar] [CrossRef]
- Gwee, M.C.; Nirthanan, S.; Khoo, H.E.; Gopalakrishnakone, P.; Kini, R.M.; Cheah, L.S. Autonomic effects of some scorpion venoms and toxins. Clin. Exp. Pharmacol. Physiol. 2002, 29, 795–801. [Google Scholar] [CrossRef]
- Nencioni, A.L.A.; Beraldo Neto, E.; Freitas, L.A.D.; Dorce, V.A.C. Effects of Brazilian scorpion venoms on the central nervous system. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 3. [Google Scholar] [CrossRef] [Green Version]
- Possani, L.D.; Merino, E.; Corona, M.; Bolivar, F.; Becerril, B. Peptides and genes coding for scorpion toxins that affect ion-channels. Biochimie 2000, 82, 861–868. [Google Scholar] [CrossRef]
- Quintero-Hernández, V.; Jiménez-Vargas, J.M.; Gurrola, G.B.; Valdivia, H.H.; Possani, L. Scorpion venom components that affect ion-channels function. Toxicon 2013, 76, 328–342. [Google Scholar] [CrossRef] [Green Version]
- Almaaytah, A.; Albalas, Q. Scorpion venom peptides with no disulfide bridges: A review. Peptides 2014, 51, 35–45. [Google Scholar] [CrossRef]
- Lecomte, C.; Sabatier, J.M.; Van Rietschoten, J.; Rochat, H. Synthetic peptides as tools to investigate the structure and pharmacology of potassium channel-acting short-chain scorpion toxins. Biochimie 1998, 80, 151–154. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, R.; Reinhart, P.H.; White, M.M. Charybdotoxin block of Shaker K+ channels suggests that different types of K+ channels share common structural features. Neuron 1988, 10, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Possani, L.D.; Becerril, B.; Delepierre, M.; Tytgat, J. Scorpion toxins specific for Na+-channels. Eur. J. Biochem. 1999, 264, 287–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cestèle, S.; Yarov-Yarovoy, V.; Qu, Y.; Sampieri, F.; Scheuer, T.; Catterall, W.A. Structure and function of the voltage sensor of sodium channels probed by a beta-scorpion toxin. J. Biol. Chem. 2006, 281, 21332–21344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezanilla, F.; Stefani, E. Gating currents. Methods Enzym. 1998, 293, 331–352. [Google Scholar] [CrossRef] [Green Version]
- Stefani, E.; Bezanilla, F. Cut-open oocyte voltage-clamp technique. Methods Enzym. 1998, 293, 300–318. [Google Scholar]
- Carbone, E.; Wanke, E.; Prestipino, G.; Possani, L.D.; Maelicke, A. Selective blockage of voltage-dependent K+ channels by a novel scorpion toxin. Nature 1982, 296, 90–91. [Google Scholar] [CrossRef]
- Olamendi-Portugal, T.; Restano-Cassulini, R.; Riaño-Umbarila, L.; Becerril, B.; Possani, L.D. Functional and immuno-reactive characterization of a previously undescribed peptide from the venom of the scorpion Centruroides limpidus. Peptides 2017, 87, 34–40. [Google Scholar] [CrossRef]
- García-Guerrero, I.A.; Cárcamo-Noriega, E.; Gómez-Lagunas, F.; González-Santillán, E.; Zamudio, F.Z.; Gurrola, G.B.; Possani, L.D. Biochemical characterization of the venom from the Mexican scorpion Centruroides ornatus, a dangerous species to humans. Toxicon 2020, 173, 27–38. [Google Scholar] [CrossRef]
- Rodríguez-Rangel, S.; Bravin, A.D.; Ramos-Torres, K.M.; Brugarolas, P.; Sánchez-Rodríguez, J.E. Structure-activity relationship studies of four novel 4-aminopyridine K+ channel blockers. Sci. Rep. 2020, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.F.; Garcia y Perez, L.G.; el Ayeb, M.; Kopeyan, C.; Bechis, G.; Jover, E.; Rochat, H. Purification and chemical and biological characterizations of seven toxins from the Mexican scorpion, Centruroides suffusus suffusus. J. Biol. Chem. 1987, 262, 4452–4459. [Google Scholar] [CrossRef] [PubMed]
- Espino-Solis, G.P.; Estrada, G.; Olamendi-Portugal, T.; Villegas, E.; Zamudio, F.; Cestele, S.; Possani, L.D.; Corzo, G. Isolation and molecular cloning of beta-neurotoxins from the venom of the scorpion Centruroides suffusus suffusus. Toxicon 2011, 57, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.; Villegas, E.; Espino-Solis, G.P.; Rodriguez, A.; Paniagua-Solis, J.F.; Sandoval-Lopez, G.; Possani, L.D.; Corzo, G. Antimicrobial peptides from arachnid venoms and their microbicidal activity in the presence of commercial antibiotics. J. Antibiot. 2013, 66, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, T.M.; Quick, M.W.; Sakai, T.T.; Krishna, N.R. Expression of functional recombinant scorpion beta-neurotoxin Css II in, E. coli. Peptides 2000, 21, 767–772. [Google Scholar] [CrossRef]
- Smith, J.J.; Alphy, S.; Seibert, A.L.; Blumenthal, K.M. Differential phospholipid binding by site 3 and site 4 toxins. Implications for structural variability between voltage-sensitive sodium channel domains. J. Biol. Chem. 2005, 280, 11127–11133. [Google Scholar] [CrossRef] [Green Version]
- Bosmans, F.; Martin-Eauclaire, M.-F.; Tytgat, J. Differential effects of five ‘classical’ scorpion β-toxins on rNav1.2a and DmNav1 provide clues on species-selectivity. Toxicol. Appl. Pharmacol. 2007, 218, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Schiavon, E.; Pedraza-Escalona, M.; Gurrola, G.B.; Olamendi-Portugal, T.; Corzo, G.; Wanke, E.; Possani, L.D. Negative-shift activation, current reduction and resurgent currents induced by β-toxins from Centruroides scorpions in sodium channels. Toxicon 2012, 59, 283–293. [Google Scholar] [CrossRef]
- Saucedo, A.L.; del Rio-Portilla, F.; Picco, C.; Estrada, G.; Prestipino, G.; Possani, L.D.; Delepierre, M.; Corzo, G. Solution structure of native and recombinant expressed toxin CssII from the venom of the scorpion Centruroides suffusus suffusus, and their effects on Nav1.5 sodium channels. Biochim. Et Biophys. Acta 2012, 1824, 478–487. [Google Scholar] [CrossRef]
- Park, J.; Oh, J.H.; Kang, H.K.; Choi, M.C.; Seo, C.H.; Park, Y. Scorpion-Venom-Derived Antimicrobial Peptide Css54 Exerts Potent Antimicrobial Activity by Disrupting Bacterial Membrane of Zoonotic Bacteria. Antibiotics 2020, 9, 831. [Google Scholar] [CrossRef]
- Tuxpan-Pérez, A.; Ibarra-Valencia, M.A.; Estrada, B.E.; Clement, H.; Corrales-García, L.L.; Espino-Solis, G.P.; Corzo, G. Antimicrobial and Immunomodulatory Effects of Selected Chemokine and Antimicrobial Peptide on Cytokine Profile during Salmonella Typhimurium Infection in Mouse. Antibiotics 2022, 11, 607. [Google Scholar] [CrossRef]
- Dai, C.; Ma, Y.; Zhao, Z.; Zhao, R.; Wang, Q.; Wu, Y.; Cao, Z.; Li, W. Mucroporin, the first cationic host defense peptide from the venom of Lychas mucronatus. Antimicrob. Agents Chemother. 2008, 52, 3967–3972. [Google Scholar] [CrossRef] [Green Version]
- 131-I-TM-601 Study in Adults with Recurrent High-Grade Glioma—Phase 2. Available online: https://clinicaltrials.gov/ct2/show/NCT00114309 (accessed on 26 March 2023).
- King, J.V.L.; Emrick, J.J.; Kelly, M.J.S.; Herzig, V.; King, G.F.; Medzihradszky, K.F.; Julius, D. A Cell-Penetrating Scorpion Toxin Enables Mode-Specific Modulation of TRPA1 and Pain. Cell 2019, 178, 1362–1374.e16. [Google Scholar] [CrossRef]
- Hakim, M.A.; Jiang, W.; Luo, L.; Li, B.; Yang, S.; Song, Y.; Lai, R. Scorpion Toxin, BmP01, Induces Pain by Targeting TRPV1 Channel. Toxins 2015, 7, 3671–3687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.Y.; Mi, Z.M.; Cheng, G.F.; Shen, W.Q.; Xiao, X.; Liu, X.M.; Liang, X.T.; Yu, D.Q. Purification and characterization of a new peptide with analgesic effect from the scorpion Buthus martensi Karch. J. Pept. Res. 2004, 64, 33–41. [Google Scholar] [CrossRef]
- Guan, R.J.; Wang, C.G.; Wang, M.; Wang, D.C. A depressant insect toxin with a novel analgesic effect from scorpion Buthus martensii Karsch. Biochim. Et Biophys. Acta Protein Struct. Mol. Enzymol. 2001, 1549, 9–18. [Google Scholar] [CrossRef]
- Zeng, X.C.; Wang, S.X.; Zhu, Y.; Zhu, S.Y.; Li, W.X. Identification and functional characterization of novel scorpion venom peptides with no disulfide bridge from Buthus martensii. Peptides 2004, 25, 143–150. [Google Scholar] [CrossRef]
- Cao, Z.; Di, Z.; Wu, Y.; Li, W. Overview of scorpion species from china and their toxins. Toxins 2014, 6, 796–815. [Google Scholar] [CrossRef] [Green Version]
- Harrison, P.L.; Abdel-Rahman, M.A.; Miller, K.; Strong, P.N. Antimicrobial peptides from scorpion venoms. Toxicon 2014, 88, 115–137. [Google Scholar] [CrossRef]
- Fan, Z.; Cao, L.; He, Y.; Hu, J.; Di, Z.; Wu, Y.; Li, W.; Cao, Z. Ctriporin, a new anti–methicillin–resistant Staphylococcus aureus peptide from the venom of the scorpion Chaerilus tricostatus. Antimicrob. Agents Chemother. 2011, 55, 5220–5229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roger, S.; Rollin, J.; Barascu, A.; Besson, P.; Raynal, P.I.; Iochmann, S.; Lei, M.; Bougnoux, P.; Gruel, Y.; Le Guennec, J.Y. Voltage-gated sodium channels potentiate the invasive capacities of human non-small-cell lung cancer cell lines. Int. J. Biochem. Cell Biol. 2007, 39, 774–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roger, S.; Rollin, J.; Barascu, A.; Besson, P.; Raynal, P.I.; Iochmann, S.; Lei, M.; Bougnoux, P.; Gruel, Y.; Le Guennec, J.Y. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discov. 2013, 3, 1364–1377. [Google Scholar]
- Bechohra, L.; Laraba-Djebari, F.; Hammoudi-Triki, D. Cytotoxic activity of Androctonus australis venom and its toxic fractions on human lung cancer cell line. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong-ngam, P.; Roytrakul, S.; Sritanaudomchai, H. BmKn-2 scorpion venom peptide for killing oral cancer cells by apoptosis. Asian Pacific journal of cancer preven tion. Asian Pac. J. Cancer Prev. 2015, 16, 2807–2811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dardevet, L.; Rani, D.; Aziz, T.A.; Bazin, I.; Sabatier, J.M.; Fadl, M.; Brambilla, E.; De Waard, M. Chlorotoxin: A helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins 2015, 7, 1079–1101. [Google Scholar] [CrossRef] [Green Version]
- BenAissa, R.; Othman, H.; Villard, C.; Peigneur, S.; Mlayah-Bellalouna, S.; Abdelkafi-Koubaa, Z.; Marrakchi, N.; Essafi-Benkhadir, K.; Tytgat, J.; Luis, J. Srairi-Abid N AaHIV a sodium channel scorpion toxin inhibits the proliferation of DU145 prostate cancer cells. Biochem. Biophys. Res. Commun. 2019, 521, 340–346. [Google Scholar] [CrossRef]
- Shao, J.H.; Cui, Y.; Zhao, M.Y.; Wu, C.F.; Liu, Y.F.; Zhang, J.H. Purification, characterization, and bioactivity of a new analgesic-antitumor peptide from Chinese scorpion Buthus martensii Karsch. Peptides 2014, 53, 89–96. [Google Scholar] [CrossRef]
- Satitmanwiwat, S.; Changsangfa, C.; Khanuengthong, A.; Promthep, K.; Roytrakul, S.; Arpornsuwan, T.; Saikhun, K.; Sritanaudomchai, H. The scorpion venom peptide BmKn2 induces apoptosis in cancerous but not in normal human oral cells. Biochem. Biophys. Res. Commun. 2016, 84, 1042–1050. [Google Scholar] [CrossRef]
- Khamessi, O.; Ben Mabrouk, H.; ElFessi-Magouri, R.; Kharrat, R. RK1, the first very short peptide from Buthus occitanus tunetanus inhibits tumor cell migration, proliferation and angiogenesis. Biochem. Biophys. Res. Commun. 2018, 499, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lansu, K.; Gentile, S. Potassium channel activation inhibits proliferation of breast cancer cells by activating a senescence program. Cell Death Dis. 2013, 4, e652. [Google Scholar] [CrossRef] [Green Version]
- Perez-Neut, M.; Rao, V.R.; Gentile, S. hERG1/Kv11.1 activation stimulates transcription of p21waf/cip in breast cancer cells via a calcineurin-dependent mechanism. Oncotarget 2016, 7, 58893–58902. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, G. Insights into Antimicrobial Peptides from Spiders and Scorpions. Protein Pept. Lett. Publ. Lett. 2016, 23, 707–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Melo, E.T.; Estrela, A.B.; Santos, E.C.; Machado, P.R.; Farias, K.J.; Torres, T.M.; Carvalho, E.; Lima, J.P.; Silva-Júnior, A.A.; Barbosa, E.G.; et al. Structural characterization of a novel peptide with antimicrobial activity from the venom gland of the scorpion Tityus stigmurus: Stigmurin. Peptides 2015, 68, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Q.; Hou, X.; Wang, L.; Zhang, Y.; Xi, X.; Wang, H.; Zhou, M.; Duan, J.; Wei, M.; Chen, T.; et al. AaeAP1 and AaeAP2: Novel Antimicrobial Peptides from the Venom of the Scorpion, Androctonus aeneas: Structural Characterisation, Molecular Cloning of Biosynthetic Precursor-Encoding cDNAs and Engineering of Analogues with Enhanced Antimicrobial and Anticancer Activities. Toxins 2015, 7, 219–237. [Google Scholar] [PubMed] [Green Version]
- Machado, R.J.A.; Estrela, A.B.; Nascimento, A.K.L.; Melo, M.M.A.; Torres-Rêgo, M.; Lima, E.O.; Rocha, H.A.O.; Carvalho, E.; Silva-Junior, A.A.; Fernandes-Pedrosa, M.F. Characterization of TistH, a multifunctional peptide from the scorpion Tityus stigmurus: Structure, cytotoxicity and antimicrobial activity. Toxicon 2016, 119, 362–370. [Google Scholar] [CrossRef]
- Guilhelmelli, F.; Vilela, N.; Smidt, K.S.; de Oliveira, M.A.; da Cunha Morales Álvares, A.; Rigonatto, M.C.L.; da Silva Costa, P.H.; Tavares, A.H.; de Freitas, S.M.; Nicola, A.M. Activity of Scorpion Venom-Derived Antifungal Peptides against Planktonic Cells of Candida spp. and Cryptococcus neoformans and Candida albicans Biofilms. Front. Microbiol. 2016, 7, 1844. [Google Scholar] [CrossRef] [Green Version]
- Santussi, W.M.; Bordon, K.C.F.; Rodrigues Alves, A.P.N.; Cologna, C.T.; Said, S.; Arantes, E.C. Antifungal Activity against Filamentous Fungi of Ts1, a Multifunctional Toxin from Tityus serrulatus Scorpion Venom. Front. Microbiol. 2017, 8, 984. [Google Scholar] [CrossRef] [Green Version]
- Conde, R.; Zamudio, F.Z.; Rodríguez, M.H.; Possani, D. Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett. 2000, 471, 165–168. [Google Scholar] [CrossRef] [Green Version]
- Carballar-Lejarazú, R.; Rodríguez, M.H.; de la Cruz Hernández-Hernández, F.; Ramos-Castañeda, J.; Possani, L.D.; Zurita-Ortega, M.; Reynaud-Garza, E.; Hernández-Rivas, R.; Loukeris, T.; Lycett, G.; et al. Recombinant scorpine: A multifunctional antimicrobial peptide with activity against different pathogens. Cell. Mol. Life Sci. 2008, 65, 3081–3092. [Google Scholar] [CrossRef]
- Gao, B.; Xu, J.; Rodriguez, M.d.C.; Lanz-Mendoza, H.; Hernández-Rivas, R.; Du, W.; Zhu, S. Characterization of two linear cationic antimalarial peptides in the scorpion Mesobuthus eupeus. Biochimie 2010, 92, 350–359. [Google Scholar] [CrossRef]
- Flores-Solis, D.; Toledano, Y.; Rodríguez-Lima, O.; Cano-Sánchez, P.; Ramírez-Cordero, B.E.; Landa, A.; de la Vega, R.C.R.; del Rio-Portilla, F. Solution structure and antiparasitic activity of scorpine-like peptides from Hoffmannihadrurus gertschi. FEBS Lett. 2016, 590, 2286–2296. [Google Scholar] [CrossRef]
- Borges, A.; Silva, S.; Op den Camp, H.J.M.; Velasco, E.; Alvarez, M.; Alfonzo, M.J.M.; Jorquera, A.; De Sousa, L.; Delgado, O. In vitro leishmanicidal activity of Tityus discrepans scorpion venom. Parasitol. Res. 2006, 99, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Scholl, U.I.; Goh, G.; Stölting, G.; de Oliveira, R.C.; Choi, M.; Overton, J.D.; Fonseca, A.L.; Korah, R.; Starker, L.F.; Kunstman, J.W.; et al. Somatic and germline CACNA1D cal cium channel mutations in aldosterone-producing adeno mas and primary aldosteronism. Nat. Genet. 2013, 45, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhao, J.; Qiao, W.; Chen, K. Recent advances in diagnosis and treatment of gliomas using chlorotoxin-based bioconjugates. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 385–405. [Google Scholar] [PubMed]
- Diss, J.K.; Stewart, D.; Pani, F.; Foster, C.S.; Walker, M.M.; Patel, A.; Djamgoz, M.B. A potential novel marker for human prostate cancer: Voltage-gated sodium channel expression in vivo. Prostate Cancer Prostatic Dis. 2005, 8, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, T.; Kubota, N.; Tsutsumi, T.; Oguri, A.; Imuta, H.; Jo, T.; Oonuma, H.; Soma, M.; Meguro, K.; Takano, H.; et al. Eicosapentaenoic acid inhibits volt age gated sodium channels and invasiveness in prostate cancer cells. Br. J. Pharmacol. 2009, 156, 420–431. [Google Scholar] [CrossRef] [Green Version]
- Al-Asmari, A.K.; Islam, M.; Al-Zahrani, A.M. In vitro analysis of the anticancer properties of scorpion venom in colorectal and breast cancer cell lines. Oncol. Lett. 2016, 11, 1256–1262. [Google Scholar] [CrossRef] [Green Version]
- El-Ghlban, S.; Kasai, T.; Shigehiro, T.; Yin, H.X.; Sekhar, S.; Ida, M.; Sanchez, A.; Mizutani, A.; Kudoh, T.; Murakami, H.; et al. Chlorotoxin-Fc fusion inhibits release of MMP-2 from pancreatic cancer cells. BioMed Res. Int. 2014, 2014, 152659. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zhao, Z.; Zhou, D.; Chen, Y.; Hong, W.; Cao, L.; Yang, J.; Zhang, Y.; Shi, W.; Cao, Z.; et al. Virucidal activity of a scorpion venom peptide variant mucroporin-M1 against measles, SARS-CoV and influenza H5N1 viruses. Peptides 2011, 32, 1518–1525. [Google Scholar] [CrossRef]
- Zhao, Z.; Hong, W.; Zeng, Z.; Wu, Y.; Hu, K.; Tian, X.; Li, W.; Cao, Z. Mucroporin-M1 Inhibits Hepatitis B Virus Replication by Activating the Mitogen-activated Protein Kinase (MAPK) Pathway and Down-regulating HNF4α in Vitro and in Vivo. J. Biol. Chem. 2012, 287, 30181–30190. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Lyu, P.; Xi, X.; Ge, L.; Mahadevappa, R.; Shaw, C.; Kwok, H.F. Triggering of cancer cell cycle arrest by a novel scorpion venom-derived peptide—Gonearrestide. J. Cell. Mol. Med. 2018, 22, 4460–4473. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Zhang, R.; Hong, W.; Cheng, Y.; Wang, H.; Lang, Y.; Ji, Z.; Wu, Y.; Li, W.; Xie, Y. Histidine-rich Modification of a Scorpion-derived Peptide Improves Bioavailability and Inhibitory Activity against HSV-1. Theranostics 2018, 8, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Deng, Y.Q.; Zou, P.; Wang, Q.; Dai, Y.; Yu, F.; Du, L.; Zhang, N.N.; Tian, M.; Hao, J.N.; et al. A peptide-based viral inactivator inhibits Zika virus infection in pregnant mice and fetuses. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Ji, Z.; Li, F.; Xia, Z.; Guo, X.; Gao, M.; Sun, F.; Cheng, Y.; Wu, Y.; Li, W.; Ali, S.A.; et al. The Scorpion Venom Peptide Smp76 Inhibits Viral Infection by Regulating Type-I Interferon Response. Virol. Sin. 2018, 33, 545–556. [Google Scholar] [CrossRef]
- El-Bitar, A.M.; Sarhan, M.M.; Aoki, C.; Takahara, Y.; Komoto, M.; Deng, L.; Moustafa, M.A.; Hotta, H. Virocidal activity of Egyptian scorpion venoms against hepatitis C virus. Virol. J. 2015, 12, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabihollahi, R.; Pooshang Bagheri, K.; Keshavarz, Z.; Motevalli, F.; Bahramali, G.; Siadat, S.D.; Momen, S.B.; Shahbazzadeh, D.; Aghasadeghi, M.R. Venom components of Iranian scorpion Hemiscorpius lepturus inhibit the growth and replication of human immunodeficiency virus 1 (HIV-1). Iran. Biomed. J. 2016, 20, 259–265. [Google Scholar]
- Deng, S.Q.; Chen, J.T.; Li, W.W.; Chen, M.; Peng, H.J. Application of the Scorpion Neurotoxin AaIT against Insect Pests. Int. J. Mol. Sci. 2019, 20, 3467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, D. A new approach to insect-pest control—combination of neurotoxins interacting with voltage sensitive sodium channels to increase selectivity and specificity. Invertebr. Neurosci. 1997, 3, 103–116. [Google Scholar] [CrossRef]
- Attarde, S.S.; Pandit, S.V. Scorpion venom as therapeutic agent-current perspective. Int. J. Curr. Pharm. Res. 2016, 7, 59–72. [Google Scholar]
- Crest, M. Kaliotoxin, a novel peptidyl inhibitor of neur onal BK-type Ca(2þ)-activated K þ channels characterized from Androctonus mauretanicus mauretanicus venom. J. Biol. Chem. 1992, 267, 1640–1647. [Google Scholar] [CrossRef]
- Chen, R.; Chung, S.H. Engineering a potent and specific blocker of voltage-gated potassium channel Kv1.3, a target for autoimmune diseases. Biochemistry 2012, 51, 1976–1982. [Google Scholar] [CrossRef]
- Adi-Bessalem, S.; Hammoudi-Triki, D.; Laraba-Djebari, F. Pathophysiological effects of Androctonus australis hector scorpion venom: Tissue damages and inflammatory response. Exp. Toxicol. Pathol. 2008, 60, 373–380. [Google Scholar] [CrossRef]
- Adi-Bessalem, S.; Hammoudi-Triki, D.; Laraba-Djebari, F. Scorpion Venom Interactions with the Immune System; Gopalakrishnakone, P., Possani, L.D.F., Schwartz, E., Rodríguez de la Vega, R.C., Eds.; Scorpion Venoms; Springer: Dordrecht, The Netherlands, 2015; pp. 87–107. [Google Scholar]
- Pucca, M.B.; Cerni, F.A.; Cordeiro, F.A.; Peigneur, S.; Cunha, T.M.; Tytgat, J.; Arantes, E.C. Ts8 scorpion toxin inhibits the Kv4.2 channel and produces nociception in vivo. Toxicon 2016, 119, 244–252. [Google Scholar] [CrossRef]
- Hmed, B.N.; Serria, H.T.; Mounir, Z.K. Scorpion peptides: Potential use for new drug development. J. Toxicol. 2013, 2013, 958797. [Google Scholar] [CrossRef]
- Lu, X.; Lu, D.; Scully, M.F.; Kakkar, V.V. Integrins in drug targeting-RGD templates in toxins. Curr. Pharm. Des. 2006, 12, 2749–2769. [Google Scholar] [CrossRef] [PubMed]
- McLane, M.A.; Joerger T Mahmoud, A. Disintegrins in health and disease. Front. Biosci. A J. Virtual Libr. 2008, 13, 6617–6637. [Google Scholar] [CrossRef] [PubMed]
- Hasan, H.F.; Radwan, R.R.; Galal, S.M. Bradykinin-potentiating factor isolated from Leiurus quinquestriatus scorpion venom alleviates cardiomyopathy in irradiated rats via remodelling of the RAAS pathway. Clin. Exp. Pharmacol. Physiol. 2020, 47, 263–273. [Google Scholar] [CrossRef]
- Rocha-Resende, C.; Leão, N.M.; de Lima, M.E.; Santos, R.A.; Pimenta, A.M.C.; Verano-Braga, T. Moving pieces in a cryptomic puzzle: Cryptide from Tityus serrulatus Ts3 Nav toxin as potential agonist of muscarinic receptors. Peptides 2017, 98, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.M.; Lan, Z.D.; Wang, M.; Liu, B.; Liu, X.Q.; Fei, H.; Xu, L.G.; Xia, Q.C.; Wang, C.G.; Wang, D.C.; et al. Molecular characterization of a new excitatory insect neurotoxin with an analgesic effect on mice from the scorpion Buthus martensi Karsch. Toxicon 1999, 37, 1165–1180. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ji, Y. Antihyperalgesia effect of BmK AS, a scorpion toxin, in rat by intraplantar injection. Brain Res. 2002, 952, 322–326. [Google Scholar] [CrossRef]
- Joseph, B.; George, J. Scorpion toxins and its applications. Int. J. Toxicol. Pharmacol. Res. 2012, 4, 57–61. [Google Scholar]
- Xie, J.; Herbert, T.P. The role of mammalian target of rapamycin (mTOR) in the regulation of pancreatic b-cellmass: Implications in the development of type-2 diabetes. Cell. Mol. Life Sci. CMLS 2012, 69, 1289–1304. [Google Scholar] [CrossRef] [PubMed]
- Bouafir, Y.; Ait-Lounis, A.; Laraba-Djebari, F. Improvement of function and survival of pancreatic betacells in streptozotocin-induced diabetic model by the scorpion venom fraction F1. Toxin Rev. 2016, 36, 1–8. [Google Scholar]
- Elshater, A.-E.; Salman, M.; Abd-Elhady, A. Physiological studies on the effect of a bradykinin potentiating factor (BPF) isolated from scorpion venom on the burnt skin of alloxan-induced diabetic Guinea pigs. Egypt. Acad. J. Biol. Sci. C Physiol. Mol. Biol. 2011, 3, 5–15. [Google Scholar] [CrossRef]
- Wang, C.G.; He, X.L.; Shao, F.; Liu, W.; Ling, M.H.; Wang, D.C.; Chi, C.W. Molecular characterization of an anti-epilepsy peptide from the scorpion Buthus martensi (Karsch). Eur. J. Biochem. 2001, 268, 2480–2485. [Google Scholar] [CrossRef] [Green Version]
- Villetti, G.; Bregola, G.; Bassani, F.; Bergamaschi, M.; Rondelli, I.; Pietra, C.; Simonato, M. Preclinical evaluation of CHF3381 as novel antiepileptic agent. Neuropharmacology 2001, 40, 866–878. [Google Scholar] [CrossRef]




| Uses | References |
|---|---|
| Analgesic | [93,94,95,96,97,98] |
| Antibacterial | [83,89,90,99,100] |
| Anticancer | [101,102,103,104,105,106,107,108,109,110,111] |
| Antifungal | [112,113,114,115,116,117] |
| Antiparasitic | [118,119,120,121,122] |
| Antitumoral | [123,124,125,126,127,128] |
| Antiviral | [129,130,131,132,133,134,135,136] |
| Insect pests | [137,138] |
| Treatment for autoimmune diseases | [139,140,141,142,143,144] |
| Treatment for cardiovascular diseases | [145,146,147,148] |
| Treatment for chronic pain | [149,150,151,152] |
| Treatment for diabetes | [153,154,155] |
| Treatment for epilepsy | [156,157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Ponce, E.; Rodríguez-Rangel, S.; Martinez, R.; Alvarado, A.; Ruiz-Baca, E.; Miranda, P.; Sánchez-Rodríguez, J.E.; Lopez-Rodriguez, A. Scorpions, Science and Folklore in Durango City. Diversity 2023, 15, 743. https://doi.org/10.3390/d15060743
Gonzalez-Ponce E, Rodríguez-Rangel S, Martinez R, Alvarado A, Ruiz-Baca E, Miranda P, Sánchez-Rodríguez JE, Lopez-Rodriguez A. Scorpions, Science and Folklore in Durango City. Diversity. 2023; 15(6):743. https://doi.org/10.3390/d15060743
Chicago/Turabian StyleGonzalez-Ponce, Eduardo, Sofia Rodríguez-Rangel, Raymundo Martinez, Adrian Alvarado, Estela Ruiz-Baca, Pablo Miranda, Jorge E. Sánchez-Rodríguez, and Angelica Lopez-Rodriguez. 2023. "Scorpions, Science and Folklore in Durango City" Diversity 15, no. 6: 743. https://doi.org/10.3390/d15060743
APA StyleGonzalez-Ponce, E., Rodríguez-Rangel, S., Martinez, R., Alvarado, A., Ruiz-Baca, E., Miranda, P., Sánchez-Rodríguez, J. E., & Lopez-Rodriguez, A. (2023). Scorpions, Science and Folklore in Durango City. Diversity, 15(6), 743. https://doi.org/10.3390/d15060743






